Abstract
Links between child maltreatment and low-grade inflammation in adulthood are well documented, but these studies often rely on adults to report retrospectively on experiences of childhood abuse. Further, these findings raise questions about whether exposure to childhood maltreatment needs time to “incubate,” only giving rise to nonresolving inflammation in adulthood, or whether heightened inflammation may be observable in childhood, closer in time to the maltreatment exposure. The present study examined this question in a sample of 155 low-income children (ages 8-12), half of whom had been exposed to maltreatment. Trained coders evaluated case reports to classify maltreatment based on timing and exposure type. Blood samples from children assessed C-reactive protein and cytokines, which were used to form a composite of low-grade inflammation. Analyses revealed a marginally significant Maltreatment Exposure × Sex interaction, which suggested that maltreatment exposure was associated with higher inflammation for girls but not boys. Additionally, analyses focused the accumulation of maltreatment experiences (through multiple forms of maltreatment or across multiple time points) revealed that girls with greater diversity in their maltreatment experiences and those who experienced maltreatment at multiple time points were at greatest risk. Finally, examination of timing of first onset of maltreatment suggested that girls whose exposures occurred before the age of five had the highest low-grade inflammation. These findings add new evidence linking maltreatment to inflammation in childhood, which could increase risk for mental and physical health problems across the lifespan.
Keywords: child maltreatment, inflammation, sex differences
1. Introduction
Child maltreatment is a serious public health concern that poses significant mental and physical health burdens on its victims (Cicchetti & Toth, 2005) as well as substantial economic burdens on society as a whole (Fang, Brown, Florence, & Mercy, 2012). Estimates suggest that nearly 700,000 children were victimized in the United States in 2018, and over half were under six years old at the time of the abuse (U.S. Dept. of Health and Human Services, 2020). Alarmingly, childhood maltreatment is most often perpetrated by primary caregivers, with almost 80% of cases involving abuse with one or more parents (U.S. Dept. of Health and Human Services, 2018).
Decades of research highlight the psychological, social, and academic sequelae for children who are victims of maltreatment, and recent meta-analyses support the notion that maltreatment experiences have a pervasive and toxic influence on a wide variety of developmental outcomes across the lifespan (e.g., Fry et al., 2018; Infurna et al., 2016; Liu, 2019). Victims of maltreatment are less likely to graduate from high school (Fry et al., 2018), are more likely to experience depressive episodes (Infurna et al., 2016), have more problematic relationships (Rogosch, Cicchetti, & Aber, 1995), and are at greater risk for substance use problems (Simpson & Miller, 2002), relative to non-abused individuals. In addition, evidence suggests that maltreatment is associated with physical health problems, including obesity (Danese & Tan, 2014) and perceived health (Min, Minnes, Kim, & Singer, 2013). One meta-analysis suggested that child abuse was associated with a range of physical health outcomes in adulthood, including neurological and musculoskeletal problems, cardiovascular disease, respiratory problems, gastrointestinal issues, and metabolic dysregulation (Wegman & Stelter, 2009). Maltreatment has also been linked to premature mortality (Chen, Turiano, Mroczek, & Miller, 2016).
Several studies have focused on possible biological mediators that might help explain, in part, why maltreatment is associated with physical health problems and premature mortality. Inflammation has received attention as a possible mechanism, which is perhaps not surprising given its role in a number of mental and physical health problems (Ridker, 2007; for a review, see Ehrlich, Miller, & Chen, 2016). Some theories suggest that inflammation may explain some of the short- and long-term consequences of early maltreatment (see Danese & McEwen, 2012; Nusslock & Miller, 2016). Several studies have linked childhood maltreatment with elevated levels of inflammatory markers in adulthood (e.g., Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016; Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, 2013). For example, in the Dunedin Longitudinal Study, childhood maltreatment (a composite of observed maternal rejection at age 3, parent-reported harsh discipline at ages 7 and 9, repeated caregiver changes through age 11, and physical abuse retrospectively reported at age 26) predicted higher levels of C-reactive protein (CRP), a biomarker of low-grade inflammation, at age 32 (Danese et al., 2009; Danese, Pariante, Caspi, Taylor, & Poulton, 2007). Similarly, older adults who reported abuse during childhood had significantly higher circulating levels of another inflammatory biomarker, interleukin (IL)-6, and marginally higher tumor necrosis factor (TNF)-α levels relative to adults who did not report being abused as children (Kiecolt-Glaser et al., 2011). Interestingly, one study of women found that retrospectively reported sexual abuse, but not physical abuse, during adolescence was associated with heightened CRP and IL-6 in adulthood (Bertone-Johnson, Whitcomb, Missmer, Karlson, & Rich-Edwards, 2012). Studies utilizing retrospective reports of harsh, chaotic, and unsupportive family environments have also revealed associations between these difficult early environments and heightened inflammatory activity in adolescence and adulthood (Miller & Chen, 2010; Slopen et al., 2010; Taylor et al., 2006).
