Abstract
Tick-borne encephalitis virus (TBEV) is one of the most common zoonotic vector-borne infections in Europe. An appropriate awareness is crucial to react quickly and efficiently to protect humans from this pathogen. From winter 2017 until spring 2018 serum samples were collected from 71 small ruminant flocks (3174 animals) in five German federal states. The sera were examined for TBEV antibodies by ELISA and serum neutralization test. In the TBEV risk areas, there was a coincidence in 14 districts between seropositive small ruminants and the occurrence of human TBE cases in 2017. In eight districts, the TBEV infection could not be detected in small ruminants although human cases were reported. In contrast, in five districts, small ruminants tested TBEV seropositive without notified human TBE cases in 2017. A changing pattern of TBEV circulation in the environment was observed by the absence of antibodies in a defined high-risk area. In the non-TBE risk areas, seropositive small ruminants were found in five districts. In two districts with a low human incidence the infection was missed by the small ruminant sentinels. An intra-herd prevalence of 12.5% was determined in a goat flock in the non-TBE risk area in 2017, two years prior the first autochthone human case was reported. All sheep and goats in this flock were examined for TBEV antibodies for three years. Individual follow-up of twelve small ruminants was possible and revealed mostly a short lifespan of TBEV antibodies of less than one year. The probability to identify TBEV seropositive sheep flocks was enhanced in flocks kept for landscape conservation or which were shepherded (p < 0.05). Our preliminary observations clearly demonstrated the successful utilization of small ruminants as sentinel animals for TBEV.
Keywords: Sheep, Goat, Tick-borne encephalitis virus, Sentinels, Public health, Surveillance
Highlights
-
•
A new TBEV hot spot was identified by screening of small ruminants.
-
•
Naturally acquired TBEV antibodies lasted less than one year in sheep and goats.
-
•
Reduced circulation of TBEV in the environment was indicated by small ruminants.
-
•
Landscape conservation and shepherding increased the chances to detect TBEV antibodies.
1. Introduction
Zoonotic vector-borne diseases are rapidly spreading in Europe and an increasing awareness is vital to react quickly and efficiently to protect humans from infections. One option to achieve this is by establishing surveillance programs using animals as sentinels for early warning [1,2]. Small ruminants are often kept under extensive husbandry conditions worldwide and have close contacts to vectors including ticks and insects carrying emerging zoonotic pathogens e. g. tick-borne encephalitis virus (TBEV), Crimean-Congo haemorrhagic fever virus (CCHFV) or West Nile virus (WNV) [[3], [4], [5]]. These pathogens induce a detectable humoral immune response but usually do not cause clinical signs in small ruminants. Therefore, sheep and goats are suitable sentinel animals to monitor such infections. Unlike most reservoir animals and vectors, small ruminants are easy to sample. The normally well-known precise geographic location of small ruminants provides information about the spatial distribution of the pathogen. This plays a crucial role for the occurrence and distribution of emerging zoonotic diseases and the timely implementation of measures to raise the awareness in the public health sector.
TBEV is one of the most common zoonotic vector-borne infections in Europe and is mainly transmitted by Ixodes ricinus through a tick bite [6]. In 2001, TBE was made a statutorily notifiable disease in humans through the German Protection against Infection Act (Infektionsschutzgesetz). Risk areas have been designated based on the incidence of infected humans and are defined as an area where the incidence is significantly (p < 0.05) higher during a five-year period in the district or within the district region (involving the district plus neighbouring districts) than the expected incidence of 1 illness/100,000 inhabitants [7]. The incidence in the two most affected German federal states, Baden-Wuerttemberg and Bavaria, undulates between 0.7 and 2.0 cases/100,000 inhabitants [8]. Clinical presentation of TBEV infection in humans was recently summarized by Ruzek and colleagues [6] in detail. In contrast to humans, TBEV rarely cause symptoms in small ruminants [[9], [10], [11]].
There is an increasing number of reports of clinical TBEV cases in humans associated with the consumption of raw milk products from infected small ruminants [[12], [13], [14]]. Virus shedding through milk was reported for six days in sheep and up to 23 days in goats [10,15]. Therefore, it is more promising to determine TBEV antibodies in serum or milk samples than detecting the virus in milk itself. An additional limit in TBEV surveillance is that the natural foci are extremely small and the virus is hardly found in ticks [[16], [17], [18]].
