Abstract
Necroptosis is a caspase-independent, lytic form of programmed cell death whose errant activation has been widely implicated in many pathologies. The pathway relies on the assembly of the apical protein kinases, RIPK1 and RIPK3, into a high molecular weight cytoplasmic complex, termed the necrosome, downstream of death receptor or pathogen detector ligation. The necrosome serves as a platform for RIPK3-mediated phosphorylation of the terminal effector, the MLKL pseudokinase, which induces its oligomerization, translocation to, and perturbation of, the plasma membrane to cause cell death. Over the past 10 years, knowledge of the post-translational modifications that govern RIPK1, RIPK3 and MLKL conformation, activity, interactions, stability and localization has rapidly expanded. Here, we review current knowledge of the functions of phosphorylation, ubiquitylation, GlcNAcylation, proteolytic cleavage, and disulfide bonding in regulating necroptotic signaling. Post-translational modifications serve a broad array of functions in modulating RIPK1 engagement in, or exclusion from, cell death signaling, whereas the bulk of identified RIPK3 and MLKL modifications promote their necroptotic functions. An enhanced understanding of the modifying enzymes that tune RIPK1, RIPK3, and MLKL necroptotic functions will prove valuable in efforts to therapeutically modulate necroptosis.
Subject terms: Kinases, Proteomics, Cell biology
Facts
Post-translational modifications of the RIPK1 and RIPK3 kinases and MLKL pseudokinase regulate their localization, activity, conformation, oligomerization, and protein interactions
To date, principally activating modifications have been reported for RIPK3 and MLKL
Most of the reported post-translational modifications in the necroptosis pathway affect the apical kinase, RIPK1
RIPK1 post-translational modifications govern whether it participates in NF-κB, apoptotic or necroptotic signaling
Open questions
Are RIPK3 and MLKL disarmed by enzymes that remove activating post-translational marks?
What is the function of MLKL ubiquitylation?
Do kinases other than RIPK3 phosphorylate MLKL to promote necroptotic death, and what are their identities?
How do RIPK3 and MLKL modifications control their protein interactions and prompt cell death?
Introduction
Necroptosis is a caspase-independent, pro-inflammatory programmed cell death pathway that was first described towards the end of the 20th century [1–5]. The term ‘necroptosis’ was subsequently coined [6] to connote the better-understood programmed cell death pathway, apoptosis, to convey that necroptosis is similarly triggered by specific signals and is tightly regulated. Morphologically, apoptotic cells are marked by cell shrinkage, chromatin condensation, and cellular fragmentation [7] and, as a mechanism of normal cell development and homeostasis, it is considered immunologically silent [8]. On the contrary, necroptosis is characterized by organelle swelling, plasma membrane rupture and, more importantly, leakage of intracellular components known as damage-associated molecular patterns (DAMPs), which propagate secondary inflammatory responses [9, 10]. In keeping with its pro-inflammatory nature, necroptosis has likely evolved as an innate immunity mechanism to complement apoptosis as a means of eliminating pathogens, such as in scenarios where pathogen-encoded proteins block apoptosis [11, 12]. The idea that necroptosis arose as an ancestral host defense mechanism is supported by the discovery of viral and bacterial proteins that block necroptosis at various steps of the signaling pathway in animals [13–23], and the recent discovery of convergently-evolved pathway effectors that contribute to plant host defense [24].
Necroptosis is effected by receptor-interacting serine/threonine protein kinase (RIPK)-3 [25–27] and the terminal effector, the mixed-lineage kinase domain-like (MLKL) pseudokinase [28–30] (Fig. 1). Ligand binding to a variety of death receptors, such as tumor necrosis factor (TNF) receptor 1 (TNFR1), can induce necroptosis by prompting association and activation of their respective cytoplasmic adaptor proteins. In cellular contexts where the cellular inhibitors of apoptosis E3 ligase family (cIAPs) [31–33] or its downstream protein kinase TAK1 [34] and the extrinsic apoptosis effector, Caspase-8 [35–37], are disarmed or depleted, death receptor ligation promotes autophosphorylation of RIPK1 kinase and assembly into high molecular weight complexes with RIPK3. Such an assembly depends on the formation of functional amyloid via their RIP homotypic interaction motifs (RHIM) [38, 39]. Subsequently, RIPK3-mediated phosphorylation provokes the killing activity of MLKL [28, 30, 40] by prompting translocation to, and disruption of, the plasma membrane [40–44]. While the precise mechanisms underlying MLKL activation, translocation, and membrane compromise are still emerging (reviewed in ref. [45, 46]), the phosphorylation of MLKL on its pseudokinase domain activation loop (S345 in mouse; S358 in human MLKL) is considered the hallmark of its activation and has been widely used as a biomarker for necroptotic death [28, 40, 47, 48]. However, necroptosis also depends on multiple downstream checkpoints that can be blocked by necrosulfonamide (NSA) [40, 49], ESCRT-III [50], Golgi/microtubule/actin trafficking pathway inhibitors [51], and MLKL killer/four-helix bundle (4HB) domain binding monobodies [52], indicating that additional steps beyond MLKL phosphorylation itself are required to induce cell death.
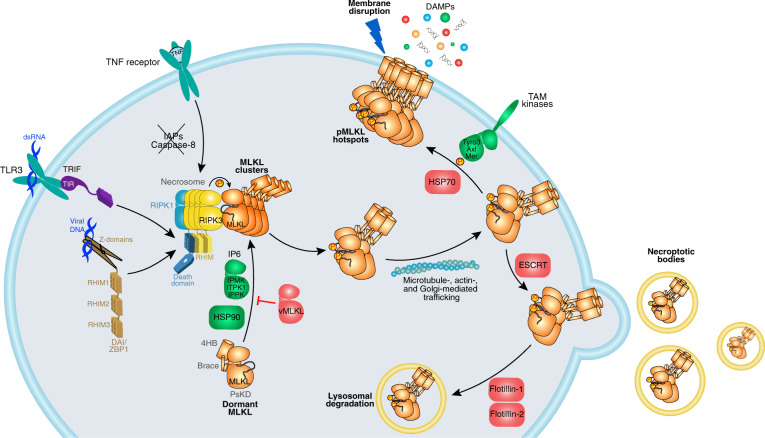
Fig. 1. Overview of the necroptosis pathway.
Necroptosis can be induced by various receptors (TNF-receptor, Toll-like receptors (TLRs), Z-DNA-binding protein 1 (ZBP1)/DAI) upon binding to their respective ligands. TNF-induced necroptosis requires the absence of cIAP and Casp8 activity. Under such conditions, RIPK1 and RIPK3 oligomerize as a RHIM-mediated amyloid fibril, resulting in RIPK3 activation by autophosphorylation, the recruitment of MLKL and its activation via RIPK3-mediated phosphorylation. Phosphorylated MLKL is thought to be activated by undergoing a conformational change to expose the killer 4HB domain [30, 43, 178, 186] and assemble into oligomers as necrosomal clusters before being trafficked to the plasma membrane via microtubule-, actin-, and Golgi-dependent mechanisms [51]. Activated MLKL accumulates into hotspots at the plasma membrane and, when a threshold is surpassed, disrupts the plasma membrane to cause death and the release of DAMPs [51]. This pathway is tightly regulated by various accessory components (positive regulators in green; negative regulators in red). Among these, certain inositol phosphates [196, 197], the chaperone HSP90 [198–200], and TAM kinases [187] promote necroptosis by positively regulating MLKL activation, translocation, and clustering at plasma membrane respectively. Conversely, necroptosis has been shown to be negatively regulated by blocking MLKL recruitment to RIPK3 (viral MLKL homolog [19]), preventing MLKL accumulation at plasma membrane (HSP70 [201]), and the removal of membrane bound MLKL (ESCRT [50, 202], flotillins [203]).
Therapeutic targeting of necroptosis in human disease
As an innate immune mechanism, necroptosis plays a key role in protection against pathogens in humans [13–17, 19, 21–23, 53, 54]. On the other hand, necroptosis has attracted much attention due to its dysregulation in a cornucopia of human inflammatory diseases. Hypo- and hyper-activation of necroptosis, as well as dysregulation of the balance between apoptosis and necroptosis, can contribute to disease pathologies (reviewed in ref. [55, 56]). Consequently, errant necroptosis has been implicated in the pathology of inflammatory syndromes [57], atherosclerosis [58], cardiac [59] and kidney [60] ischemia/reperfusion injury, sepsis [61–63], inflammatory bowel disease [64], neurodegenerative diseases [65], and cancer [66], although some of these attributions remain the matter of debate [62, 67–72]. In addition, damage caused by some bacterial infections have been proposed to be attributable to excessive necroptosis and inflammation [73, 74]. Taken together, ample evidence suggests that restricting necroptosis will serve as a useful therapeutic strategy for the treatment of various infectious and non-infectious inflammatory diseases.
Targeting the post-translational modifications that promote necroptotic cell death is an attractive avenue for clinical intervention in many diseases. To date, most effort in targeting the pathway has been focused on RIPK1 kinase—the apical, instigating kinase in necroptosis signaling—with several inhibitors progressing into early phase clinical trials (as reviewed in ref. [75]). The rationale for targeting RIPK1 is clear; patient variants in RIPK1 that prevent cleavage and thus negative regulation by Casp8 were reported to underlie an inflammatory disease termed CRIA (cleavage-resistant RIPK1-induced autoinflammatory syndrome) [37, 76, 77]. Furthermore, colitis patients harboring inactivating substitutions in NEMO exhibit elevated RIPK1 kinase activity and cell death [78], providing further support that targeting RIPK1 and the modifying enzymes that promote its necroptotic activity could be therapeutically advantageous. There has been some debate about whether targeting the downstream kinase, RIPK3, could be a viable therapeutic strategy, because in some contexts RIPK3 inhibitors and kinase-inactivating mutations appear to favor a kinase domain conformation that promotes constitutive apoptosis [79, 80], rather than simply inhibiting the catalytic activity of RIPK3 to prevent the phosphorylation and activation of the terminal effector, MLKL [81]. It is foreseeable that targeting MLKL could be advantageous because of MLKL’s specific role in executing necroptosis, although the only selective agent reported to date, NSA, is a covalent modifier of MLKL and is thus not suitable for clinical development [28]. Recent studies suggest that small molecules that target RIPK1, RIPK3, and MLKL simultaneously may prove an effective strategy in targeting necroptotic diseases pharmacologically [63].
Activation of necroptosis signaling downstream of receptors
A number of cell surface receptors can trigger necroptosis upon binding to their respective ligands, including TNFR1, interferon receptors (IFNRs), Toll-like receptors (TLRs), and DAI/ZBP1 (Fig. 1). While the adaptor proteins involved in each receptor complex vary depending on the type of receptor, they all signal through the necrosome to cause necroptotic death. The exact composition of the necrosome may also vary, but the core components comprise RIPK1, RIPK3, and MLKL. Necrosome assembly and MLKL activation occurs downstream of various stimuli, of which the TNFR1 pathway is the most well-studied. It is presumed that other death receptors function analogously, although like TLRs [82], it is possible distinct adaptor proteins connect the receptor signals to the necrosome. Activated IFNRs signal via JAK kinases and their cognate STAT proteins, leading to a transcriptional program that results in synthesis of necrosome modulator proteins [83]. An understanding of the connection between IFNR signaling and necroptosis is still in its infancy, but was reported to involve PKR kinase and the DAI/ZBP1 sensor protein [83, 84]. DAI/ZBP1 is best understood as a multi-RHIM-containing DNA sensing protein that detects, and is activated by, viral dsDNA. DAI/ZBP1 was proposed to activate RIPK3 directly via a RHIM–RHIM interaction [22] to promote MLKL phosphorylation and necroptotic cell death, which can be attenuated by RIPK1 sequestration of DAI/ZBP1 [85, 86].
Recent studies have revealed the existence of multiple signaling checkpoints that control necroptosis [19, 28, 30, 40, 42, 46, 51, 52, 87–89]. At the heart of these regulatory checkpoints lie many post-translational modifications that can promote (Fig. 2) or attenuate (Fig. 3) signaling flux via the necroptosis pathway. Here, we review our current understanding of the modifications that influence the activity, localization, and stability of the RIPK1, RIPK3, and MLKL: the core proteins of the necroptosis machinery.