Only a handful of studies have examined associations between maltreatment and inflammation in children and adolescents (e.g., Danese et al., 2011). For example, in a study of 40 children (18 of whom experienced maltreatment), maltreatment status was not associated with salivary IL-1β or CRP (Tyrka, Parade, Valentine, Eslinger, & Seifer, 2015). Similarly, Cicchetti, Handley, and Rogosch (2015) did not find an association between maltreatment status and salivary CRP in a sample of nearly 500 youth (approximately half of whom had been maltreated). These studies used salivary markers of inflammation, however, which predominately reflect immunologic activity in the mouth, rather than tissues where diseases of interest develop (e.g., the brain, lungs, and heart). This is especially true for markers that do not readily pass from serum to saliva (e.g., CRP). Evidence from some studies, however, suggests that salivary markers of inflammation are modestly related to markers in serum, at least when levels in blood are expected to be high (e.g., Byrne et al., 2013).
Some evidence, however, suggests that exposure to victimization (including maltreatment) across childhood is associated with serum markers of inflammation at age 18 (Baldwin et al., 2018). In this large longitudinal sample of twins, researchers identified a main effect of victimization on CRP (measured in dried blood spots) at age 18 that was qualified by a significant interaction with child sex. Follow-up analyses revealed that this association was significant for females but not for males. Although speculative, Baldwin and colleagues note that some research suggests that females may be more vulnerable than males to the toxic effects associated with early life adversity (e.g., Chen et al., 2016), although data from animal models suggests that these sex differences may not be straightforward (Ganguly & Brenhouse, 2015).
We were interested in building on the findings of Baldwin et al. (2018) to explore how exposure to maltreatment in childhood might be associated with inflammation in childhood. Whereas Baldwin et al. created a composite measure of victimization experiences (a score that reflected children’s exposure to domestic violence, bullying by peers, and maltreatment), in the present study, we focused specifically on children’s maltreatment experiences. We obtained detailed reports of official case records, which allowed us to extract information about what types of maltreatment children experienced (i.e., neglect, emotional maltreatment, physical abuse, or sexual abuse) as well as the developmental timing of when these exposures occurred (ranging from infancy through late childhood). This extensive information allows us to capture detailed portraits of children’s maltreatment experiences, which in turn may shed light on which features of those experiences are most likely to be associated with inflammation. Further, Baldwin et al. examined CRP in late adolescence and found that childhood victimization was associated with inflammation by this period of development (an advance over the majority of the literature that has focused on inflammation in adulthood). Our sample includes assessments of multiple markers of low-grade inflammation in late childhood—an earlier developmental period that provides an opportunity to start evaluating questions about how early in development these proposed links between maltreatment and inflammation become evident. In addition, we included a panel of cytokines as well as CRP in our analysis, which is notable because, although CRP is a proxy marker for inflammation and may predict future health problems, it is not causally involved in disease (e.g., Pepys, 2005; Timpson et al., 2005). The cytokines included in our analysis (IL-6, IL-8, IL-10, and TNF-α) have pleiotropic and coordinated effects within the body, including both the activation and inhibition of other cellular responses to pathogens, and together play an important role in the innate immune response (see Cohen & Cohen, 1996, for a review).
In the present study, we explored several research questions related to the ways in which maltreatment might confer risk for elevations in low-grade inflammation among low-income children. Across analyses, we tested for main effects of maltreatment and the role of children’s sex as a possible moderator, given evidence described earlier from Baldwin and colleagues (2018) as well as animal studies (Ganguly & Brenhouse, 2015) that negative outcomes associated with maltreatment may differ by sex. First, we evaluated the association between maltreatment exposure and children’s low-grade inflammation. We hypothesized that children who experienced maltreatment would have higher levels of inflammation than nonmaltreated youth. Second, we examined questions surrounding the extent to which the accumulation of maltreatment experiences—including diversity of types of maltreatment and chronicity across multiple time points—were linked to children’s inflammation. Like Copeland et al. (2012), who found that cumulative depressive episodes were linked to later CRP, we predicted that youth who had more than one type of maltreatment experience or multiple periods of maltreatment exposure would have elevations in their low-grade inflammation. Finally, we conducted exploratory analyses to determine whether the age of first onset of maltreatment exposure mattered for children’s low-grade inflammation.
2. Methods
2.1. Participants
Participants included 155 children ranging in age from 8-12 yr (Mage = 10.1, SD = .97; 52.3% male) who attended a summer day camp research program (see Cicchetti & Manly, 1990, for more information about the camp procedures). This program was designed for school-aged low-income children and included both maltreated children (n = 78) and nonmaltreated children (n = 77). The sample was racially diverse (72.9% Black, 18.7% White, 8.4% biracial or other race).
To recruit children who had experienced maltreatment, a DHS liaison examined Child Protective Services reports to identify families with a child in the targeted age range who had been maltreated. The DHS liaison contacted a random group of eligible families and explained the camp and research involvement. Interested families consented to having their contact information shared with our project staff. Participating families were representative of those receiving services through DHS.
