Abstract
With a robust rise in the number of COVID-19 cases, the World Health Organization (WHO) has declared COVID-19 as a pandemic on 11th March 2020. COVID-19 pandemic has invited global researchers from various biomedical and biotechnological researchers to plan various treatment modalities for combating this pandemic crisis. At present, there is the unavailability of specific treatment modality; however, researchers have thrown light into the exploration of mesenchymal stem cells (MSCs) to therapeutically perquisite in ameliorating immune-mediated progressive worsening in COVID-19 infected patients. Cellular therapy (CT) has revolutionized the treatment of untreatable diseases with a better clinical and functional outcome. Placenta, being considered as medical waste, contains a variety of stem cells, and hence placenta-derived MSCs (P-MSCs) owe potentiality for extrapolation to combat COVID-19 pandemic. The usage of P-MSCs in combating the COVID-19 pandemic has plausible challenges in terms of isolation, harvesting, expansion, characterization, and involvement of ethical concerns. This article provides an insight into dealing COVID-19 pandemic with P-MSCs as cell-based therapy embracing immunomodulatory and immune-privileged potentials and future prospects. Advocating prospective randomized controlled clinical trials ethically will concretely supplement for its efficacy and safety concerns.
Keywords: Coronavirus, COVID-19, cellular therapy (CT), placenta-derived mesenchymal stem cells (P-MSCs)
Introduction
Towards the end of 2019, numerous cases popped out with symptoms of seasonal flu in Wuhan city of China wherein the detailed investigation unveiled the history of exposure in the Hunan Seafood market. In January 2020, the aetiological factor responsible for such health deterioration was identified as coronavirus disease which was the subset of Sarbecovirus (1) and 79.6% genetically identical to the genome of SARS-CoV (2). On a later date, the World Health Organization (WHO) named the disease as SARS-CoV-2 virus-associated disease and agent factor as SARS-CoV-2. Slowly on due course, a large number of countries got infected with COVID-19 infection, and hence on 11th March 2020, WHO declared COVID-19 as a pandemic disease which is of public health importance. The natural course of the COVID-19 was traced out with evolving symptoms, signs, and clinical presentation. To date, no proper treatment in terms of drugs or vaccines is available for treating COVID-19 patients. Quarantine and social distancing have been adapted as the core principles of deterrence. The clinicians, biomedical experts, and researchers from varying specialties across the globe have been mobilized for tracing a solution for the COVID-19 pandemic.
According to the International Society for Stem Cell Research (ISSCR), no stem cell-based therapies have been approved for the treatment of COVID-19. Recently due to the evolution of cell-based therapy, stem cell and regenerative medicine in various fields have thrown a light on mesenchymal stem cells (MSCs) as one of the therapeutic approaches for treating COVID-19 (3). MSCs have the intrinsic ability to regenerate the damaged tissues to maintain the naïve homeostasis. MSCs possess the intrinsic ability to curb the infection with pre-defined cytokines, chemokines, and growth factors. On this horizon, placental stem cells with MSC activity play a vital role in curbing COVID-19 pneumonia with improved lung compliance (4). Being considered as medical waste, the placenta serves as a temporary immune-tolerant unit to maintain the pregnancy and feto-maternal circuit. The placenta prevails as an immune-privileged organ and hence prevents immunological rejection (5,6). Over decades, researchers have scrutinized the stem cell activity of various cells present in the placenta for therapeutic applications and biomedical research. With recent advances in cell-based therapy and auto-bio-banking, awareness among the public and researchers gained significant importance over the placenta, placental cells, and placental extracts. The lung injury associated with COVID-19 is due to the aberrant, uncontrolled inflammatory response leading to a surge in pro-inflammatory mediators (IL-6, IL-1, TNF-α, and interferon) produced by lymphocytes, dendritic cells, and macrophages resulting in a phenomenon known as ‘cytokine storm’ (7-12). There is a strong correlation between cytokine levels and the associated lung injury, acute respiratory distress syndrome (ARDS), and the poor prognosis in COVID-19 infection (13). This research article provides an overview of how placental stem cells render immune privilege in COVID-19 patients and serves as a beacon of hope to dispense therapeutically in attenuating this cytokine storm phenomenon in these patients; provided prospective randomized controlled clinical trials adduce concretely for its efficacy and safety respectively.