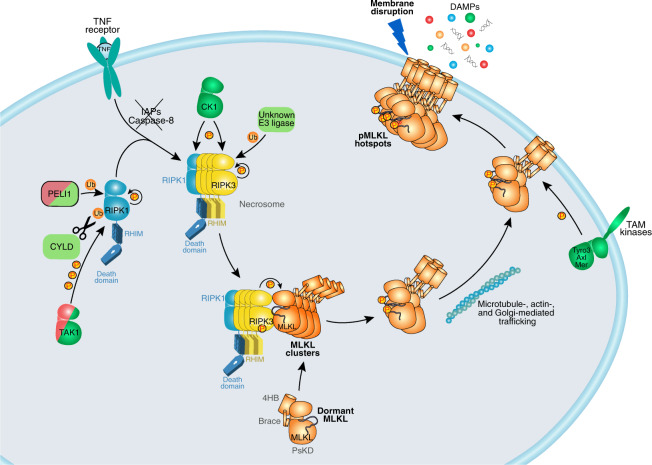
Fig. 2. Post-translational modifications positively regulate necroptosis.
RIPK1 is positively regulated by K63-linked ubiquitination (PELI1), removal of M1-linked ubiquitin chains (CYLD), and autophosphorylation. RIPK3 autophosphorylation is required for its interaction with, and phosphorylation of, MLKL. This is promoted by RIPK3 ubiquitination at K5, as well as CK1-mediated phosphorylation in some contexts. TAM kinase-mediated phosphorylation was proposed to promote MLKL clustering at plasma membrane into hotspots.
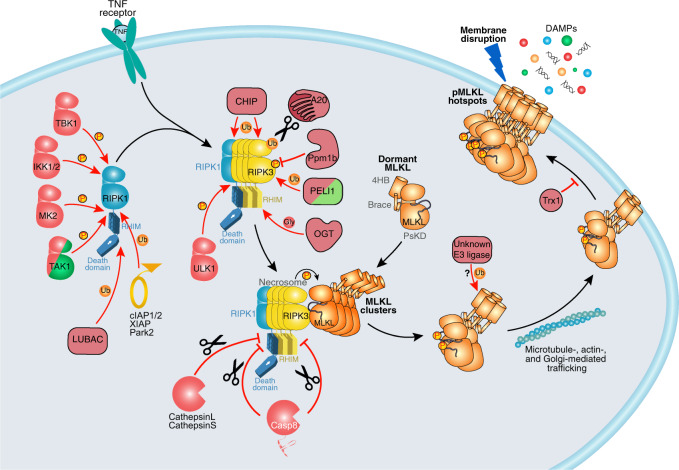
Fig. 3. Post-translational modifications negatively regulate necroptosis.
Many components of TNFR1 complex I that contribute to pro-survival signaling negatively regulate necroptosis via RIPK1. These include inhibitory phosphorylation by kinases TBK1, IKK1/2, MK2, TAK1, and ULK1; as well as ubiquitylation by E3 ligases LUBAC, cIAP1/2, XIAP, and Park2. CHIP- and A20-mediated ubiquitylation targets RIPK1 and RIPK3 for degradation. The Ppm1b phosphatase subtly attenuates necroptosis by removing RIPK3 autophosphorylation. PELI1-mediated ubiquitination is proposed to target RIPK3 towards proteasomal degradation. Cleavage of RIPK1 by cathepsins, RIPK1, and RIPK3 by Casp8 inhibits necroptosis. MLKL is ubiquitylated by unknown E3 ligases. Reduction of disulfide bonds by Trx1 was proposed to prevent MLKL from assembling into higher-order species.
Overview of the necroptosis signaling pathway
As a hub for cell death signaling, RIPK1 is involved in the regulation of both pro-survival NF-κB activation, apoptosis, and necroptosis (reviewed in ref. [90]). Upon TNF ligation, RIPK1 along with other adaptor proteins and other signaling components are recruited to the cytosolic death domain of TNFR1 to form the TNF-receptor-associated complex I, which serves as a platform for signaling via the pro-survival NF- κB pathway [91, 92]. RIPK1 is heavily ubiquitylated in complex I, which serves to confine RIPK1 to this death receptor-nucleated complex to suppress cell death and promote inflammatory gene expression. When one of the checkpoints in complex I fails or is negated, it can lead to RIPK1-dependent apoptosis in certain scenarios [93], but apoptosis can also be promoted via RIPK1 kinase-independent mechanisms [94]. The role of RIPK1 in necroptosis, however, is complicated because, depending on cellular context, both positive (necroptosis-promoting) and negative (necroptosis-attenuating) regulatory functions for RIPK1 have been reported. Murine cells expressing kinase-inactive RIPK1 are protected from TNF-induced necroptosis, implicating its kinase activity in driving necroptosis via the TNF pathway [95]. Because RIPK1 knock-out mice die perinatally [96], yet mice bearing either of two distinct kinase-inactive mutant RIPK1 knock-in alleles (K45A or D138N) are viable and fertile [80, 95], it can be concluded that RIPK1’s scaffolding function is important in restricting necroptosis. This idea is supported by the observation that cells lacking RIPK1 undergo increased spontaneous RIPK3-dependent death [95, 97–99], indicating that depending on cellular context, RIPK1 can either promote or negate RIPK3 activation and therefore cell death.
Although RIPK1 S161 autophosphorylation appears to precede necrosome formation [86, 100, 101] and drives RIPK1-RIPK3 interaction, it remains unclear how interactions among RHIM proteins are regulated [38, 39]. Studies using RIPK1 and RIPK3 fused to dimerization domains indicate that RIPK1-RIPK3 interaction alone is insufficient to cause necroptosis in mouse cells, while RIPK3-RIPK3 homo-interaction is both necessary and sufficient to induce necroptosis [94, 102, 103]. More recently, attention has turned to the possibility that, in addition to the RHIM, the RIPK3 kinase domain contributes to dimerization (Fig. 4b), as would be expected considering the importance of RIPK3 phosphorylation to induction of necroptosis [81]. These studies point towards a model where RIPK1 seeds the amyloid fibril by RIPK1-RIPK3 interaction, which is insufficient to propagate necroptotic signals. When more RIPK3 molecules are recruited to the necrosome, RIPK3-RIPK3 homo-interaction presumably enables RIPK3 autophosphorylation, which is a known prerequisite for RIPK3 engagement and activation of MLKL by phosphorylation [28, 104, 105]. RIPK3-MLKL interaction is mediated by their respective kinase and pseudokinase domains, as demonstrated by the crystal structure of murine RIPK3-MLKL complex [105], but curiously this interaction can only be detected biophysically when mixing recombinant proteins of the human, but not the murine, system [19, 30, 89]. Nevertheless, RIPK3-MLKL interaction is clearly necessary for necroptotic signaling; mutations to RIPK3 [28, 104] or MLKL [89] at the interaction interface, or binding of viral MLKL orthologs [19] to RIPK3, can block necroptotic signaling in both human and murine cell lines. Furthermore, overexpressed human RIPK3 and MLKL have been observed pre-associate under basal conditions in the cytoplasm [28], before higher order necrosomes assemble in the perinuclear space, and recruit RIPK3-MLKL subcomplexes [51, 106]. Taken together, although RIPK3 autophosphorylation, oligomerization, and interaction with MLKL are critical for necroptosis signaling, the interplay among these events remains unclear.
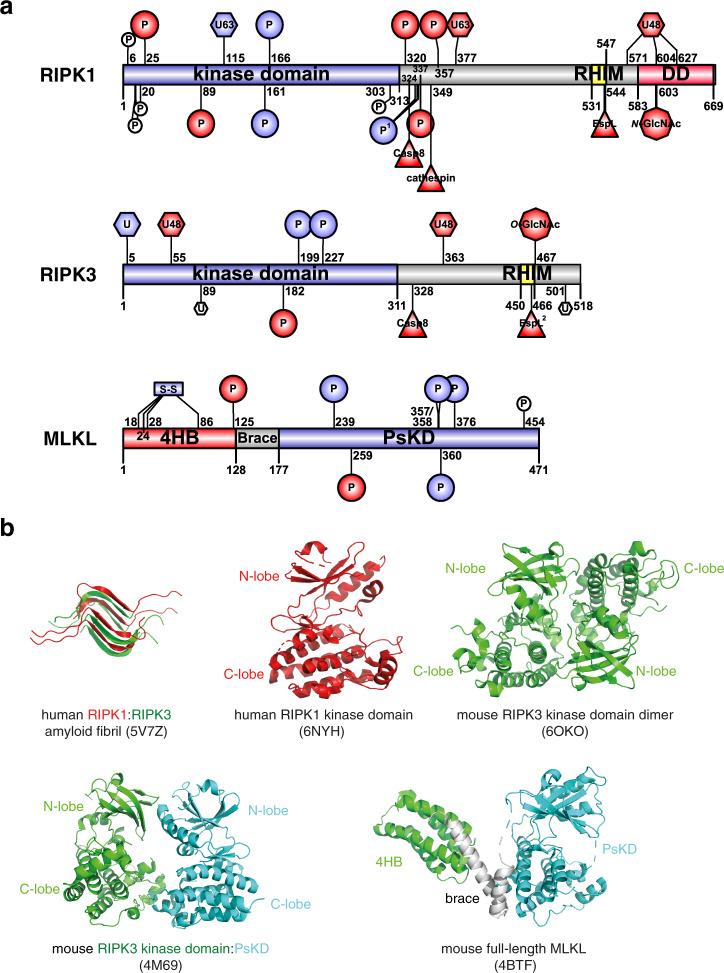
Fig. 4. Domain architecture and known post-translational modification sites of the necrosome components.
a Residues are numbered based on human ortholog sequence. Modifications are colored based on proposed effect to necroptotic cell death. Necroptosis-promoting (positive regulatory) events are colored blue; negative events are colored red. Modifications that were observed but have no known function in necroptosis are in white. Sequence diagrams were generated using IBS [204]. 4HB four-helix bundle domain, PsKD pseudokinase domain, RHIM RIP homotypic interaction motif, DD death domain. b Representative structures of RIPK1 and RIPK3 component domains and full-length MLKL (PDB accession numbers: 5V7Z [38]; 6NYH [71]; 6OZO [205]; 4M69 [105]; 4BTF [30]).
MLKL consists of an N-terminal four-helix bundle domain (4HB), followed by a two-helix brace region and the C-terminal pseudokinase domain (Fig. 4), designated based on the topology observed in the full-length mouse MLKL crystal structure [30]. The MLKL 4HB is a lipid-binding domain [40, 44, 89, 107], which executes necroptosis by permeabilizing the plasma membrane. The underlying lytic mechanism, such as whether MLKL oligomers assemble into a transmembrane pore or merely perturb the lipid bilayer, remains heavily debated in the absence of structural studies of the 4HB embedded in a lipid environment (reviewed in ref. [88]). While the 4HB appears to be the direct executioner of plasma membrane rupture, the C-terminal pseudokinase domain acts to suppress the lytic function of the 4HB domain [43]. The pseudokinase domain is regulated by interaction with RIPK3 and RIPK3-mediated phosphorylation, which has led to the proposal that this domain acts as a molecular switch that can be toggled by phosphorylation by RIPK3 [30, 89, 108] and possibly other kinases [47]. Following activation, MLKL assembles into oligomers and subsequently into higher order assemblies, which are trafficked to the plasma membrane via Golgi-microtubule-actin-dependent mechanisms [43, 51, 89, 108]. It has been proposed that when the accumulation of MLKL at the plasma membrane surpasses a critical threshold level at focal “hotspots”, membrane integrity is compromised and cell death ensues [51]. The activities of each of the core elements of the necroptosis machinery, RIPK1, RIPK3, and MLKL, can be tuned by post-translational modifications. Below we describe these events and evaluate evidence for their roles in promoting or negating necroptosis signaling.
Post-translational modifications of RIPK1
Phosphorylation
RIPK1 is heavily regulated with post-translational modifications. A number of phosphosites that influence RIPK1’s catalytic activity and thus engagement in death signaling have been reported (Fig. 4a, Table 1). These include autophosphorylation sites, which mark its activation towards necroptosis, as well as several phosphosites by accessory kinases, which were proposed to inhibit RIPK1’s kinase activity.