Examining sera from sheep and goats for TBEV antibodies was found to be a suitable tool for the early detection of virus circulation in the environment before human cases occurred [[17], [18], [19], [20]]. In the present study, the occurrence of TBEV antibodies was determined in 71 small ruminant flocks kept in Germany. In order to evaluate the usage of animal data for TBEV surveillance, the number of human TBE cases based on the German district level were used as a comparison. Factors which might influence the detection of TBEV antibodies in small ruminants were available from interviews with the sheep farmers and were additionally analysed. The longevity of TBEV antibodies in naturally infected sheep and goats were identified by conducting an individual follow-up study for three years in eight goats and four sheep in the same flock.
2. Material & methods
2.1. Animals
Serum samples from small ruminants were available from a Q fever study conducted from winter 2017 until spring 2018 [21]. In total, 71 sheep flocks were visited in five federal states of Germany: Schleswig-Holstein (SH), Lower Saxony (LS), North Rhine-Westphalia (NRW), Baden-Wuerttemberg (BW) and Bavaria (BAV). These states have the largest sheep populations within Germany. The participating farms were selected based on the owners' willingness to contribute to the study. Within the flocks, the number of required samples to estimate the intra-herd prevalence of TBEV antibodies was calculated on the assumption of 3% expected prevalence [17], 95% confidence interval, 80% power and 5% precision. A maximum of 44 animals per flock were sampled. If goats were kept on the same farm, their sample size was calculated under the same assumptions, independently of the number of sampled sheep. In 2017, the northern states (SH, LS, NRW) were defined as non-TBE risk areas according to the Robert Koch-Institute [22]. Whereas almost the entire areas of the southern states (BW, BAV) were known as TBEV risk areas [22] (Fig. 1). In total, 36 small ruminant flocks (1396 sheep and 323 goats) from 27 districts located in the TBE risk area and 35 small ruminant flocks (1331 sheep and 124 goats) from 22 districts in the non-TBE risk area were involved in the present study. Information on the husbandry system, tick infestation observed by the animal owners and treatment against ectoparasites within the last 12 months was available due to interviews with the farm managers which were conducted for the Q fever study [23]. These factors were also analysed with regard to TBEV infection in small ruminants. The incidence of reported human TBE cases in 2017 was taken as a reference for comparison with the data received from the animal analysis [24].
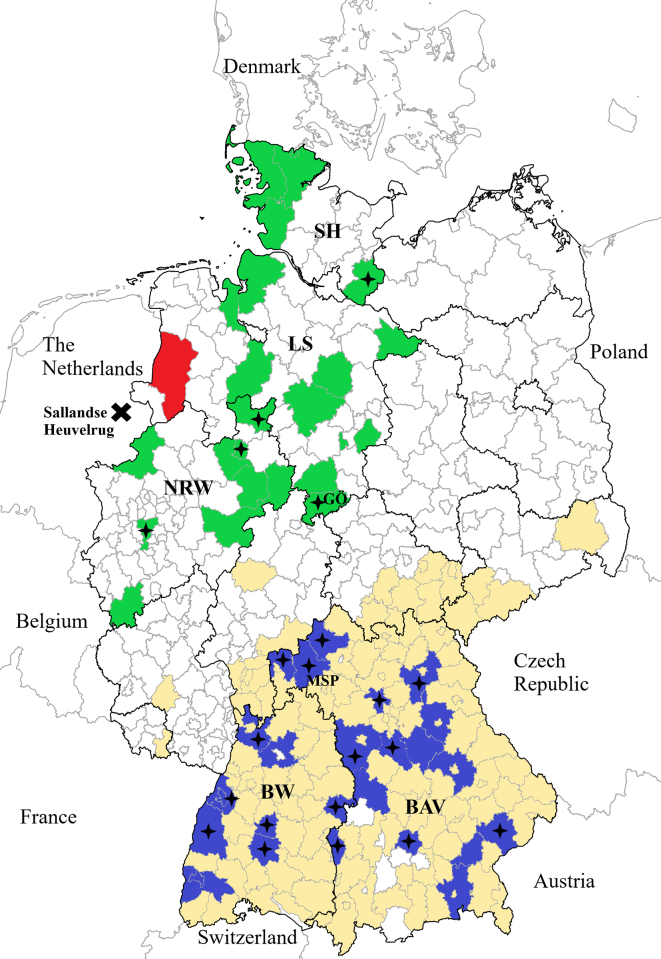
Fig. 1.
Administrative districts of Germany sectioned according to the risk for humans to acquire TBEV infection and the location of districts with small ruminant flocks participating in this TBEV antibody study.