Placenta and its stem cells
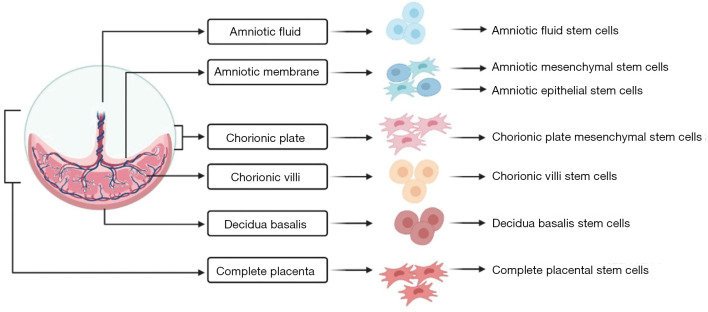
The placenta is a temporary immunological organ that maintains the pregnancy without any immunological rejection. In post-partum, the placenta weighs around 500 grams and possesses a discoid shape in structure. In course of pregnancy, the placenta changes its morphology from the immature placenta in the first and second trimester of pregnancy to the mature placenta in the third trimester. Though immature placenta faces a lot of ethical concerns over its usage in therapeutic applications, researchers are interested in the mature placenta which possess various compartments of stem cells as shown in Figure 1.
Figure 1.
Forms of placental stem cells.
Being primitive and pluripotent, stem cell-like cells in the placenta has high potentiality for self-renewal and differentiation than MSCs from other sources (14). Placenta-derived MSCs (P-MSCs) represent a homogeneous population of cells with an intrinsic ability for homing and cellular lineage priming potential. They show low immunological properties in both in-vivo and in-vitro studies. They exhibit a property of high proliferative rate in culture than bone marrow-derived MSCs (BM-MSCs) (15). P-MSCs possess low telomerase activity and hence they are safe in regenerating the tissues of interest.
Various forms of placental MSCs
Amniotic membrane-derived MSCs (AMe-MSCs)
AMe-MSCs are of amniotic epithelial stem cells (AE-SCs) amniotic MSCs (A-MSCs). AMe-MSCs are pluripotent and are isolated from the fibroblastic layer of the amniotic membrane. AMe-MSCs suppress inflammation and enhance immunomodulation. They promote angiogenesis, inhibit oxidative stress, stimulates remyelination, and regulates matrix metalloproteinases (16). AMe-MSCs express high levels of a cell adhesion molecule surface markers than the chorionic plate or the decidua cells. AMe-MSCs possess a low level of differentiation capacity than chorionic plate-derived MSCs (CP-MSCs) and chorionic villous-derived MSCs (CV-MSCs) (17).
Amniotic fluid-derived MSCs (AF-MSCs)
An alternative approach could result from the use of MSCs derived from extra-embryonic tissues, which possess the advantage of being isolated from tissues normally discarded after birth, hence exempt from ethical concern, such as amniotic fluid, umbilical cord, and placenta (18). Though heterogeneous populations, AF-MSCs are composed of fetal-derived-differentiated and undifferentiated progenitor cells (19). AF-MSCs are less differentiated with superior replicative lifespan and proliferating potential and more pluripotent than BM-MSCs (20). Due to the paracrine nature of cytokines and chemokines in AF-MSCs, they induce vasculogenesis, angiogenesis, and osteogenesis. AF-MSCs does not form teratoma in-vivo (21). AF-MSCs demonstrated no karyotypic aberrations or transformation potential in-vitro and no tumorigenic effect in-vivo (22).
Chorionic plate-derived MSCs
Though isolated from the chorionic plate of the placenta, CP-MSCs are of multipotent in nature. The chorionic plate is composed of the amnion, extra-amniotic mesenchymal cells, cytotrophoblast, and syncytiotrophoblast (23). CP-MSC possesses preserved homing and priming potential. CP-MSCs reveal a higher capacity to inhibit T-cell proliferation and superior angiogenic potential than other placental stem cells (24). CP-MSCs expresses higher genes for differentiation into adipogenic, osteogenic, and hepatogenic lineages than Wharton’s jelly-derived MSCs (WJ-MSCs) (25).