Table 1.
Post-translational modifications of RIPK1.
| Domain | Residuea | PTM | Modifying enzyme | Effect on necroptosis | Proposed mechanism | Ortholog studied | Cell modelb | Mouse model | Recombinant protein | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Mouse | ||||||||||
| Kinase | S6 | S6 | Phosphorylation | IKK1/2 | No effect | – | Mouse | MEF, MDF | No | No | [121] |
| Kinase | S14/15 | S14/15 | Phosphorylation | Auto | No effect | – | Human | 293T | No | No | [109] |
| Kinase | S20 | – | Phosphorylation | Auto | No effect | – | Human | 293T | No | No | [109] |
| Kinase | S25 | S25 | Phosphorylation | IKK1/2 | ↓ Inhibit RIPK1 S166 autophosphorylation and cell death | Inhibit kinase activity | Mouse | MEF, MDF, BMDM | Yes | Yes | [121] |
| Kinase | S89 | S89 | Phosphorylation | Unknown | ↓ Attenuates kinase activity | Unknown | Human | Jurkat | No | No | [87] |
| Kinase | S161 | S161 | Phosphorylation | Auto | no effect | – | Human | Jurkat | No | No | [87] |
| ↑ Drives RIPK1-RIPK3 interaction | Conformational change | Mouse | L929 | No | No | [100] | |||||
| No effect | – | Mouse | BMDM, MDF, MEF | No | No | [101] | |||||
| Kinase | S166 | S166 | Phosphorylation | Auto | ↑ Drives RIPK1-RIPK3 interaction | Conformational change | Mouse | BMDM, MDF, MLF, MEF | Yes | No | [86, 101] |
| Intermediate | S320 | S321 | Phosphorylation | TAK1 | ↓ Attenuates complex II formation, RIPK1 S166 autophosphorylation, and RIPK1-dependent cell death | Inhibit kinase activity | Mouse | L929, MEF | No | No | [124] |
| MK2 | ↓ Attenuates complex II formation and RIPK1-dependent cell death | Inhibit kinase activity | Human, mouse | MEF, BMDM, BT549, HT29 | Yes | Yes | [122] | ||||
| No effect | – | Mouse | MEF, BMDM | Yes | Yes | [123] | |||||
| Intermediate | S331 | S332 | Phosphorylation | TAK1 | ↑ Hyperphosphorylation promotes RIPK3 interaction and necroptosis | Unknown | Mouse | L929, MEF | No | No | [124] |
| Intermediate | S333 | S334 | Phosphorylation | ||||||||
| Intermediate | S335/T337 | S336 | Phosphorylation | ||||||||
| MK2 | ↓ Attenuates complex II formation and RIPK1-dependent cell death | Inhibit kinase activity | Mouse | MEF, BMDM | Yes | Yes | [123] | ||||
| Intermediate | S357 | S356 | Phosphorylation | ULK1 | ↓ Attenuates complex II formation and RIPK1-dependent cell death | Unknown | Mouse, human | L929, MEF, 293T | No | Yes | [125] |
| Intermediate | K377 | K376 | K63-linked ubiquitylation | cIAP1/2 | ↓ Inhibits complex II formation and cell death | Prevent RIPK1 dissociation from complex I; recruit LUBAC, TAK1, IKK1/2, and NF-κB activation | Mouse | MEF | Yes | No | [127, 129–131] |
| K377 | K376 | Park2 | ↓ Delayed necroptosis | Recruit LUBAC, TAK1, IKK1/2 and NF-κB and MAPK activation | Human, mouse | MEF, 293T, 661W | No | No | [132] | ||
| K377 | K376 | Deubiquitylation (K63-linked) | A20 | ↓ Inhibits complex II formation and cell death | Targets RIPK1 for proteasomal degradation | Mouse, human | MEF, K562, A549, mouse T cells | Yes | No | [147, 148, 151–154, 184] | |
| – | Unknown | Unknown | K48-linked ubiquitylation | ||||||||
| – | Unknown | Unknown | M1-linked ubiquitylation | LUBAC (HOIL, HOIP, SHARPIN) | ↓ Inhibits TNF-induced cell death | Recruit TAK1, IKK1/2, A20, CYLD, and NF-κB activation | Mouse | MEF | Yes | No | [114, 134–141] |
| – | Unknown | Unknown | Deubiquitylate K63- and M1-linked Ub | CYLD | ↑ Promote necrosome formation and necroptosis | Promote RIPK1 kinase activity | Mouse, human | MEF, HT29, L929, BMDM | Yes | No | [142, 143, 149, 150] |
| Kinase | K115 | K115 | K63-linked ubiquitylation | PELI1 | ↑ Required for necrosome formation | Promote RIPK1 kinase activity | Mouse, human | MEF, HT29 | Yes | No | [162, 163] |
| Intermediate | K571 | – | K48-linked ubiquitylation | CHIP | ↓ Inhibits TNF-induced necroptosis | Targets RIPK1 for lysosomal degradation | Mouse, human | MEF, L929, HT29 | Yes | No | [156–158] |
| Death | K604 | K589 | |||||||||
| Death | K627 | K612 | |||||||||
| – | Unknown | Unknown | K11-linked ubiquitylation | UbcH5, cIAP1 | Unknown | Promote K11-linked polyubiquitination which recruits NEMO | Human | 293T, HeLa S3, HT1080, HT29 | No | Yes | [133] |
| Intermediate | D324 | D325 | Cleavage | Casp8 | ↓ Inhibits necroptosis | Protease cleavage | Human, mouse | HT29, MEF, L929, BMDM | Yes | Yes | [35–37, 76, 171] |
| Intermediate | L349 | L348 | Cleavage | Cathepsin | ↓ Attenuates necroptosis | Protease cleavage | Mouse | BMDM | No | Yes | [175] |
| RHIM | Y544 | H533 | Cleavage | EspL | ↓ (Pathogen) inhibits necroptosis | Protease cleavage | Mouse | MDF, BMDM | Yes | Yes | [17] |
| Death | R603 | R588 | N-GlcNAc | NleB | ↓ (Pathogen) inhibits TNF-induced cell death | Inactivates death domain-mediated interactions | Mouse, human | 293T, HeLa, MEF, HT29 | Yes | Yes | [20, 21] |
aEquivalent residues in human and mouse orthologs are inferred by sequence homology. Bold indicates the ortholog residue with direct evidence of such post-translational modification.
bCell line abbreviations: MEF mouse embryonic fibroblasts, MDF mouse dermal fibroblasts, 293T human embryonic kidney cell line HEK293T, BMDM bone marrow-derived macrophages, Jurkat human T lymphocyte Jurkat cells, L929 mouse fibrosarcoma cells, MLF mouse lung fibroblasts, BT549 human breast cancer cells, HT29 human adenocarcinoma cells, 661W mouse cone photoreceptor cells, K562 human chronic myelogenous leukemia cells, A549 human adenocarcinomic alveolar basal epithelial cells, HeLa (S3) human adenocarcinoma cells, HT1080 human fibrosarcoma cells.
Autophosphorylation upregulates RIPK1 activity
The primary function of RIPK1’s kinase domain is generally accepted to be autophosphorylation [100, 101, 109]. RIPK1 only exhibits modest kinase activity, and RIPK1-mediated phosphorylation of RIPK3 or other substrates has not been convincingly shown experimentally. Therefore, RIPK1 does not promote necroptosis via a classical phosphorylation cascade, but instead, relies on RIPK1 autophosphorylation for pathway initiation. The autophosphorylation sites S161 and S166 have emerged as biomarkers for RIPK1 activation, but the biological functions of these phosphorylation events remain the subject of debate [86, 100, 101, 109]. A recent study failed to detect S161 phosphorylation in endogenous RIPK1, suggesting that the contribution of this phosphorylation event to RIPK1 regulation might depend on the cellular and stimulus context [101]. Studies of phosphoablating mutations have yielded conflicting results. While S161A reconstituted necroptosis similarly to wild-type RIPK1 [87], subsequent work [100] argued that S161A is not a faithful mimetic of unmodified S161 because it disrupts the S161-D156 hydrogen bond observed in the structure of RIPK1 [110], and allows the activation loop to adopt an activated kinase conformation. On the contrary, studies of S161N RIPK1 support a functional role for S161 phosphorylation, because this mutant both failed to reconstitute necroptosis and prevented its co-localization with RIPK3 [100].
While S166 is widely used as a biomarker for RIPK1 activation [109, 111], phosphorylation of this site appears to be insufficient for necroptosis. It was proposed that S166 may not be a necroptosis-specific phosphorylation event, because it was detected in both TNF and TZ (TNF plus pan-caspase inhibitor Z-VAD-FMK) treatments, and this autophosphorylation did not require RIPK1 ubiquitination [86]. Recent studies of mice bearing a S166A RIPK1 knock-in allele indicates that S166 phosphorylation promotes and is coincident with, but insufficient to cause, cell death [101]. These studies raise the possibility that autophosphorylation of additional sites may be promoted by S166 phosphorylation for RIPK1 to reach its full kinase activity.
IKK1/2, TAK1, MK2, and ULK1-mediated phosphorylation downregulates RIPK1
RIPK1 was reported to be phosphorylated by IKK1/2, TAK1, and MK2 to regulate its kinase activity. These phosphorylation sites are located either on the N-lobe of the kinase domain, or in the intermediate region located between the kinase domain and RHIM (Fig. 4a, b), each resulting in inhibition of its kinase activity. TAK1 and IKK1/2 protein kinases are recruited to the TNFR1 complex I via the ubiquitin chains added by cIAP proteins and linear ubiquitin chain assembly complex (LUBAC, consisting of HOIL/HOIP/SHARPIN) [112] to RIPK1 [92, 113–117], where they are best known for stimulation of the NF-κB pathway. IKK1/2-mediated phosphorylation of RIPK1 within TNFR1 complex I was reported to protect cells from RIPK1-dependent apoptosis and necroptosis independent of NF-κB signaling [118]. RIPK1-dependent apoptosis is mediated by the cytosolic complex II (Casp8, FADD, cFLIP, and RIPK1), which forms after RIPK1 dissociates from complex I and drives Casp8 activation [119, 120]. Additionally, IKK1/2 enzymatic activity was reported to prevent RIPK1 integration into complex II via phosphorylation of S25 in the kinase domain of RIPK1 [121]. Introduction of the phosphomimetic S25D mutation into RIPK1 reduced kinase activity and S166 phosphorylation, thereby accounting for the protective role served by the introduction of this mutation, and S25 phosphorylation, in cells and in mice [121]. The nature of the S25 substitution appears to play an important role, however, because S25E mouse RIPK1 could reconstitute signaling in Ripk1−/− L929 cells [100]. These findings raise the possibility that some substitutions at this site may compromise RIPK1 kinase domain structure and/or protein interactions, rather than simply emulating phosphorylation. Another N-lobe inhibitory phosphosite, S89, was identified similarly by mutational analyses, where S89A increased human RIPK1 kinase activity while S89D attenuated it [87], though the identity of the kinase that modifies this site remains of outstanding interest. In concert, S6 in RIPK1 was also reported to be phosphorylated by IKK1/2, but no functional role was attributed to this phosphorylation event [121].
Both TAK1 and MK2 were proposed to phosphorylate S321 of mouse RIPK1 and the equivalent residue in human RIPK1, S320, to attenuate RIPK1 kinase activity and, accordingly, cell death [122–124]. Because TAK1 and MK2 function within the same signaling cascade, the relative contribution of each kinase to this modification is currently debated [122–124]. These studies concordantly found that S321/S320 phosphorylation in complex I attenuated complex II formation and cell death in a cIAP1/2-dependent manner downstream of TNF signaling. In addition to phosphorylation of S321, each of S332, S334 and S336 were identified as phospho-sites in RIPK1, with S336 an additional substrate of MK2 [123, 124], suggesting a role for multiple phosphorylation events within the intermediate region in promoting necroptosis. The observation that S336A mutation, but not S321A, sensitized mouse embryonic fibroblasts (MEFs) to cell death led to the proposal that phosphorylation of S336 has a more prominent role in restricting RIPK1-dependent cell death [123].
