Abstract
Background:
Glucose tolerance abnormalities including cystic fibrosis related diabetes (CFRD) are common in patients with cystic fibrosis (CF). The underlying pathophysiology is not fully understood. Emerging evidence suggests that CFTR dysfunction may directly or indirectly impact β-cell function, offering the potential for improvement with CFTR modulator therapy. In small pilot studies, treatment with ivacaftor improved insulin secretion in patients with the G551D CFTR mutation. In the current study, we examined the impact of lumacaftor/ivacaftor therapy on glucose tolerance and insulin secretion in patients with CF who were homozygous for the F508del mutation.
Methods:
39 subjects from the PROSPECT Part B study who had been prescribed lumacaftor/ivacaftor by their CF care team at a CF Foundation’s Therapeutic Development Network center were recruited. Subjects underwent 2-hour oral glucose tolerance tests (OGTTs) at baseline prior to first dose of lumacaftor/ivacaftor, and at 3, 6 and 12 months on therapy. OGTT glucose, insulin and c-peptide parameters were compared.
Results:
Compared to baseline, OGTT fasting and 2 hour glucose levels, glucose area under the curve, insulin area under the curve and time to peak insulin level were not significantly different at 3, 6 and 12 months on lumacaftor/ivacaftor therapy. Similarly, C-peptide levels were no different.
Conclusions:
Lumacaftor/ivacaftor therapy did not improve insulin secretion or glucose tolerance in patients with CF who were homozygous for the F508del mutation.
Keywords: Cystic fibrosis, CFRD, Insulin secretion, Orkambi
1. Background
Lumacaftor/ivacaftor (brand name Orkambi) is a combination drug that was approved by the US FDA and the European Medicines Agency in 2015 to treat people with cystic fibrosis (CF) who have two copies of the F508del cystic fibrosis transmembrane conductance regulator (CFTR) mutation. Ivacaftor increases the activity of the CFTR protein at the surface of epithelial cells, while lumacaftor improves CFTR folding and trafficking to the cell surface [1,2]. PROSPECT (A Multicenter Longitudinal Study of CFTR-dependent Disease Profiling in Cystic Fibrosis), was an observational post-marketing study conducted in the United States to evaluate multiple outcomes of lumacaftor/ivacaftor therapy, with Part B including subjects who had two copies of the F508del mutation [3]. GIFT (Glucose and Insulin Functional Testing) was a substudy of Part B, designed to assess the impact of lumacaftor/ivacaftor on glucose tolerance and insulin secretion in this population.
Glucose tolerance abnormalities are a well-known feature of CF. Cystic fibrosis related diabetes (CFRD) occurs in 15-20% of adolescents and more than half of adults [4]. The majority of CF patients who do not have overt diabetes have abnormal glucose tolerance [5]. While CFRD does not usually develop before puberty, it has its roots in childhood and impaired glucose tolerance and indeterminate glycemia are common in 6-9 year old children with CF [6].
While there are many factors that lead to development of diabetes in CF, the primary pathologic feature is progressive insulin insufficiency [5]. Even patients with normal glucose tolerance by oral glucose tolerance testing (OGTT) display abnormalities in insulin secretion. It has been postulated that the basic CFTR defect is involved in the etiology of CFRD, either through a direct role in β-cell insulin secretion or indirectly via its impact on islet inflammation and exocrine pancreatic disease. Over time, as fibrosis of the exocrine pancreas progressively disrupts and destroys islets, and as unrelenting inflammatory stress occurs related to chronic pulmonary and sinus infection, the functional impact of CFTR on insulin secretion in the remaining islets potentially becomes more critical.
A proof-of-principle study in 5 CF patients with the G551D CFTR mutation treated for 1 month with ivacaftor provided preliminary evidence that drugs that substantially improve CFTR function may increase insulin secretion in CF [7]. Similar evidence of improved early insulin secretion after ivacaftor therapy was found in a study of 12 individuals with the G551D mutation (8). GIFT was developed to examine whether the lumacaftor/ivacaftor combination would impact insulin secretion and glucose tolerance in patients homozygous for the common CF mutation F508del.
