Highlights
-
•
Neoplasia of the cervix is one of the most frequent in women.
-
•
Immunological impairment in cervical intraepithelial neoplasia and cancer have been reported.
-
•
In-situ expression of cytokines and leukocytes during the cervical neoplasia occurs.
-
•
Some of these cytokines can prevent or induce the progression of the neoplasm.
-
•
Possible role in therapeutics of cervical cancer by cytokine manipulations need to be evaluated.
Keywords: Immunopathology, Uterine cancer, Cytokines, Leukocytes, Progression
Abstract
Neoplasia of the cervix represents one of the most common cancers in women. Clinical and molecular research has identified immunological impairment in squamous intraepithelial cervical lesions and cervical cancer patients. The in-situ expression of several cytokines by uterine epithelial cells and by infiltrating leukocytes occurs during the cervical intraepithelial neoplasia and cervical cancer. Some of these cytokines can prevent and others can induce the progression of the neoplasm. The infiltrating leukocytes also produce cytokines and growth factors relate to angiogenesis, chemotaxis, and apoptosis capable of modulating the dysplasia progression. In this review we analyzed several interleukins with an inductive effect or blocking effect on the neoplastic progression. We also analyze the genetic polymorphism of some cytokines and their relationship with the risk of developing cervical neoplasia. In addition, we describe the leukocyte cells that infiltrate the cervical uterine tissue during the neoplasia and their effects on neoplasia progression.
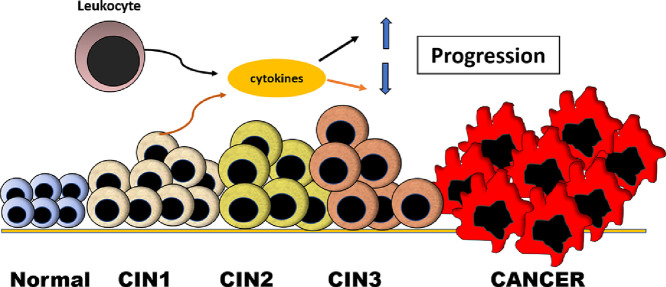
Graphical abstract
Leukocyte and uterine tissue cytokines effects on the progression of neoplasia. During the progression of uterine neoplasia both leukocyte cytokines and cytokines produced by the neoplastic tissue can have opposite or synergistic effects on the progression of intraepithelial neoplasia.
Introduction
Cervical cancer ranks third in cancer incidence worldwide and is the most frequent gynecological cancer in developing countries [1, 2]. The relationship between the immune system and cancer has been studied for many years. Rudolf Virchow [3] proposed this relationship centuries ago. Thus, immune factors such as infiltrating cells, types of cytokines, and other molecules related to the immune system have been characterized as predictive factors in the evolution of cancers. It is generally accepted that inflammation and its cells and soluble mediators are involved in the evolution of cancerous lesions, some inducing progression and others inducing inhibiting of tumor evolution [4, 5]. There are well known mechanisms during inflammation that lead to tumor growth. These include DNA damage and the disruption of the extracellular matrix by reactive oxygen species [6] and metalloproteinases [7] respectively, and the stimulation of tumor growth by cytokines such as IL-1B [8] and IL-8 [9]. In addition, the immune system during inflammation leads to modifications of both the tumor cells and the microenvironment. In this regard, cytokines such as vascular endothelial growth factor (VEGF) induce neoangiogenesis, along with disruption of the extracellular matrix, tumor cell migration, and metastasis [10]. The main cells that infiltrate cancerous tumors and modify their evolution are monocytes/macrophages (Mn/MΦ) and lymphocytes. Mn/MΦ, which represent the major component of tumor infiltrates, produce high amounts of cytokines such as IL-1β, IL-6, IL-23, and TNF-α, which are important in tumor modulation during inflammatory processes [10, 11].
Besides the relationship between inflammation and tumorigenesis, there are other cells of the immune system that produce mediators capable of modulating tumor evolution. The expression of tumor antigens on cancerous cells induces the formation of CD8+ lymphocyte clones with cytotoxic activity and capable of slowing tumor growth, and together with the activity of CD4+ lymphocytes represent the most important anti-tumor activity. In this regard, CD8+ lymphocytes induce tumor cell apoptosis using granzymes [12] and CD4+ lymphocytes produce cytokines and chemokines such as IFN-γ, IL-12, CXCL9 and CXCL10 inducing activation and accumulation of CD8+ lymphocytes [13, 14]. Tumor cells in response to these anti-tumor activities can produce mediators that induce immunosuppressive effects on the immune system. The production of cytokines such as IL-10 and TGF-B, can inhibit the cytotoxicity and proliferation of T-lymphocytes [15]. Tumoral cells can also express ligands such as PDL1 and PD-L2 which when binding to PD-1 receptors on T lymphocytes inhibit their activity [16, 17]. Tolerance to tumor antigens mediated by the activity of CD4+ Treg lymphocytes and myeloid suppressor cells has also been demonstrated [15]. In this way, this interconnected network of cells (lymphoid, myeloid, endothelial, lymphatic, and stromal cells) represents the tumor microenvironment, that is involved in anti-tumor and pro-tumor responses by the activation or inhibition of immune system, respectively.
Approximately one third of cancerous tumors are linked to inflammation induced by microorganisms. In this respect and focusing on this review the human papillomavirus (HPV) is highly involved in the induction of cervical and neck cancer [18, 19]. HPV is a small DNA virus that can infect the skin and respiratory and anogenital tract with the ability to induce cancers [20]. According to their ability to promote malignancy are classified into low- risk and high-risk viruses. Viral proteins E6 and E7 have been evaluated for their ability to induce alterations in the proliferation and differentiation of cells that invade. High-risk HPV types 16 and 18 are involved in 99% of cervical cancers [20]. After viral cell invasion the proteins E6 and E7, bind and inactivate the tumor-suppressor gene product p53, and the retinoblastoma tumor-suppressor protein (pRb), respectively [21], these proteins are required for malignant conversion. These facts support the hypothesis that E6 and E7 are related to the onset and maintenance of human cervical cancers.
In addition to the pro-tumor effect induced by HPV, the immune system response to this infection occurs. Both the innate and adaptive immune systems are involved in the defense against HPV infection and the induced cervical neoplasia [22, 23]. Viral protein-specific CD4+ T lymphocytes interact with both early and late viral peptides in the context of their binding to the histocompatibility complex class II (HLA II) on antigen-presenting cells (dendritic cells, Mn/MΦ) [22, 23]. Activation of CD4+ T cells leads to the production of various cytokines that can inhibit or promote HPV infection and neoplastic lesions. In this regard, Th1 cytokines (INF-gamma, IL-2 and TNF-beta) have a beneficial effect because they reduce neoplastic lesions, while Th2 cytokines (IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13) produce a deleterious effect promoting neoplastic lesions; however, IL-4 and IL-5 can generate development of B-lymphocytes and induce strong antibody production [24].
Cytolytic T lymphocytes (CD8+) play an important role in HPV infection and cervical neoplasia, both by destroying the cells that possess the viral antigens in conjunction with HLA class I and by destroying cells presenting neoplastic antigens. The cytotoxic action of CD8+ cells requires signals sent by dendritic cells and activated CD4+ cells [25]. The HPV viral proteins E6 and E7 appear to be very important in the action of CD8+ cytolytic cells. However, more studies are needed to determine the role of CD8+ cytotoxic lymphocytes in the regression of cervical neoplasia [26]. These immune factors are reflected in cervical biopsies from patient with HPV infection, and from patients with different degrees of cervical intraepithelial neoplasia (CIN) and cervical cancer. In this regard, histological analysis of cervical lesions shows that HPV infection is restricted to the cervical epithelium, and high infiltration of Mn/MΦ, NK cells, and CD4+ and CD8+ T lymphocytes into the epithelial layer is observed. This infiltration is accompanied by increased expression of adhesion molecules (E-selectin and VCAM) in blood vessel endothelial cells and the production of chemokines such as RANTES [27, 28]. In cases of CIN and cervical cancer, a reduction of Langerhans cells is seen in both cases. These epithelial dendritic cells are important as antigen presenting cells, necessary for the activation of T lymphocytes. The absence or decrease of these cells may result in T-lymphocytes with a greater capacity for tolerance than for cytolytic activity [29, 30]. Diminished Langerhans' cells have been associated with HPV infection [31].
The evolution of CIN is unpredictable and histopathological analyses cannot predict it. Analysis of biomarkers may be useful to determine progression or regression of the lesions. These biomarkers include viral factors (viral genotype, viral DNA methylation), host factors (human leukocyte antigen, markers of lymphoproliferation, amplification of telomerase and epigenetic effects induced by HPV and cellular factors (Ki-67, p53 and pRb) [32]. Cervical cancer is a major gynecological problem in developing and underdeveloped countries. Despite the significant advancement in early detection and treatment modalities, several patients often show poor prognosis and significantly high mortality rates especially recurrent cervical cancer [33]. The development of noninvasive biomarker with the potential to provide more specific tumor characterization before treatment begins or during therapy may permit clinicians to administer a more individualized anti-cancer treatment. At present, new biomarker techniques have appeared for an efficient diagnosis of cervical neoplasia. Radiomics is a mathematical-statistical procedure extracting information from medial images, which has the potential for prediction of staging, histological type, node status, relapse, and survival in patients with cervical cancer [34]. Deoxyribonucleic and ribonucleic acids have been used as biomarkers in the diagnosis and prognosis of cervical neoplasia. In this regard, several DNA methylation markers such as ANKRD18CP, C13ORF18, EPB41L3, JAM3, SOX1 and ZSCAN1 have high sensitivity and specificity to detect CIN2 [35]. Other host-cell DNA methylation markers (ASCL1, LHX8, ST6GALNAC5, GHSR, SST and ZIC1) have high sensitivity and specificity for CIN3 and cancer of HPV-positive women [36]. The efficacy of high-risk human papillomavirus (HR-HPV) and DNA image cytometry (DNA-ICM) status have been used to identify CIN2 and CIN3 [37]. MicroRNA (miRNA) are involved in carcinogenesis and response to viral infections. Human papillomaviruses induce aberrant expression of many cellular miRNAs, this dysregulation could be used as a marker in early diagnosis of HR-HPV infection, CIN and cervical cancer [38]. Aberrant expression of lncRNAs (transcripts) has been used as a key factor in cervical tumorigenic process [39]. Recently it has been realized that non-coding RNAs (ncRNA), including long noncoding RNAs (LncRNAs), microRNAs, circular RNAs and piRNAs (PIWI-interacting RNAs), can all play a role in gynecological cancer and several clinical trials are underway looking at these biomarkers and therapeutic roles [40]. For histological diagnosis of high-grade cervical lesions, p16INK4a immunostaining has proven to be useful. Therefore, p16INK4a immuno-cytology may be applicable as a favorable technology for cervical cancer screening [41].