Because maltreating families primarily have low socioeconomic status (National Incidence Study; Sedlak et al., 2010), we recruited nonmaltreated children from families with similar demographics. The DHS liaison identified families who were receiving Temporary Assistance to Needy Families but had no history of CPS involvement. The DHS liaison contacted a random group of these families and explained the camp and research involvement. Interested families consented to having their contact information shared with our project staff. Of note, families who received preventive DHS services due to risk for maltreatment were not included in this study. During initial enrollment, research assistants interviewed mothers of children in this group using the Maternal Child Maltreatment Interview (Cicchetti, Toth, & Manly, 2003) to confirm that the child did not have any prior unreported incidents that would have been classified as maltreatment.
For all families, we obtained informed consent from parents for their participation and for their child’s participation in the summer camp research program, as well as for examination of any Department of Human Services (DHS) family records. Children provided informed assent. All study procedures were approved by the university Institutional Review Board.
2.2. Procedures
2.2.1. Day camp study design.
Children were invited to participate in a five-day summer camp, which included a research component (see Cicchetti & Manly, 1990). Children were randomly assigned to groups of 10 same-sex and same-age peers (half of each group included maltreated children). Groups were led by three trained camp counselors, who were unaware of children’s maltreatment statuses and study hypotheses. Each camp day lasted 7 hr and included both recreational activities and research assessments conducted by trained research assistants.
2.3. Measures
2.3.1. Maltreatment Classification System (MCS).
The Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993) was used to capture detailed information about children’s exposure to maltreatment. This system utilizes DHS records about investigations and findings regarding maltreatment experiences in families. Based on operational definitions, raters make independent judgments and classify the maltreatment exposures by (a) subtypes that each child experienced, (b) frequency of the occurrence, (c) severity, and (d) developmental period in which the exposure occurred (infancy [0-18 months], toddlerhood [19-35 months], preschool [36-59 months], early school age [ages 5 to 7], or later school age [ages 8-12]). Coding is based on all available information in DHS records and does not rely on DHS determinations.
Maltreatment subtype experiences included neglect, emotional maltreatment, physical abuse, and sexual abuse. Neglect experiences reflect a failure of caregivers to provide for the child’s basic physical needs (food, clothing, shelter, medical treatment). Neglect can also include a lack of supervision, moral-legal neglect (e.g., involving the child in a crime), and educational neglect. Emotional maltreatment involves extreme thwarting of a child’s basic emotional needs for psychological safety and security. Evidence of emotional maltreatment includes belittling and ridiculing the child, extreme negativity and hostility, abandonment, and suicidal or homicidal threats. Individuals who commit physical abuse engage in nonaccidental physical injury to the child, and can be identified by bruising, welts, burns, broken bones, and reports of choking. Finally, sexual abuse involves attempted or actual sexual contact between the child and caregiver for purposes of the adult’s sexual satisfaction or financial benefit. Examples of sexual abuse include exposure to pornography or adult sexual activity, sexual touching and fondling, and forced intercourse with the child.
The MCS is the gold standard assessment of maltreatment, and its reliability and validity has been established (e.g., Bolger, Patterson, & Kupersmidt, 1998; Dubowitz et al., 2005; English et al., 2005; Manly, 2005; Manly, Kim, Rogosch, & Cicchetti, 2001; Smith & Thornberry, 1995). Trained research staff and a clinical psychologist who had achieved adequate reliability prior to the start of this study coded DHS records. Kappas for the presence of each maltreatment subtype ranged from 0.90 to 1.00.
In the sample used in the present analyses, 66.7% of the maltreated children experienced emotional maltreatment, 55.1% were victims of neglect, 34.6% were victims of physical abuse, and 7.7% experienced sexual abuse. Consistent with other samples of maltreatment, many children experienced more than one subtype of maltreatment (51.3%; M = 1.64, SD = .74), and 34.6% of children experienced maltreatment across multiple developmental periods (M = 1.36, SD = .48).
2.3.2. Low-grade Inflammation.
All children provided non-fasting blood samples via antecubital venipuncture in the early- to mid-afternoon on day four of the camp week. Samples were collected in EDTA-treated Vacutainers, which were frozen at −80 °C until analysis. At that time the samples were thawed, the plasma was harvested, and five biomarkers of low-grade inflammation were measured: C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α). CRP was measured in duplicate by high-sensitivity immunoturbidimetric assay on a Roche/Hitachi cobas c502 analyzer. The average intra- and inter-assay coefficients of variation were 2.5% and 5.6%, respectively. This assay’s lower limit of detection is 0.2 mg/L. Undetectable CRP values (n = 80) were replaced with .19mg/L. The cytokines were measured in duplicate by electrochemiluminescence on a SECTOR Imager 2400A (MesoScale Discovery) with a Human Pro-Inflammatory 4-Plex Ultra-Sensitive assay (MesoScale Discovery), following instructions provided by the manufacturer (Fu et al, 2010). The kit’s lower limits of detection range from 0.10 pg/mL (IL-8) to 0.80 pg/mL (IL-10). Across runs, the average intra-assay coefficients of variation were 6.72% (IL-6), 1.99% (IL-8), 15.01% (IL-10), and 3.00% (TNF-α). All inter-assay coefficients of variation were < 8%.
We removed extreme values (> 3SD from the mean), which included four IL-10 values (scores above 4.56pg/mL), four IL-6 values (scores above 5.43pg/mL), and two CRP values (scores above 6.08mg/L). We then log-transformed variables that had skewness values of 2.0 or greater, which included IL-6, IL-8, and CRP. The five biomarkers were then standardized and used to create a mean composite of low-grade inflammation (Cronbach’s alpha = .68).