2. Methods
2.1. Subjects
Subjects in PROSPECT Part B were homozygous for the F508del mutation and had been prescribed lumacaftor/ivacaftor by their CF care team at one of the multiple CF centers that form the CF Foundation’s Therapeutic Development Network (3). Part B subjects age six years and older with and without CFRD were offered the choice of participating in the GIFT substudy. The substudy was approved by human subject committees at each institution where the work was performed, and all subjects gave informed consent/assent as appropriate for age. Acute illness or systemic steroid treatment in the 28 days prior to baseline were exclusion criteria, as were organ transplantation, treatment with oral hypoglycemic agents, or antibody-proven type 1 diabetes. Patients were enrolled for up to 12 months. “Study end” refers to the last study visit completed.
2.2. OGTT protocol
A standard two-hour OGTT was performed at baseline prior to the first dose of lumacaftor/ivacaftor, and at 3, 6 and 12 months on therapy. After an overnight fast, an intravenous line was placed for blood draws, and patients consumed 1.75 gr/kg (max 75 gram) dextrose (GlucoCrush, CardinalHeath, Dublin, Ohio). Blood was drawn prior to and at 30, 60, 90, and 120 minutes post glucose ingestion. Patients on overnight enteral tube feedings withheld the feeding the night before the OGTT. All patients with CFRD were on insulin therapy at baseline. For the OGTT, they continued their usual basal insulin (insulin glargine or detemir for those on multiple daily injections and rapid-acting insulin at basal rates for those on continuous infusion pumps), but withheld all rapid-acting insulin boluses for at least 6 hours prior to the OGTT.
2.3. Analytic methods
Plasma samples were collected on ice and immediately centrifuged at 4°C, separated, and stored at −20°C. Deidentified frozen samples were shipped on dry ice to the central lab at Fairview University Diagnostic Laboratories in Minneapolis, MN for determination of glucose, insulin and c-peptide levels, and analysis and calculation of glucose tolerance and metabolic status. Insulin and c-peptide levels were measured by chemiluminescent immunoassay and glucose by a glucose oxidase method.
2.4. Statistics
Logarithmic transformation was applied to the OGTT glucose levels and insulin parameters at baseline, and 3, 6, and 12 months after starting lumacaftor/ivacaftor therapy to ensure normal distribution of the transformed data. The means of the log-transformed data were compared between baseline, 3, 6, and 12 months using one-way ANOVA for each variable, including fasting glucose levels, 2-hour glucose levels, glucose area under the curve, insulin area under the curve, and time to peak insulin level during the OGTT.
3. Results
3.1. Subjects
Thirty-nine subjects were recruited from 16 centers. Subject characteristics are present in the Table. At baseline subjects were 22±10 years of age (range 12-51) and 51% were female. Of the 39 subjects who participated in GIFT, 30 completed 12 months in the study, 4 completed 6 months, and 5 completed 3 months. Reasons for dropout before 12 months included discontinuing lumacaftor/ivacaftor or refusing further OGTTs.
Between baseline and study end, clinical parameters changed as follows: sweat chloride decreased from 101±10 to 82±18 mmol/L (p=<0.001); FEV1 (%pred) was unchanged from 85±22 to 87±22 (p=0.89); BMI percentile (age ≤18) was unchanged from 63±25 to 69±20 (p=0.31); and BMI (kg/m2, age>18) increased from 22.6±2.5 to 23.8±3.0 (p = 0.03).
3.2. Glucose tolerance category
As expected in a CF population, there was modest fluctuation over time between OGTT categories (categorical distributions were not statistically different). At baseline, 15 (38%) had CFRD, 6 (15%) had IGT, 9 (23%) had INDET, and 9 (23%) had NGT. At study end (last completed OGTT visit), glucose tolerance category improved in 9 (23%), deteriorated in 8 (21%) and did not change in 22 (56%) subjects. Thirty-three percent of subjects with NGT had deterioration in glucose tolerance status, half of subjects with INDET or IGT improved while half stayed the same or deteriorated, and 2 (13%) subjects with CFRD demonstrated an improved glucose tolerance category at study end (Table 1, Table 2).