The in-situ localization of factors that modulate the progression of CIN to cancer in the cervical tissues have been extensively studied [31]. Immunological and other factors associated or not with HPV infection have been shown in cervical biopsies from women with different degrees of cervical injury. Genital infection with certain strains of HPV is associated with a high risk of malignant transformation, and HPV-associated CIN can become invasive cancer. Host factors are critical in regulating tumor growth by expressing different modulating factors including immune response [42]. This review focuses on the possible in situ role of cytokines and leukocytes on the evolution of cervical neoplasia (Table 1).
Table 1.
Effect of cytokines on the evolution of cervical intraepithelial neoplasia.
| Cervical neoplasia | Cytokines |
|---|---|
| Prevent progression | IL-2, INF-γ, IL-12, IL-21, IL-10, IL-32, IL-37, and TGF-β1 |
| Induce progression | IL-1, IL-6, IL-8, IL-10, IL-17, IL-32, TGF-β1, MCP-1, and RANTES |
| Dual effect | IL-10, IL-32, and TGF-β1 |
In-situ cervical cytokines in CIN and cervical cancer
Cytokines are low molecular weight proteins which have a complex regulatory influence on inflammation and immunity. They have a role in development of immune and inflammatory response involving hematopoietic cells, lymphoid cell, and various pro-inflammatory and anti-inflammatory cells [43]. About 200 cytokines are recognized to date. Cytokines are categorized on the basis from which they are produced either from Th1 cells (Interleukin-2: IL-2, interferon-gamma: IFN-γ, tumor necrosis alpha: TNF-α), Th2 cells (IL-4, IL-5, IL-6, IL-9, IL-13), Th17 (IL-17, IL-22, IL-21,IL-25) and T regulatory cells (Treg: IL-10, IL-35, TGF-β) [44]. According to their secretion, cytokines can be lymphokines (secreted by T cells and regulate the immune response), proinflammatory (amplify and perpetuate the inflammatory process), anti-inflammatory (negatively modulate the inflammatory response), growth factors (promote cell survival) and chemokines (chemotactic for inflammatory cells) [45]. In addition to inflammation, immunity and infections, cytokines have now expanded their domain to atherosclerosis and cancer [46].
Chronic inflammation is associated with cancers including cervical cancer. During the inflammation, the upregulation of cytokines and the presence of immune cells in cervical tissue modulate the incidence of inflammation and the progression or regression of cervical dysplasia [47]. The production of cytokines by various cervical cells and by infiltrating leukocytes can be triggered by antigenic stimuli, including HPV infection. In this regard, significantly lower levels of IL-1α, IL-2, IL-4 and TNF-α are detected in cervical samples obtained from HPV infected patients with low-grade dysplasia when compared to samples obtained from high-grade dysplasia [48]. HPV is capable of inducing down-regulation in the expression of interferon and upregulation of IL10 and transforming growth factor (TGF-β1) to produce a local immunosuppressive environment, which, along with altered tumor surface antigens, forms an immunosuppressive network that inhibits the antitumor immune response [49]. However, controversial findings regarding to the role of HPV have been reported. In this regard, patients with precancerous lesions of the uterine cervix showed prevail of type Th1 cytokines (IL-2 and IFN-γ) in relation to Th2 (IL-4 and IL-6), whether they are HPV positive or negative [50], suggesting that the type of immune response may be independent of HPV infection. Immunological in situ events can be reflected in the microenvironment, since high levels of IL-12p40, IL-10, TGF-β1, TNF-α and IL-1β have been found in cervicovaginal washing fluid from patients with cervical cancer infected by HPV [51]. In general, the production of cytokines in the cervical tissue during the malignant transformation can induce, suppress, or have both effects on the progression of the neoplasm.
Interferon-gamma
The involvement of the immune response in the progression of human uterine cervix cancer has been documented. The in-situ presence of cytokines in cervical tissues from patients with CIN and cervical cancer support this hypothesis. The Th1 cytokines IL-2 and IFN-γ are immunostimulatory thus capable of limiting tumor growth. The Th2 cytokines interleukin 4 (IL-4) and interleukin 10 (IL-10) are immunoinhibitory and are thus capable of stimulating tumor growth [52]. IFN-γ expression and IFN-γ mRNA were reported increased in HPV-positive high-grade CIN, suggesting the inducer effect of this infection on IFN-γ production [53, 54]. In this regard, HPV persistent infection induced an immunologic dissonance with decreasing expression of Th1 cytokines (INF-γ), that is aggravated with the progression of the disease [52]. In addition, IFN-γ treatment is an effective therapeutic method to reduce CIN lesions [55, 56].
Interleukin-1
The involvement of IL-1 in tumorigenesis, cancer progression, metastasis, and even in the response to cancer treatment (i.e., chemotherapy, surgery, or radiation) has been studied extensively. IL-1 dysregulation has been shown to be associated with almost all types of human malignancies [57]. IL-1 plays an important role in inflammation and inflammation plays a role in the pathogenesis of cancer in two different pathways, an intrinsic pathway, where genetic mutations activate oncogenes and cause neoplasia, and an extrinsic pathway, where an inflammatory environment increases the susceptibility to cancer [58]. The expression of IL-1 is higher in CIN compared to healthy cervix uteri tissue [59], and it is even higher in CIN3 compared to CIN1 [48, 59]. IL-1 can induce normal and tumor cell growth [60]. In CIN with a co-existent HPV infection, an increased number of cells expressing IL-1 are seen, because HPV16, −18 stimulates IL-1 release in keratinocytes, and IL1 stimulates proliferation of immortal and malignant cervical epithelial cells [60]. Single and IL-1 gene-cluster polymorphisms have been suggested to be associated with cervical cancers [61]. However, some studies show increase in the risk of progression of pre-neoplastic lesions to cervical tumor in women with lower IL-1β gene expression [62].
Interleukin 2
IL-2 is a cytokine produced by T cells during an immune response, it is necessary for the growth, proliferation, and differentiation of naïve T cells into effector T cells. This cytokine is used as a therapeutic cytokine in cancer [63, 64]. The expression of IL-2 and its receptor in cervical cancer cells have been documented. Previous studies have shown an inverse relationship between IL-2 expression and CIN progression in patients with cervical dysplasia; as the degree of cervical lesions increased, the expressions of IL-2 decreased, and IL-10 increased [65]. This association could be a potential factor that influence the pathogenicity of HPV infection and the occurrence and development of cervical lesions [65, 66]. This inverse association between degree of CIN and IL-2 has also been demonstrated in vaginal immune factor [67]. Different concentrations of IL-2 could activate effector cells of the immune system cells. Low concentrations of IL-2 may promote the regulatory microenvironment and tumor growth. High concentrations of IL-2 activate immune system cells, such as CD8+, CD4+, and γδ T cells and NK cells with capacity to eliminate tumor cells [68]. The anti-tumoral effect of IL-2 is based not only on the ability of this cytokine to stimulate cellular-mediated immunity, but also because of its direct effects on tumor cells [69].
Interleukin-6
Interleukin 6 (IL-6) is produced in response to infections and tissue injuries and contributes to host defense through the stimulation of acute phase responses, hematopoiesis, and immune reactions. IL-6 plays a pathological effect on chronic inflammation and autoimmunity [70]. IL-6 has recently shown to act in vitro as a growth factor for cervical carcinoma cell lines, and increased IL-6 gene and protein expression has been reported associated to severity of cervical neoplasia and in invasive cervical cancer, suggesting a role in the pathogenesis of the uterine cervix neoplasia and in advanced neoplastic cervical lesions [71]. This effect of IL-6 may be associated to the capacity of this cytokine to promote tumor angiogenesis and the development of cervical cancer [71]. Production of IL-6 might also contribute to a local immunosuppressive effect in cervical dysplasia, silencing an autocrine IL-6 response and preventing constitutive production of the mononuclear cell-attracting chemokine (MCP-1). Both mechanisms might help the tumor to escape from the immune system [72]. In addition, dysplasia severity has been shown associated with IL-6 levels and inversely associated with IL-2 levels (an anti-tumor cytokine) [73]. Circulating levels of this cytokine have been associated to the degree of cervical dysplasia in patients with persistent low-grade squamous intraepithelial lesion, suggesting an additional biomarker for early cervical neoplasia [74].
Interleukin-8
Interleukin-8 (IL-8), a proinflammatory chemokine, induces neutrophil chemotaxis and degranulation and it is a regulatory factor within the tumor microenvironment. IL-8 activates multiple intracellular signaling after interactions with G protein-coupled receptors (CXCR1 and CXCR2) on cell surface [75]. Increased expression of IL-8 and/or its receptors has been characterized in cancer cells, endothelial cells, infiltrating neutrophils, and tumor-associated macrophages [76]. Therefore, IL-8 serves an important function in chronic inflammation and cancer development. Enhanced expression of IL-1β and IL-8 indicates (Th2 inflammatory response) has been reported in uterine cervical region associated to the progression of CIN lesions [77]. The upregulation of IL-8 in cervical cancers correlates with angiogenesis, monocyte/macrophage infiltration and it is a prognostic indicator of cancer evolution [78]. In vitro analysis shows that exogenous IL-8 was capable of inducing IL-8 receptors (IL-8RA, IL-8RB) on Hela cells and increasing migratory and proliferative efficiencies of those cells [79]. In addition to, blockade of IL-8 with an antibody or small hairpin RNA demonstrated significant anti-tumor effects in a xenograft model and in cellular cultures [80].