2.3.3. Body Mass Index (BMI).
Weight and height were measured on a single occasion using standard procedures. Weight was measured using a digital scale. To measure children’s height, children stood against a wall, and trained research assistants used a standard measuring tape. BMI was calculated using the standard formula (weight divided by height squared).
3. Results
3.1. Sample Characteristics and Preliminary Analyses
Children in the maltreated and non-maltreated groups were comparable on age, sex, and BMI. The groups differed by race, however, such that there were more African American children in the nonmaltreated group relative to the maltreated group, X2 (3) = 11.89, p = .008.
As is common in pediatric samples, 80 children had undetectable CRP values. Children with undetectable versus detectable values did not differ by child sex X2 (1) = 2.78, p = .10 or maltreatment status X2 (1) =.01, p = .94, however.
3.2. Statistical approach
Below, we present three series of analyses. First, we used analyses of covariance (ANCOVA) to examine differences in low-grade inflammation as a function of sex and maltreatment status. Second, we considered the role of cumulative exposure as a factor that may relate to low-grade inflammation. We conceptualized cumulative exposure in two ways. First, we examined the diversity of maltreatment exposures (either within the same developmental period or at different points in time). Then, we examined the extent to which children experienced maltreatment across developmental periods, thereby providing an assessment of the chronicity of maltreatment. Finally, we examined whether children’s inflammation differed as a function of the age of onset for their first maltreatment experience. All analyses control for age and BMI.
3.3. Maltreatment Exposure and Low-Grade Inflammation
We first tested group differences in children’s low-grade inflammation and whether boys and girls differed in this association. Although there were no main effects of maltreatment or sex (ps > .10), there was a marginally significant Maltreatment × Sex interaction predicting the inflammation composite (F[1, 148] = 3.54, p = .062; see Figure 1). Post-hoc probing of the estimated marginal means revealed that girls who were maltreated had marginally higher levels of inflammation than non-maltreated girls (Mdiff = .255, p = .09) and had significantly higher levels than boys who were maltreated (Mdiff = .369, p = .014). Maltreated and nonmaltreated boys did not differ in their levels of inflammation, however (p = .33).
Figure 1.
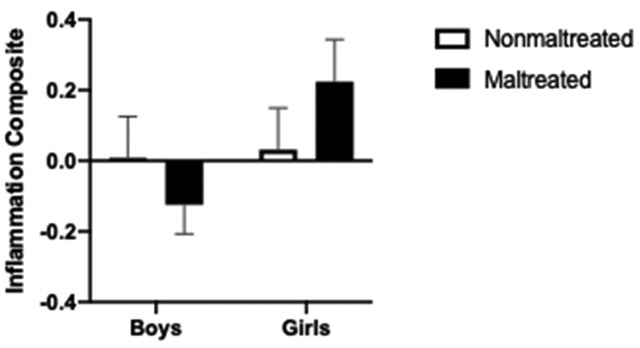
Standardized inflammation composite scores as a function of maltreatment and child sex. Maltreated girls had marginally higher inflammation composite scores than girls who were not maltreated and significantly higher inflammation composite scores than boys who were maltreated
3.4. Diversity of Maltreatment Exposures and Low-Grade Inflammation
We next examined whether children who experienced multiple forms of maltreatment (i.e., emotional maltreatment, neglect, physical abuse, or sexual abuse) would have higher levels of inflammation compared to children who experienced only one type of maltreatment. Although the main effect of Diversity of Maltreatment exposure was not significant (F[2, 146] = .799, p = .45), analyses revealed a significant Diversity of Maltreatment × Sex interaction (F[2, 146] = 6.325, p = .002, see Figure 2). For girls, those who were exposed to two or more forms of maltreatment had higher inflammation compared to girls who were not maltreated (Mdiff = .54, p = .003) and had higher inflammation compared to girls who experienced one form of maltreatment (Mdiff = .57, p = .012). Nonmaltreated girls’ low-grade inflammation did not differ from girls who had experienced only one type of maltreatment (Mdiff = .03, p = .86). Among children who experienced multiple forms of maltreatment, girls had higher inflammatory composite scores than boys (Mdiff = .79, p < .001). Boys’ inflammation scores did not differ as a function of the number of types of maltreatment they experienced (all ps > .06).
Figure 2.