Districts within TBEV risk areas are coloured in yellow, non-risk areas are uncoloured. This classification is based on human TBE cases notified between 2002 and 2017 [22].
In total, 71 small ruminant farms in five German federal states (Schleswig-Holstein: SH, Lower Saxony: LS, North Rhine-Westphalia: NRW, Baden-Wuerttemberg: BW, Bavaria: BAV) participated in the study. The district Emsland, coloured in red, is the first TBEV risk area in the federal state of LS since 2019 (in this district no small ruminants were sampled).
Districts with participating small ruminant flocks within the TBEV risk areas are coloured in blue, districts with participating small ruminant flocks within the non-TBEV risk areas are coloured in green. The black stars indicate the localisation of the flocks with seropositive TBEV small ruminants, GÖ = district of Göttingen, MSP = district of Main-Spessart, and Sallandse Heuvelrug a TBEV hot spot in the Netherlands. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
TBEV antibodies were also determined in a dairy small ruminant flock in the non-TBE risk area [Lower Saxony, Göttingen (GÖ)]. Beside milk production, the flock was also kept for landscape conservation with sedentary husbandry. Only new breeding males entered the flock and females were recruited exclusively from their own off-spring. The entire flock participate in the Caprine Arthritis Encephalitis Virus and Maedi-Visna-Virus sanitation programme and has been sampled regularly to obtain the unaffected status. All serum samples collected in this context from July 2017 to July 2020 were examined for TBEV antibodies. An individual animal follow-up of the TBEV antibody activity was possible for four sheep and eight goats which tested inconclusive or positive by the TBEV ELISA and confirmed by the SNT at least once. As part of the follow-up, milk samples were collected in July 2020 from 70 goats and 39 sheep and examined for TBEV RNA.
2.2. Diagnostic methods
The serum samples were screened using a commercial TBEV antibody ELISA (Immunozym® FSME IgG all Species, PROGEN Biotechnik GmbH, Heidelberg, Germany) according to the manufacturer's instructions (sensitivity 97%, specificity 99%). The manufacturer specified samples with >126 Vienna Units (VIEU) / ml as positive, values of <63 VIEU / ml as negative, values between 63 and 126 VIEU / ml were considered as inconclusive.
The serum neutralization test (SNT) is considered to be the gold standard for the detection of TBEV antibodies [25]. Therefore, samples tested as inconclusive or positive by ELISA were analysed by a modified SNT [26] to confirm the ELISA results. For this purpose, BHK-21 cells were seeded in a 96 well plate at 8000 cells per well 24 h prior to infection. Serum samples were heat-inactivated at 56 °C for 30 min. Starting at a 1:10 dilution, the serum samples were serially diluted in duplicates as follows: 1:40, 1:160 and 1:640 in DMEM (+2% foetal bovine serum, 1% penicillin, 1% streptomycin and 1% glutamine). TBE virus strain Neudoerfl corresponding to a final MOI of 0.05 was added to each diluted sample before incubating for one hour at room temperature. After incubation, the BHK-21 culture supernatants were replaced by the serum samples with the virus. The cells were incubated at 37 °C and 5% CO2 and monitored for cytopathic effects (CPE) for one week. The highest serum dilution with no visible CPE was defined as antibody titre (reciprocal of the dilution). Each serum was tested in duplicates. Serum samples with antibody titres of ≥1:40 were counted as positive and included in a further analysis. A mouse monoclonal antibody with neutralizing activity (kindly provided by Dr. Matthias Niedrig) was included on each plate as a positive control.
Milk samples were subjected to viral RNA extraction in pools of five using the Qiagen Viral RNA Mini Kit (Qiagen, Hilden, Germany) and tested for the presence of TBEV RNA by qRT-PCR following the protocol of Schwaiger & Cassinotti [27].
2.3. Statistical analyses
Fisher's exact tests were used to investigate the influence of goats, performing landscape conservation, husbandry system, tick infestation and treatment against ectoparasites on the presence of TBEV antibodies in sheep flocks in Germany (GraphPad Prism 8, Cypress, USA). Odds ratio (OR), a 95% confidence interval (CI) and p values were calculated for variables. Results p < 0.05 were considered as significant.
3. Results
The distribution of TBEV positive flocks examined from winter 2017 until spring 2018 and the human incidence per district in 2017 is provided in detail in the supplementary tables A.1 and A.2.