Table 1.
GIFT substudy subject characteristics at baseline and after 12 months of lumacaftor/ivacaftor therapy. Mean ± SD.
| Baseline | *12 Months | |
|---|---|---|
| Age | 22.19 ± 9.76 | 21.92 ± 9.00 |
| Gender M/F, n | 19 / 20 | 16 / 14 |
| Genotype, n (%) | ||
| • ΔF508/ΔF508 | 39 (100) | 30 (100) |
| OGTT Category, n (%) | ||
| • NGT | 9 (23) | 12 (40) |
| • INDET | 9 (23) | 5 (16.7) |
| • IGT | 6 (15) | 5 (16.7) |
| • CFRD | 15 (38) | 8 (26.7) |
| BMI | ||
| • Age >18: BMI kg/m2 | 22.58 ± 2.46 | 23.76 ± 3.05 |
| • Age ≥18: BMI percentile | 63.38 ± 24.56 | 68.60 ± 20.33 |
| %predFEV1 | 85.08 ± 22.11 | 87.17 ± 21.58 |
| Sweat Chloride (mmol/L) | 100.76 ± 9.79 | 81.50 ± 18.27 |
NGT=normal glucose tolerance, INDET=indeterminate glycemia, IGT=impaired glucose tolerance, CFRD= CF-related diabetes.
Baseline summaries are based on the 39 subjects enrolled with OGTT baseline measure; 12-month summaries are based on 30 subjects who had OGTT measures at 12 months.
Table 2.
Change in OGTT category from baseline.
| Total Subject Number | OGTT Category Improved N, (%) | OGTT Category Stayed the Same N, (%) | OGTT Category Worsened N, (%) | |
|---|---|---|---|---|
| Total Cohort | 39 | 9 (23%) | 24 (62%) | 6 (15%) |
| CFRD (on insulin therapy) | 15 | 2 (13%) | 13 (87%) | NA |
| Non-diabetic (NGT, INDET, IGT) | 24 | 7 (29%) | 11 (46%) | 6(25%) |
| NGT | 9 | NA | 6 (67%) | 3 (33%) |
| IGT | 6 | 3 (50%) | 1(17%) | 2 (33%) |
| INDET + IGT | 15 | 7 (47%) | 5 (33%) | 3 (20%) |
NGT=normal glucose tolerance, INDET=indeterminate glycemia, IGT=impaired glucose tolerance, CFRD= CF-related diabetes.
3.3. Glucose levels
The distribution of glucose parameters per time point are shown in Fig. 1. The p-values for the ANOVA tests comparing the means at baseline, 3, 6, and 12 months for fasting glucose levels, 2-hour glucose levels, and glucose area under the curve during the OGTT were 0.74, 0.26, and 0.67, respectively. While these values represent the group as a whole, there were also no significant differences noted when data were evaluated by the glucose tolerance category at baseline (NGT, IGT, INDET, CFRD–data not shown). Thus, lumacaftor/invacaftor did not significantly impact glucose levels between baseline, 3, 6, and 12 months in this population.
Fig. 1.
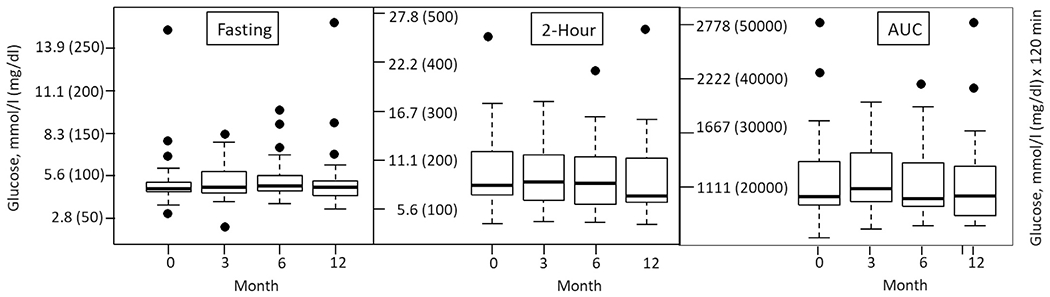
Box plots of OGTT glucose levels at baseline and 3, 6, and 12 months after starting lumacaftor/ivacaftor therapy. Left: fasting glucose levels; Middle: 2 hour glucose levels; Right: Glucose area under the curve. The boundaries of the boxes indicate the interquantile range (IQR), with the bold line in the middle indicating the median. The dots are outliers, which are defined as beyond the range between the top and bottom lines. Data are presented for all 39 subjects, at each time point they completed.