Interleukin-10
IL-10 is a cytokine with multiple, pleiotropic effects in immunoregulation and inflammation. It downregulates the expression of Th1 cytokines, mayor histocompatibility complex (MHC) class II antigens, and co-stimulatory molecules on macrophages [81]. The up-regulated expression of IL-10 can inhibit immune responses may be inducing an immunosuppressive environment by upregulating HLA-G expression and downregulating HLA class I expression [82]. In general, the higher levels of IL-10 suggest a potential down-modulation of tumor-specific immune responses to HPV-infected lesions. This phenomenon appears to provide a tumor 'progressive' microenvironment in these patients with cervical neoplasia [83]. Bypassing the local immunological defense reactions in the cervix is one of the prerequisites for HPV infections to progress to CIN. IL-10 over-expression along with HPV infection is one of the independent covariates of CIN2/3, creating a microenvironment that favors progressive cervical disease and immune evasion by HPV [84].
Interleukin-17
IL-17 is a cytokine with diverse functions in host defense and in the pathology of autoimmune disorders, chronic inflammatory diseases, and cancer. IL-17-producing cells may play a role in antitumor immunity. However, recently Th17 cells and their cytokines have been implicated in both pro and anti- tumorigenic processes. Expression of IL-17 and IL-23 (a major inducer of IL-17) has been reported elevated in human HPV-infected patients with cervical epithelial hyperplasia suggesting an immunosuppressive role for IL-17 in HPV-associated epithelial hyperplasia [85]. According with this, increased levels of IL-17 in supernatant of cervical tissue homogenate from patients with CIN infected by high-risk HPV showed the tendency to progression of CIN with high-risk HPV infection [86]. Other studies show association of IL-17 with other molecules in cervical dysplasia. Human leukocyte antigen G (HLA-G) and IL-17 expression in specimens with CIN 1 has been reported, suggesting that these molecules have a contribution towards cervical progression. These data suggest that HLA-G and IL-17 expression may be an early marker for assessing the progression of cervical lesions [87]. Increased number of IL-17-positive cells and MTA1 (metastasis associated 1) expression in CIN and cervical cancer compared to normal cervical tissues have been reported. The number of IL-17-positive cells was positively correlated with MTA1 suggesting progression of dysplasia. Analysis in cellular cultures show that IL-17 upregulates MTA1 mRNA and protein expression to promote HeLa and DU-145 cells migration and invasion [88]. In addition, IL-17A was observed upregulated in the cervical mucus from patients with cervical cancer and CIN associated with more severe cervical neoplasia [89].
Interleukin-21 and interleukin-12
Interleukin-21(IL-21) stimulates cytotoxicity and IFN-γ production in natural killer (NK) cells suggesting effective anti-tumor action) [90]. Interleukin-12 (IL-12) is a potent stimulator of T-cell function. IL-12 is known to stimulate effector cell populations, such as cytotoxic T cells and natural killer cells with anti-tumor effect [91, 92]. Cervical cancer cells are known to produce an extensive range of cytokines and chemokines, including IL-12 [93]. IL-21 and IL-12 have been known to be effective antitumor agents, enhancing the cytotoxicity of peripheral blood mononuclear cells in patients with CIN3 and cervical cancer and the possible mechanisms may be due to down-regulated Treg and Th17 cell differentiation [94]. IL-12 treatment in women with cervical cancer and HPV infection improved lymphoproliferative responses to HPV in a clinical trial, suggesting specific activation of lymphocytes by this cytokine [95].
Interleukin-32
Interleukin 32 (IL-32) was initially identified in activated natural killer (NK) and T cells, and its expression is strongly augmented by microbes, mitogens, and other pro-inflammatory cytokines. IL-32 is an amplifier of inflammation through its stimulatory effects on pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α. Since IL-32 expression is increased in inflammatory diseases and amplifies inflammatory cytokines, it is conceivable that IL-32 is strongly associated with various cancers related to inflammation [96]. IL-32 is present at substantial levels in cervical cancer tissues and in HPV-positive cervical cancer cells. The high-risk variant of HPV induces IL-32 expression via E7-mediated cyclooxygenase 2 stimulation [97]. IL-32 may indirectly promote tumor angiogenesis via inducing other cytokines and growth factors that it is crucial in the pathogenesis and progression of cancer [98].
Interleukin-33
Interleukin 33 (IL-33) is a dual function cytokine that also acts as a nuclear factor. IL-33 resides in the nucleus of the cells and upon tissue damage, necrosis, or injury, it is quickly released into extracellular space where it binds to its receptor suppression of tumorigenicity 2 (ST2) on the membrane of target cells to potently activate a Th2 immune response. The IL-33/ST2 axis is emerging as a potent modulator of the microenvironment. By recruiting a cohort of immune cells, it can remodel the microenvironment to promote malignancy or impose tumor regression [99], [100], [101]. It has shown the essential protective anti-viral immunity role of IL-33 that it is upregulated by IFN-γ. IL-33 may play a role in the pathogenesis of HPV induced cervical neoplasia by the effect of IL-33/ST2 on HPV infection. In this regard, diminished expression of IL-33 and IL33/ST2 has been reported in progression of CIN and carcinogenesis mediated by HPV infection. This defective anti-viral immunity may be related to diminished local production of IFN-γ [102, 103].
Interleukin 37
Interleukin (IL-37), a new IL-1 family member, is expressed in various normal cells and tissues and is regulated by inflammatory stimuli and pro-cytokines via different signal transduction pathways. This cytokine is expressed in a variety of cancers, chronic inflammatory and autoimmune disorders, and exerts anti-inflammatory effects [104]. Growing evidence has indicated that IL-37 is a potential anticancer molecule that mainly plays an inhibiting role in different kinds of cancers. Analysis of the effect of IL-37 on Hela cell cultures show this cytokine as a potential anticancer cytokine. IL-37 upregulated Bim (Bcl-2 family member Bim is required for apoptosis) in cervical cancer cells promoting apoptosis [105]. In addition, IL-37 suppressed cell proliferation and invasion of cervical cancer (Hela cells) and STAT3 is involved in this process. These data suggest that IL-33 is an anticancer cytokine in cervical neoplasia [106].
Transforming growth factor beta1
Transforming growth factor β1 (TGF-β1) is a multifunctional cytokine that plays important roles in cervical neoplasia formation, invasion, progression, and metastasis. TGF-β1 functions as a tumor inhibitor in precancerous lesions and early-stage cancers of cervix whereas as a tumor promoter in later stage. This switch from a tumor inhibitor to a tumor promoter might be due to various alterations in TGF-β signaling pathway, such as mutations or loss of expression of TGF-β receptors and Smad proteins. Additionally, the oncoproteins of HPV have been shown to stimulate TGF-β1 expression, which in turn suppresses host immune surveillance. Thus, in addition to driving tumor cell migration and metastasis, TGF-β1 is believed to play a key role in promoting HPV infection by weakening host immune defense [107]. Cervical carcinomas consist of tumor cell nests surrounded by varying amounts of intra-tumoral stroma containing different quantities and types of immune cells. TGF-β1 is involved in the formation of stroma and extracellular matrix and in immunosuppression. An inverse relationship between TGF-β1 mRNA expression in tumor cells and the extent of the tumor infiltrate has been demonstrated suggesting decreased neoplasia progression [108]. Alterations of the TGF-β1 signaling pathway by HPV-16 E7 protein plays an important role during the development of cervical cancer by immuno-inhibition and stimulation of tumor cell proliferation through the TGF-β1 /Smads signaling pathway [109, 110]. It has been attributed a beneficial effect to beta-carotene in relation to the risk of cervical cancer. In this respect, patients in the highest quartiles of dietary beta-carotene intakes had significantly lower cervical cancer risks than those in the lowest quartiles [111].
Chemokines
Chemokines play a role in tumor-inflammation and angiogenesis that could be involved in cervical tumor progression. IL-8 is a chemokine that was previously explained in topic 2.5. This cytokine can be expressed in CIN in conjunction with other chemokines. In this regard, monocyte chemoattractant protein-1 (MCP-1) and IL-8 expression were found increased in CIN accompanied by an increment of lymphocyte infiltration co-expressing CD3/MCP-1 and CD3/IL-8 according to the CIN evolution [112]. The presence of an eosinophilic infiltrate in patients with cervical squamous carcinoma has been shown to correlate with a worse overall survival, suggesting a less effective immune response in these cases. Type 2 cytokines such as IL-4 and IL-5 known to attract eosinophilic granulocytes, have been reported up-regulated in cervical cancer, findings that might explain the worse clinical outcome seen in cervical cancer patients with an eosinophilic tumor infiltrate [113, 114]. The expression of chemokines has also been reported in liquid based cervical samples. In this regard, increased IL-8 (neutrophil granulocyte attractant) was found in liquid based cervical samples and associated with cervical cancer [115]. Examination of co-expression patterns of chemokines in relation to HPV infection status in the cervical mucus from patients with cervical cancer and CIN showed upregulation of RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted) and MCP-1 associated with more severe cervical neoplasia [89]. Fig. 1 shows the trend of the different cytokines in the modulation of cervical dysplasia progression.
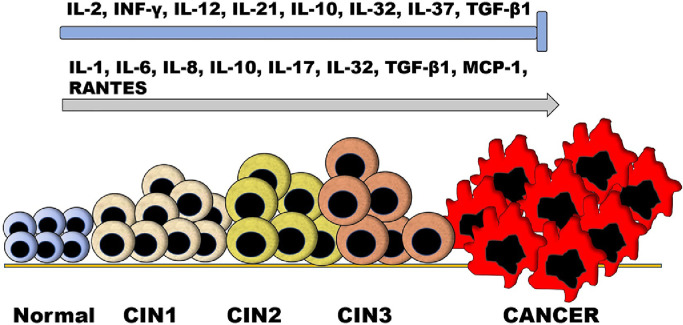
Fig. 1.