Taken together, IKK1/2, TAK1, and MK2 phosphorylation of RIPK1 kinase represent multiple mechanisms by which necroptosis can be suppressed during pro-survival NF-κΒ signaling. In addition to these, the autophagy-initiating kinase ULK1 was recently proposed to add another layer of regulation by phosphorylating an additional site, S357, C-terminal to the kinase domain [125]. However, ULK1 activity only modestly impacted necroptosis, suggesting a non-obligate function of S357 phosphorylation in tuning necroptosis.
Ubiquitylation
RIPK1 is heavily ubiquitylated in the TNFR1 complex I, which serves as a signaling platform for the regulation of cell survival, apoptotic and necroptotic death. Several types of ubiquitylation have been observed on RIPK1, including Met1-, K11-, K48-, and K63-linked ubiquitylation (reviewed in ref. [126]) (Table 1). A number of E3 ligases and deubiquitinases have been proposed to mediate ubiquitin modifications. The best characterized examples are of the complex I components, cIAP1/2, LUBAC, A20, and CYLD. However, few ubiquitylation sites have been characterized, and evidence connecting ubiquitylation sites and ubiquitin-modifying enzymes is scarce. Recently, several ubiquitin-modifying enzymes have been implicated as positive and negative regulators of RIPK1-dependent cell death.
Downregulation of RIPK1-mediated cell death by ubiquitylation
cIAP-mediated K63-linked ubiquitylation of RIPK1 is well-established to suppress RIPK1-mediated cell death [127] (and reviewed in ref. [128]). These ubiquitylation events enable recruitment of complex I components, including LUBAC, TAB1/2, TAK1, NEMO, and IKK1/2, to activate the pro-survival NF-κB pathway and inhibit cell death. Recently, K63-linked ubiquitylation on K376 of mouse RIPK1 (equivalent to K377 of human RIPK1) was identified as an important mechanism to suppress cell death [129–131]. The RIPK1 K376R mutation caused embryonic lethality in knock-in mice, which was attributed to increased kinase activity and elevated sensitivity to RIPK1-dependent cell death. These studies support a role of K376 ubiquitylation in suppressing RIPK1 kinase activity, although formal evidence that K376 is the direct substrate of cIAP1/2 is still lacking. While the substrate site(s) in RIPK1 remain unknown, another E3 ligase, Parkin (Park2), was reported to bind RIPK1 in complex I and perform similar functions to cIAP1/2 in some contexts [132]. Loss of Park2 had only a modest impact on necroptosis, which suggests that Park2 performs an auxiliary role for cell death, the details of which await further investigation. Additionally, cIAP1 facilitates K11-linked polyubiquitylation of RIPK1 during TNF-induced NF-κB signaling. This action is dependent on the UbcH5 family of E2 enzymes [133]. Although K11-linked polyubiquitylation is more commonly known to be a degradation signal, the observation of NEMO binding to this K11-linked polyubiquitin chain led to the proposal of a possible non-degradative signaling role [133], whose biological effect on necroptotic cell death awaits further characterization.
LUBAC negatively regulates RIPK1-mediated cell death, but the precise mechanisms and ubiquitylation sites remain unknown. Downstream of cIAP1/2, LUBAC is recruited to complex I where it catalyzes M1-linked (linear) ubiquitylation of TNFR1, TRADD, and RIPK1. Such LUBAC-mediated ubiquitylation reinforces complex I, enables recruitment of the NEMO/IKK1/IKK2 complex to drive NF-κB activation, and negatively regulates RIPK1-mediated cell death [114, 134–141]. Much like deubiquitylation of the cIAP-mediated K63-linked ubiquitin chain from RIPK1, removal of M1-linked ubiquitin chains by CYLD is required for RIPK1-dependent cell death to proceed [142, 143]. Consistent with these in vitro observations, mice with impaired LUBAC components display severe inflammation or embryonic lethality that could be rescued by co-deletion of Casp8 with RIPK3 or MLKL in some cases [141, 144–146]. Paradoxically, LUBAC is required in complex I to recruit A20 and CYLD, each of which exhibit opposing effects on necroptosis [142, 143, 147–150]. They further modify the ubiquitylation of RIPK1 to exert pro-death effects that are countered by M1-linked ubiquitylation [143].
A20 is a bifunctional ubiquitin-modifying enzyme that restricts NF-κB activation and apoptosis following TNF- and TLR-stimulation [147, 148]. The N-terminal domain of A20 is a deubiquitinase that removes K63-linked ubiquitin chains from RIPK1, while the C-terminal zinc finger (ZnF7) domain functions as a ubiquitin ligase that polyubiquitylates RIPK1 with K48-linked ubiquitin chains to target it for proteasomal degradation. Accordingly, perinatal lethality and widespread tissue inflammation phenotype were observed in A20-deficient mice [151]. A20 is recruited to LUBAC-mediated M1-linked ubiquitylation sites via its ZnF7 domain, and A20 binding also protects M1-linked ubiquitin chains from deubiquitylation or degradation [152, 153]. On the other hand, contrary to the anti-inflammatory roles proposed by the above studies, A20 was proposed to bind cytosolic complex II via a ZnF7-dependent mechanism and potentiate apoptotic cell death [154]. However, why this promotes cell death instead of inhibiting it and whether this recruitment to the necrosome is dependent on re-ubiquitylation of necrosomal RIPK1 by HOIP remains to be established [155].
Carboxyl-terminus of Hsp70-interacting protein (CHIP) is an E3 ligase that introduces K48-linked ubiquitin chains on K571, K604, and K627 of RIPK1, leading to its lysosomal degradation [156–158]. CHIP-deficiency led to increased levels of RIPK1 and increased sensitivity to TNF-induced necroptosis in cell lines and provoked an inflammatory postnatal lethal phenotype in mice. These data support a role for CHIP as a negative regulator of necroptosis.
Optineurin (OPTN) is a ubiquitin-binding protein that has been implicated in regulating necroptosis by regulating turnover of RIPK1 [65]. OPTN-depletion increased expression levels of RIPK1, RIPK3 and MLKL, and sensitized cultured cells to necroptosis. Consequently, OPTN was proposed as a negative regulator of necroptosis; however, since OPTN is not a E3 ligase itself, the mechanism by which OPTN promotes RIPK1 modification and regulation is unclear.
Upregulation of RIPK1-mediated necroptosis by ubiquitin-modifying enzymes
CYLD is another deubiquitinase that is recruited to complex I downstream of LUBAC [143], but unlike A20, CYLD is recruited to LUBAC via the adaptor SPATA2 [159]. CYLD removes K63- and M1-linked ubiquitin chains from RIPK1 and TRAFs, thereby promoting formation of cytosolic RIPK1-nucleated signaling platforms, including the necrosome, which directs necroptosis [142, 143, 149, 150]. Whether CYLD catalyzes deubiquitylation in complex I or the necrosome is a matter of debate [143, 149], but since LUBAC was shown to ubiquitylate RIPK1 in both complex I and the necrosome, it is possible CYLD can be recruited to both complexes. Furthermore, cleavage of CYLD by Casp8 was proposed as a mechanism to hold aberrant necroptosis in check downstream of death receptor stimulation [160]. However, knock-in mice containing the CYLD D215A allele, a form of CYLD that cannot be cleaved by Casp8, were viable [37], suggesting this mechanism may be dispensable during homeostasis. Taken together, several lines of evidence support a role for CYLD as a positive regulator of necroptosis, although it is not essential in all cell types and therefore likely performs an auxiliary role in tuning necroptotic signaling [161].
Several additional ubiquitin-modifying enzymes have been proposed to function as regulators of necroptosis. PELI1, an E3 ligase known to be involved in mediating TLR3/4 signaling, was shown to K63-linked ubiquitylate RIPK1 on K115 during TNF-induced necroptosis in a RIPK1 kinase-dependent manner [162, 163]. PELI1 deficiency was reported to abrogate the RIPK1-RIPK3 interaction and necroptosis, suggesting PELI1 functions as an essential positive regulator of necroptosis. Paradoxically, PELI1 was reported to exhibit the opposite effect in the context of RIPK3 ubiquitylation by targeting RIPK3 for degradation [164]. Therefore, the net effect of PELI1 on necroptotic signaling remains unclear. In a subsequent study, RIPK1 K115R knock-in mice were found to be viable with only modestly elevated responsiveness to TNF-induced death [129], suggesting a less prominent role for this ubiquitination site during steady state. Lastly, APC11, an E3 ligase of the APC/C complex that targets its substrates for degradation via K11- and K48-linked polyubiquitination, was identified in an siRNA knockdown screen as a RIPK1, and therefore necroptosis, activator [93]. Further biochemical and in vivo data are required to confirm the mechanism and physiological relevance by which APC11 regulates necroptosis.
Other post-translational modifications
It is well-established that necroptosis is suppressed by Casp8, whose cellular inhibitors and substrates also regulate necroptosis by extension. Activated Casp8 in cytosolic complex II cleaves human RIPK1 at D324 [35, 37, 76, 77, 165] (mouse RIPK1 D325) and possibly RIPK3 at D328 [36] (mouse RIPK3 D333), thereby severing their kinase domains from the RHIM oligomerization modules to limit the capacity of RIPK1 and RIPK3 to assemble into the necrosome and execute necroptosis [166]. Inhibition or deficiency of Casp8 or its binding partner, the FADD adaptor protein, enables necroptosis to ensue [167, 168]. The catalytic activity of Casp8 is tightly controlled by two cFLIP isoforms [169, 170]. cFLIPL is a pseudoprotease that forms a heterodimer with Casp8 and inhibits apoptosis. Formation of this heterodimer prevents the Casp8 autoprocessing that is required for apoptosis to proceed, while retaining cleavage activity towards RIPK1 and potentially RIPK3 to block necroptosis [171]. cFLIPS on the other hand inhibits Casp8-mediated cleavage of RIPK1 and RIPK3 in addition to inhibition of Casp8 autoprocessing, and thus functions as a pro-necroptotic factor [32]. XIAP is another direct inhibitor of apoptotic caspases. Similar to cIAP1/2, XIAP is an E3 ligase of the inhibitor of apoptotic protein (IAP) family, but its mechanism is more enigmatic. Loss of XIAP confers some sensitivity to TNF- and TLR-induced cell death in macrophages and dendritic cells [172, 173], but in neutrophils this requires co-deletion with cIAP1/cIAP2 [174]. Furthermore, an aforementioned positive regulator of necroptosis, CYLD, has also been shown to be a Casp8 substrate whose cleavage was proposed to limit TNF-induced necroptosis [160].
It appears likely that proteolytic post-translational modifications more broadly function to regulate necroptotic flux. Cathepsin B and S were reported to cleave RIPK1 during TNF- or TLR-induced necroptosis in macrophages [175]. Knocking down the expression of cathepsins resulted in decreased RIPK1 cleavage and slightly increased sensitivity to necroptosis. Further in vivo data will be useful to confirm the physiological relevance of cathepsins as necroptosis regulators. Enteropathogenic Escherichia coli (EPEC) also encode a cysteine protease, EspL, that cleaves RHIM-containing proteins (RIPK1, RIPK3, TRIF, and ZBP1/DAI) [17], as well as an arginine glycosylation enzyme, NleB, which inhibits the interactions among the same proteins via N-acetylglucosamine modifications [20, 21]. These represent mechanisms by which post-translational modifications are utilized by pathogens to restrict necroptosis and evade the associated host immune response. Collectively, these studies highlight that, while among the necroptotic machinery, our knowledge of the post-translational modifications that regulate RIPK1 is the most advanced, it is likely that additional modifications that tune RIPK1’s activity, localization, protein interactions, and stability remain still to be discovered.
Post-translational modifications of RIPK3
Phosphorylation
To date, all known phosphorylation events in RIPK3 have been reported to promote necroptotic signaling (Fig. 2, Table 2). RIPK3 autophosphorylation is a crucial event for induction of cell death by necroptosis, and is thought to arise following the self-association of RIPK3 protomers within the necrosome [25, 26]. Although inhibition of RIPK1 kinase activity also inhibits RIPK3 phosphorylation [25, 80, 111, 163], no phosphorylation of RIPK3 by RIPK1 was observed in in vitro kinase assays [25]. These data support the idea that RIPK1 activates RIPK3 via a scaffolding function [25], which seeds the formation of higher-order RIPK3 complexes. This is further supported by multiple reports of overexpression of RIPK3 driving autophosphorylation and necroptosis [27, 94, 176] because a surfeit of RIPK3 will obviate the need for RIPK1 to nucleate higher-order RIPK3 assemblies of increased local concentration for autophosphorylation. In keeping with this idea, activation of RIPK3 can also mediated by the RHIM-containing proteins TRIF [82] and ZBP1 [22, 84], even though neither is a kinase, supporting the notion that the activation of RIPK3 does not require direct phosphorylation by RIPK1.
