Abstract
Significant improvements in genetics, nutrition, and food efficiency have had a great impact on the rapid growth of broilers, notably with increases in muscle mass. However, with rapid growth, the broiler industry has been negatively impacted by the increased incidence of myopathies, including white striping. White striping affects the pectoralis major muscle of broilers, particularly the larger breasts of rapidly growing modern commercial broiler lines. In this study, we documented the growth process of commercial broiler chickens from hatching to market weight at 6 wk. Gross pathology and histopathology analyses were performed on pectoralis major muscle collected weekly from birds culled from 1 to 6 wk. The severity of both gross and histologic pathologies in the breast muscle increased over time. White striping was initially observed at week 2, with a rise in the incidence and severity through the sixth week. Mild histopathology was noted in week 2, characterized by macrophage infiltration and limited phagocytosis of the muscle. Muscle condition deteriorated with age and weight gain, with more prevalent macrophages, phagocytosis, and interstitial fibroblasts. By week 5 and 6, there was severe myopathy including regions of obliterated muscle tissue. Linear regression models show a positive correlation between white striping, gross pathology, and histopathology relative to weight and age.
Key words: broiler, pectoralis major, white striping, pathology, histopathology
Introduction
Optimizations in genetic selection and in nutrition have improved commercial broiler growth rate and feed efficiency, thus resulting in increases in breast yield and in declines in days to market (Zuidhof et al., 2014). However, the commercial benefit of a fast growing broiler chicken with a large breast is reduced with the occurrence of skeletal muscle myopathies that compromise meat quality—in particular the pectoralis major, the predominant muscle for the industry (Petracci et al., 2015). One commercially detrimental myopathy is white striping (WS) which occurs when intramuscular striations form that run parallel to muscle fibers, mainly in the breasts; these myopathic lesions decrease protein content, increase fat, and affect consumer acceptance for the fillets (Kuttappan et al., 2012; Petracci et al., 2013; Soglia et al., 2016). The growth of the pectoralis major muscle exceeds vascular support for homeostasis, including satellite cell-mediated repair mechanisms. This unsustainable pressure on muscle metabolism initiates degenerative features associated with muscle deterioration (Petracci et al., 2015). Histological changes are associated with WS, including vacuolar degeneration, perivascular necrosis, fibrosis, and infiltration of macrophages and connective tissue (Kuttappan et al., 2013; Sihvo et al., 2014; Soglia et al., 2016).
We hypothesized that the rapid growth of commercial broilers was associated with increased incidence of pathology, particularly WS, detected in gross pathology and histopathology. We examined gross pathological and histological features arising in birds culled each week over the 6-week growth process from hatching to market weight. Equations were devised to predict the level of WS and pathology with growth.
Materials and methods
Growing Conditions and Weighing Birds
Commercial Cobb 500 broiler chickens (120) were raised for up to 6 wk, in accordance with a University of California Davis Institutional Animal Care and Use Committee–approved protocol. Chicks were received on the day of hatching, weighed, and randomly assigned to open floor pens (4 foot × 6 foot), 10 birds/pen. Birds were kept on a 23/1 h light–dark cycle for the first 2 d and thereafter a 16/8 h light–dark cycle. Birds were kept at 30°C over the first few days; then room temperatures were decreased by 2°C–3°C weekly until reaching 20°C, which was maintained for the remainder of the study. Birds were provided an ad libitum corn/soy commercial diet as starter (week 1 and 2), grower (week 3 and 4), and finisher (week 5 and 6) as was previously described (Sachs et al., 2019). Because live weight is predictive of breast weight, live birds were weighed weekly (Silva et al., 2006).
Culling, Sample Collection, and White Striping Examination
Up to 20 birds, from 2 random pens, were sacrificed at 7, 14, 21, 28, 35, and 42 d via CO2 inhalation and cervical dislocation. The skin was pulled back from the breast to expose the muscle. Pectoralis major muscles were observed for the presence of WS and characterized as normal (absent), moderate (observed but <1 mm thick), or severe (>1 mm thick), and scored as 0, 1, or 2, respectively, by 2 observers coming to a consensus (Kuttappan et al., 2012). The muscles were photographed for gross pathology examination; samples were collected from the same anterior portion of the left breast of each bird during weekly necropsy. Samples were stored in 10% neutral buffered formalin at 22°C; after 7 d, formalin was replaced with 70% ethanol.
Gross Pathology Assessment
Photographs of the pectoralis major muscle of each broiler were examined and ranked by 3 observers for gross pathology. Final ranking was based on consensus. Birds were assigned a muscle pathology rank from 0 to 4 as described by Griffin et al. (2018): 0, no presence of WS; 1, presence of WS only; 2, presence of surface hemorrhaging near the sternal apex; 3, presence of intramuscular hemorrhaging near the sternal apex; and 4, ischemia.