Effect of cytokines on the progression of cervical neoplasia. In general, cytokines have the effects of slowing or preventing the progression of the neoplasm, inducing its progression, or having a dual effect according to the circumstances. Cytokines that prevent progression of the malignancy (┬) include: IL-2, INF-γ, IL-12, IL-21, IL-10, IL-32, IL-37, and TGF-β1; those that induce progression (↑): IL-1, IL-6, IL-8, IL-10, IL-17, IL-32, TGF-β1, MCP-1, and RANTES; and those that may have a dual effect: IL-10, IL-32, and TGF-β1.
Cytokine genetic polymorphisms
Results from cytokine genetic polymorphisms and cervical dysplasia studies are sometimes conflicting, and the association is not always clear. Polymorphisms in regulatory and coding regions of cytokine genes have been associated with susceptibility to cervical neoplasia. In this regard, it has been reported that TNFA -308A allele is associated with susceptibility to HPV infection and IL18 -607A allele confer protection against HPV infection [116]. TGF-β1 −509T allele confers marginal protection for early stage 1B but risk for stage II of cervical cancer [117]. Several studies suggest that some polymorphic sites change the cytokines levels and influence the cancer development in HPV infected patients. Evaluation of functional polymorphisms at +874 (T/A) IFNγ and +1188 (A/C) IL-12B genes in cervical smears samples did not show significant differences between CIN patients and control groups on IFN-γ allelic polymorphism [118]. Resistance to apoptosis through the Fas pathway might enable many cancers to escape the immune system. A possible role for the IL-10 gene (for the A-allele of the IL-10-592 polymorphism) in CIN progression and squamous cell cervical cancer has been reported. In addition, a role for the Fas gene (polymorphism at position −670 of the Fas promotor) in the development of adenocarcinoma of the cervix has also been reported [119]. IL-10 -1082 gene polymorphism has been shown as a marker of genetic susceptibility to cervical cancer among Japanese women [120]. IL-6-597A/G and TNF-α-308G/A polymorphisms showed increased risk of cervical cancer [121]. Single and IL-1 gene-cluster polymorphisms have been suggested to be associated with cervical cancers [61]. Plasma IL-1β level and IL-1β C-511T polymorphism may consider as candidate biomarkers for cervical cancer in Egyptian women [121, 122]. However, the IL1β gene, encoding IL-1β cytokine, contains several single nucleotide polymorphisms and IL1B -511 C/C genotypes were significantly associated with a decreased risk of cervical cancer in korean population [123]. IL-12A rs568408 polymorphism contributed to increasing risk of cervical cancer, providing evidence that IL-12 polymorphisms may play a potential role in cancer risk [124].
Circulating cytokine positive leukocytes
In situ cytokines events in cervical tissues can be reflected in the circulation. In this regard, circulating mononuclear leukocytes from HPV 16 and 18 status patients showed decline levels of IL-2 in high-grade CIN and cancer patients, whereas IFN-γ levels were decreased only in patients with advanced cancer of cervix. In addition, an increase in the levels of IL-4 and IL-10 in leukocytes was found in all cancer cervix and CIN3 patients, as compared to those with early CIN grades and healthy controls. The type 2 and type 1 cytokine levels were significantly correlated with HPV status [125]. In other studies, the progress of CIN2 in patients infected by HPV was accompanied with increasing levels of IFN-γ, IL-10 and TNFα positive leukocytes in the circulation [126].
In situ presence of leukocytes in human cervical intraepithelial neoplasia and cervical cancer
Extensive studies have been conducted on the role of cytokines in CIN and cervical cancer [127]. The presence of cytokines in association with both infiltrating and cervical tissue cells is an important aspect of the pathogenesis of CIN and cervical cancer. Tumors commonly are infiltrated by leukocytes. The immune cell infiltration into the cancer tissue included increased numbers of monocyte/macrophages, helper T cells (CD4+), and CD25+ lymphocytes compared to benign tissue [128]. Chemokines are cytokines which induce chemotaxis on many cell types which play a crucial role in inflammatory processes and in tumor associated angiogenesis, as well as in tumor progression. The production of attractants for leukocytes such as MCP-1, IL-8 and macrophage inflammatory proteins (MIP) has been identified in tumor tissues of patients with different neoplasms [129, 130]. In this regard, increased expressions of MCP-1 and IL-8 in CIN were observed accompanied by increment of lymphocyte infiltration co-expressing CD3/MCP-1 and CD3/IL-8. CD3/MCP-1 cell percentage was found decreased and CD3/IL-8 percentage increased according to the CIN evolution, suggesting tumor progression [131]. MCP-1 and IL-8 positive lymphocytes may potentially contribute to the overall concentration of chemokines produced during cervical dysplasia and enhance leukocyte attraction.
The inflammatory microenvironment has been linked to the progression of cervical neoplasia. Th17 lymphocyte subtype produces the anti-inflammatory cytokine IL-17 that can be involved in the progression of these neoplasms. It has been shown that CCL20 is related to the decreased of Th17 lymphocytes in cervical neoplasia suggesting a role of this molecule in the neoplasm progression [132]. In addition, the expression of CCL20 is directly linked to the expression of dendritic cells. Thus, viral proteins such as E6 and E7 from HPV that can decrease the expression of CCL20, decrease the presence of dendritic cells in cervical neoplasia and therefore are important factors in tumor progression [133]. However, the expression of chemokines such as CXCL9/10/11 is highly correlated with high expression of dendritic cell subtypes (CD141/BDCA3+cDC1) in squamous cell carcinoma primary tumors [134].
The association of angiogenesis and leukocyte infiltration in CIN has also been reported. Increased expression of vascular endothelial growth factor (VEGF) co-expressed in lymphocytes (CD3/VEGF) and in monocyte-macrophages was observed related to the progression of CIN. A significant increment of CD3/VEGF lymphocytes was found in CIN3 and monocyte-macrophages expressing VEGF in CIN2 and 3 [112]. The expression of VEGF in lymphocytes and monocytes/macrophages is suggestive of VEGF production by these leukocytes and may play an important role in the induction of angiogenesis by these cells. In this regard, angiogenesis is a complex process that plays an important role in the development of many types of cancer. The role of angiogenesis in cervical neoplasia is related to HPV- inhibition of p53 and stabilization of hypoxia-inducible factor-1α. Both mechanisms are able to increase expression of VEGF [135]. Activation of VEGF promotes endothelial cell proliferation and migration, favoring formation of new blood vessels and increasing permeability of existing blood vessels [135]. Associated with those findings, increased micro vessel density was reported in CIN3 and cervical cancer [136, 137] (Fig. 2).
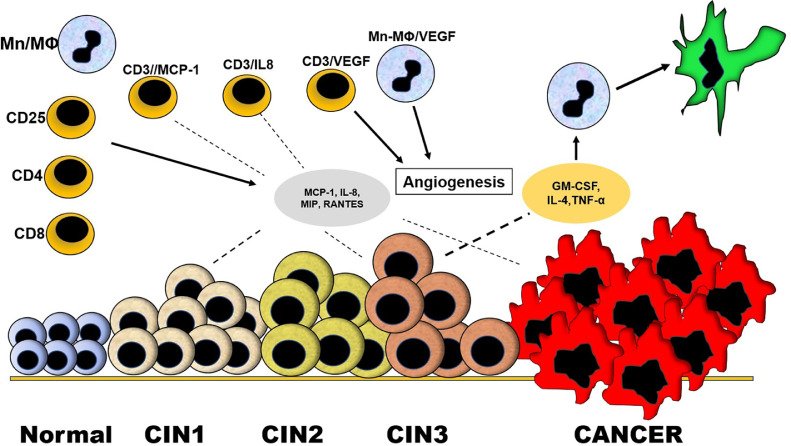
Fig. 2.
Infiltration of leukocytes into the neoplastic cervical tissues. During cervical dysplasia, cytokines capable of attracting leukocytes (chemokines) and inducing leukocyte infiltration can be produced. These infiltrating cells can be mononuclear cells with phenotypes of CD8, CD4, CD25 and monocytes/macrophages (Mn/MΦ). Some of the infiltrating cells may be producers of MCP-1 and IL-8 (CD3/MCP-1, CD3/IL-8) and contribute to the overall production of these chemokines during the neoplasia. Other infiltrating cells can produce vascular endothelial growth factor (CD3/VEGF, Mn/MΦ/VEGF) and contribute to angiogenesis facilitating the dysplasia progression. In situ production of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4 and TNF-α by cervical tissue during the cervical dysplasia can induce differentiation of monocytes/macrophages into dendritic cells, relevant cells against HPV infection, the main inducer of cervical dysplasia.
Cytokines can act in situ to induce differentiation of infiltrating mononuclear leukocytes to dendritic cells. In this regard, mononuclear cells obtained from patients with low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL) and cervical cancer showed different grade of differentiation to dendritic cells according to the extent of cervical lesions (high percentage in HSIL and low percentage in LSIL) when they are stimulated by granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4 and TNF-α [138]. The existence of dendritic cells in the cervical tissue together with the differentiation of monocytes/macrophages to dendritic cells may be relevant in the in situ and systemic response against HPV infection, the main inducer of cervical dysplasia [139]. Therapeutic attempts have been made to alter presence and function of infiltrating leukocytes in the cervix neoplasm. In this regard, intralesional IFN-α 2b treatment in patients with CIN 2/3 resulted with a reduction in CD4+ and CD8+ T lymphocyte infiltration [140] (Fig. 2).