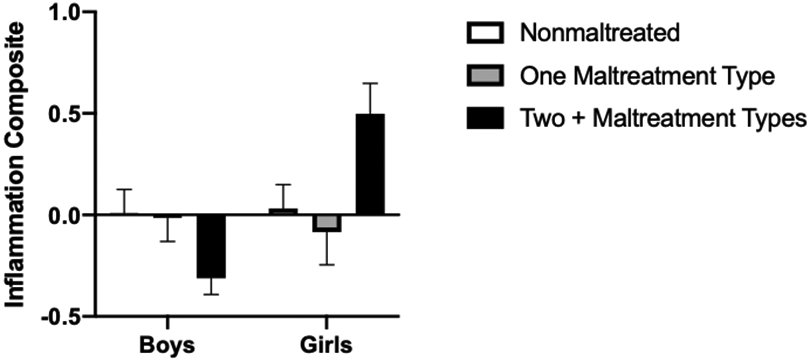
Standardized inflammation composite scores as a function of child sex and the diversity in maltreatment exposure types. Maltreated girls who experienced multiple forms of maltreatment had significantly higher inflammation composite scores than nonmaltreated girls or girls who experienced one form of maltreatment
3.5. Chronicity of Maltreatment Exposure and Low-Grade Inflammation
Similarly, we examined whether children who experienced maltreatment across multiple developmental periods (i.e., infancy, toddlerhood, preschool, early childhood, or later childhood) showed higher levels of inflammation relative to nonmaltreated children and children who experienced maltreatment at one time point only. We note here that this index overlapped with the diversity of exposure index described above. Specifically, of the 27 children who experienced maltreatment at two or more periods, only one child experienced the same maltreatment type at each time point. Conversely, of the 39 children who experienced two or more types of maltreatment, 13 children experienced this treatment during one developmental period, and 26 children experienced maltreatment at two or more time periods.
Although the main effect of Maltreatment Chronicity was not significant (F[2, 143] = 1.574, p = .21), analyses revealed a significant Maltreatment Chronicity × Sex interaction (F[2, 143] = 7.09, p = .001). For girls, those who were exposed to maltreatment at two or more time periods had higher inflammation compared to girls who were not maltreated (Mdiff = .79, p = .001) or who experienced maltreatment in one period only (Mdiff = .70, p = .004). Nonmaltreated girls’ inflammatory composites did not differ from girls who had been maltreated during one time period (Mdiff = .08, p = .60). Among children who experienced maltreatment at two or more time periods, girls had higher inflammatory composite scores than boys (Mdiff = 1.06, p < .001). Boys’ inflammation scores did not differ as a function of the number of developmental periods when they experienced maltreatment (all ps > .09).
3.6. Age of First Onset of Maltreatment and Low-Grade Inflammation
Lastly, we conducted exploratory analyses to evaluate whether the age of onset of children’s first maltreatment exposure was associated with low-grade inflammation. Using the MCS scoring information, we categorized children based on their maltreatment exposure as early onset, occurring between infancy and preschool (roughly between the ages of 0-4 yr; n = 51) versus childhood onset (approximately ages 5-12; n = 24). Although there was no main effect of age of first onset, analyses highlighted a significant Maltreatment Onset Age × Sex interaction (F[2, 148] = 3.81, p = .024; see Figure 3). Among girls, those who had their first maltreatment experience early (that is, before age 5) had higher inflammatory composite scores than girls who were not maltreated (Mdiff = .46, p = .009) and had marginally higher inflammatory composite scores than girls whose exposure occurred at age 5 or later (Mdiff = .41, p = .08). Girls whose first maltreatment exposure occurred at age 5 or later did not differ from nonmaltreated girls in their levels of inflammation (Mdiff = .06, p = .79). Among children who were first exposed to maltreatment before the age of 5, girls had higher levels of inflammation than boys (Mdiff = .53, p = .004). Boys’ inflammation scores did not differ as a function of age at first onset (all ps > .11).
Figure 3.
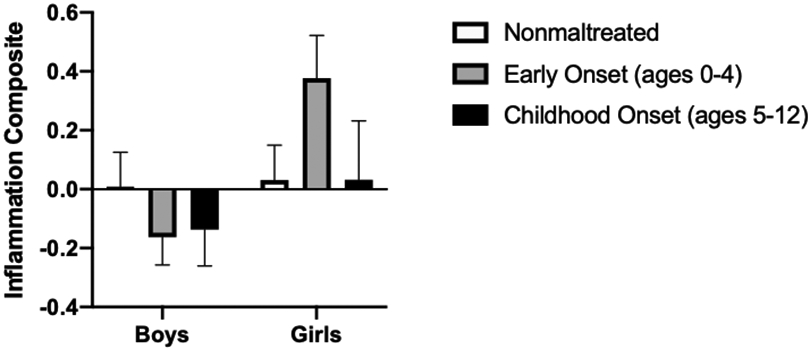
Standardized inflammation composite scores as a function of child sex and the age of first onset of maltreatment exposure. Among girls, those who had their first maltreatment experience before age 5 had higher inflammatory composite scores than girls who were not maltreated and had marginally higher inflammatory composite scores than girls whose exposure occurred at age 5 or later. Boys’ inflammation scores did not differ as a function of age at first onset
4. Discussion
The present study is among the first to examine whether maltreatment exposures are associated with low-grade inflammation in childhood. In contrast to previous studies of children that have focused on saliva, we measured inflammatory biomarkers in circulation. This approach better reflects inflammatory activity in the tissues where common health problems develop, and is validated by large-scale prospective studies linking circulating values to subsequent major depression, diabetes, heart disease, and premature mortality (Danesh, Collins, Appleby, & Peto, 1998; Libby, Ridker, & Hansson, 2009; Ridker, 2007; Yeh & Willerson, 2003). We found that childhood maltreatment—for girls—was associated with marginally higher levels of low-grade inflammation in late childhood. Subsequent analyses evaluated differences in inflammation as a function of the diversity and chronicity of exposures, and again we found a consistent pattern in which, for girls only, the experience of multiple forms and repeated instances of maltreatment was associated with higher inflammation. Finally, our exploratory analyses focused on the age of onset suggested that girls’ greatest risk for elevated inflammation emerged when they experienced maltreatment early in life—before the age of 5. One challenge with these analyses, however, is that it is difficult to distinguish between early onset from greater accumulation of maltreatment experiences across childhood. Indeed, in our sample, children who experienced maltreatment before age five had, on average, 1.84 maltreatment exposures, whereas children who were maltreated after age 5 had 1.25 documented exposures (t[73] = 3.66, p < .001). Given the exploratory nature and small Ns in these groups, we consider these findings preliminary until substantiated in subsequent projects.