3.4. Insulin levels
The distribution of the insulin area under the curve during the OGTT and the time to peak insulin level (a crude estimate of early insulin secretion) per time point, are shown in Fig. 2. The p-values for the ANOVA tests comparing the means of these two parameters at baseline, 3, 6, and 12 months were 0.82 and 0.33, respectively. Similar to glucose levels, there were no significant differences noted when data were evaluated by glucose tolerance category at baseline (data not shown). C-peptide responses mirrored insulin responses (data not shown). Thus, lumacaftor/invacaftor did not significantly impact insulin secretion parameters between baseline, 3, 6, and 12 months.
Fig. 2.
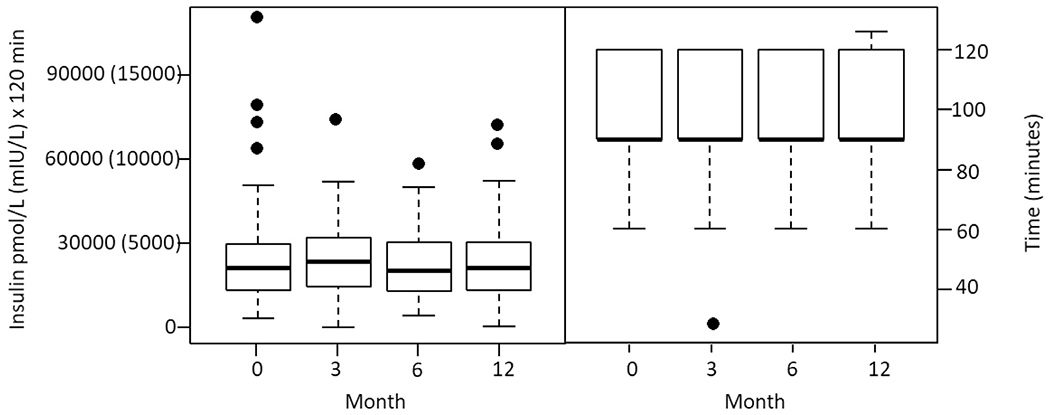
Box plots of OGTT insulin parameters at baseline and 3, 6, and 12 months after starting lumacaftor/ivacaftor therapy. Left: Insulin area under the curve; Right: Time to peak insulin level during the OGTT.
3.5. Diabetes management
For those with CFRD, insulin therapy was managed by their primary teams during the course of the study. No patient reported significant hypoglycemia. It is unknown whether insulin dosage was reduced in the two patients whose OGTT improved.
4. Discussion and conclusion
This study examined the impact of lumacaftor/ivacaftor therapy on glucose tolerance and insulin secretion in patients with CF and two copies of the F508del CFTR mutation. We hypothesized that, similar to the impact of ivacaftor on patients with the G551D mutation, lumacaftor/ivacaftor would improve glucose metabolic parameters in this population. Our hypothesis proved to be incorrect. During up to 12 months of therapy, there was no significant improvement in glucose tolerance in any of the four glucose tolerance categories. Lumacaftor/ivacaftor did not significantly impact glucose, insulin or c-peptide levels between baseline, 3, 6, and 12 months.
While overt diabetes does not usually occur before puberty in CF, abnormalities in insulin secretion and glucose tolerance occur very early in life. Yi and colleagues reported that abnormal glucose metabolism was already present in 39% of children with CF between the ages of 3 months to 5 years [9]. In CF ferret and pig models, abnormalities in glucose tolerance and insulin secretion are seen in the neonatal period [10,11]. In newborn CF pigs, the abnormalities in glucose tolerance were not associated with reduced islet cell mass, suggesting that functional β-cell defects play a role in the pathogenesis of CFRD (10). These studies imply a role for CFTR in insulin secretion, raising the possibility that correction of CFTR might impact endocrine pancreatic function.