Immune therapy
In general, cervical cancer (CC) immune therapy can be divided into four groups: immune checkpoint blockade, adoptive cell transfer therapy, cytokine treatment and therapeutic vaccines. Cancer cells can escape from the immune balance by provoking an immune-suppressive state and tumor growth. Checkpoint blockades aim to break microenvironment immune suppression. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CLTA-4) are the most promising immune checkpoints targeted in CC [141, 142]. The expression levels of PD-1/PD-L1 and CTLA-4 are high in CC, and dendritic cells and T cells express high levels of PD-1 and PDL1 in CIN samples [143, 144]. Blockade of PD-1/PD-L1 interrupted immune suppression in CC, by increasing subset of T cells, CD8+FoxP3+CD25+T cells and decreasing immune-suppressive Tregs cells [145]. In addition, the combination of PD-1/PD-L1 and CTLA-4 may enhance the therapeutic efficiency [146].
Antigen- specific T cell immunotherapy could be used to attack tumor evasion and may have many potential benefits. Thus, by optimizing dendritic cells maturation and adding appropriate cytokines, it is possible to obtain oncoproteins E6 and E7-specific T cells [147]. Chimeric antigen receptor T cells have been used in CC cells and achieved positive results [148]. Autologous cytokine-induced killer cell transfusion in CC patients improved immune function and life quality [149].
Cytokine therapies have been used for cancer treatment. Their specific functions in CC are not clear. However, IL-2 and IL-6 confer protection to CC cells against apoptosis [150, 151]. Immunoactive TNF- α and immunosuppressive IL-10 are associated with CC susceptibility [152].
Various therapeutic vaccines are being developed, including DNA, RNA and peptide vaccines. All of these vaccine types exerted antitumor effects through activating the T cell response, especially CD8+ cells [153, 154]. Several clinical trials have indicated that therapeutic vaccines plus immune checkpoint inhibitors or radiotherapy together induced an immune response in premalignant lesions and CC [155].
Conclusions
The in-situ upregulation of cytokines in the cervical tissue plays a very important role in the progression or in the reduction of cervical neoplasia. These cytokines can generally be expressed in conjunction with other cytokines and their interaction can be linked to a result regarding the evolution of cervical neoplasia. Neoplastic tissue can produce chemokines that induce the infiltration of leukocytes, which can produce various types of cytokines that modulate the neoplasm progression. Factors such as HPV infection can interact with the immune response and influence the progression of the neoplasm. Knowledge of the effects of each cytokine during cervical dysplasia is important for the therapeutic use of these molecules.
Funding
This study did not receive any funding.
Availability of data and materials
Not applicable.
Ethic approval
Not applicable.
Consent for publication
Not applicable.
Authors' contributions
Data acquisition: Jesús A Mosquera, Yenddy N Carrero and Diana E Callejas. Article preparation: Jesús A Mosquera, Yenddy N Carrero and Diana E Callejas. Article editing Jesus A Mosquera. Article review: All authors.
Declaration of Competing Interest
The authors declare no competing interest.
Acknowledgement
Not applicable.
Biographies
Yenddy N. Carrero. Medical Faculty, Zulia University, Maracaibo, Venezuela. PhD in Immunology from Alcala de Henares University, Spain.
Diane E. Callejas. Medical Faculty, Zulia University, Maracaibo, Venezuela. PhD in Immunology from Alcala de Henares University, Spain.
Jesus A. Mosquera. Medical Faculty, Zulia University, Maracaibo, Venezuela. Medical doctor. Inmunolopathology. Washintong University. St. Louis MO. USA.
Contributor Information
Yenddy N. Carrero, Email: yenddycarrero@yahoo.es.
Diana E. Callejas, Email: callejas.diana60@gmail.com.
Jesús A. Mosquera, Email: mosquera99ve@yahoo.com.
References
- 1.Moshkovich O., Lebrun-Harris L., Makaroff L., Chidambaran P., Chung M., Sripipatana A., Lin S.C. Challenges and opportunities to im-prove cervical cancer screening rates in US Heath centers through patient-centered medical home transformation. Adv. Prev. Med. 2015;2015 doi: 10.1155/2015/182073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benard V.B., Thomas C.C., King J., Massetti G.M., Doria-Rose V.P., Saraiya M. Vital signs: cervical cancer incidence, mortality, and screening – United States, 2007- 2012. MMWR Morb. Mortal. Wkly. Rep. 2014;63:1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal M., LaRusso N.F., Burgart L.J., Gores G.J. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 7.Shuman-Moss L.A., Jensen-Taubman S., Stetler-Stevenson W.G. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am. J. Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue H., Lin B., Ni P., Xu H., Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta- analysis. J. Gastroenterol. Hepatol. 2010;25:1604–1617. doi: 10.1111/j.1440-1746.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 9.Haghnegahdar H., Du J., Wang D., Strieter R.M., Burdick M.D., Nanney L.B., Cardwell N., Luan J., Shattuck-Brandt R., Richmond A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J. Leukoc. Biol. 2000;67(1):53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkwill F. Cancer and the chemokine network. Nat. Rev. Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 11.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mlecnik B., Tosolini M., Charoentong P., Kirilovsky A., Bindea G., Berger A., Camus M., Gillard M., Bruneval P., Fridman W-H., Pagès F., Trajanoski Z., Galon J. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138(4):1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 14.de Chaisemartin L., Goc J., Damotte D., Validire P., Magdeleinat P., Alifano M., I.Cremer W-H.Fridman, Sautès-Fridman C., Dieu-Nosjean M.-C. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 15.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 16.Freeman G.J., Long A.J., I.wai Y, Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., Horton H.F., Fouser L., Carter L., Ling V., Bowman M.R., Carreno B.M., Collins M., Wood C.R., Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castle P.E., Hillier S.L., Rabe L.K., Hildesheim A., Herrero R., Bratti M.C., Sherman M.E., Burk R.D., Rodriguez A.C., Alfaro M., Hutchinson M.L., Morales J., Schiffman M. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papilloma virus (HPV) Cancer Epidemiol. Biomark. Prev. 2001;10(10):1021–1027. [PubMed] [Google Scholar]
- 19.Rothenberg S.M., Ellisen L.M. The molecular pathogenesis of head and neck squamous cell carcinoma. J. Clin. Invest. 2012;122:1951–1957. doi: 10.1172/jci59889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brianti P., De Flammineis E., Mercuri S.R. Review of HPV-related diseases and cancers. New. Microbiol. 2017;40:80–85. [PubMed] [Google Scholar]
- 21.Hoppe-Seyler K., Bossler F., Braun J.A., Herrmann A.L., Hoppe-Seyler F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018;26:158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Stanley M. Immunology of HPV Infection. Curr. Obstet. Gynecol. Rep. 2015;4:195–200. doi: 10.1007/s13669-015-0134-y. [DOI] [Google Scholar]
- 23.Wollenberg B. Cancer Immunology and HPV. Recent Results. Cancer Res. 2017;206:243–248. doi: 10.1007/978-3-319-43580-0_19. [DOI] [PubMed] [Google Scholar]
- 24.Deligeoroglou E., Giannouli A., Athanasopoulos N., Karountzos V., Vatopoulou A., Dimopoulos K. HPV infection: immunological aspects and their utility in future therapy. Infect. Dis. Obstet. Gynecol. 2013;2013 doi: 10.1155/2013/540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melief C.J.M., van Der Burg S.H., Toes R.E.M., Ossendorp F., Offringa R. Effective therapeutic anticancer vaccines based on precision guiding of cytolytic T lymphocytes. Immunol. Rev. 2002;188:177–182. doi: 10.1034/j.1600-065x.2002.18816.x. [DOI] [PubMed] [Google Scholar]
- 26.Nilges K., Höhn H., Pilch H., Neukirch C., Freitag K., Talbot P.J. Human papillomavirus type 16 E7 peptide-directed CD8+ T cells from patients with cervical cancer are cross-reactive with the coronavirus NS2 protein. J, Virol. 2003;77:5464–5474. doi: 10.1128/jvi.77.9.5464-5474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi Y.W., Kang M.C., Seo Y.B., Namkoong H., Park Y., Choi D.H., Suh Y.S., Lee S-W., Sung Y.C., Jin H-T. Intravaginal Administration of Fc-Fused IL7 Suppresses the Cervicovaginal Tumor by Recruiting HPV DNA Vaccine-Induced CD8 T Cells. Clin. Cancer Res. 2016;22:5898–5908. doi: 10.1158/1078-0432.CCR-16-0423. [DOI] [PubMed] [Google Scholar]
- 28.de Méndez M.T., Bosch A.L. Abnormal immunoexpression of cell adhesion molecules (CAMs) in cervical cancer. Int. J. Surg. Pathol. 2011;19:733–742. doi: 10.1177/1066896909343435. [DOI] [PubMed] [Google Scholar]