In the TBEV risk areas (BW, BAV), 78 sheep and 22 goats tested positive for TBEV antibodies. The occurrence of antibodies to TBEV in small ruminant flocks coincided with the incidence of human TBE cases in 14 districts. There were no detectable antibodies against TBEV in sheep and goats in eight districts, although human cases were notified in these areas in 2017 (human incidence in 2017: 0.29–10.7). In contrast, in five districts with zero human incidence in 2017, small ruminants tested positive with an intra-herd prevalence between 2.3% and 25%.
Antibodies against TBEV were determined in six sheep and four goats in non-TBE risk areas (SH, LS, NRW). Only one sheep (n = 499) in SH was positive. In LS, an intra-herd prevalence of 12.5% (n = 32) was found in one goat herd (GÖ) but human TBE cases were not notified in 2017. All other examined flocks in this federal state were negative, though a low human incidence was indicated in one participating district in 2017 (Celle = 0.56). In NRW, an intra-herd prevalence of 6.8% (n = 44) was detected in a sheep flock which is in line with the occurrence of human cases (incidence 0.32). In the district of Borken both flocks tested negative although a human incidence of 0.27 was reported in 2017. In contrast, in two districts (Mettmann and Bielefeld) with no human TBE cases in 2017, one sheep was identified with TBEV antibodies in each of both flocks (n = 18, n = 44).
The probability to identify TBEV seropositive sheep flocks was enhanced in flocks kept for landscape conservation or which were shepherded (p < 0.05) (Table 1). The presence of goats, observed tick infestation and ectoparasitic treatment did not play a critical role for TBEV infection in small ruminant flocks (p ≥ 0.05).
Table 1.
Risk factors on herd level for an infection with tick-borne encephalitis virus detected by serum neutralization test in 71 sheep flocks in Germany (2017/2018).
| Variable | Category | Apparent prevalence of positive farms/farms total (%) | Odds ratio (OR) | 95% confidence interval (CI) | p value |
|---|---|---|---|---|---|
| Keeping of goats within the sheep flock | Yes | 13/27 (48.15) | 2.79 | 0.96–7.45 | 0.07 |
| No | 11/44 (25) | ||||
| Animals were kept for landscape conservation | Yes | 22/48 (45.83) | 8.89 | 2.00–40.76 | 0.003 |
| No | 2/23 (8.70) | ||||
| Husbandry system | Shepherding | 13/23 (56.52) | 4.37 | 1.40–13.34 | 0.008 |
| Sedentary | 11/48 (22.92) | ||||
| Tick infestation | Yes | 22/56 (39.29) | 4.21 | 0.99–19.88 | 0.07 |
| No | 2/15 (13.33) | ||||
| Treatment against ectoparasites within the last 12 months | Yes | 15/44 (34.09) | 1.03 | 0.36–2.83 | >0.99 |
| No | 9/27 (33.33) |
In order to evaluate the presence of TBEV and the persistence of antibodies, all adult sheep and goats of the TBEV-positive flock in LS (GÖ) were included in the three-year retrospective investigation. A constant intra-herd prevalence of at least 2.2% in sheep and 8.3% in goats was determined in the sheep and goat flock respectively (Table 2). The persistence of TBEV antibody titres varied between the animals from detectable only once to three times in succession (Table 2). None of the 109 milk samples collected from the sheep and goats in July 2020 tested positive for TBEV by qRT-PCR.
Table 2.
Intra-herd prevalence of the dairy sheep and goat flock located in the non-tick-borne encephalitis virus (TBEV) risk area (Lower Saxony, Göttingen, GÖ). Individual follow-up of TBEV antibody titres detected by serum neutralization test of eight goats and four sheep within a period of three years. Titres ≥1:40 are classified as positive and negative results are indicated with ‘-’.