Table 2.
Post-translational modifications of RIPK3.
| Domain | Residuea | PTM | Modifying enzyme | Effect on necroptosis | Proposed mechanism | Ortholog studied | Cell modelb | Mouse model | Recombinant protein | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Mouse | ||||||||||
| Kinase | T182 | T187 | Phosphorylation | Auto | Both ↑ and ↓, net effect unclear | Promotes RIPK3 kinase activity; but also recruits PELI1 which targets RIPK3 for proteasomal degradation | Human | HT29, 293T | No | Yes | [164] |
| Kinase | S199 | S204 | Phosphorylation | Auto | ↑ Promotes necroptosis | Promotes RIPK3 kinase activity | Human, mouse | MEF, Jurkat, HT29 | No | Yes | [87] |
| Kinase | S227 | T231/S232 | Phosphorylation | Auto | ↑ Promotes necroptosis | Promotes interaction with MLKL | Mouse, human | MEF, Jurkat, HT29 | No | Yes | [28, 87] |
| S227 | T231/S232 | CK1α, CK1δ, and CK1ε | Human | HeLa | No | Yes | [181] | ||||
| S227 | T231/S232 | Dephosphorylation | Ppm1b | ↓ Restrict necroptosis | Antagonize essential phosphorylation | Human, mouse | 293T, L929, HeLa | Yes | Yes | [183] | |
| Kinase | K5 | K5 | Deubiquitination | A20 | ↓ Inhibits complex II formation and cell death | A20 deubiquitinate K63-linked ubiquitin chain of RIPK3 at K5, which is required for necrosome formation | Mouse | T cell, MEF | No | No | [184] |
| Kinase | K55 | K56 | K48-linked ubiquitination | CHIP | ↓ Attenuates necroptosis | Targets RIPK3 for lysosomal degradation | Mouse, human | MEF, L929, HT29 | Yes | No | [156] |
| Kinase | K89 | – | K48-linked ubiquitination | CHIP | – | – | – | – | – | – | [156] |
| Intermediate | K363 | – | K48-linked ubiquitination | CHIP | ↓ Attenuates necroptosis | Targets RIPK3 for lysosomal degradation | Mouse, human | MEF, L929, HT29 | Yes | No | [156] |
| – | K48-linked ubiquitination | PELI1 | ↓ Attenuates necroptosis | Targets RIPK3 for proteasomal degradation | Mouse, human | MEF, HT29 | No | No | [164] | ||
| Intermediate | K501 | – | K48-linked ubiquitination | CHIP | – | – | – | – | – | – | [156] |
| Intermediate | D328 | D333 | Cleavage | Casp8 | ↓ Inhibits necroptosis | Protease cleavage | Human | HeLa, 293T | No | Yes | [36, 171] |
| RHIM | N462 | Y453 | Cleavage | EspL | ↓ (Pathogen) inhibits necroptosis | Protease cleavage | Mouse | MDF, BMDM | Yes | Yes | [17] |
| RHIM | T467 | Unknown | O-GlcNAc | OGT | ↓ Attenuates necroptosis | Steric hinderance prevent RHIM-mediated RIPK1-RIPK3 interaction | Human, mouse | 293T, L929, THP-1, BMDM | Yes | No | [185] |
aEquivalent residues in human and mouse orthologs are inferred by sequence homology. Bold indicates the ortholog residue with direct evidence of such post-translational modification.
bCell line abbreviations: HT29 human adenocarcinoma cells, 293T human embryonic kidney cell line HEK293T, MEF mouse embryonic fibroblasts, Jurkat human T lymphocyte Jurkat cells, HeLa human adenocarcinoma cells, L292 mouse fibrosarcoma cells, BMDM bone marrow-derived macrophages, THP-1 human monocyte-like cells.
Three autophosphorylation sites have been discovered within human RIPK3, which are proposed to contribute to RIPK3’s function in necroptotic signaling. S227 in human RIPK3 and the counterpart in mouse RIPK3, T231/S232, are located in the kinase C-lobe and are the best characterized RIPK3 phosphosites (Fig. 4a, b), with verified functions in both human and mouse cells [28, 87, 104]. S227 phosphorylation is dispensable for human RIPK3 kinase activity, but is critical for recruitment of MLKL and subsequent induction of necroptosis. Introduction of the phosphoablating mutation, S227A, into human RIPK3 completely abrogated necroptotic cell death [28, 104]. In contrast to human RIPK3, mouse RIPK3 has an additional, adjacent phosphosite, T231, which was reported to contribute to mouse RIPK3’s necroptotic activity [104]. The additional requirement for T231 phosphorylation likely contributes to the inability of mouse and human RIPK3 to respectively bind and activate mouse and human MLKL [104, 177, 178]. Nevertheless, human RIPK3 S227 and mouse RIPK3 T231/S232 phosphorylation are widely used as biomarkers for RIPK3 activation and the instigation of necroptosis. Although a role for Casein Kinase (CK)1α in regulating necroptosis was previously suggested by its reported binding and phosphorylation of RIPK1 [179], recently CK1γ2 and γ3 [180], CK1α, CK1δ, and CK1ε [181] were additionally proposed to phosphorylate S227 of human RIPK3 within the necrosome. In contrast, CK1γ2 was proposed to serve an entirely opposite function by binding and negatively-regulating RIPK3 activity, and therefore necroptosis, in the testes [182]. The relative contribution to RIPK1 and RIPK3 regulation by CK1 proteins, the contexts under which regulatory function occurs, whether different CK1 isoforms serve different functions, and whether they are positive or negative regulatory events, remain of outstanding interest. Dephosphorylation of the counterpart of human RIPK3 S227 in mouse RIPK3, T231/S232, was reported to be catalyzed by the Ppm1b phosphatase to restrict necroptosis, with Ppm1b deletion leading to a modest increase in RIPK3 phosphorylation, death of cultured cells, and tissue damage in the TNF-induced sterile sepsis mouse model [183]. This raises the possibility that other phosphatases may antagonize phosphorylation of the necrosome and act as additional negative regulators of necroptosis.
Beyond S227 and T231/S232 in the C-lobes of human and mouse RIPK3, respectively, the phosphorylation of additional sites has been reported to promote necroptosis. Phosphorylation of human RIPK3 S199 was also proposed to be required for necroptotic cell death in HT29 cells [26], although subsequent studies indicate S199 phosphorylation is dispensable [104]. Alanine substitution of the mouse RIPK3 counterpart of S199, S204, on the other hand compromised mouse RIPK3 kinase activity and necroptosis when introduced in Ripk3−/− fibroblasts [87]. While substitution of mouse RIPK3 S204 with the phosphomimetic residue, Asp, did not lead to constitutive killing, this mutant could reconstitute necroptotic signaling in Ripk3−/− fibroblasts to a comparable extent to wild-type RIPK3. In contrast to wild-type mouse RIPK3, treatment of RIPK1 with small-molecule inhibitor, Nec-1, did not block S204D mouse RIPK3-mediated death following necroptotic stimulation [87]. These findings support the idea that mouse RIPK3 S204 phosphorylation is necessary for necroptosis, and that mimicking phosphorylation alleviates the need for RIPK1 activity to promote RIPK3 activity. Precisely why RIPK1 activity is required for mouse RIPK3 to become phosphorylated on S204 is unclear, because RIPK1 is not known to phosphorylate RIPK3. It remains of interest to deduce whether Nec-1 binding to RIPK1 leads to an unproductive conformation or if it is the blockade of RIPK1 activity and autophosphorylation that thwarts RIPK3 interaction and activation.
It was proposed that phosphorylation of a site at the C-terminal end of the human RIPK3 activation loop, T182, was required to prime S227 phosphorylation and therefore promote RIPK3 necroptotic activity [164]. Phosphorylation of T182 was reported to be dependent on RIPK3 kinase activity, so it is presumed to be an autophosphorylation site. While the cellular contexts under which T182 phosphorylation are required for RIPK3 activation are not fully understood, it was proposed that this modification is required for interaction with PELI1, an E3 ubiquitin ligase that catalyzes K48-linked polyubiquitination to target RIPK3 for proteasomal degradation [164]. Hence, this is a possible mechanism by which phosphorylated, and therefore activated, RIPK3 could be selectively targeted for degradation to restrict necroptosis.
Ubiquitylation
RIPK3 is regulated by E3 ubiquitin ligases, including A20, CHIP, and PELI1, which are also known to ubiquitylate RIPK1 and negatively regulate necroptosis. Because RIPK3 ubiquitylation at K5 by an unknown E3 ligase under basal conditions is required to support the formation of the necrosome and promote necroptosis, the removal of K63-linked ubiquitin chains at this site by A20 restricts necroptosis [184]. It is notable that although A20 restricts RIPK1 and RIPK3 necroptotic activities, the underlying mechanisms appear to differ. A20 ubiquitylation of RIPK1 was reported to target RIPK1 for proteasomal degradation, however A20 ubiquitylation of RIPK3 has not been observed and, instead, RIPK3 negative regulation appears to rely on K5 deubiquitylation.
The cellular levels of RIPK3 appear to be regulated by K48-linked ubiquitylation by the E3 ubiquitin ligases, CHIP and PELI1, which induces RIPK3 degradation to suppress necroptosis [156, 164]. CHIP was reported to ubiquitylate RIPK3 to regulate protein levels via lysosomal degradation [156], similar to CHIP’s function as a regulator of RIPK1. While human RIPK3 is ubiquitylated at K55, K89, K363, and K501 by CHIP, it appears that K55 and K363 are the critical sites for RIPK3 regulation. Although CHIP-mediated ubiquitylation of RIPK3 led to lysosomal targeting, PELI1-mediated K48-ubiquitylation of K363, a site also modified by CHIP, targeted RIPK3 to the proteasome for degradation [164]. CHIP- or PELI1-depletion in human and mouse cells increased RIPK3 levels and correspondingly increased sensitivity to TNF-induced necroptosis, supporting their functions as negative regulators of RIPK3 and therefore necroptosis. However, the net effect of PELI1 on necroptosis is unclear, because PELI1 is also reported to regulate RIPK1 to promote RIPK1-RIPK3 interaction and necroptosis [162, 163].
Other post-translational modifications
Similar to RIPK1, RIPK3 cleavage by Casp8 during apoptotic conditions is proposed to serve as a mechanism to restrict necroptosis to favor the non-death outcomes of TNF signaling [36, 171]. Casp8 was proposed to cleave human RIPK3 at D328 in the intermediate region between the kinase and RHIM domains, which is a similar position to RIPK1 cleavage site (Fig. 4). While this cleavage was not observed in human cells overexpressing the D328A mutant RIPK3 [36], the precise physiological significance of RIPK3 cleavage by Casp8 is unclear.
O-GlcNAc transferase (OGT) of the hexosamine biosynthetic pathway was reported to mediate O-linked β-N-GlcNAcylation of mouse RIPK3 on T467, adjacent to the RHIM motif [185]. This modification was proposed to inhibit the interaction between RIPK1 and RIPK3, possibly due to steric hindrance of interactions between RHIM domains. The universality of this regulatory mechanism between species, tissues and necroptotic stimulation remains unclear at present. Like the reported ubiquitylation sites in RIPK3, the OGT substrate residue in mouse RIPK3, T467, is poorly conserved amongst RIPK3 orthologs. Therefore, it remains to be established whether adjacent sites could be targeted by OGT and other glycosylation enzymes in other species to regulate RIPK3’s necroptotic function.