Collectively, these findings provide support for the hypothesis that childhood maltreatment may give rise to low-grade inflammation, and these associations may appear earlier in development than previously observed. Given inflammation’s role in many chronic diseases of aging, as well as mental health outcomes in children and adults (e.g., Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Miller & Cole, 2012), these findings shed light on a possible mechanism that may explain, in part, how experiences like maltreatment in childhood may forecast chronic mental and physical health problems by midlife. These findings further suggest that maltreatment’s association with inflammation does not lie dormant for decades, only to emerge in adulthood. Rather, the findings suggest the possibility that these traumatic experiences have a more immediate impact on inflammatory signaling. This question deserves greater attention in future research, as do questions about timing of exposure and duration of effect. Because we collected blood samples at only one time point, our conclusions regarding these issues must be considered tentative. In this regard, our analyses do suggest the possibility that, for girls, exposure to maltreatment in childhood is associated with higher inflammation prior to the onset of adolescence. Whether this effect of maltreatment exposure in childhood is associated with sustained inflammation across adolescence and into adulthood remains unknown, however. Such longitudinal investigations will be important in order to evaluate whether maltreatment exposure puts individuals at risk for chronic mental and physical health problems. Also important in future studies will be the use designs that permit stronger causal inferences; the discordant twin approach used in Baldwin et al. (2018) is particularly useful in this regard.
Our use of the Maltreatment Classification System is a particular strength of the study that merits further discussion. By evaluating official case files with explicit operational definitions of multiple child maltreatment parameters, which detailed children’s experiences and events in the family, we were able to delineate more precise characterizations of children’s maltreatment exposures than would have been available with primary case determinations alone, Further, the detailed coding of records provided more precise information regarding frequency, timing, and maltreatment subtypes. This information is independent of biases related to parent-report or retrospective recall. As noted earlier, because of the distribution of maltreatment subtypes in our sample, we were unable to examine whether subtypes of abusive experiences were individually linked to children’s inflammation. Larger sample sizes will be important in future investigations to make more conclusive statements about whether there is some specificity in the types of maltreatment exposure and developmental timing of that exposure that is most strongly linked to inflammation, and whether the consequences of these variations in exposures differ for girls and boys.
Nevertheless, these findings provide an important step in linking maltreatment exposure to inflammation in childhood—a period of development generally considered to be an exceptionally healthy time in life. In fact, researchers looking to connect social experiences to inflammation in childhood often struggle with “floor effects” because the distribution of inflammatory markers is so restricted (Miller & Chen, 2010). Children in this sample were ostensibly healthy and free of chronic disease (and only 17% of the sample was considered overweight), yet approximately 25% of the sample had CRP values of 1.0mg/L or greater—values that the American Heart Association considers “moderate risk” for heart disease (Ridker, 2003). As noted earlier, caution is warranted here because we are relying on a one-time assessment of children’s CRP and other inflammatory biomarkers, so it is possible that we captured a temporary elevation that was soon mitigated. Longitudinal studies with multiple assessments of inflammatory markers will be informative for examining the extent to which maltreatment is associated with chronic elevations. Further, future studies that include multiple assessments of both maltreatment and inflammation across development would allow researchers to test more nuanced models about the timing of hypothesized links between maltreatment and inflammation (e.g., how soon does inflammation rise following exposure?). Similarly, multiple assessments would also allow for consideration of the developmental trajectories of inflammation across childhood (e.g., following maltreatment exposure, does inflammation rise gradually or is there a rapid spike in levels, and is this increase maintained over time?).
Another priority for future research is to unpack the possible explanations for the sex differences observed in the present study. In this sample, girls and boys did not differ in overall maltreatment exposure, number of subtypes, or number of developmental periods in which they experienced maltreatment. Sex differences in outcomes following maltreatment exposure have been reported (e.g., Chen et al., 2016; Gallo, Munhoz, Loret de Mola, & Murray, 2018; Godinet, Li, & Berg, 2014; Hagborg, Tidefors, & Fahlke, 2017), although findings appear to be inconsistent, and our understanding of why these differences occur has been lacking. Frequently, researchers include children’s sex as a control variable but do not consider possible sex differences in the negative outcomes associated with maltreatment. We speculate that sex differences in coping may be one possible explanation for the patterns observed in the present study, although we were unable to test this hypothesis in the present study.