Tissue Preparation, Staining, and Histological Analysis
Fixed pectoralis major muscle samples were trimmed to 4 mm × 6 mm x 4 mm blocks with skin retained along the long surface and fibers running parallel to a short side. Blocks were infiltrated with paraffin and embedded (Sakura Tissue-Tek VIP 5 and TEC EC 4710). Blocks were cut into 4-μm sections on a rotary microtome (Leica RM2255). Sections were floated in a warm water bath and mounted on precleaned Superfrost Plus glass slides. Sections were stained with freshly filtered Harris hematoxylin for 4 min, followed by 0.25% Eosin-Y for 1 s. Sections were also stained using Masson's Trichrome Stain (Polysciences Catalog # 25088). Images of stained sections were captured using a BX43F microscope fitted with a DP80 digital and cellSens Dimension software v.1.12 (Olympus). Images of stained sections were categorized for the degree of muscle pathology, based on the incidence of myopathic abnormalities and severity of muscle damage. Distinct histological changes were used as determinants in developing a grading system with sections graded as: normal (0), mild (1), moderate (2), or severe (3), by 3 observers coming to a consensus while observing the incidence of myopathic abnormalities on the H&E and Trichrome slides (Kuttappan et al., 2013).
Statistics
Weights of culled broilers were compared using ANOVA analyses with Tukey's multiple-comparison tests. White striping scores, pathology ranks, and histopathology scores of the culled birds were analyzed using Goodman and Kruskal's gamma nonparametric measure of correlation. ANOVA analyses were performed to use cull week or cull weight to predict WS score, pathology rank, and histopathology score in linear regression models; to use regression, variables were considered continuous. Pearson's correlation coefficients were calculated to measure the strength of a linear association between cull weight, cull week, WS score, pathology rank, and histopathology score. An ANOVA analysis with multiple comparison testing was performed on the weights of the birds surviving each week to determine pen and sex effects. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA) and jamovi v.1.2 (https://www.jamovi.org) software.
Results and discussion
Weights, Striping Scores, and Gross Pathology Ranks
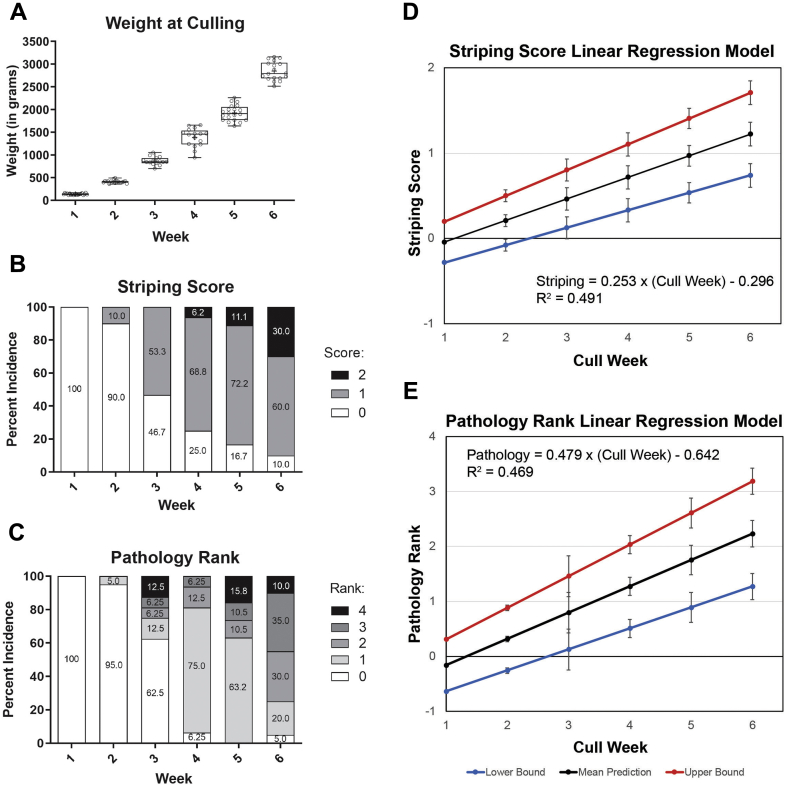
Hatched broiler chicks weighed 38 ± 2 g at the start of the study. Broilers culled at week 1 weighed 135 ± 17 g, while broilers culled at week 6 weighed 2,790 ± 273 g (Figure 1A). The average weekly growth of the birds that survived from hatching until the end of the study was 105%. When grossly examining WS, the initial onset was observed at week 2 and increased thereafter both in quantity and striping score severity (Figure 1B). The first documented case of WS during week 2 was categorized as moderate (WS 1), whereas severe WS (WS 2) was detected starting in week 4 and increased in prevalence until week 6. The number of broilers with normal breast tissue decreased each week such that by week 6 the frequencies of normal, moderate, and severe striping were 10, 60, and 30%, respectively. Gross inspection of breast muscle revealed an increasing trend in the severity of the pathology rank as broilers aged and grew in weight. Pathology was first observed during week 2 with 10% of broilers evaluated as rank 1, whereas pathology was present in over 90% of broilers in week 4, 5 and 6 (Figure 1C).