Chorionic villous-derived MSCs
Portmann-Lanz et al. isolated CV-MSCs from the placenta. He defined that isolation of chorionic villous as a complex from the entire placenta. Chorionic villous cells mimic the properties of MSCs and are composed of stromal fibroblasts, endothelial cells, and macrophages (26). CV-MSCs possess a greater pluripotent potential and retarded aging phenotype than BM-MSCs (27). Barlow et al., observed a faster proliferation rate and long term growth abilities of CV-MSCs than BM-MSCs (28).
Decidual stem cells (DSCs)
Despite embryo implantation and placentation by decidua, it is composed of heterogeneous, multipotent stromal cells, glandular cells, and leukocytes. They stain positive for MHC-II, CD29, -73, and -90 and negative for CD34, -45, -56, and -16. DSCs differentiate into various lineages of germ cells (29). Decidual stromal cells alter the levels of metalloproteinases in the environment and induce the secretion of IL-6 and -8 and TNF-α. DSCs enhance angiogenesis (30). DSCs serve as a potential source of treatment for ischemic diseases and neurodegenerative diseases. Decidual stem cells had shown higher proliferation capacity compared to MSCs from the amniotic membrane or chorionic plate (17).
Umbilical cord blood cells (UBCs)
From the last two decades, UBCs gained significance among stem cell researchers and clinicians for treating diseases of bone marrow and inborn error of metabolism. Cord blood cells do not require human leukocyte antigen (HLA) matching and shown less incidence of graft versus host disease (GVHD) responses. Cord blood cells can be banked and used on a later date as “off-the-shelf” (31). The four forms of stem cells identified in UC are (I) whole umbilical cord-derived MSCs (UC-MSCs), (II) umbilical cord Wharton’s jelly (UC-WJ), umbilical cord artery (UCA), and umbilical cord vein (UCV) MSCs (obtained a result of mincing after removing umbilical vessels), (III) UC lining and sub-amnion-derived MSCs and (IV) UC perivascular stem cells (UC-PVC) (32). These cord blood cells possess plastic adherent morphology in cultures and co-cultures. They possess cell surface antigenic markers such as CD73, -90, and -105. These UBCs finally differentiate into osteoblasts, chondroblasts, and adipocytes in-vitro. Human umbilical cord matrix (UCM) cells are isolated from the Wharton’s jelly of human cords. Various researchers reported that UCM cells possess the properties of MSC and hence they defined UCM cells as MSC-like cells (33). Though shows pleiotropism, UCM cells express the pluripotency gene markers such as Oct-4, Nanog, and Sox-2. UCM cells can be isolated and harvested easily, clonally expandable, and genetically engineered and express CD44 (85% cells) (33).
Wharton’s jelly stromal cells (WJ-SCs)
Umbilical vessels covered by mucoid connective tissue are called Wharton’s jelly which contains collagen and proteoglycans (34). Wang et al., reported the robust evidence of WJ-SCs expressing MSC-like activity (35). WJ-SC express fibroblast-like morphology in cultures. They possess relatively high evidence of pluripotent markers on their cell surface. They express HLA-G antigen which shows the evidence of immune privileged nature of cells. WJ-SC can be obtained in a non-invasive method and are potential for cellular banking.
Immunological cross-talks of placental MSCs
The placenta represents the immunological window between the fetal and maternal immune system. The expression of cell surface antigens among various cells with MSC-like activity and HLA system are the most important factors regulating the immune functions of MSCs. As discussed earlier, the placenta being the least immunological organ, trophoblasts express reduced major histocompatibility complex (MHC) reactivity and apoptosis-inducing mechanisms (36). Trophoblastic cells possess non-classical MHC such as HLA-E, -F, and -G. HLA-G inhibits natural killer cell (NK cell) migration and T lymphocytic proliferation by NKR2B4 receptors (37).