Clinical results from small pilot studies in humans provided early evidence that CFTR may play a reversible role in insulin secretion abnormalities. Five CF patients with the G551D mutation underwent oral and intravenous glucose tolerance testing before and after 4 weeks of daily therapy with ivacaftor; four of the five showed improved OGTT and IVGTT insulin responses, with 66-178% greater stimulated insulin secretion [7]. In another study, improved early insulin secretion was found four months after starting ivacaftor therapy in 12 individuals with the G551D mutation [8]. These promising early studies did not answer the controversial question of whether CFTR is directly involved in insulin secretion, or, alternatively, whether the beneficial effect of increased CFTR activity on the β-cell is via indirect mechanisms such as improved clinical and nutritional status and reduced systemic inflammation.
A direct role is suggested by several laboratory studies reporting CFTR expression and channel activity in rodent and human islets [12–16]. Studies have also provided evidence that CFTR channels play a role in insulin exocytosis and regulation of membrane potential in β-cells, and that CFTR deficiency may lead to defects in insulin secretion [14,15,17]. In vitro, silencing of CFTR in MIN6 β-cells (a pancreatic insulinoma cell line) leads to significantly reduced insulin secretion [13]. CFTR deficiency has also been reported to contribute to dysregulation in glucagon secretion [16]. These experimental data support the concept that CFTR is present in α- and β-cells and may play a direct role in insulin secretion.
However, not all studies support this hypothesis. In a thorough set of experiments, Hart et al. [18] reported that CFTR deletion from mouse β-cells did not affect glucose tolerance. They also found minimal CFTR mRNA expression and no detectable CFTR protein in human islet cells. They concluded that β-cell loss and dysfunction is due to fibrotic and inflammatory disruption of the islet external environment and that it is intra-islet inflammation, rather than the direct effect of a CFTR mutation in the β-cell, that affects insulin secretion.
Whether the impact of CFTR on pancreatic endocrine function is direct or indirect, one might expect that normalization of CFTR activity would halt ongoing damage and potentially lead to metabolic improvement, such as was seen in the ivacaftor pilot studies [7,8]. However, two recent small pilot studies evaluating lumacaftor/ivacaftor treatment of F508del homozygous patients did not find improvement in OGTT parameters or in glucose levels measured by continuous glucose monitoring; these studies included 5 patients evaluated before and 6-8 weeks after treatment with the modulator [19], and nine adolescents assessed after about 6 months of treatment [20]. The current study with a much larger number of subjects demonstrates that lumacaftor/ivacaftor does not improve β-cell function or glucose tolerance in this population.
The most likely explanation for why there is no observed influence of lumacaftor/ivacaftor on OGTT glucose or insulin levels is that there is insufficient improvement in CFTR activity with this particular drug in the F508del homozygous population to make a measurable difference in islet function. In the 4% of CF patients who carry the G551D mutation, ivacaftor is highly effective at restoring CFTR activity, improving sweat chloride measurements (a surrogate measure for CFTR function) from a mean of ~100 mmol/L at baseline to ~50 mmol/L, a 50% improvement and, importantly, one that decreases sweat chloride levels to below the 60 mmol/L threshold required for the diagnosis of CF [21]. In contrast, only modest sweat chloride improvements have been noted with lumacaftor/ivacaftor in F508del homozygous patients. In the current PROSPECT cohort, mean sweat chloride levels only improved by about 20%, from ~100 mmol/L at baseline to ~80 mmol/L at 12 months [3]. Cell culture studies are consistent with these findings. While ivacaftor has been shown to increase G551D CFTR activity to about half of that observed in normal human bronchial epithelial cells [22], the combination of lumacaftor and ivacaftor restores F508del CFTR activity to only about 10-30% of wild-type CFTR levels [23]. Thus, we postulate that the CFTR modulating activity of lumacaftor/ivacaftor in the F508del homozygous population is insufficient to significantly improve pancreatic insulin secretion.