Figure 1.
Weights, striping scores, and gross pathology of birds culled each week. (A) A box plot displays weights with whiskers representing range, the box representing 25th–75th interquartile, the median line within the, and the “+” demarcating the mean. Individual bird weights are plotted as dots. Every comparison was significant (ptukey < 0.0001, one-way ANOVA with Tukey multiple-comparison test). White striping scores (B) and gross muscle pathology ranks (C) are displayed as stacked bars of percentage incidence. Scores analyzed using Goodman and Kruskal's gamma test. For striping scores, gamma = 0.8647, Z = 9.14, P < 0.001 (n = 111). For gross muscle pathology ranks, gamma = 0.8152, Z = 8.28, P < 0.001 (n = 111). Linear regression descriptions of the incidence of white striping (D) and the gross pathology ranks (E) relative to cull week include red and blue lines for equations of the upper and lower bounds of the 95% confidence interval for the intercept and unstandardized regression coefficient. Linear regression equations and their goodness-of-fit measure values are provided.
Linear regression equations were calculated to best fit the data to describe striping (Figure 1D) and gross pathology (Figure 1E) by cull week. The linear regression model for WS score demonstrated that until week 3, the average broiler did not experience WS. However, by week 4, the upper bound of the 95% confidence interval passed WS score 1—a point with moderate striping. The linear regression model that plotted gross pathology rank by cull week conveyed that until week 2 there was no presence of WS even within the upper bound below muscle pathology rank 1; however, by week 4, broilers demonstrated WS with surface hemorrhaging near the sternal apex as the upper bound reached muscle pathology rank 2.
Histopathology
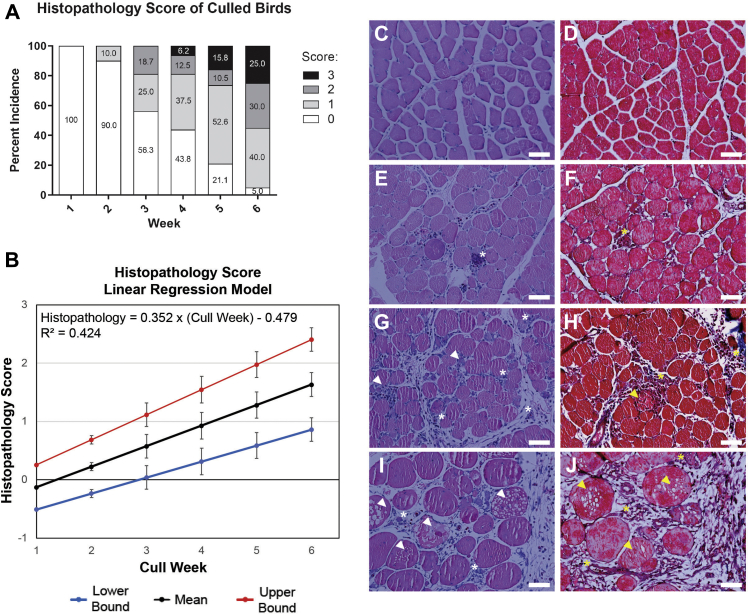
Histopathological scoring allowed for comparisons to be made across broiler and week as birds were culled (Figure 2A). Over the first 2 wk, young birds generally had normal histology (score 0; Figures 2C and 2D). However, histopathological findings in pectoralis major muscle tissue samples were first documented at week 2; ten percent of the broilers exhibited myopathic abnormalities classified as mild—demonstrating some macrophage infiltration and limited phagocytosis of muscle fibers (Figures 2E and 2F). Broilers characterized with moderate pathology had more than a few macrophages phagocytizing muscle, as well as the appearance of fibrotic tissue, and pronounced presence of adipocytes throughout the muscle (Figures 2G and 2H). Pectoralis major muscle with a severe phenotype had ample macrophage infiltration evident, resulting in distinct changes in the skeletal muscle architecture with complete obliteration of muscle fibers (Figures 2I and 2J). After week 2, the incidence of myopathic abnormalities increased each week until the end of the study at week 6 (Figure 2A). Normal muscle histology deteriorated with time with more prevalent macrophages, phagocytosis, and interstitial fibroblasts. The first documented case of broilers classified as moderate (score 2) was observed during week 3. Severe damage (score 3) was first observed in week 4. By week 6, 95% of the broilers had some form of pathological abnormality. A linear regression equation calculated to best fit the histopathology data (Figure 2B) revealed that the upper bound reached a histopathology score 1 during week 3. The upper bounds represent how one might conservatively approach detection of WS.