Post-translational modifications of MLKL
Phosphorylation
Phosphorylation of the activation loop of pseudokinase domain of MLKL by RIPK3 is considered a hallmark of MLKL activation [28, 30, 40, 47, 48] (Figs. 2 and 4, Table 3). Phosphorylation of the activation loop of the pseudokinase domain (S345 of murine MLKL [30]; T357/S358 of human MLKL [28]) by RIPK3 precedes necroptotic death in both human and murine cell lines. Phosphorylation is proposed to trigger a conformational change in the MLKL pseudokinase domain [30, 178, 186], which is communicated via a two-helix connector termed the brace region to the N-terminal 4HB domain [43, 177]. These steps are presumed to induce exposure of the 4HB domain, which enables MLKL translocation to the plasma membrane where the 4HB domain permeabilizes the membrane to cause cell death. Broadly, these principles are thought to be common to MLKL activation across species, although the underlying mechanisms appear to have diverged between MLKL orthologs [30, 89, 108, 178]. Principally, the precise function of activation loop phosphorylation appears to differ between species. In mouse cells, mutations to mimic phosphorylation of the MLKL activation loop at S345 suffice to induce cell death, whereas those in rat MLKL lead to a partial activation, and those in human and horse MLKL do not [178]. These findings have led to the proposal that MLKL activation loop phosphorylation may serve a range of functions in promoting MLKL activation, from triggering the conformational interconversion of mouse MLKL to the active form with unleashed 4HB domain to inducing dissociation from RIPK3 to enable membrane translocation and disruption in the case of human MLKL. The underlying molecular basis is that endogenous mouse RIPK3 and MLKL do not appear to stably interact [30], leading to the model that mouse RIPK3 can transiently interact with and phosphorylate mouse MLKL [46, 88]. In human MLKL, on the other hand, phosphomimetic substitutions of T357/S358 failed to reconstitute necroptosis in human cell lines and recombinant human MLKL harboring these substitutions exhibited deficits in human RIPK3 binding [89], raising the question whether human MLKL requires RIPK3 interaction or phosphorylation of other sites to execute necroptosis.
Table 3.
Post-translational modifications of MLKL.
| Domain | Residuea | Modification | Modifying enzyme | Effect on necroptosis | Proposed mechanism | Ortholog studied | Cell modelb | Mouse model | Recombinant protein | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Mouse | ||||||||||
| 4HB | S125 | S124 | Phosphorylation | Unknown | —Inconclusive | – | Human, mouse | 293T, MDF | No | No | [47, 188, 189] |
| Brace | – | S158 | Phosphorylation | Unknown | ↓ Inhibits necroptosis | Unknown | Mouse | MDF | No | No | [47] |
| PsKDc | S239 | S228 | Phosphorylation | RIPK3 | ↑ Modestly enhances necroptosis | Unknown | Mouse | MDF | No | No | [47] |
| PsKD | S259 | S248 | Phosphorylation | RIPK3 | ↓ Inhibits necroptosis | Unknown | Mouse | MDF | No | No | [47] |
| PsKD | T357/S358 | S345 | Phosphorylation | RIPK3 | ↑Promotes necroptosis | Promotes MLKL oligomerization and membrane translocation | Human, mouse | HeLa, NIH 3T3, HT29 | No | Yes | [28, 30, 40, 47, 48] |
| PsKD | S360 | S347 | Phosphorylation | RIPK3 | ↑Promotes necroptosis slightly | Unknown | Mouse | MEF | No | No | [30, 48] |
| PsKD | – | T349 | Phosphorylation | RIPK3 | No effect | – | Mouse | MEF | No | No | [30, 48] |
| PsKD | T374 | S360 | Phosphorylation | Unknown | Unknown | – | Human | HeLa | No | No | [188, 189] |
| PsKD | Y376 | Y363 | Phosphorylation | TAM kinases | ↑Promotes necroptosis | Promotes MLKL oligomerization at the plasma membrane | Human, mouse | HT29, MEF, Jurkat, L929 | Yes | No | [187] |
| PsKD | S454 | S441 | Phosphorylation | Unknown | No effect | Mediates myelin breakdown independent from necroptosis pathway | Mouse | Schwann cells | Yes | No | [190] |
| 4HBc | C18 | C18 | Disulfide bond | Unknown | ↑Promotes necroptosis | Mediates polymer formation | Human, mouse | HT29, L929 | No | Yes | [193, 195] |
| 4HB | C24 | C24 | Disulfide bond | Unknown | ↑Promotes necroptosis | Mediates polymer formation | Human, mouse | HT29, L929 | No | Yes | [193, 195] |
| 4HB | C28 | C28 | Disulfide bond | Unknown | ↑Promotes necroptosis | Mediates polymer formation | Human, mouse | HT29, L929 | No | Yes | [193, 195] |
| 4HB | C18/C24/C28 | C18/C24/C28 | Disulfide bond | Unknown | ↑Promotes necroptosis | Possible structural function | Mouse | MDF | No | No | [43] |
| 4HB | C86 | – | Disulfide bond | Unknown | ↑Promotes necroptosis | Mediates polymer formation | Human, mouse | HT29, L929 | No | Yes | [193] |
| – | Disulfide bond reduction | Thioredoxin-1 | ↓ Suppress necroptosis | Prevents polymer formation | Human | HeLa, HT29, 293T | No | Yes | [194] | ||
aEquivalent residues in human and mouse orthologs are inferred by sequence homology. Bold indicates the ortholog residue with direct evidence of such post-translational modification.
bCell line abbreviations: MDF mouse dermal fibroblasts, HeLa human adenocarcinoma cells, NIH-3T3 immortalized mouse embryonic fibroblasts, HT29 human adenocarcinoma cells, MEF mouse embryonic fibroblasts, Jurkat human T lymphocyte Jurkat cells, L929 mouse fibrosarcoma cells, 293T human embryonic kidney cell line HEK293T.
c4HB four-helix bundle domain, PsKD pseudokinase domain.
Beyond the activation loop, additional phosphorylation sites in mouse MLKL were identified as mouse RIPK3 substrates by mass spectrometry [30, 47, 48]. Further residues adjacent to S345 in the mouse MLKL activation loop residues, S347 and T349, are also phosphorylated by mouse RIPK3 [30, 48]. S347 phosphorylation appears to serve an auxiliary role in activating MLKL, while T349 phosphorylation does not measurably contribute [48]. Non-activation loop residues of mouse MLKL were also found to be phosphorylated, including S228 and S248 in the MLKL N-lobe by RIPK3, as well as S158 in the brace region by an unknown kinase [47]. Mouse MLKL bearing phospho-ablating and phosphomimetic mutations at these sites could reconstitute necroptotic signaling in Mlkl−/− fibroblasts, indicating that phosphorylation of these sites is not essential to MLKL activation, but instead likely tunes the necroptotic signal. S228A, S228E, and S158A mouse MLKL mutants induced stimulus-independent cell death, which was aggravated by deletion of RIPK3, leading to the proposal that RIPK3 binding may also act as a negative regulator of necroptosis [47]. These phosphosites are conserved in human MLKL, except S158, which is replaced with Asn, and it remains of interest whether the orthologous residues (and other phosphosites) perform similar functions in the regulation of human MLKL killing function.
Members of the TAM (Tyro3, Axl, and Mer) receptor tyrosine kinases are known to activate multiple pro-survival and proliferation signaling cascades, such as PI3K, MAPK, and Rac pathways, but curiously, they were recently reported to mediate necroptosis by phosphorylating Y376 of human MLKL [187]. Phosphorylation of Y376 was proposed to serve as a secondary cue to induce MLKL oligomerization at the plasma membrane, although the precise chronology remains an open question after MLKL was observed to form oligomers prior to membrane translocation [51, 52]. While a human MLKL Y376F mutant failed to reconstitute necroptosis in HeLa cells overexpressing RIPK3 [187], it remains to be established under what circumstances Y376 is solvent exposed. In human MLKL structures, Y376 is located in the short helix at the C-terminal end of the activation loop and performs a structural role within the C-lobe of the pseudokinase domain. Thus, it is of interest to understand the conditions that lead to its exposure for phosphorylation and the ubiquity of this phenomenon.
Additional phosphosites in MLKL have been identified by mass spectrometry, although these have not yet been attributed functions in necroptotic signaling and nor have the responsible kinases been identified. S125 and T374 in human MLKL were phosphorylated in a cell cycle dependent manner [188, 189], while mouse MLKL phosphorylation on S441 was attributed a role in provoking MLKL-directed myelin breakdown in Schwann cells [190]. The occurrence of these events raises the possibility that additional, as yet unidentified sites are likely to be phosphorylated within MLKL in different cellular contexts upon exposure to different stimuli. It is also noteworthy that some species, including chickens, express MLKL, but not RIPK3 [19, 191, 192], raising the possibility that, in addition to RIPK3, other kinases may phosphorylate MLKL to provoke its necroptotic activity. Currently, however, the existence and identities of these kinases, and the stimuli and cellular contexts that might provoke their activity toward MLKL, are unknown.
Ubiquitylation
Ubiquitylation of MLKL has been observed following necroptotic stimulation in bone marrow-derived macrophages in a TRIF-dependent manner [172]. However, the function, underlying mechanisms, ubiquitylation sites, and the responsible E3 ligases remain unclear to date.
Other post-translational modifications
MLKL has been reported to form a disulfide-dependent amyloid-like polymer during necroptosis in cells [193], with thioredoxin-1 (Trx-1) proposed to suppress necroptosis by reducing MLKL 4HB domain cysteines to prevent oligomer formation [194]. However, the precise function and the ubiquity of MLKL amyloid forms remain unclear. It is notable that disulfide-bonded MLKL tetramers have become a widely used diagnostic for MLKL activation by non-reducing SDS-PAGE, although formation of disulfide bonds between MLKL protomers in cells prior to lysis has not been clearly demonstrated. Accordingly, these oligomers, which represent a small proportion of cellular MLKL in most studies, could well represent disulfide linkages that arise as a consequence of cell lysis owing to the proximity of MLKL protomers to one another within the necrosome. Therefore, the physiological function of MLKL oligomers formed from intersubunit disulfide bonds requires further investigation.
Conclusions and open questions
Among the post-translational modifications reported to regulate the core necroptotic proteins—RIPK1, RIPK3, and MLKL—the preponderance is reported to target the apical effector, RIPK1. In addition to RIPK1 having been studied for many years more than RIPK3 and MLKL, RIPK1 participates in pro-inflammatory TNF signaling, and cell death by apoptosis and necroptosis. Consequently, many post-translational modifications serve to divert RIPK1’s involvement between these different signaling conduits, whereas those in RIPK3 and MLKL direct their activation in cell death signaling. To date, the bulk of post-translational modifications in RIPK3 and MLKL are reported to activate their necroptotic functions, and consequently it remains to be discovered whether negative regulatory modifications exist and whether the activating post-translational modifications can be defused with other modifying enzymes, such as deubiquitinases and phosphatases. Thus far, only A20 has been identified as a deubiquitinase and Ppm1b a phosphatase for RIPK3, while none have been reported for MLKL. As our understanding of differences in signaling events between species, cell lines and necroptotic stimuli grows, we expect that future studies will reveal further post-translational modifications within RIPK3 and MLKL and unearth negative regulators to remove post-translational marks and halt the progression of necroptotic death. Such studies will reveal further checkpoints in the signaling pathway, which could be targeted therapeutically to counter necroptotic pathologies.
Funding
We are grateful for scholarship support for YM (Melbourne Research Scholarship and AINSE PGRA scholarship) and thank the Australian National Health and Medical Research Council for fellowship (PEC, 1079700; JMM, 1172929), grant (1124735, 1124737), and infrastructure (IRIISS 9000587) support, with additional support from the Victorian Government Operational Infrastructure Support scheme.
Author contributions
Critical evaluation of published studies: all authors; writing—original draft: YM; writing—review and editing: all authors.
Compliance with ethical standards
Conflict of interest
PEC and JMM contribute to a project developing necroptosis inhibitors in collaboration with Anaxis Pharma. The other authors declare no conflict of interest.
Ethics statement
Not applicable.