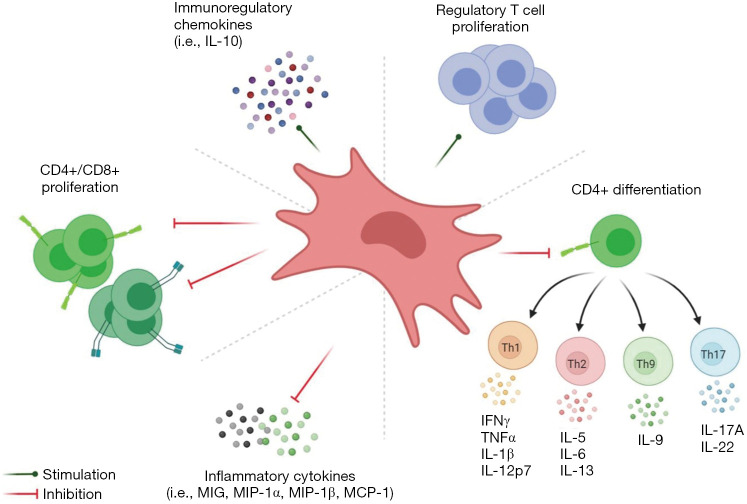
In the natural course of pregnancy, due to an immune-privileged environment, the upregulation of T helper 1 cells occur which secrete anti-inflammatory cytokines (IL-4, -5, -9, -10 and -13) and downregulation of T helper 2 cells occur which secrete pro-inflammatory cytokines (IL-1 and -6, INF-γ). P-MSCs inhibit the proliferation of T lymphocytes due to the abundance expression of indolamine 2,3-dioxygenase (IDO) and immunomodulatory cytokines (as shown in Figure 2) (38,39). P-MSCs regulate the maturation of Treg cells than other MSCs. The downregulation of FoxP3 expression along with the co-culturing of P-MSC leads to inhibition of T cell proliferation which enhances the immunosuppressive activity of P-MSCs (40). P-MSCs induce T lymphocyte anergy and Treg cells through soluble factors and cell-cell interactions, induce T cell apoptosis by IDO, INF-γ, and HLA-G system and modulation of antigen-presenting cell functions by down-regulating MHC class II expression and co-stimulatory molecules.
Figure 2.
Immunological facets of P-MSCs. P-MSCs, placenta-derived mesenchymal stem cell.
By RT-PCR method, Lee et al. determined that all P-MSCs expressed markers of stem cells and three germ layers. P-MSCs exposed immunomodulatory effects when co-cultured with activated T-cells in a dose-dependent manner. The expression of HLA-ABC, HLA-G, IL-2, -4 and -13 and GM-CSF were found in a dose-dependent manner in CP-MSCs when compared to other MSCs (41). TNF-α induce the production of the immunosuppressive prostaglandins by 100 folds the baseline in MSCs (41).
Placental stem cells and COVID-19
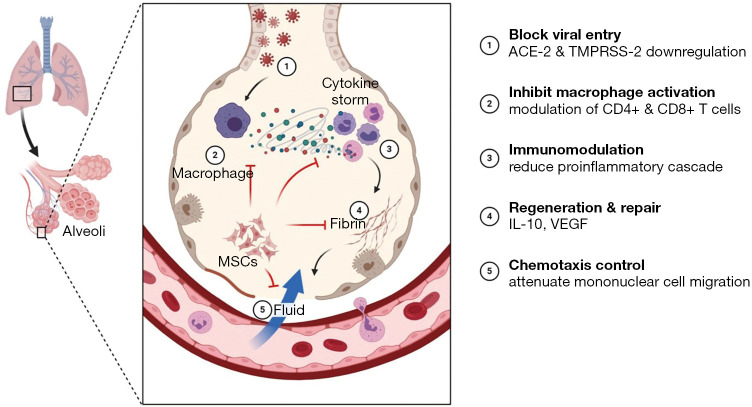
SARS-CoV-2 is a positive-sense, ssRNA virus with 79.6% similar sequence to SARS-CoV, which accounts for the largest genomic specifications among RNA viruses (2). The viral particles gain entry to pulmonary alveolar epithelial cells through ACE-2 receptors, which are not only present in the alveolar epithelium but also present in cardiac myocytes, hepatic, and renal parenchymal cells. Due to the presence of ACE-2 receptors in various organs, the clinical syndromal complex leads to multi-organ dysfunction (42,43). The clinical syndrome proceeds with a dry cough, watery nasal discharge, fever, mild to severe dyspnoea. Once viral load replicates exponentially, epithelial and endothelial breach leads to increased vascular permeability induces cytokine storm in the micro-alveolar environment. The immunobiology of COVID-19 disease correlates with the onset of cytokine storm, where the pro-inflammatory cytokines (IFN-α and γ, IL-1β, -6, -12, -8 and -33, TNF-α, TGF-β) accumulates in abundance (44). COVID-19 affected individuals with underlying systemic illness worsen the immunological barrier among the cells in the individual. Hence, the affected individual develops ARDS, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndromes (MODS), and succumbs to death.