There are several limitations to this study. Twenty-three percent of subjects did not complete the full 12 months, raising questions about their overall level of adherence to the protocol and to ivacaftor/lumacaftor therapy. The non-randomized study design is a limitation. We cannot rule out that more subtle improvements might have been seen over a longer study period.
Since this manuscript was submitted a new report came out of France with a different conclusion [24]. Forty patients age 24±10 years with IGT (78%) or newly diagnosed untreated CFRD without fasting hyperglycemia (22%) were followed for one year on ivacaftor/lumacaftor therapy. They found that 58% of subjects showed improvement and 43% no change in glucose tolerance; no patient worsened. The primary difference between our studies is in the patient population. They excluded patients requiring insulin therapy for CFRD and those with NGT. Abnormal glucose tolerance was defined by the 2 hour and not the 1 hour glucose, so subjects with INDET were not included. If we look at the six patients in our study with IGT, three (50%) showed improvement. If we combine the 15 subjects with INDET and IGT (since these two categories have equal risk of progression to CFRD), we similarly see that 47% showed improvement, 33% stayed the same, and 20% worsened. Thus, looking at our study and the study out of France, there is potentially a modest improvement in glucose tolerance in the subset of patient who start out with IGT or INDET.
There are several medications newly available or currently in the developmental pipeline with greater impact on CFTR function than lumacaftor/ivacaftor. The triple combination of elexacaftor/tezacaftor/ivacaftor [25–27] has produced robust improvements in sweat chloride levels in F508del heterozygous and homozygous populations, similar to the improvement seen with ivacaftor treatment of patients with G551D mutations. There are other drugs in development which might lead to similar or even bigger improvements. Given the greater CFTR functional recovery with newer compounds, it will be important to investigate their impact on insulin secretion and CFRD. It is plausible to speculate that the greatest impact of modulator drugs might occur if effective therapy was initiated very early in life, at a time when pancreatic β-cell mass is still relatively preserved. In this situation, CF-related diabetes could theoretically become a preventable complication of CF.
In summary, our results show that lumacaftor/invacaftor therapy did not improve insulin secretion or glucose tolerance in patients with CF who were ΔF508del homozygous, although we cannot rule out a modest effect in those with IGT. The unimpressive effect may be related to the fact that lumacaftor/ivacaftor had limited impact on CFTR activity, below that needed to restore CFTR-dependent β-cell function. Future research with new modulator therapies that result in greater CFTR recovery would provide more insights into the role of CFTR in β-cell function in humans.
Acknowledgments
Funding
This work was funded by the Cystic Fibrosis Foundation (SAGEL14K1). The Cystic Fibrosis Foundation had no role in the study design, data analysis, or manuscript writing.
Abbreviations
- AUCgluc
area under the 2 hour OGTT curve, glucose
- AUCins
area under the 2 hour OGTT curve, insulin
- CFRD
cystic fibrosis related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- GIFT
Glucose and Insulin Functional Testing, a substudy of PROSPECT
- IGT
Impaired glucose tolerance
- INDET
Indeterminate glycemia
- NGT
Normal glucose tolerance
- OGTT
Oral glucose tolerance test
- PROSPECT
A Two-Part Multicenter Prospective Longitudinal Study of CFTR-Dependent Disease Profiling in Cystic fibrosis
Footnotes
Declaration of Competing Interest
Amir Moheet has no declarations of interest. Daniel Beisang has no declarations of interest. Lin Zhang has no declarations of interest. Scott D. Sagel received grant funding from the CFF to conduct this study. Jill M. VanDalfsen has no declarations of interest. Sonya L. Heltshe has no declarations of interest. Carla Frederick has no declarations of interest. Michelle Mann has no declarations of interest. Nicholas Antos has no declarations of interest. Joanne Billings has no declarations of interest. Steven M. Rowe provided consulting services for Vertex Pharmaceuticals, and received research product for investigator initiated research and trial support from that company. Antoinette Moran served on a medical advisory board for Vertex Pharmaceuticals. She received grant funding from the CFF to conduct this study.