| Sampling Date (mm/yyyy) | 07/2017 | 12/2017 | 07/2018 | 07/2019 | 07/2020 | |
|---|---|---|---|---|---|---|
| Species | Intra-herd prevalence | |||||
| Dairy goats (n) | 14.8% (54) | 13.6% (66) | 8.3% (72) | 9.0% (71) | 9.6% (73) | |
| Dairy sheep (n) | 7.7% (39) | 2.2% (46) | 2.3% (43) | 2.8% (67) | 2.8% (71) | |
| Species | Animal ID | TBEV antibody titres | ||||
| Goat | 6244 | 1:40 | – | 1:40 | 1:40 | – |
| 6245 | – | – | – | – | 1:160 | |
| 6246 | – | 1:100 | 1:40 | – | 1:40 | |
| 6257 | 1:40 | 1:160 | 1:40 | – | – | |
| 18826 | – | – | 1:40 | 1:40 | – | |
| 18842 | – | 1:40 | – | – | – | |
| 18844 | 1:40 | – | – | – | – | |
| 18929 | 1:40 | 1:400 | – | – | 1:40 | |
| Sheep | 6354 | 1:40 | – | 1:40 | 1:40 | 1:160 |
| 18837 | – | – | 1:160 | – | 1:40 | |
| 18942 | – | 1:40 | – | 1:40 | – | |
| 18948 | 1:40 | – | – | 1:40 | – | |
4. Discussion
In sera of sheep and goats sampled in the TBEV risk and non-risk areas antibodies against TBEV were detected, although in some districts no human TBE cases were registered in 2017. Specifically, the district of Göttingen (GÖ) is known as a non-TBE risk area but TBEV antibodies were detected in this district in an extensively managed dairy sheep and goat flock in 2017. The first autochthone human case in this district was reported two years later [7] and it might be possible that there is a link between the human TBE case and the seropositive small ruminant flock. Single animals had undulating titres and different sheep and goats showed a high antibody response (≥1:160). A reinfection within the three-year investigation period cannot be excluded. Antibody titres of ≥1:120 in goats indicate a very recent infection and the probable presence of an active foci [19]. Therefore, a continuously TBEV infection in the flock was ongoing. One positive animal is sufficient to induce an alimentary TBEV infection in several humans by consuming raw milk products [28], and raw milk consumption from the present flock may be a great risk for the public. However, testing of 109 milk samples from July 2020 for viral RNA revealed no active infections and also no virus secretions in any of the animals at the time of sampling. It is desirable to collect ticks on the pastures of the small ruminant flock for direct virus detection. However, this is labour-intensive, expensive and is of little success despite the occurrence of seropositive animals [19,29] and acquired human infections [29,30]. Therefore, weekly collected bulk tank milk (BTM) samples examined for TBEV by PCR could lead to an early detection of the virus and limits the area for tick collection to a region recently grazed by small ruminants. However, the virus concentration might be diluted in the BTM depending on the numbers of milking and virus excreting animals. Therefore, the use of BTM samples for TBEV monitoring has to be validated in the future.
Sheep and goats vaccinated with a human TBEV vaccine showed detectable antibodies for 28 months but these animals were immunized four times [31]. To our best knowledge, this is the first report about an individual follow-up of sheep and goats naturally infected with TBEV over several years in a non-TBEV risk area. According to our findings, the antibody response to naturally acquired TBEV infections might be short and lasted less than one year in most sheep and goats. Reinfection might happen in single animals which showed a longer duration or an undulate antibody response. The short lifespan of TBEV antibodies enables the possibility to monitor changing patterns of the pathogen's existence in TBEV risk areas even in the absence of human cases, particularly if vaccination coverage in humans improves. For instance, Klaus and colleagues [17] analysed samples from three sheep flocks in the Main-Spessart (MSP) district revealing a detection rate of 9% to 43% within the flocks. In the current study almost all animals tested negative from the same district. This changing pattern of the occurrence of TBEV in a risk area has already been observed for the human incidence in the neighbouring districts of MSP [8].
The numbers of TBEV positive small ruminants were higher in the TBEV risk area compared to the non-risk area. This is in line with a previous conducted study and coincides with the incidence of human TBE cases [8,17]. The reasons for the existence of TBEV mainly restricted to southern Germany and the virus dissemination to non-TBE risk areas, are still under investigation [8,32]. Rodents, birds and larger wild mammals like deer and foxes may distribute infected ticks [16,32]. There are concerns about the further spreading of TBEV from southern Germany to the central federal states. However, northern European countries next to Germany like Denmark and the Netherlands also have an increasing distribution of TBEV [[33], [34], [35]]. Although, TBEV antibodies were not detected in small ruminants from both districts next to Denmark in the current study, a spreading from north to south cannot be ruled out. Moreover, the Dutch TBEV hot spot, the Sallandse Heuvelrug region, is close to the border to the German federal states LS and NRW. The German district Emsland is located close to the Dutch TBEV hot spot area and was declared as the first TBE risk area in northern Germany since 2019 [7,35]. Moreover, single human TBE cases were reported in districts (e. g. Borken in NRW) close to the Netherlands. These facts support the requirement for a TBEV surveillance in northern parts of Germany to be prepared for further spreading of the virus.