- 29.Patel S., Chiplunkar S. Host immune responses to cervical cancer. Curr. Opin. Obstet. Gynecol. 2009;21:54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi A., Weinberg V., Darragh T., Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogénesis. Mucosal Immunol. 2008;1:412–420. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 31.Matthews K., Leong C.M., Baxter L., Inglis E., Yun K., Bäckström B.T. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J. Virol. 2003;77:8378–8385. doi: 10.1128/jvi.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeneman M.M., Kruitwagen R.F., Nijman H.W., Slangen B.F., Van Gorp T., Kruse A.J. Natural history of high-grade cervical intraepithelial neoplasia: a review of prognostic biomarkers. Expert. Rev. Mol. Diagn. 2015;15:527–556. doi: 10.1586/14737159.2015.1012068. [DOI] [PubMed] [Google Scholar]
- 33.Adiga D., Eswaran S., Pandey D., Sharan K., Prasada Kabekkodu S. Molecular landscape of recurrent cervical cancer. Crit. Rev. Oncol. Hematol. 2020;157 doi: 10.1016/j.critrevonc.2020.103178. [DOI] [PubMed] [Google Scholar]
- 34.Ai Y., Zhu H., Xie C., Jin X. Radiomics in cervical cancer: Current applications and future potential. Crit. Rev. Oncol. Hematol. 2020;152 doi: 10.1016/j.critrevonc.2020.102985. [DOI] [PubMed] [Google Scholar]
- 35.Li N., Hu Y., Zhang X., Liu Y., He Y., van der Zee A.G.J., Schuuring E., Wisman G.B.A. DNA methylation markers as triage test for the early identification of cervical lesions in a Chinese population. Int. J. Cancer. 2018;144(4):746–754. doi: 10.1002/ijc.31897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dick S., Verhoef L., De Strooper L.M., Ciocănea-Teodorescu I., Wisman G.B.A., Meijer C.J., Bleeker M.C., Steenbergen R.D., Heideman D.A. Evaluation of six methylation markers derived from genome-wide screens for detection of cervical precancer and cancer. Epigenomics. 2020;12(18):1569–1578. doi: 10.2217/epi-2019-0331. [DOI] [PubMed] [Google Scholar]
- 37.Costa A.F., Pogere A., Farina Pasinato A.P.B., Monteiro Bello E.J., Casimiro Onofre A.S., de Miranda Onofre F.B. DNA ploidy measurement and human papillomavirus in abnormal cervical cytology. Cytopathology. 2021;32(2):180–186. doi: 10.1111/cyt.12943. [DOI] [PubMed] [Google Scholar]
- 38.Pisarska J., Baldy-Chudzik K. MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer. J. Clin. Med. 2020;9:3668. doi: 10.3390/jcm9113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Carvalho Galvão M.L.T., Coimbra E.C. Long noncoding RNAs (lncRNAs) in cervical carcinogenesis: New molecular targets, current prospects. Crit. Rev. Oncol. Hematol. 2020;156 doi: 10.1016/j.critrevonc.2020.103111. [DOI] [PubMed] [Google Scholar]
- 40.Razavi Z.S., Tajiknia V., Majidi S., Ghandali M., Mirzaei H.R., Rahimian N., Hamblin M.R., Mirzaei H. Gynecologic cancers and non-coding RNAs: Epigenetic regulators with emerging roles. Crit. Rev. Oncol. Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103192. [DOI] [PubMed] [Google Scholar]
- 41.Song F., Du H., Xiao A., Wang C., Huang X., Yan P., Liu Z., Qu X., Belinson J.L., Wu R. Evaluating the Performance of p16 INK4a Immunocytochemistry in Cervical Cancer Screening. Cancer Manag. Res. 2020;12:9067–9075. doi: 10.2147/CMAR.S273079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen P.A., Jhingran A., Oaknin A., Denny L. Cervical Cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 43.Deverman B.E., Patterson P.H. Cytokines and CNS Development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Zídek Z., Anzenbacher P., Kmonickoval E. Current status and challenges of cytokine pharmacology. Br. J. Pharmacol. 2009;157:342–361. doi: 10.1111/j.1476-5381.2009.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Invest. 2008;118v:3546–3560. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Germano G., Allavena P., Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Hemmat N., Bannazadeh Baghi H. Association of Human Papillomavirus Infection and Inflammation in Cervical Cancer. Pathog. Dis. 2019;77:ftz048. doi: 10.1093/femspd/ftz048. [DOI] [PubMed] [Google Scholar]
- 48.Daniilidis A., Koutsos J., Oikonomou Z., Nasioutziki M., Hatziparadisi K., Tantanasis T. Cytokines of cervical mucosa and human papilloma virus infection of the cervix: A descriptive study. Acta Cytol. 2016;60:58–64. doi: 10.1159/000445161. [DOI] [PubMed] [Google Scholar]
- 49.Torres-Poveda K., Bahena-Román M., Madrid-González C., Burguete-García A.I., Bermúdez-Morales V.H., Peralta-Zaragoza O., Madrid-Marina V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014;5(4):753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardo-Govea T., Callejas D., Núñez-Troconis J., Araujo M., Costa L., Pons H., Delgado M., Monsalve F. [Gamma interferon (IFN-gamma), tumor necrosis factor alpha (TNF-alpha) and interleukins 2, 4 and 6 (IL-2, IL-4, IL-6) in cervical-uterine cells of intraepithelial neoplasia: a preliminary report] Invest. Clin. 2005;46(1):5–13. [PubMed] [Google Scholar]
- 51.Tjiong M.Y., van der Vange N., ter Schegget J.S., Burger M.P., ten Kate F.W., Out T.A. Cytokines in cervicovaginal washing fluid from patients with cervical neoplasia. Cytokine. 2001;14:357–360. doi: 10.1006/cyto.2001.0909. [DOI] [PubMed] [Google Scholar]
- 52.Lin W., Niu Z., Zhang H., Kong Y., Wang Z., Yang X. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int. J. Clin. Exp. Pathol. 2019;12:3604–3612. [PMC free article] [PubMed] [Google Scholar]
- 53.Colín-Ferreyra M.C., Mendieta-Zerón H., Romero-Figueroa M.S., Martínez-Madrigal M., Martínez-Pérez S., Domínguez-García M.V. [Expression of gamma interferon during HPV and chlamydia trachomatis infection in cervical samples] Enferm. Infecc. Microbiol. Clin. 2015;33:105–109. doi: 10.1016/j.eimc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Hu T., Yang P., Zhu H., Chen X., Xie X., Yang M., Liu S., Wang H. Accumulation of invariant NKT cells with increased IFN-γ production in persistent high-risk HPV-infected high-grade cervical intraepithelial neoplasia. Diagn. Pathol. 2015;10:1–10. doi: 10.1186/s13000-015-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sikorski M., Bieda T., Bobek M., Zrubek H. Dynamics of cervical langerhans cell counts in the course of HPV-positive CIN treatment with the use of human recombinant interferon gamma. Eur. J. Gynaecol. Oncol. 2005;26:294–298. [PubMed] [Google Scholar]
- 56.Sikorski M., Zrubek H. Recombinant human interferon gamma in the treatment of cervical intraepithelial neoplasia (CIN) associated with human papillomavirus (HPV) infection. Eur. J. Gynaecol. Oncol. 2003;24:147–150. [PubMed] [Google Scholar]
- 57.Mantovani A., Barajon I., Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018;281:57–61. doi: 10.1111/imr.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 59.Mhatre M., McAndrew T., Carpenter C., Burk R.D., Einstein M.H., Herold B.C. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm. Dis. 2012;39:591–597. doi: 10.1097/OLQ.0b013e318255aeef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae J.Y., Kim E.K., Yang D.H., Zhang X., Park Y.J., Lee D.Y., Che C.M., Kim J. Reciprocal interaction between carcinoma-associated fibroblasts and squamous carcinoma cells through interleukin-1α induces cancer progression. Neoplasia. 2014;16(11):928–938. doi: 10.1016/j.neo.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu S., Hu G., Chen J., Xie G. Interleukin 1b and interleukin 1 receptor antagonist gene polymorphisms and cervical cancer: a meta-analysis. Int. J. Gynecol. Cancer. 2014;24:984–990. doi: 10.1097/IGC.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 62.Matamoros J.A., Ferreira da Silva M.I., Freire de Moura P.M.M., Gomes Leitão M.C., Campos Coimbra E. Reduced expression of IL-1β and IL-18 proinflammatory interleukins increases the risk of developing cervical cancer. Asian Pac. J. Cancer Prev. 2019;20:2715–2721. doi: 10.31557/APJCP.2019.20.9.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malek T.R. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 64.Marabondo S., Kaufman H.L. High-dose interleukin-2 (IL-2) for the treatment of melanoma: safety considerations and future directions. Expert. Opin. Drug Saf. 2017;16:1347–1357. doi: 10.1080/14740338.2017.1382472. [DOI] [PubMed] [Google Scholar]
- 65.Mindioli R., Callejas D., Nunez-Montiel J., Mosquera J. Increased IL-2, IL-2 receptor and IL-10 positive cells in premalignant lesions of uterine cervix. Invest. Clin. 2008;49:533–545. [PubMed] [Google Scholar]
- 66.Zheng J.J., Song J.H., Yu C.X., Wang F., Wang P.C., Meng J.W. Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health. 2019;19:1–8. doi: 10.1186/s12905-019-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng J.W., Song J.H. Association between interleukin-2, interleukin-10, secretory immunoglobulin A and immunoglobulin G expression in vaginal fluid and human papilloma virus outcome in patients with cervical lesions. Oncol. Letters. 2019;18:5543–5548. doi: 10.3892/ol.2019.10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valle-Mendiola A., Gutiérrez-Hoya A., Lagunas-Cruz M.C., Weiss-Steider B., Soto-Cruz I. Pleiotropic effects of IL-2 on cancer: Its role in cervical cancer. Mediat. Inflamm. 2016;2016 doi: 10.1155/2016/2849523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casana P.H., Hernandez H., Arana M.J. Interleukin-2 inhibits proliferation of HPV-associated tumor cells and halts tumor growth in vivo. Biochem. Biophys. Res. Commun. 2002;299:818–824. doi: 10.1016/s0006-291x(02)02715-8. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei L.H., Kuo M.L., Chen C.A., Cheng W.F., Cheng S.P., Hsieh F.J. Interleukin-6 in cervical cancer: the relationship with vascular endothelial growth factor. Gynecol. Oncol. 2001;82:49–56. doi: 10.1006/gyno.2001.6235. [DOI] [PubMed] [Google Scholar]