Footnotes
Edited by G. Melino
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 2.Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, et al. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1995;47:67–75. [PubMed] [Google Scholar]
- 3.Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9:801–8. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 4.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–30. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 7.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heckmann BL, Tummers B, Green DR. Crashing the computer: apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ. 2019;26:41–52. doi: 10.1038/s41418-018-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 10.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Nailwal H, Chan FKM. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019;26:4–13. doi: 10.1038/s41418-018-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson JS, Murphy JM. Down the rabbit hole: Is necroptosis truly an innate response to infection? Cell Microbiol. 2017;19:e12750. doi: 10.1111/cmi.12750. [DOI] [PubMed] [Google Scholar]
- 13.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–51. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Kaiser WJ, Mocarski ES. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol. 2015;204:439–48. doi: 10.1007/s00430-015-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler H, Cotsmire S, Langland J, Kibler KV, Kalman D, Upton JW, et al. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc Natl Acad Sci. 2017;114:11506–11. doi: 10.1073/pnas.1700999114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson JS, Giogha C, Muhlen S, Nachbur U, Pham CL, Zhang Y, et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steain M, Baker M, Pham CLL, Shanmugam N, Gambin Y, Sierecki E, et al. Varicella zoster virus encodes a viral decoy RHIM to inhibit cell death. PLoS Pathog. 2020;16:e1008473. doi: 10.1371/journal.ppat.1008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie EJ, Sandow JJ, Lehmann WIL, Liang LY, Coursier D, Young SN, et al. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep. 2019;28:3309–3319.e5. doi: 10.1016/j.celrep.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 20.Pollock GL, Oates CVL, Giogha C, Wong Fok Lung T, Ong SY, Pearson JS, et al. Distinct roles of the antiapoptotic effectors NleB and NleF from enteropathogenic Escherichia coli. Infect Immun. 2017;85:e01071–16. [DOI] [PMC free article] [PubMed]
- 21.Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–6. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- 22.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–7. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher-Etherington A, Nobre L, Nightingale K, Antrobus R, Nichols J, Davison AJ, et al. Human cytomegalovirus protein pUL36: a dual cell death pathway inhibitor. Proc Natl Acad Sci USA. 2020;117:18771–9. doi: 10.1073/pnas.2001887117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdi LK, Huang M, Zhang X, Nakano RT, Kopp LB, Saur IML, et al. Discovery of a family of mixed lineage kinase domain-like proteins in plants and their role in innate immune signaling. Cell Host Microbe. 2020;28:813–824.e6. [DOI] [PubMed]
- 25.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Zhang DW, Shao J, Lin J, Zhang N, Lu B-J, Lin S-C, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–53. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Webster JD, Dugger DL, Goncharov T, Roose-Girma M, Hung J, et al. Ubiquitin ligases cIAP1 and cIAP2 limit cell death to prevent inflammation. Cell Rep. 2019;27:2679–2689.e3. doi: 10.1016/j.celrep.2019.04.111. [DOI] [PubMed] [Google Scholar]
- 32.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–72. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- 34.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20:1381–92. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Devin A, Rodriguez Y, Liu Z. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–26. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng S, Yang Y, Mei Y, Ma L, Zhu D-e, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–31. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 38.Mompean M, Li W, Li J, Laage S, Siemer AB, Bozkurt G, et al. The structure of the necrosome RIPK1-RIPK3 Core, a human hetero-amyloid signaling complex. Cell. 2018;173:1244–1253.e10. doi: 10.1016/j.cell.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–50. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Li W, Ren J, Huang D, He WT, Song Y, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–21. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA. 2014;111:15072–7. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Petrie EJ, Hildebrand JM, Murphy JM. Insane in the membrane: a structural perspective of MLKL function in necroptosis. Immunol Cell Biol. 2017;95:152–9. doi: 10.1038/icb.2016.125. [DOI] [PubMed] [Google Scholar]
- 46.Murphy JM. The killer pseudokinase mixed lineage kinase domain-like protein (MLKL). Cold Spring Harb Perspect Biol. 2020;12:a036376. [DOI] [PMC free article] [PubMed]
- 47.Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, Hall C, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochemical J. 2015;471:255–65. doi: 10.1042/BJ20150678. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23:76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murai S, Yamaguchi Y, Shirasaki Y, Yamagishi M, Shindo R, Hildebrand JM, et al. A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat Commun. 2018;9:4457. doi: 10.1038/s41467-018-06985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300.e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samson AL, Zhang Y, Geoghegan ND, Gavin XJ, Davies KA, Mlodzianoski MJ, et al. MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat Commun. 2020;11:3151. doi: 10.1038/s41467-020-16887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrie EJ, Birkinshaw RW, Koide A, Denbaum E, Hildebrand JM, Garnish SE, et al. Identification of MLKL membrane translocation as a checkpoint in necroptotic cell death using Monobodies. Proc Natl Acad Sci USA. 2020;117:8468–75. doi: 10.1073/pnas.1919960117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson J, Loh Z, Ullah MA, Lynch JP, Werder RB, Collinson N, et al. Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells. Am J Respir Crit Care Med. 2020;201:1358–71. doi: 10.1164/rccm.201906-1149OC. [DOI] [PubMed] [Google Scholar]
- 54.Shubina M, Tummers B, Boyd DF, Zhang T, Yin C, Gautam A, et al. Necroptosis restricts influenza A virus as a stand-alone cell death mechanism. J Exp Med. 2020;217:e20191259. [DOI] [PMC free article] [PubMed]
- 55.Molnar T, Mazlo A, Tslaf V, Szollosi AG, Emri G, Koncz G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019;10:860. doi: 10.1038/s41419-019-2094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4:e128834. [DOI] [PMC free article] [PubMed]
- 57.Hildebrand JM, Kauppi M, Majewski IJ, Liu Z, Cox AJ, Miyake S, et al. A missense mutation in the MLKL brace region promotes lethal neonatal inflammation and hematopoietic dysfunction. Nat Commun. 2020;11:3150. doi: 10.1038/s41467-020-16819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasheed A, Robichaud S, Nguyen MA, Geoffrion M, Wyatt H, Cottee ML, et al. Loss of MLKL (mixed lineage kinase domain-like protein) decreases necrotic core but increases macrophage lipid accumulation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40:1155–67. doi: 10.1161/ATVBAHA.119.313640. [DOI] [PubMed] [Google Scholar]
- 59.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–16. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 60.Muller T, Dewitz C, Schmitz J, Schroder AS, Brasen JH, Stockwell BR, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74:3631–45. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–18. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–76. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierotti CL, Tanzer MC, Jacobsen AV, Hildebrand JM, Garnier JM, Sharma P, et al. Potent inhibition of necroptosis by simultaneously targeting multiple effectors of the pathway. ACS Chem Biol. 2020;15:2702–13. doi: 10.1021/acschembio.0c00482. [DOI] [PubMed] [Google Scholar]
- 64.Pierdomenico M, Negroni A, Stronati L, Vitali R, Prete E, Bertin J, et al. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol. 2014;109:279–87. doi: 10.1038/ajg.2013.403. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–8. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34:757–774.e7. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dermentzaki G, Politi KA, Lu L, Mishra V, Perez-Torres EJ, Sosunov AA, et al. Deletion of Ripk3 prevents motor neuron death in vitro but not in vivo. eNeuro. 2019;6:eneuro.0308–18.2018. [DOI] [PMC free article] [PubMed]
- 68.Dominguez S, Varfolomeev E, Brendza R, Stark K, Tea J, Imperio J, et al. Genetic inactivation of RIP1 kinase does not ameliorate disease in a mouse model of ALS. Cell Death Differ. 2020. 10.1038/s41418-020-00625-7. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 69.Wang T, Perera ND, Chiam MDF, Cuic B, Wanniarachchillage N, Tomas D, et al. Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ. 2020;27:1728–39. doi: 10.1038/s41418-019-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alvarez-Diaz S, Preaudet A, Samson AL, Nguyen PM, Fung KY, Garnham AL, et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2020; 10.1038/s41418-020-00673-z. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 71.Patel S, Webster JD, Varfolomeev E, Kwon YC, Cheng JH, Zhang J, et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2020;27:161–75. doi: 10.1038/s41418-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webster JD, Kwon YC, Park S, Zhang H, Corr N, Ljumanovic N, et al. RIP1 kinase activity is critical for skin inflammation but not for viral propagation. J Leukoc Biol. 2020;107:941–52. [DOI] [PMC free article] [PubMed]
- 73.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen S, Seidelin JB, LaCasse EC, Nielsen OH. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci Signal. 2020;13:eaax8295. [DOI] [PubMed]
- 76.Lalaoui N, Boyden SE, Oda H, Wood GM, Stone DL, Chau D, et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature. 2020;577:103–8. doi: 10.1038/s41586-019-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577:109–14. doi: 10.1038/s41586-019-1830-y. [DOI] [PubMed] [Google Scholar]
- 78.Vlantis K, Wullaert A, Polykratis A, Kondylis V, Dannappel M, Schwarzer R, et al. NEMO prevents RIP kinase 1-mediated epithelial cell death and chronic intestinal inflammation by NF-kappaB-dependent and -independent functions. Immunity. 2016;44:553–67. doi: 10.1016/j.immuni.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 81.Raju S, Whalen DM, Mengistu M, Swanson C, Quinn JG, Taylor SS, et al. Kinase domain dimerization drives RIPK3-dependent necroptosis. Sci Signal. 2018;11:eaar2188. [DOI] [PMC free article] [PubMed]
- 82.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci USA. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang D, Liang Y, Zhao S, Ding Y, Zhuang Q, Shi Q, et al. ZBP1 mediates interferon-induced necroptosis. Cell Mol Immunol. 2020;17:356–68. doi: 10.1038/s41423-019-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–8. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–33. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 87.McQuade T, Cho Y, Chan FK. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem J. 2013;456:409–15. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrie EJ, Czabotar PE, Murphy JM. The structural basis of necroptotic cell death signaling. Trends Biochem Sci. 2019;44:53–63. doi: 10.1016/j.tibs.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Petrie EJ, Sandow JJ, Jacobsen AV, Smith BJ, Griffin MDW, Lucet IS, et al. Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun. 2018;9:2422. doi: 10.1038/s41467-018-04714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA. 2019;116:9714–22. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ting AT, Pimentel-Muiños FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–96. [PMC free article] [PubMed] [Google Scholar]
- 92.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 93.Amin P, Florez M, Najafov A, Pan H, Geng J, Ofengeim D, et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl Acad Sci USA. 2018;115:E5944–E5953. doi: 10.1073/pnas.1806973115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, et al. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ. 2014;21:1600–12. doi: 10.1038/cdd.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA. 2014;111:7753–8. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 97.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–88. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 98.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kearney CJ, Cullen SP, Clancy D, Martin SJ. RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J. 2014;281:4921–34. doi: 10.1111/febs.13034. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laurien L, Nagata M, Schunke H, Delanghe T, Wiederstein JL, Kumari S, et al. Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat Commun. 2020;11:1747. doi: 10.1038/s41467-020-15466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, et al. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21:1511–21. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, et al. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–20. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen W, Zhou Z, Li L, Zhong C-Q, Zheng X, Wu X, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288:16247–61. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5:70–78. doi: 10.1016/j.celrep.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 106.Samson AL, Fitzgibbon C, Patel KM, Hildebrand JM, Whitehead LW, Rimes JS, et al. A toolbox for imaging RIPK1, RIPK3 and MLKL in mouse and human cells. bioRxiv. 2020. 10.1101/2020.10.26.356063. [DOI] [PMC free article] [PubMed]
- 107.Su L, Quade B, Wang H, Sun L, Wang X, Rizo J. A plug release mechanism for membrane permeation by MLKL. Structure. 2014;22:1489–1500. doi: 10.1016/j.str.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanzer MC, Matti I, Hildebrand JM, Young SN, Wardak A, Tripaydonis A, et al. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ. 2016;23:1185–97. doi: 10.1038/cdd.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie T, Peng W, Liu Y, Yan C, Maki J, Degterev A, et al. Structural basis of RIP1 inhibition by necrostatins. Structure. 2013;21:493–9. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 111.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–80. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tokunaga F, Iwai K. LUBAC, a novel ubiquitin ligase for linear ubiquitination, is crucial for inflammation and immune responses. Microbes Infect. 2012;14:563–72. doi: 10.1016/j.micinf.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 113.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 114.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 115.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–48. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 116.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105:11778–83. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 118.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, et al. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 119.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 120.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 121.Dondelinger Y, Delanghe T, Priem D, Wynosky-Dolfi MA, Sorobetea D, Rojas-Rivera D, et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat Commun. 2019;10:1729. doi: 10.1038/s41467-019-09690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaco I, Annibaldi A, Lalaoui N, Wilson R, Tenev T, Laurien L, et al. MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol Cell. 2017;66:698–710.e5. doi: 10.1016/j.molcel.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dondelinger Y, Delanghe T, Rojas-Rivera D, Priem D, Delvaeye T, Bruggeman I, et al. MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat Cell Biol. 2017;19:1237–47. doi: 10.1038/ncb3608. [DOI] [PubMed] [Google Scholar]
- 124.Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8:359. doi: 10.1038/s41467-017-00406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu W, Wang X, Berleth N, Deitersen J, Wallot-Hieke N, Bohler P, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31:107547. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 126.Peltzer N, Darding M, Walczak H. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 2016;26:445–61. doi: 10.1016/j.tcb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 127.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–24. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lalaoui N, Vaux DL. Recent advances in understanding inhibitor of apoptosis proteins. F1000Res. 2018;7:F1000 Faculty Rev-1889.