The background of husbandry systems is of critical importance for reliable surveillance data but this information is missing in many studies. Despite the occurrence of milk-borne TBE cases [[12], [13], [14]] not all dairy herds are at risk and suitable for surveillance. Specifically, several commercial dairy goat flocks are kept under intensive indoor conditions and this might have led to a general low detection rate in the past [17,29,36]. In the present study, all goats were kept under extensive husbandry conditions and co-grazed with sheep. The apparent prevalence of TBEV positive flocks was higher in sheep flocks with goats compared to pure sheep flocks but this difference was not significant (p = 0.07). Moreover, landscape conservation or shepherding enhanced the probability to detect TBEV antibodies in sheep flocks probably due to the very frequent access to the natural habitat of I. ricinus like forest edges, hedges and thickets. Hence, TBEV surveillance programmes should focus on these types of sheep farming.
The SNT is considered as gold standard for estimating the sensitivity and specificity of TBEV ELISAs [25,37]. Different flaviviruses occur in the small ruminant population including Louping ill virus and West Nile virus and may cross-react in the TBEV ELISA producing false-positive results [31,38,39]. To the authors best knowledge, other flaviviruses than TBEV were not present in the German sheep population during the investigation period from winter 2017 until spring 2018 and before that time. Klaus and colleagues [37] confirmed the high specificity of the TBEV IgG all species ELISA with the SNT but the ELISA's sensitivity was lower than the SNT and some positive serum samples were left unrevealed. Nevertheless, analysing serum samples with an ELISA is a suitable tool to screen large samples sizes for TBEV antibodies but confirmation with the SNT is necessary [20,37].
The authors are aware that a direct comparison between animals' intra-herd prevalence and incidence of human cases is limited. The data collection and processing in veterinary and human medicine is different and both figures give only an indication about the occurrence of TBEV in the districts in 2017. In the current study, the numbers of examined flocks and animals are not representative for the district because the samples were taken predominately for a Q fever study. Consequently, some TBEV infections were missed by small ruminants particularly in the risk area because of the extremely small natural foci of the pathogen. Nevertheless, our preliminary observations clearly demonstrated the successful utilization of small ruminants as sentinel animals for TBEV.
5. Conclusion
Small ruminants are suitable sentinel animals for unveiling new TBEV hot spots and monitoring the presence of the virus in the environment independently from human cases. The use of the annually collected small ruminants' serum samples for the legally required brucellosis monitoring (Council Directive 91/68/EEC of 28 January 1991) would be a time-saving, low-cost and easy way to perform a TBEV surveillance. Data from animal surveillance must be combined with data from human notification programmes in order to implement an integrated surveillance, which is in line with the One Health approach [2,29].
Funding
This work was supported by the Federal Ministry of Education and Research (BMBF) under Grant 01KI1726B and Grant 01KI17219 as part of the Zoonotic Infectious Diseases Research Network; Lower Saxony State Office for Consumer Protection and Food Safety under Grant P54-34. The APC was funded by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding program Open Access Publishing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank Clara Schoneberg for providing the figure. Special thanks to the animal owners for their willingness to contribute to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100227.