In the horizon of COVID-19, P-MSCs act at ACE-2 and TMPRSS-2 receptors level and block further entry of the contagion into pulmonary alveolar cells. P-MSCs attenuates cytokine storm induced by virus particles and shifts the equilibrium to an anti-inflammatory environment and thereafter further restrict the entry of mononuclear cells into the damaged alveolar epithelium. P-MSCs enhances the immunomodulatory, anti-inflammatory, and immuno-privileged activities in the damaged alveolar cells. Hence, the repair and regeneration of those cells ensue and further pulmonary compliance improvises (as shown in Figure 3).
Figure 3.
Schematic representation of intervention with MSCs in COVID-19. MSCs, mesenchymal stem cell.
Discussion
The concept of “cellular therapy” (CT) and “cell-based therapy” has become more comprehensive and revolutionized among stem cell researchers to salvage various disorders with minimal intervention and maximum functional outcome. “CT” is defined as the usage of one’s own cells without any breach in the cellular biology to regenerate and rejuvenate the functions of sub-functional and non-functional cells or tissues. US-FDA coined CFR 21 section 1271 for dealing with the cellular and cell-based therapy which contains the regulations for cGTP2 and investigational new drugs (45). The acceptable forms of CT are autologous, allogenic, and syngeneic. CT targets at (I) exponential increase in the cell of interest, (II) ideal timing of cell supply and (III) minimal invasiveness of the cell-based treatment (45).
CT gives a ray of hope among clinicians for incurable and pandemic diseases. There are various sources of stem cells, progenitor and immature cells, and recombinant engineered cells. Based on the nature of disease and target organs or tissues, CT protocol has to be manipulated. Under CFR 21, section 1271 lay the criteria for the regulation of CT under section 361 which states that human cells, tissues, and cellular and tissue-based products (HCT/Ps) are (I) minimally manipulated, (II) intended for homologous use, (III) must not involve any combination of articles with cells or tissues and (IV) must/must not have any systemic effects/dependent on any metabolic activity of cells for its primary function either for autologous or allogenic or reproductive use (46).
At a glance, P-MSCs have a cluster of advantages over other sources as (I) perennially available with limited ethical issues as being discarded due to medical waste following childbirth, (II) procured without invasive procedure unlike other sources, (III) yields cells in larger quantities from a single-sourced placenta, (IV) can be banked for multiple future therapeutic purposes, (V) bestowed with low immunogenicity profile whereby protects from immune destruction rapidly and attenuates local activation of immunity, and (VI) protected from SARS-CoV-2 infection (4). Among the various sources of P-MSCs, UBCs are the most potential sources of cells with maximum stem cell activity in terms of treatment for hematological and non-hematological diseases (47). The available CT products from UBCs are Hemacord, Allocord, Clevecord, and Ducord. In 2011, the FDA approved Hemacord and Allocard for cellular and cell-based therapy for various disorders (48,49).
ARDS is a clinical condition which is characterized by the alveolar-epithelial barrier disruption, interstitial edema, and sequestration of inflammatory cells in the pulmonary parenchyma that result in acute respiratory failure. Various researchers demonstrated the usage of MSCs and secretomes to combat ARDS (50-54). In a paracrine fashion, MSCs secrete IL-4, -8, and -10 in a dependent fashion, attenuates polymorphonuclear cells influx, and downregulates the production of inflammatory cytokines in the pulmonary parenchyma. With the due release of various growth factors, MSCs upregulate AT-II cells regeneration and proliferation, disruption of endothelial cell apoptosis, and repair of disrupted alveolar-epithelial barrier in ARDS (55,56). MSCs resolves ongoing inflammation and oedematous stage by secreting pro-resolving mediator lipoxin A4 (57). Zheng et al., concluded that allogeneic adipose tissue-derived MSCs through intravenous route prove a safe and optimal approach in ARDS management (54). Monsel et al., determined the administration of MSCs through intra-tracheal route attenuates inflammation and edema of pulmonary parenchyma by clearing the microbes in a lipocalin-2-dependent manner (58).