Figure 2.
Histopathological findings of birds culled each week. (A) Percentage incidence of myopathic abnormalities in the pectoralis major muscle for broilers culled each week was reported for histopathology scores and represented as a stacked bar graph analyzed using Goodman and Kruskal's gamma test (gamma = 0.778, Z = 6.841, P < 0.001, n = 111). (B) Linear regression of the histopathology scores is provided. Red and blue lines represent linear equations of the upper and lower bounds of the 95% confidence interval for the intercept and unstandardized regression coefficient. Linear regression equations and their goodness-of-fit measure values are provided. Pectoralis major muscle histopathology was examined for each broiler culled; representative images (H&E and Masson's trichrome, respectively) depict scores: 0, normal (C,D); 1, mild (E,F); 2, moderate (G,H); 3, severe (I,J). Asterisks (∗) represent examples of macrophage infiltration; arrowheads represent instances of macrophages phagocytizing muscle fibers or degeneration of muscle fibers. Scale bar: 50 microns.
Examining Correlations in the Data
Data for WS scores, pathology ranks, histopathology scores, cull week, and weight at cull week were analyzed for correlations. Cull weight and cull week were nearly perfectly positively correlated (Pearson's r = 0.973, P < 0.001). White striping score, pathology rank, and histopathology score were all highly positively correlated with cull weight and week. For example, Pearson's r values were 0.701, 0.685, and 0.651 (P < 0.001) for WS score, gross pathology rank, and histopathology score, respectively, relative to cull week.
Interpreting Findings
In this study, WS had a direct correlation with age and weight, suggesting that broiler growth rate contributes to WS prevalence. The severity of both gross and histological pathologies in pectoralis major muscle increased after the initial onset of WS. It has been hypothesized that the fibrous tissue surrounding muscle is not able to withstand the stress of increasing size and changes in intramuscular pressure without muscle deterioration (Griffin et al., 2018). This study provided evidence to support this hypothesis as the severity of the gross and histological pathology worsened each week. As initial pathological changes occurred, we observed macrophage infiltration of the tissue, which further exacerbated the progression of myodegeneration. Moreover, histology demonstrated that with age and weight gain, there was an increased frequency of myopathic abnormalities contributing to distinctive pathophysiological mechanisms of fat deposition, muscle fiber degeneration, vacuolation, and myodegeneration. Collectively our 3 linear regression models demonstrated that between weeks 2 and 4 the broiler's pectoralis major muscle experienced damage that was detectable both histopathologically and grossly. Thus, future studies examining specific pathological mechanisms should be performed within the week 2 to 4 time frame with more days added in between to better resolve pathological changes—histologically or molecularly—seen temporally.
Some limitations of this study should be noted. A group of broilers were culled each week, so that different broilers were examined each week instead of following pathology, including striping, within each individual broiler over the 6 wk. The same broilers could have been used week to week if targeted muscle biopsies were performed. Moreover, pens used in the study were a fixed size; whole pens of birds were collected such that effective pen size per bird would remain constant throughout the trial. A pen effect on weight only occurred in one comparison—week 1 for pens 1 and 2 (ANOVA pTukey = 0.018).
Acknowledgments
The authors gratefully acknowledge funding from the U.S. Poultry & Egg Association (Project #713, MJM & AJK), and support from the University of California Davis Department of Animal Science and College of Agricultural and Environmental Sciences, and the California Agricultural Experiment Station for funding research affiliated with USDA Multi-State Project NC 1184. The authors thank Foster Farms for the donation of the broiler chicks. They are grateful for the guidance from Mrs. Kristy Portillo, the Avian Facility staff, and Mr. David Gall, all within the Department of Animal Science.
Disclosures
All authors have no competing interests to declare.
References
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F.C., Mckee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.I., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds. Poult. Sci. 2015;71:363–374. [Google Scholar]
- Sachs N.J., Hampton A.R., Foster K.K., Pechanec M.Y., Henderson J.D., King, King A.J., Mienaltowski, Mienaltowski M.J. The effects of an alternative diet regimen with natural methionine ingredients on white striping breast myopathy in broiler chickens. Poult. Sci. 2019;98:413–421. doi: 10.3382/ps/pey327. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the Pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Silva S.R., Pinheiro V.M., Guedes C.M., Mourão J.L. Prediction of carcase and breast weights and yields in broiler chickens using breast volume determined in vivo by real-time ultrasonic measurement. Br. Poult. Sci. 2006;47:694–699. doi: 10.1080/00071660601038776. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., E. Babini, Di Nunzio M., Mazzoni M., Sirri F., C, Cavani, Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]