Appendix A. Supplementary data
Supplementary material
References
- 1.Neo J.P.S., Tan B.H. The use of animals as a surveillance tool for monitoring environmental health hazards, human health hazards and bioterrorism. Vet. Microbiol. 2017;203:40–48. doi: 10.1016/j.vetmic.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scotch M., Odofin L., Rabinowitz P. Linkages between animal and human health sentinel data. BMC Vet. Res. 2009;5:15. doi: 10.1186/1746-6148-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster I., Mertens M., Mrenoshki S., Staubach C., Mertens C., Brüning F. Sheep and goats as indicator animals for the circulation of CCHFV in the environment. Exp. Appl. Acarol. 2016;68:337–346. doi: 10.1007/s10493-015-9996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selim A., Abdelhady A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop. Anim. Health Prod. 2020 doi: 10.1007/s11250-020-02339-x. [DOI] [PubMed] [Google Scholar]
- 5.Imhoff M., Hagedorn P., Schulze Y., Hellenbrand W., Pfeffer M., Niedrig M. Review: sentinels of tick-borne encephalitis risk. Ticks Tick Borne Dis. 2015;6:592–600. doi: 10.1016/j.ttbdis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Ruzek D., Avšič Županc T., Borde J., Chrdle A., Eyer L., Karganova G. Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019;164:23–51. doi: 10.1016/j.antiviral.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Robert Koch-Institut FSME-Risikogebiete in Deutschland (Stand: Januar 2020) Epidemiol. Bull. 2020;8:3–19. doi: 10.25646/6510. [DOI] [Google Scholar]
- 8.Hellenbrand W., Kreusch T., Böhmer M.M., Wagner-Wiening C., Dobler G., Wichmann O. Epidemiology of tick-borne encephalitis (TBE) in Germany, 2001–2018. Pathogens. 2019;8:42. doi: 10.3390/pathogens8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulsen K.M., Granquist E.G., Okstad W., Vikse R., Stiasny K., Andreassen A.K. Experimental infection of lambs with tick-borne encephalitis virus and co-infection with Anaplasma phagocytophilum. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balogh Z., Egyed L., Ferenczi E., Bán E., Szomor K.N., Takács M. Experimental infection of goats with tick-borne encephalitis virus and the possibilities to prevent virus transmission by raw goat milk. Intervirology. 2012;55:194–200. doi: 10.1159/000324023. [DOI] [PubMed] [Google Scholar]
- 11.Böhm B., Schade B., Bauer B., Hoffmann B., Hoffmann D., Ziegler U. Tick-borne encephalitis in a naturally infected sheep. BMC Vet. Res. 2017;13:267. doi: 10.1186/s12917-017-1192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockmann S., Oehme R., Buckenmaier T., Beer M., Jeffery-Smith A., Spannenkrebs M. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Eurosurveillance. 2018;23:17–00336. doi: 10.2807/1560-7917.ES.2018.23.15.17-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerlik J., Avdičová M., Štefkovičová M., Tarkovská V., Pántiková Valachová M., Molčányi T. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: analysis of tick-borne encephalitis outbreaks in Slovakia during 2007–2016. Travel Med. Infect. Dis. 2018;26:37–42. doi: 10.1016/j.tmaid.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ilic M., Barbic L., Bogdanic M., Tabain I., Savic V., Kosanovic Licina M.L. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick Borne Dis. 2020;11 doi: 10.1016/j.ttbdis.2020.101513. [DOI] [PubMed] [Google Scholar]
- 15.Gresikova M. Recovery of the tick-borne encephalitis virus from the blood and milk of subcutaneously infected sheep. Acta Virol. 1958;2:113–119. [PubMed] [Google Scholar]
- 16.Dobler G., Hufert F., Pfeffer M., Essbauer S. Tick-borne encephalitis: From microfocus to human disease. In: Mehlhorn H., editor. Progress in Parasitology. Springer; Berlin: 2011. pp. 323–331. [Google Scholar]
- 17.Klaus C., Beer M., Saier R., Schau U., Moog U., Hoffmann B. Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus - epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tick Borne Dis. 2012;3:27–37. doi: 10.1016/j.ttbdis.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Stefanoff P., Pfeffer M., Hellenbrand W., Rogalska J., Rühe F., Makówka A. Virus detection in questing ticks is not a sensitive indicator for risk assessment of tick-borne encephalitis in humans. Zoonoses Public Health. 2013;60:215–226. doi: 10.1111/j.1863-2378.2012.01517.x. [DOI] [PubMed] [Google Scholar]