Previous studies on MSC use in diseases with underlying inflammatory or autoimmune reactions showed that those studies used various MSC sources, doses, and routes of administration (59-61). Therefore, MSC use in COVID-19 might take advantage of the knowledge of optimal and safe doses of MSC from a certain source. As the use of MSCs is intended to alleviate ARDS due to cytokine storm, the optimal route is supposed to be intravenous. A tracing study in mice, which used an in-vivo imaging system, revealed that 24 h after injection, most of the MSCs will be trapped in the lungs (30–60%), and liver (5–15%) (62). A study on the clinical trial. gov from 2008 through 2018, which consisted of 914 MSC trials, showed that 43% used the intravenous (IV) route. From the 914 trials, 16 had published their results. Most of the 16 published results showed that improved outcomes were attained using minimal effective dose (MED) between 70–190 million MSCs/patient/dose for IV route. However, four trials, which reported a dose-response data, showed a narrower MED of 100–150 million MSCs/patient, while higher or lower IV doses were less effective (63).
A more direct route, such as on-site transplantation into the bronchial tree and alveoli, theoretically may place the MSCs in the real battlefield, but the fact that in ARDS there is an accumulation of proteinaceous and fibrin exudate inside alveoli, and abundant macrophages infiltrating air spaces (64), this approach may be counterproductive as the MSCs may stay in the lumen of the bronchial tree and cannot reach the battlefield. Moreover, the vehicle solution to suspend the MSCs may prevent oxygenation in alveoli and aggravate the condition. Thereby, the route of MSC administration for pulmonary pathology has to be optimized. With the above available evidence, it is clear that the usage of P-MSCs for combating ARDS is caused by COVID-19.
In the United States, a randomized, placebo-controlled, interventional and double-blinded phase II clinical trial (NCT04389450) is on-going to evaluate the safety and efficacy of intramuscular allogenic placental cells ex-vivo expanded MSCs for the treatment of severe COVID-19. A total of 140 candidates are recruited for the trial with due consent and ethical consideration. The candidates in the biological intervention group will receive allogeneic ex-vivo expanded placental mesenchymal-like adherent stromal cells and in the placebo group will receive a placebo solution for injection. The study is expected to get completed within September 2021 with a primary outcome measure of the number of ventilator-free days within 28 days of injection and secondary outcome measures of all causes of mortality within 28 days of injection and duration of mechanical ventilation within 8 weeks of injection. The results of this study are awaited for its clinical effects (65).
Implementation of P-MSCs as a treatment for COVID-19 has a few limitations. They are as follows (I) standardization of isolation and harvesting protocols, (II) dose, frequency, and route of MSC delivery, (III) autologous or allogenic preparation protocols, (IV) ethical concern in selection and utility among a wide array of sources of MSCs and (V) randomized controlled trials to be conducted with aforementioned sources of placental stem cells.