In summary, research with adult samples suggests that the extent to which individuals are exposed to maltreatment in childhood is related to mild, chronic inflammation in adulthood. The present study adds to this literature and further suggests that, at least for girls, exposure to maltreatment is associated with inflammation much earlier in development during childhood. Future research should investigate why this effect is specific to girls, and whether any protective factors might mitigate these links.
Acknowledgments
This research was supported by MH083979 from the National Institute on Mental Health, DP2 MD013947 from the Common Fund, DA027827 from NIDA and HL122328 from NHLBI. This research was supported by the Jacobs Foundation (Early Career Research Fellowship 2018 1288 07).
Footnotes
Conflicts of Interest
All authors report no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Baldwin JR, Arseneault L, Caspi A, Fisher HL, Moffitt TE, Odgers CL, … Danese A (2018). Childhood victimization and inflammation in young adulthood: A genetically sensitive cohort study. Brain, Behavior, and Immunity, 67, 211–217. doi: 10.1016/j.bbi.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, & Cicchetti D (1993). Defining child maltreatment: The interface between policy and research. In Cicchetti D & Toth SL (Eds.), Child abuse, child development, and social policy (pp. 7–73). Norwood, NJ: Abex. [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V (2016. ). Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21, 642–649. doi: 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, & Rich-Edwards JW (2012). Inflammation and early-life abuse in women. American Journal of Preventive Medicine, 43, 611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ, & Kupersmidt JB (1998). Peer relationships and self-esteem among children who have been maltreated. Child Development, 69, 1171–1197. doi: 10.1111/j.1467-8624.1998.tb06166.x [DOI] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson M, Reynolds EC, Walsh KA, Laughton K, Waloszek J … Allen NB (2013). Acute phase protein and cytokine levels in serum and saliva: A comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behavior, and Immunity, 34, 164–175. doi: 10.1016/j.bbi.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Chen E, Turiano NA, Mroczek DK, & Miller GE (2016). Association of reports of childhood abuse and all-cause mortality rates in women. JAMA Psychiatry, 73, 920–927. doi: 10.1001/jamapsychiatry.2016.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Handley ED, & Rogosch FA (2015). Child maltreatment, inflammation, and internalizing symptoms: Investigating the roles of C-reactive protein, gene variation, and neuroendocrine regulation. Development and Psychopathology, 27, 553–566. doi: 10.1017/S0954579415000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Manly JT (1990). A personal perspective on conducting research with maltreating families: Problems and solutions. In Brody GH & Sigel I (Eds.), Methods of family research: Biographies of research projects (Vol. 2, pp. 87–133). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cicchetti D, & Toth SL (2005). Child maltreatment. Annual Review of Clinical Psychology, 1, 409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, & Manly JT (2003). Maternal Maltreatment Classification Interview. Unpublished manuscript. [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, & Grassi-Oliveira R (2013). Childhood maltreatment and inflammatory markers: A systematic review. Acta psychiatrica Scandinavica, 129, 180–192. doi: 10.1111/acps.12217 [DOI] [PubMed] [Google Scholar]
- Cohen MC, & Cohen S (1996). Cytokine function: A study in biologic diversity. American Journal of Clinical Pathology, 105, 589–598. doi: 10.1093/ajcp/105.5.589 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L Worthman C, Angold A, & Costello EJ (2012). Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biological Psychiatry, 71, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, … Arseneault L (2011). Biological embedding of stress through inflammation processes in childhood. Molecularly Psychiatry, 16, 244–246. doi: 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, … Caspi A (2009). Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatric Adolescent Medicine, 163, 1135–1143. doi: 10.1001/archpediatrics.2009.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104, 1319–1324. doi: 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & Tan M (2014). Childhood maltreatment and obesity: Systematic review and meta-analysis. Molecular Psychiatry, 19, 544–554. doi: 10.1038/mp.2013.54 [DOI] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiological Behavior, 106, 29–39. doi: 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, & Peto R (1998). Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. Journal of the American Medical Association, 279, 1477–1482. doi: 10.1001/jama.279.18.1477 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz H, Pitts SC, Litrownik AJ, Cox CE, Runyan D, & Black MM (2005). Defining child neglect based on child protective services data. Child Abuse & Neglect, 29, 493–511. doi: 10.1016/j.chiabu.2003.09.024 [DOI] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, & Chen E (2016). Childhood adversity and adult physical health. In Cicchetti D (Ed.), Developmental Psychopathology (3rd ed., pp. 1–42). Hoboken, NJ: Wiley. [Google Scholar]