- 72.Hess S., Smola H., de Silva U.S., Hadaschik D., Kube D., Baldus S.E., Flucke U., Pfister H. Loss of IL-6 receptor expression in cervical carcinoma cells inhibits autocrine il-6 stimulation: abrogation of constitutive monocyte chemoattractant protein-1 production. J. Immunol. 2000;165(4):1939–1948. doi: 10.4049/jimmunol.165.4.1939. [DOI] [PubMed] [Google Scholar]
- 73.Li B., Zhang L., Zhao J., Tan G., Zhang W., Zhang N., Tian J., Qu P. The value of cytokine levels in triage and risk prediction for women with persistent high-risk human papilloma virus infection of the cervix. Infect. Agent Cancer. 2019;14:16. doi: 10.1186/s13027-019-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paradkar P.H., Agashe S.V., Joshi J.V., Jagtap S.S., Affandi M.Z., Vaidya R.A. Serum IL-6 and micrometry of pap smears in women with cervical low-grade intraepithelial lesions. Asian Pac. J. Cancer Prev. 2010;11:989–992. [PubMed] [Google Scholar]
- 75.Alfaro A., Sanmamed M.F., Rodríguez-Ruiz M.E., Teijeira A., Oñate C., González A., Ponz M., Schalper K.A., Pérez-Gracia J.L., Melero I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017;60:24–31. doi: 10.1016/j.ctrv.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Waugh D.J., Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 77.Iwata T., Fujii T., Morii K., Saito M., Sugiyama J., Nishio H., Morisada T., Tanaka K., Yaguchi T., Kawakami Y., Aoki D. Cytokine profile in cervical mucosa of Japanese patients with cervical intraepithelial neoplasia. Int. J. Clin. Oncol. 2015;20(1):126–133. doi: 10.1007/s10147-014-0680-8. [DOI] [PubMed] [Google Scholar]
- 78.Fujimoto J., Sakaguchi H., Aoki I., Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 79.Jia L., Li F., Shao M., Zhang W., Zhang C., Zhao X., Luan H., Y.Qi P.Zhang, Liang L., Jia X., Zhang K., Lu Y., Yang Z., Zhu X., Zhang Q., Du J., Wang W. IL-8 is upregulated in cervical cancer tissues and is associated with the proliferation and migration of HeLa cervical cancer cells. Oncol. Letters. 2018;15(1):1350–1356. doi: 10.3892/ol.2017.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu S., Shang H., Cui L., Zhang Z., Zhang Y., Li Y., Wu J., Li R-K., Xie J. Targeted blockade of interleukin-8 abrogates its promotion of cervical cancer growth and metastasis. Mol. Cell. Biochem. 2013;375:69–79. doi: 10.1007/s11010-012-1529-y. [DOI] [PubMed] [Google Scholar]
- 81.Pestka S., Krause C.D., Sarka D., Walter M.R., Shi Y., Fisher P.B. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 82.Rodríguez J.A., Galeano L., Palacios D.M., Gómez C., Serrano M.L., Bravo M.M., Combita A.L. Altered HLA class I and HLA-G expression is associated with IL-10 expression in patients with cervical cancer. Pathobiology. 2012;79(2):72–83. doi: 10.1159/000334089. [DOI] [PubMed] [Google Scholar]
- 83.Ali K.S., Ali H.Y., Jubrael J.M. Concentration levels of IL-10 and TNFα cytokines in patients with human papilloma virus (HPV) DNA⁺ and DNA⁻ cervical lesions. J. Immunotoxicol. 2012;9:168–172. doi: 10.3109/1547691X.2011.642419. [DOI] [PubMed] [Google Scholar]
- 84.Syrjänen S., Naud P., Sarian L., Roteli-Martins C., Longatto-Filho A., Tatti S., Branca M., Eržen M., Hammes L.S., Costa S., Syrjänen K. Immunosuppressive cytokine Interleukin-10 (IL-10) is up-regulated in high-grade CIN but not associated with high-risk human papillomavirus (HPV) at baseline, outcomes of HR-HPV infections or incident CIN in the LAMS cohort. Virchows Arch. 2009;455:505–515. doi: 10.1007/s00428-009-0850-7. [DOI] [PubMed] [Google Scholar]
- 85.Gosmann C., Mattarollo S.R., Bridge J.A., Frazer I.H., Blumenthal A. IL-17 suppresses immune effector functions in human papillomavirus-associated epithelial hyperplasia. J. Immunol. 2014;193:2248–2257. doi: 10.4049/jimmunol.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xue J., Wang Y., Chen C., Zhu X., Zhu H., Hu Y. Effects of Th17 cells and IL-17 in the progression of cervical carcinogenesis with high-risk human papillomavirus infection. Cancer Med. 2018;7:297–306. doi: 10.1002/cam4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miranda L.N., Reginaldo F.P., Souza D.M., Soares C.P., Alves Silva T.G., Ferreira Rocha K.B., Nunes Jatobá C.A., Donadi E.A., Leon Andrade J.M., Silveira Gonçalves A.K., Oliveira Crispim J.C. Greater expression of the human leukocyte antigen-G (HLA-G) and interleukin-17 (IL-17) in cervical intraepithelial neoplasia: analytical cross-sectional study. Sao Paulo Med. J. 2015;133(4):336–342. doi: 10.1590/1516-3180.2013.7170009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo N., Shen G., Zhang Y., Moustafa A.A., Ge D., You Z. Interleukin-17 promotes migration and invasion of human cancer cells through upregulation of mta1 expression. Front. Oncol. 2019;9:46. doi: 10.3389/fonc.2019.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Otani S., Fujii T., Kukimoto T., Yamamoto N., Tsukamoto T., Ichikawa R., Nishio E., Iwata A. Cytokine expression profiles in cervical mucus from patients with cervical cancer and its precursor lesions. Cytokine. 2019;120:210–219. doi: 10.1016/j.cyto.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Park Y.K., Shin D.J., Cho D., Kim S.K., Lee J.J., Shin M-G., Ryang D-W., Lee J-S., Park M-H., Yoon J.H., Jegal Y.J. Interleukin-21 increases direct cytotoxicity and IFN-γ production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res. 2012;32:839–846. [PubMed] [Google Scholar]
- 91.Strobl H. Molecular mechanisms of dendritic cell sublineage development from human hematopoietic progenitor/stem cells. Int, Arch, Allergy Immunol. 2003;131:73–79. doi: 10.1159/000070921.0. [DOI] [PubMed] [Google Scholar]
- 92.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 93.Zijlmans H.J., Fleuren G.J., Baelde H.J., Eilers P.H., Kenter G.G., Gorter A. Role of tumor-derived proinflammatory cytokines GM-CSF, TNF-alpha, and IL-12 in the migration and differentiation of antigen-presenting cells in cervical carcinoma. Cancer. 2007;109:556–565. doi: 10.1002/cncr.22428. [DOI] [PubMed] [Google Scholar]
- 94.Tian Y., Yuan C., Ma D., Zhang Y., Liu Y., Zhang W., Hou F., Cui B. IL-21 and IL-12 inhibit differentiation of Treg and TH17 cells and enhance cytotoxicity of peripheral blood mononuclear cells in patients with cervical cancer. Int. J. Gynecol. Cancer. 2011;21(9):1672–1678. doi: 10.1097/IGC.0b013e3182358955. [DOI] [PubMed] [Google Scholar]
- 95.Wadler S., Levy D., Frederickson H.L., Falkson C.I., Wang Y., Weller E., Burk R., Ho G., Kadish A.S. Eastern Cooperative Oncology Group, A phase ii trial of interleukin-12 in patients with advanced cervical cancer: Clinical and immunologic correlates. Eastern Cooperative Oncology Group Study E1E96. Gynecol. Oncol. 2004;92(3):957–964. doi: 10.1016/j.ygyno.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 96.Han S., Yang Y. Interleukin-32: Frenemy in Cancer? BMB Rep. 2019;52:165–174. doi: 10.5483/BMBRep.2019.52.3.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S., Kim J.H., Kim H., Kang J.W., Kim S.H., Yang Y., Kim J., Park J.S., Park S.N., Hong J.T., Yoon D.-Y. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology. 2011;132(3):410–420. doi: 10.1111/j.1365-2567.2010.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan H., He D., Huang X., Zhang E., Chen Q., Xu R., Liu X., Zi F., Cai Z. Role of interleukin-32 in cancer biology. Oncol. Letters. 2018;16(1):41–47. doi: 10.3892/ol.2018.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liew F.Y., Girard J.P., Turnquist H.R. Interleukin-33 in health and disease. Nature Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 100.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nature Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., M.Moshrefi J.Qin, Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 102.Kulhan N.G., Kulhan M., Aydin M., Nayki U., Nayki C., Ulug P. Could interleukin-33 and its suppressor of tumorigenicity 2 (st2) receptor have a role in cervical human papillomavirus (HPV) infections? Gynecol. Endocrinol. 2019;35:796–802. doi: 10.1080/09513590.2019.1590699. [DOI] [PubMed] [Google Scholar]
- 103.Wang L., Li H., Liang F., Hong Y., Jiang S., Xiao L. Examining IL-33 expression in the cervix of HPV-infected patients: a preliminary study comparing IL-33 levels in different stages of disease and analyzing its potential association with IFN-γ. Med. Oncol. 2014;31:143. doi: 10.1007/s12032-014-0143-0. [DOI] [PubMed] [Google Scholar]