- 19.Casati Pagani S., Frigerio Malossa S., Klaus C., Hoffmann D., Beretta O., Bomio-Pacciorini N. First detection of TBE virus in ticks and sero-reactivity in goats in a non-endemic region in the southern part of Switzerland (Canton of Ticino) Ticks Tick Borne Dis. 2019;10:868–874. doi: 10.1016/j.ttbdis.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Rieille N., Klaus C., Hoffmann D., Péter O., Voordouw M.J. Goats as sentinel hosts for the detection of tick-borne encephalitis risk areas in the Canton of Valais, Switzerland. BMC Vet. Res. 2017;13:217. doi: 10.1186/s12917-017-1136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf A., Prüfer T.L., Schoneberg C., Campe A., Runge M., Ganter M. Prevalence of Coxiella burnetii in German sheep flocks and evaluation of a novel approach to detect an infection via preputial swabs at herd-level. Epidemiol. Infect. 2020;148:1–7. doi: 10.1017/S0950268820000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert Koch-Institut. FSME Risikogebiete in Deutschland (Stand: April 2018). Bewertung des örtlichen Erkrankungsrisikos. Epidemiol. Bull. 2018;17:163–173. doi: 10.17886/EpiBull-2018-022. [DOI] [Google Scholar]
- 23.Wolf A., Prüfer T.L., Schoneberg C., Campe A., Runge M., Ganter M. Risk factors for an infection with Coxiella burnetii in German sheep flocks. Epidemiol. Infect. 2020;148:1–9. doi: 10.1017/S0950268820002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert Koch-Institut Web-basierte Abfrage der Meldedaten gemäß Infektionsschutzgesetz (IfSG) 2017. https://survstat.rki.de (accessed 19 June 2020)
- 25.Holzmann H., Kundi M., Stiasny K., Clement J., McKenna P., Kunz C. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 1996;48:102–107. doi: 10.1002/(sici)1096-9071(199601)48:1<102::aid-jmv16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Orlinger K.K., Hofmeister Y., Fritz R., Holzer G.W., Falkner F.G., Unger B. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J. Infect. Dis. 2011;203 doi: 10.1093/infdis/jir122. 1556-64. [DOI] [PubMed] [Google Scholar]
- 27.Schwaiger M., Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 2003;27:136–145. doi: 10.1016/s1386-6532(02)00168-3. [DOI] [PubMed] [Google Scholar]
- 28.Balogh Z., Ferenczi E., Szeles K., Stefanoff P., Gut W., Szomor K.N. Tick-borne encephalitis outbreak in Hungary due to consumption of raw goat milk. J. Virol. Methods. 2010;163:481–485. doi: 10.1016/j.jviromet.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Alfano N., Tagliapietra V., Rosso F., Ziegler U., Arnoldi D., Rizzoli A. Tick-borne encephalitis foci in Northeast Italy revealed by combined virus detection in ticks, serosurvey on goats and human cases. Emerg Microbes Infect. 2020;9:474–484. doi: 10.1080/22221751.2020.1730246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zöldi V., Papp T., Rigó K., Farkas J., Egyed L. A 4-year study of a natural tick-borne encephalitis virus focus in Hungary, 2010–2013. EcoHealth. 2015;12:174–182. doi: 10.1007/s10393-014-0969-0. [DOI] [PubMed] [Google Scholar]
- 31.Klaus C., Ziegler U., Kalthoff D., Hoffmann B., Beer M. Tick-borne encephalitis virus (TBEV) - findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 2014;10:78. doi: 10.1186/1746-6148-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelitsch A., Wernike K., Klaus C., Dobler G., Beer M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses. 2019;11 doi: 10.3390/v11070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen N.S., Larsen S.L., Olesen C.R., Stiasny K., Kolmos H.J., Jensen P.M. Continued expansion of tick-borne pathogens: tick-borne encephalitis virus complex and Anaplasma phagocytophilum in Denmark. Ticks Tick Borne Dis. 2019;10:115–123. doi: 10.1016/j.ttbdis.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Laugesen N.G., Stenør C. Tick-borne encefalitis-associeret meningoradikuloneuritis erhvervet ved Esbjerg. Ugeskr. Laeger. 2019;181 V03190197. [PubMed] [Google Scholar]
- 35.Dekker M., Laverman G.D., de Vries A., Reimerink J., Geeraedts F. Emergence of tick-borne encephalitis (TBE) in the Netherlands. Ticks Tick Borne Dis. 2019;10:176–179. doi: 10.1016/j.ttbdis.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Klaus C., Ziegler U., Hoffmann D., Press F., Fast C., Beer M. Tick-borne encephalitis virus (TBEV) antibodies in animal sera - occurrence in goat flocks in Germany, longevity and ability to recall immunological information after more than six years. BMC Vet. Res. 2019;15:399. doi: 10.1186/s12917-019-2157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klaus C., Beer M., Saier R., Schubert H., Bischoff S., Süss J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl. Munch. Tierarztl. Wochenschr. 2011;124:443–449. doi: 10.2476/0005-9466-124-444. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert L. Louping ill virus in the UK: a review of the hosts, transmission and ecological consequences of control. Exp. Appl. Acarol. 2016;68:363–374. doi: 10.1007/s10493-015-9952-x. [DOI] [PubMed] [Google Scholar]
- 39.Rimoldi G., Mete A., Adaska J., Anderson M., Symmes K., Diab S. West Nile virus infection in sheep. Vet. Pathol. 2017;54:155–158. doi: 10.1177/0300985816653796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material