References
- [1].Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Urrutia A, Joubran J, Seepersaud S, Sussky K, Hoffman BJ, Van Goor F, Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros 2012;11:237–45. [DOI] [PubMed] [Google Scholar]
- [2].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA, Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011;108:18843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sagel SD, Khan U, Heltshe SL, Clancy JP, Borowitz D, Gelfond D, Donaldson SH, Moran A, Ratjen F, VanDalfsen JM, Rowe SM, on behalf of the PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical effectiveness of lumacaftor/ivacaftor in cystic fibrosis patients homozygous for F508del-CFTR. 2020; submitted [Google Scholar]
- [4].Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med 2015;191:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, Slovis B, Committee CCC. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care 2010;33:2677–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, Thomas W, Moran A. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 2010;11:487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, Moran A, Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes 2013;14:417–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, Stefanovski D, Hadjiliadis D, Rickels MR, Rubenstein RC. Islet hormone and incretin secretion in cystic fibrosis following 4-months of ivacaftor therapy. Am J Respir Crit Care Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, Engelhardt JF, Ode KL, Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2016;194:974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, Buchanan KM, Vanegas Calderon OG, Abu-El-Haija M, Pezzulo AA, Reznikov LR, Hoegger MJ, Rector MV, Ostedgaard LS, Taft PJ, Gansemer ND, Ludwig PS, Hornick EE, Stoltz DA, Ode KL, Welsh MJ, Engelhardt JF, Norris AW. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boom A, Lybaert P, Pollet JF, Jacobs P, Jijakli H, Golstein PE, Sener A, Malaisse WJ, Beauwens R, Expression and localization of cystic fibrosis transmembrane conductance regulator in the rat endocrine pancreas. Endocrine 2007;32:197–205. [DOI] [PubMed] [Google Scholar]
- [13].Ntimbane T, Mailhot G, Spahis S, Rabasa-Lhoret R, Kleme ML, Melloul D, Brochiero E, Berthiaume Y, Levy E, CFTR silencing in pancreatic beta-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol Endocrinol Metab 2016;310:E200–12. [DOI] [PubMed] [Google Scholar]
- [14].Edlund A, Esguerra JLS, Wendt A, Flodstrom-Tullberg M, Eliasson L, CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med 2014:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, Fok KL, Tsang LL, Yu MK, Huang WQ, Sun X, Chung YW, Jiang X, Sohma Y, Chan HC, Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun 2014;5:4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodström-Tullberg M, Eliasson L. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep 2017;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koivula FNM, McClenaghan NH, Harper AGS, Kelly C, Islet-intrinsic effects of CFTR mutation. Diabetologia 2016;59:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, Bautista A, Spigelman AF, Babon JAB, DeNicola ME, Dadi PK, Bush WS, Balamurugan AN, Brissova M, Dai C, Prasad N, Bottino R, Jacobson DA, Drumm ML, Kent SC, MacDonald PE, Powers AC. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 2018:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thomassen JC, Mueller MI, Alejandre Alcazar MA, Rietsche lE, van Konings-bruggen-Rietschel S, Effect of Lumacaftor/Ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J Cyst Fibros 2018;17:271–5. [DOI] [PubMed] [Google Scholar]
- [20].Li A, Vigers T, Pyle L, Zemanick E, Nadeau K, Sagel SD, Chan CL, Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J Cyst Fibros 2019;18:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ramsey BW, Davies J, McElvaney G, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 2011;108:18843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescua P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009;106:18825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Misgault B, Chatron E, Reynaud Q, Touzet S, Abely M, Melly L, Dominique S, Troussier F, Ronsin-Pradel O, Gerardin M, Mankikian J, Cosson L, Chiron R, Bounyar L, Porzio M, Durieu I, Weiss L, Kessler R, Kessler L. Effect of one-year lumacaftor-ivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J CystFibros, 10.1016/j.jcf2020.03.002. [DOI] [PubMed] [Google Scholar]
- [25].Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL, Group V–S. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med 2018;379:1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R. VX17-445-102 Study Group. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med 2019;7:1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, E. T, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, M SM, Sos-nay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy KS, VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019:194–148 [DOI] [PMC free article] [PubMed] [Google Scholar]