Conclusions
It is extremely well substantiated that placental cells serve as one of the sources of MSCs and the emerging evidence in COVID-19 has shown how MSCs can plausibly serve to rectify immune dysregulation as witnessed in the severely ill patient. The need of the hour demands to focus upon the techniques of isolation and expanding these cells in the required count so as to establish the potentiality of this novel CT. Advocating prospective randomized controlled clinical trials ethically will concretely supplement for its efficacy and safety concerns.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-034). The authors have no conflicts of interest to declare.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265-9. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe SM. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov 2020;5:100019. 10.1016/j.medidd.2020.100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berishvili E, Kaiser L, Cohen M, et al. Treatment of COVID-19 pneumonia: the case for placenta-derived cell therapy. Stem Cell Rev Rep 2020. [Epub ahead of print]. doi: . 10.1007/s12015-020-10004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogozhykh O, Prokopyuk V, Figueiredo C, et al. Placenta and placental derivatives in regenerative therapies: experimental studies, history, and prospects. Stem Cells Int 2018;2018:4837930. 10.1155/2018/4837930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanellopoulos-Langevin C, Caucheteux SM, Verbeke P, et al. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod Biol Endocrinol 2003;1:121. 10.1186/1477-7827-1-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest 2020;130:2620-9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020;92:791-6. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Liu H, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:203-8. [DOI] [PubMed] [Google Scholar]
- 12.Sun D, Li H, Lu X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr 2020;16:251-9. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu M. Clinical features of cytokine storm syndrome. In: Cron R, Behrens E. editors. Cytokine storm syndrome. Cham: Springer, 2019:31-42. [Google Scholar]
- 14.Macias MI, et al. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol 2010;203:495.e9-23. 10.1016/j.ajog.2010.06.045 [DOI] [PubMed] [Google Scholar]
- 15.de la Torre P, Pérez-Lorenzo MJ, Flores AI. Human placenta-derived mesenchymal stromal cells: a review from basic research to clinical applications. In: Valarmathi MT. editor. Stromal cells: structure, function, and therapeutic implications. London: IntechOpen, 2018. doi: 10.5772/intechopen.76718. [DOI] [Google Scholar]
- 16.Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, et al. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol 2019;10:238. 10.3389/fimmu.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indumathi S, Harikrishnan R, Mishra R, et al. Comparison of feto-maternal organ derived stem cells in facets of immunophenotype, proliferation and differentiation. Tissue Cell 2013;45:434-42. 10.1016/j.tice.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 18.Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 2004;18:877-91. 10.1016/j.bpobgyn.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 19.Simoni G, Colognato R. The amniotic fluid-derived cells: the biomedical challenge for the third millennium. J Prenat Med 2009;3:34-6. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Lee Y, Kim H, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 2007;40:75-90. 10.1111/j.1365-2184.2007.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rennie K, Gruslin A, Hengstschläger M, et al. Applications of amniotic membrane and fluid in stem cell biology and regenerative medicine. Stem Cells Int 2012;2012:721538. 10.1155/2012/721538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sessarego N, Parodi A, Podesta M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 2008;93:339-46. 10.3324/haematol.11869 [DOI] [PubMed] [Google Scholar]
- 23.Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004;22:649-58. 10.1634/stemcells.22-5-649 [DOI] [PubMed] [Google Scholar]
- 24.González PL, Carvajal C, Cuenca J, et al. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med 2015;4:1109-21. 10.5966/sctm.2015-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MJ, Shin KS, Jeon JH, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton's jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res 2011;346:53-64. 10.1007/s00441-011-1249-8 [DOI] [PubMed] [Google Scholar]
- 26.Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 2006;194:664-73. 10.1016/j.ajog.2006.01.101 [DOI] [PubMed] [Google Scholar]
- 27.Ventura Ferreira MS, Bienert M, Müller K, et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res Ther 2018;9:28. 10.1186/s13287-017-0757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev 2008;17:1095-107. 10.1089/scd.2007.0154 [DOI] [PubMed] [Google Scholar]
- 29.Hayati AR, Nur Fariha MM, Tan GC, et al. Potential of human decidua stem cells for angiogenesis and neurogenesis. Arch Med Res 2011;42:291-300. 10.1016/j.arcmed.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Hess AP, Hamilton AE, Talbi S, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007;76:102-17. 10.1095/biolreprod.106.054791 [DOI] [PubMed] [Google Scholar]