- 129.Kist M, Komuves LG, Goncharov T, Dugger DL, Yu C, Roose-Girma M, et al. Impaired RIPK1 ubiquitination sensitizes mice to TNF toxicity and inflammatory cell death. Cell Death Differ. 2020. 10.1038/s41418-020-00629-3. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 130.Tang Y, Tu H, Zhang J, Zhao X, Wang Y, Qin J, et al. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat Commun. 2019;10:4157. doi: 10.1038/s41467-019-12033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X, Zhang H, Xu C, Li X, Li M, Wu X, et al. Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat Commun. 2019;10:4158. doi: 10.1038/s41467-019-11839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Shan B, Liang Y, Wei H, Yuan J. Parkin regulates NF-kappaB by mediating site-specific ubiquitination of RIPK1. Cell Death Dis. 2018;9:732. doi: 10.1038/s41419-018-0770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010;29:4198–209. doi: 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 135.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 136.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–6. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 137.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–41. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Damgaard RB, Nachbur U, Yabal M, Wong WW, Fiil BK, Kastirr M, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell. 2012;46:746–58. doi: 10.1016/j.molcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 139.Fiil BK, Damgaard RB, Wagner SA, Keusekotten K, Fritsch M, Bekker-Jensen S, et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol Cell. 2013;50:818–30. doi: 10.1016/j.molcel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci USA. 2013;110:15247–52. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peltzer N, Darding M, Montinaro A, Draber P, Draberova H, Kupka S, et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112–7. doi: 10.1038/s41586-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moquin DM, McQuade T, Chan FK-M. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLOS ONE. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13:2258–72. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peltzer N, Rieser E, Taraborrelli L, Draber P, Darding M, Pernaute B, et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014;9:153–65. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 145.Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife. 2014;3:e03464. doi: 10.7554/eLife.03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumari S, Redouane Y, Lopez-Mosqueda J, Shiraishi R, Romanowska M, Lutzmayer S, et al. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. Elife. 2014;3:e03422. [DOI] [PMC free article] [PubMed]
- 147.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 148.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 149.Legarda D, Justus SJ, Ang RL, Rikhi N, Li W, Moran TM, et al. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell Rep. 2016;15:2449–61. doi: 10.1016/j.celrep.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ganjam GK, Terpolilli NA, Diemert S, Eisenbach I, Hoffmann L, Reuther C, et al. Cylindromatosis mediates neuronal cell death in vitro and in vivo. Cell Death Differ. 2018;25:1394–407. doi: 10.1038/s41418-017-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–4. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Verhelst K, Carpentier I, Kreike M, Meloni L, Verstrepen L, Kensche T, et al. A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 2012;31:3845–55. doi: 10.1038/emboj.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Polykratis A, Martens A, Eren RO, Shirasaki Y, Yamagishi M, Yamaguchi Y, et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol. 2019;21:731–42. doi: 10.1038/s41556-019-0324-3. [DOI] [PubMed] [Google Scholar]
- 154.Garcia-Carbonell R, Wong J, Kim JY, Close LA, Boland BS, Wong TL, et al. Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc Natl Acad Sci USA. 2018;115:E9192–E9200. doi: 10.1073/pnas.1810584115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Almagro MC, Goncharov T, Newton K, Vucic D. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 2015;6:e1800. doi: 10.1038/cddis.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seo J, Lee EW, Sung H, Seong D, Dondelinger Y, Shin J, et al. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol. 2016;18:291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 157.Mollah S, Wertz IE, Phung Q, Arnott D, Dixit VM, Lill JR. Targeted mass spectrometric strategy for global mapping of ubiquitination on proteins. Rapid Commun Mass Spectrom. 2007;21:3357–64. doi: 10.1002/rcm.3227. [DOI] [PubMed] [Google Scholar]
- 158.Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–40. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Elliott PR, Leske D, Hrdinka M, Bagola K, Fiil BK, McLaughlin SH, et al. SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling. Mol Cell. 2016;63:990–1005. doi: 10.1016/j.molcel.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–82. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 162.Wang H, Meng H, Li X, Zhu K, Dong K, Mookhtiar AK, et al. PELI1 functions as a dual modulator of necroptosis and apoptosis by regulating ubiquitination of RIPK1 and mRNA levels of c-FLIP. Proc Natl Acad Sci USA. 2017;114:11944–9. doi: 10.1073/pnas.1715742114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Almagro MC, Goncharov T, Izrael-Tomasevic A, Duttler S, Kist M, Varfolomeev E, et al. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ. 2017;24:26–37. doi: 10.1038/cdd.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi SW, Park HH, Kim S, Chung JM, Noh HJ, Kim SK, et al. PELI1 selectively targets kinase-active RIP3 for ubiquitylation-dependent proteasomal degradation. Mol Cell. 2018;70:920–935.e7. doi: 10.1016/j.molcel.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 165.Zhang X, Dowling JP, Zhang J. RIPK1 can mediate apoptosis in addition to necroptosis during embryonic development. Cell Death Dis. 2019;10:245. doi: 10.1038/s41419-019-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–82. doi: 10.1038/s41586-019-1752-8. [DOI] [PubMed] [Google Scholar]
- 167.Zhang H, Zhou X, McQuade T, Li J, Chan FK-M, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–6. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 170.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 171.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 174.Wicki S, Gurzeler U, Wong WW, Jost PJ, Bachmann D, Kaufmann T. Loss of XIAP facilitates switch to TNFalpha-induced necroptosis in mouse neutrophils. Cell Death Dis. 2016;7:e2422. doi: 10.1038/cddis.2016.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McComb S, Shutinoski B, Thurston S, Cessford E, Kumar K, Sad S. Cathepsins limit macrophage necroptosis through cleavage of Rip1 kinase. J Immunol. 2014;192:5671–8. doi: 10.4049/jimmunol.1303380. [DOI] [PubMed] [Google Scholar]
- 176.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, et al. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Davies KA, Tanzer MC, Griffin MDW, Mok YF, Young SN, Qin R, et al. The brace helices of MLKL mediate interdomain communication and oligomerisation to regulate cell death by necroptosis. Cell Death Differ. 2018;25:1567–80. doi: 10.1038/s41418-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Davies KA, Fitzgibbon C, Young SN, Garnish SE, Yeung W, Coursier D, et al. Distinct pseudokinase domain conformations underlie divergent activation mechanisms among vertebrate MLKL orthologues. Nat Commun. 2020;11:3060. doi: 10.1038/s41467-020-16823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Sun X, Wu J, Xu B-e, Gu C, Wang H, et al. Casein kinase 1α interacts with RIP1 and regulates NF-κB activation. Biochemistry. 2008;47:441–8. doi: 10.1021/bi7010515. [DOI] [PubMed] [Google Scholar]
- 180.Lee SY, Kim H, Li CM, Kang J, Najafov A, Jung M, et al. Casein kinase-1gamma1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis. 2019;10:923. doi: 10.1038/s41419-019-2146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hanna-Addams S, Liu S, Liu H, Chen S, Wang Z. CK1alpha, CK1delta, and CK1epsilon are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc Natl Acad Sci USA. 2020;117:1962–70. doi: 10.1073/pnas.1917112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li D, Ai Y, Guo J, Dong B, Li L, Cai G, et al. Casein kinase 1G2 suppresses necroptosis-promoted testis aging by inhibiting receptor-interacting kinase 3. eLife. 2020;9:e61564. doi: 10.7554/eLife.61564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen W, Wu J, Li L, Zhang Z, Ren J, Liang Y, et al. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat Cell Biol. 2015;17:434–44. doi: 10.1038/ncb3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–27. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li X, Gong W, Wang H, Li T, Attri KS, Lewis RE, et al. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/threonine-protein kinase 3. Immunity. 2019;50:576–590.e6. doi: 10.1016/j.immuni.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murphy JM, Lucet IS, Hildebrand JM, Tanzer MC, Young SN, Sharma P, et al. Insights into the evolution of divergent nucleotide-binding mechanisms among pseudokinases revealed by crystal structures of human and mouse MLKL. Biochemical J. 2014;457:369–77. doi: 10.1042/BJ20131270. [DOI] [PubMed] [Google Scholar]
- 187.Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, et al. TAM Kinases Promote Necroptosis by Regulating Oligomerization of MLKL. Mol Cell. 2019;75:457–468.e4. doi: 10.1016/j.molcel.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 188.Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–48. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 189.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–7. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ying Z, Pan C, Shao T, Liu L, Li L, Guo D, et al. Mixed lineage kinase domain-like protein MLKL breaks down myelin following nerve injury. Mol Cell. 2018;72:457–468.e4. doi: 10.1016/j.molcel.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 191.Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 2016;26:721–32. doi: 10.1016/j.tcb.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 192.Newton K, Manning G. Necroptosis and Inflammation. Annu Rev Biochem. 2016;85:743–63. doi: 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- 193.Liu S, Liu H, Johnston A, Hanna-Addams S, Reynoso E, Xiang Y, et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci USA. 2017;114:E7450–E7459. doi: 10.1073/pnas.1707531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reynoso E, Liu H, Li L, Yuan AL, Chen S, Wang Z. Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis. J Biol Chem. 2017;292:17514–24. doi: 10.1074/jbc.M117.799353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huang D, Zheng X, Wang Z-A, Chen X, He W-T, Zhang Y, et al. The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process. Mol Cell Biol. 2017;37:e00497–16. doi: 10.1128/MCB.00497-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dovey CM, Diep J, Clarke BP, Hale AT, McNamara DE, Guo H, et al. MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell. 2018;70:936–948.e7. doi: 10.1016/j.molcel.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McNamara DE, Dovey CM, Hale AT, Quarato G, Grace CR, Guibao CD, et al. Direct activation of human MLKL by a select repertoire of inositol phosphate metabolites. Cell Chem Biol. 2019;26:863–877.e7. [DOI] [PMC free article] [PubMed]
- 198.Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bigenzahn JW, Fauster A, Rebsamen M, Kandasamy RK, Scorzoni S, Vladimer GI, et al. An inducible retroviral expression system for tandem affinity purification mass-spectrometry-based proteomics identifies mixed lineage kinase domain-like protein (MLKL) as an heat shock protein 90 (HSP90) client. Mol Cell Proteom. 2016;15:1139–50. doi: 10.1074/mcp.O115.055350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, et al. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis. 2016;7:e2089. doi: 10.1038/cddis.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Johnston AN, Ma Y, Liu H, Liu S, Hanna-Addams S, Chen S, et al. Necroptosis-blocking compound NBC1 targets heat shock protein 70 to inhibit MLKL polymerization and necroptosis. Proc Natl Acad Sci USA. 2020;117:6521–30. doi: 10.1073/pnas.1916503117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47:51–65.e7. doi: 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 203.Fan W, Guo J, Gao B, Zhang W, Ling L, Xu T, et al. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci Signal. 2019;12:eaaw3423. doi: 10.1126/scisignal.aaw3423. [DOI] [PubMed] [Google Scholar]
- 204.Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, et al. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics. 2015;31:3359–61. doi: 10.1093/bioinformatics/btv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hart AC, Abell L, Guo J, Mertzman ME, Padmanabha R, Macor JE, et al. Identification of RIPK3 type II inhibitors using high-throughput mechanistic studies in hit triage. ACS Med Chem Lett. 2020;11:266–71. doi: 10.1021/acsmedchemlett.9b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]