- English DJ, Upadhyaya MP, Litrownik AJ, Marshall JM, Runyan DK, Graham JC, & Dubowitz H (2005). Maltreatment’s wake: The relationship of maltreatment dimensions to child outcomes. Child Abuse & Neglect, 29, 597–619. doi: 10.1016/j.chiabu.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Fang X, Brown DS, Florence CS, & Mercy JA (2012). The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse & Neglect, 36, 156–165. doi: 10.1016/j.chiabu.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D, Fang X, Elliott S, Casey T, Zheng X, Li J, Florian L, & McCluskey G (2018). The relationships between violence and childhood and educational outcomes: A global systematic review and meta-analysis. Child Abuse & Neglect, 75, 6–28. doi: 10.1016/j.chiabu.2017.06.021 [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhu J, & Van Eyk JE (2010). Comparison of multiplex immunoassay platforms. Clinical Chemistry, 56, 314–318. doi: 10.1373/clinchem.2009.135087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EAG, Munhoz TN, Loret de Mola C, & Murray J (2018). Gender differences in the effects of childhood maltreatment on adult depression and anxiety: A systematic review and meta-analysis. Child Abuse & Neglect, 79, 107–114. doi: 10.1016/j.chiabu.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Ganguly P, & Brenhouse HC (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental Cognitive Neuroscience, 11, 18–30. doi: 10.1016/j.dcn.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinet MT, Li F, & Berg T (2014). Early childhood maltreatment and trajectories of behavioral problems: Exploring gender and racial differences. Child Abuse & Neglect, 38, 544–556. doi: 10.1016/j.chiabu.2013.07.01 [DOI] [PubMed] [Google Scholar]
- Hagborg JM, Tidefors I, & Fahlke C (2017). Gender differences in the association between emotional maltreatment with mental, emotional, and behavioral problems in Swedish adolescents. Child Abuse & Neglect, 67, 249–259. doi: 10.1016/j.chiabu.2017.02.033 [DOI] [PubMed] [Google Scholar]
- Infurna MR, Reichl C, Parzer P, Schimmenti A, Bifulco A, & Kaess M (2016). Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. Journal of Affective Disorders, 190, 47–55. doi: 10.1016/j.jad.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, & Glaser R (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73, 16–22. doi: 10.1097/PSY.0b013e31820573b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, & Hansson GK (2009). Inflammation in atherosclerosis: From pathophysiology to practice. Journal of the American College of Cardiology, 54, 2129–2138. doi: 10.1016/j.jacc.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT (2019). Childhood maltreatment and impulsivity: A meta-analysis and recommendations for future study. Journal of Abnormal Child Psychology, 47, 221–243. doi: 10.1007/s10802-018-0445-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT (2005). Advances in research definitions of child maltreatment. Child Abuse & Neglect, 29, 425–439. doi: 10.1016/j.chiabu.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, & Cicchetti D (2001). Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology, 13, 759–782. [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21, 848–856. doi: 10.1177/0956797610370161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72, 34–40. doi: 10.1016/j.biopsych.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Kim H, & Singer LT (2013). Pathways linking childhood maltreatment and adult physical health. Child Abuse & Neglect, 37, 361–373. doi: 10.1016/j.chiabu.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80, 23–32. doi: 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys MB (2005). CRP or not CRP? That is the question. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 1091–1094. doi: 10.1161/01.ATV.0000169644.88847.28 [DOI] [PubMed] [Google Scholar]
- Ridker PM (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107, 363–369. doi: 10.1161/01.CIR.0000053730.47739.3C [DOI] [PubMed] [Google Scholar]
- Ridker PM (2007). Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutrition Reviews, 65, S253–259. doi: 10.1111/j.1753-4887.2007.tb00372.x [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, & Aber JL (1995). The role of child maltreatment in early deviations in cognitive and affective processing abilities and later peer relationship problems. Development and Psychopathology, 7, 591–609. doi: 10.1017/S0954579400006738 [DOI] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Green A, & Li S (2010). Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress, executive summary. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families. [Google Scholar]
- Simpson TL, & Miller WR (2002). Concomitance between childhood sexual and physical abuse and substance use problems: A review. Clinical Psychology Review, 22, 27–77. doi: 10.1016/S0272-7358(00)00088-X [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and Whites in the Midlife in the United States Survey. Psychosomatic Medicine, 72, 694–701. doi: 10.1097/PSY.0b013e3181e9c16f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, & Thornberry TP (1995). The relationship between childhood maltreatment and adolescent involvement in delinquency. Criminology, 33, 451–481. doi: 10.1111/j.1745-9125.1995.tb01186.x [DOI] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, & Seeman TE (2006). Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biological Psychiatry, 60, 819–824. doi: 10.1016/j.biopsych.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Lawlor DA, Harbord RM, Gaunt TR, Day IN, Palmer LJ, … Davey Smith G (2005). C-reactive protein and its role in metabolic syndrome: Mendelian randomization study. The Lancet, 366, 1954–1959. doi: 10.1016/S0140-6736(05)67786-0 [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Valentine TR, Eslinger NM, & Seifer R (2015). Adversity in preschool-aged children: Effects on salivary interleukin-1β. Development and Psychopathology, 27, 567–576. doi: 10.1017/S0954579415000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Institutes of Health (2020). Child Maltreatment 2018. Washington, DC: Author. [Google Scholar]
- Wegman HL, & Stetler C (2009). A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine, 71, 805–812. doi: 10.1097/PSY.0b013e3181bb2b46 [DOI] [PubMed] [Google Scholar]
- Yeh ETH, & Willerson JT (2003). Coming of age of C-reactive protein: Using inflammation markers in cardiology. Circulation, 107, 370–371. doi: 10.1161/01.CIR.0000053731.05365.5A [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