- 31.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev 2006;2:155-62. 10.1007/s12015-006-0022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells 2014;6:195-202. 10.4252/wjsc.v6.i2.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arutyunyan I, Elchaninov A, Makarov A, et al. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int 2016;2016:6901286. 10.1155/2016/6901286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 2004;22:1330-7. 10.1634/stemcells.2004-0013 [DOI] [PubMed] [Google Scholar]
- 35.Riley JK. Trophoblast immune receptors in maternal-fetal tolerance. Immunol Invest 2008;37:395-426. 10.1080/08820130802206066 [DOI] [PubMed] [Google Scholar]
- 36.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update 2003;9:347-57. 10.1093/humupd/dmg026 [DOI] [PubMed] [Google Scholar]
- 37.Jones BJ, Brooke G, Atkinson K, et al. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta 2007;28:1174-81. 10.1016/j.placenta.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Li C, Zhang W, Jiang X, et al. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res 2007;330:437-46. 10.1007/s00441-007-0504-5 [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Jung J, Cho KJ, et al. Immunomodulatory effects of placenta-derived mesenchymal stem cells on T cells by regulation of FoxP3 expression. Int J Stem Cells 2018;11:196-204. 10.15283/ijsc18031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Jung J, Lee HJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol 2012;13:219-24. 10.1016/j.intimp.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 41.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- 42.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-4. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata-Yoshikawa N, Okamura T, Shimizu Y, et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol 2019;93:e01815-8. 10.1128/JVI.01815-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golchin A, Farahany TZ. Biological products: cellular therapy and FDA approved products. Stem Cell Rev Rep 2019;15:166-75. 10.1007/s12015-018-9866-1 [DOI] [PubMed] [Google Scholar]
- 46.U.S. Department of Health and Human Services. Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue Based Products: Minimal Manipulation and Homologous Use. Guidance for Industry and Food and Drug Administration Staff. 2017. Available online: https://www.fda.gov/media/109176
- 47.Matsumoto MM, Matthews KRW. A need for renewed and cohesive US policy on cord blood banking. Stem Cell Rev Rep 2015;11:789-97. 10.1007/s12015-015-9613-9 [DOI] [PubMed] [Google Scholar]
- 48.Allison M. Hemacord approval may foreshadow regulatory creep for HSC therapies. Nat Biotechnol 2012;30:304. 10.1038/nbt0412-304 [DOI] [PubMed] [Google Scholar]
- 49.Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med 2018;7:643-50. 10.1002/sctm.17-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Hu S, Xu X, et al. The vascular endothelial growth factors-expressing character of mesenchymal stem cells plays a positive role in treatment of acute lung injury in vivo. Mediators Inflamm 2016;2016:2347938. 10.1155/2016/2347938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu S, Li J, Xu X, et al. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther 2016;7:66. 10.1186/s13287-016-0320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang B, Chen L, Wang X, et al. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int J Mol Sci 2017;18:689. 10.3390/ijms18040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015;3:24-32. 10.1016/S2213-2600(14)70291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 2014;15:39. 10.1186/1465-9921-15-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chambers DC, Enever D, Ilic N, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014;19:1013-8. 10.1111/resp.12343 [DOI] [PubMed] [Google Scholar]
- 56.Tzouvelekis A, Paspaliaris V, Koliakos G, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 2013;11:171. 10.1186/1479-5876-11-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang X, Abbott J, Cheng L, et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol 2015;195:875-81. 10.4049/jimmunol.1500244 [DOI] [PubMed] [Google Scholar]
- 58.Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med 2015;192:324-36. 10.1164/rccm.201410-1765OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regmi S, Pathak S, Kim JO, et al. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol 2019;98:151041. 10.1016/j.ejcb.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 60.Ciccocioppo R, Baumgart DC, Dos Santos CC, et al. Perspectives of the international society for cell and gene therapy gastrointestinal scientific committee on the intravenous use of mesenchymal stromal cells in inflammatory bowel disease (PeMeGi). Cytotherapy 2019;21:824-39. 10.1016/j.jcyt.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 61.Pawitan JA, Yang Z, Wu YN, et al. Towards standardized stem cell therapy in type 2 diabetes mellitus: a systematic review. Curr Stem Cell Res Ther 2018;13:476-88. 10.2174/1574888X13666180502143657 [DOI] [PubMed] [Google Scholar]
- 62.Srinivasan RC, Kannisto K, Strom SC, et al. Evaluation of different routes of administration and biodistribution of human amnion epithelial cells in mice. Cytotherapy 2019;21:113-24. 10.1016/j.jcyt.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 63.Kabat M, Bobkov I, Kumar S, et al. Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med 2020;9:17-27. 10.1002/sctm.19-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700-4. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ClinicalTrials.Gov . Available online: https://clinicaltrials.gov/ct2/show/NCT04389450