- 104.Wang L., Quan Y., Yue Y., Heng X., Che F. Interleukin-37: A crucial cytokine with multiple roles in disease and potentially clinical therapy. Oncol. Lett. 2018;15:4711–4719. doi: 10.3892/ol.2018.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ouyang P., An W., Chen R., Zhang H., Chen D., Jiang E., Zhu W., Li P., Guo H., Chen Z., Wang S. IL-37 promotes cell apoptosis in cervical cancer involving bim upregulation. Onco. Targets Ther. 2019;12:2703–2712. doi: 10.2147/OTT.S201664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang S., An W., Yao Y., Chen R., Zheng X., Yang W., Zhao Y., Hu X., Jiang E., Bie Y., Chen Z., Ouyang P., Zhang H., Xiong H. Interleukin 37 expression inhibits stat3 to suppress the proliferation and invasion of human cervical cancer cells. J. Cancer. 2015;6(10):962–969. doi: 10.7150/jca.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu H., Luo H., Shen Z., Hu X., Sun L., Zhu X. Transforming growth factor-β1 in carcinogenesis, progression, and therapy in cervical cancer. Tumour Biol. 2016;37:7075–7083. doi: 10.1007/s13277-016-5028-8. [DOI] [PubMed] [Google Scholar]
- 108.Hazelbag S., Gorter A., Kenter G.G., van den Broek L., Fleuren G. Transforming growth factor-beta1 induces tumor stroma and reduces tumor infiltrate in cervical cancer. Human Pathol. 2002;33:1193–1199. doi: 10.1053/hupa.2002.130109. [DOI] [PubMed] [Google Scholar]
- 109.Iancu I.V., Botezatu A., Goia-Ruşanu C.D., Stănescu A., Huică I., Nistor E., Anton G., Pleşa A. TGF-beta signaling pathway factors in HPV-induced cervical lesions roum. Arch. Microbiol. Immunol. 2010;69(3):113–118. [PubMed] [Google Scholar]
- 110.Xu Q., Wang S., Xi L., Wu S., Chen G., Zhao Y., Wu Y., Ma D. Effects of human papillomavirus type 16 E7 protein on the growth of cervical carcinoma cells and immuno-escape through the TGF-beta1 signaling pathway. Gynecol. Oncol. 2006;101(1):132–139. doi: 10.1016/j.ygyno.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 111.Kim J., Kim M., Lee J., Kim J-H., Son S.K., Song E-S., Lee K.B., Lee J.P., Lee J.M., Yun Y.M. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: a case control study in Korea. Nutr. Cancer. 2010;62(2):181–189. doi: 10.1080/01635580903305326. [DOI] [PubMed] [Google Scholar]
- 112.Carrero Y., Mosquera J., Callejas D., Alvarez-Mon M. In situ increased chemokine expression in human cervical intraepithelial neoplasia and cervical cancer. Pathol. Res. Pract. 2015;211(4):281–285. doi: 10.1016/j.prp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 113.Xie F., Liu L-B., Shang W-Q., Chang K-K., Meng Y-H., Mei J. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364(2):106–107. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Hazelbag S., Fleuren G.J., Baelde J.J., Schuuring E., Kenter G.G., Gorter A. Cytokine profile of cervical cancer cells. Gynecol. Oncol. 2001;83(2):235–243. doi: 10.1006/gyno.2001.6378. [DOI] [PubMed] [Google Scholar]
- 115.Osiagwu D.D., Azenabor A.E., Osijirin A.A., Awopetu P.I., Oyegbami F.R. Evaluation of interleukin 8 and interleukin 10 cytokines in liquid based cervical cytology samples. Pan. Afr. Med. J. 2019;32:148. doi: 10.11604/pamj.2019.32.148.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tavares M.C., de Lima Júnior S.F., Coelho A.V., Marques T.R.N.M., Araújo D.H.T., de A Heráclio S., Ramos Amorim M.M., de Souza P.R.E., Crovella S. Tumor necrosis factor (TNF) alpha and interleukin (IL) 18 genes polymorphisms are correlated with susceptibility to HPV infection in patients with and without cervical intraepithelial lesion. Ann. Hum. Biol. 2016;43(3):261–268. doi: 10.3109/03014460.2014.1001436. [DOI] [PubMed] [Google Scholar]
- 117.Singh H., Jain M., Mittal B. Role of tgf-beta1 (-509c>t) promoter polymorphism in susceptibility to cervical cancer. Oncol. Res. 2009;18(1):41–45. doi: 10.3727/096504009789745656. [DOI] [PubMed] [Google Scholar]
- 118.do Carmo Vasconcelos de Carvalho V., de Macêdo J.L., de Lima C.A., de Andrade Heráclio S., Amorim M., Diniz Maia M.M., Figueiredo Porto A.L., de Souza P.R.E. IFN-gamma and IL-12B polymorphisms in women with cervical intraepithellial neoplasia caused by human papillomavirus. Mol. Biol. Rep. 2012;39(7):7627–7634. doi: 10.1007/s11033-012-1597-9. [DOI] [PubMed] [Google Scholar]
- 119.Zoodsma M., Nolte I.M., Schipper M., Oosterom E., van der Steege G., de Vries E.G.E., Te Meerman G.J., van der Zee A.G.J. Interleukin-10 and Fas polymorphisms and susceptibility for (pre)neoplastic cervical disease. Int. J. Gynecol. Cancer. 2005;3:282–290. doi: 10.1111/j.1525-1438.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto K., Oki A., Satoh T., Okada S., Minaguchi T., Onuki M.A., Ochi H., Nakao S., Sakurai M., Abe A., Hamada H., Yoshikawa H. Interleukin-10 -1082 gene polymorphism and susceptibility to cervical cancer among Japanese women. Jpn. J. Clin. Oncol. 2010;40(11):1113–1116. doi: 10.1093/jjco/hyq094. [DOI] [PubMed] [Google Scholar]
- 121.Gupta M.K., Singh R., Banerjee M. Cytokine gene polymorphisms and their association with cervical cancer: A North Indian study. Egyptian J. Med. Hum. Genet. 2016;17(2):155–163. doi: 10.1016/j.ejmhg.2015.10.005. [DOI] [Google Scholar]
- 122.Al-Tahhan M.A., Etewa R.L., El Behery M.M. Association between circulating interleukin-1 beta (il-1β) levels and il-1β c-511t polymorphism with cervical cancer risk in egyptian women. Mol. Cell. Biochem. 2011;353(1-2):159–162. doi: 10.1007/s11010-011-0782-9. [DOI] [PubMed] [Google Scholar]
- 123.Kang S., Kim J.W., Park N.H., Song Y.S., Park S.Y., Kang S.B., Lee H.P. Interleukin-1 beta-511 polymorphism and risk of cervical cancer. J. Korean Med. Sci. 2007;22(1):110–113. doi: 10.3346/jkms.2007.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng Y., Wang M., Tian T., Liu K., Liu X., Zhai Y., Lin S.i, Yang P., Li S., Dai Z., Lu J. Role of interleukin-12 gene polymorphisms in the onset risk of cancer: A meta-analysis. Oncotarget. 2017;8(18):29795–29807. doi: 10.18632/oncotarget.16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharma A., Rajappa M., Saxena A., Sharma M. Cytokine profile in Indian women with cervical intraepithelial neoplasia and cancer cervix. Int. J. Gynecol. Cancer. 2007;17(4):785–879. doi: 10.1111/j.1525-1438.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 126.Abramovskikh O.S. [The prognostic significance of estimation of levels of peripheral blood cytokines in patients with cervical neoplasms] Klin. Lab. Diagn. 2012;3:35–37. [PubMed] [Google Scholar]
- 127.Paradkar P.H., Joshi J.V., Mertia P.N., Agashe S.V., Vaidya R.A. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac. J. Cancer Prev. 2014;15(9):3851–3864. doi: 10.7314/apjcp.2014.15.9.3851. [DOI] [PubMed] [Google Scholar]
- 128.Sawe R.T., Mining S.K., Ofulla A.V., Patel K., Guyah B., Chumba D., Prosperi J.R., Kerper M., Shi Z., Sandoval-Cooper M., Taylor K., Badve S., Stack M.S., Littlepage L.E. Tumor infiltrating leukocyte density is independent of tumor grade and molecular subtype in aggressive breast cancer of Western Kenya. Trop. Med. Health. 2017;45:19. doi: 10.1186/s41182-017-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pellegrino A., Vacca A., Scavelli C., Dammacco F. Chemokines and tumors. Recent Prog. Med. 2002;93(11):642–654. [PubMed] [Google Scholar]
- 130.Soria G., Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 131.Carrero Y., Callejas D., Alaña F., Silva C., Mindiola R., Mosquera J. Increased vascular endothelial growth factor expression, CD3 positive cell infiltration and oxidative stress in premalignant lesions of the cervix. Cancer. 2009;115(16):3680–3688. doi: 10.1002/cncr.24411. [DOI] [PubMed] [Google Scholar]
- 132.Walch-Rückheim B., Mavrova R., Henning M., Vicinus B., Kim Y-J., Bohle R.M., Juhasz-Böss I., Solomayer E-F., Smola S. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPβ to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res. 2015;75(24):5248–5259. doi: 10.1158/0008-5472.CAN-15-0732. [DOI] [PubMed] [Google Scholar]
- 133.Jiang B., Xue M. Correlation of E6 and E7 Levels in High-Risk HPV16 Type Cervical Lesions with CCL20 and Langerhans Cells. Genet. Mol. Res. 2015;14(3):10473–10481. doi: 10.4238/2015.September.8.8. [DOI] [PubMed] [Google Scholar]
- 134.Rotman J., Heeren A.M., Gassama A.A., Lougheed S.M., Pocorni N., Stam A.G.M., Bleeker M.C.G., Zijlmans H.J.M.A.A., Mom C.H., Kenter G.G., Jordanova E.S., de Gruijl T.D. Adenocarcinoma of the Uterine Cervix Shows Impaired Recruitment of CDC1 and CD8 + T Cells and Elevated β-Catenin Activation Compared with Squamous Cell Carcinoma. Clin. Cancer Res. 2020;26(14):3791–3802. doi: 10.1158/1078-0432.CCR-19-3826. [DOI] [PubMed] [Google Scholar]
- 135.Tomao F., Papa A., Rossi L., Zaccarelli E., Caruso D., Zoratto F., Benedetti P.P., Tomao S. Angiogenesis and antiangiogenic agents in cervical cancer. Onco. Targets Ther. 2014;7:2237–2248. doi: 10.2147/OTT.S68286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu X., Liu H., Ye M., Zhu X. Prognostic value of microvessel density in cervical cancer. Cancer Cell. Int. 2018;18:152. doi: 10.1186/s12935-018-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El Sabaa B.M., Meleiss M., Zaki I. VEGF expression and microvascular density in relation to high-risk-HPV infection in cervical carcinoma – An immunohistochemical study. Alexandria J. Med. 2012;48(1):47–57. doi: 10.1016/j.ajme.2011.12.001. [DOI] [Google Scholar]
- 138.Lopes A.M.M., Michelin M.A., Murta E.F.C. Monocyte-derived dendritic cells from patients with cervical intraepithelial lesions. Oncol. Lett. 2017;13(3):1456–1462. doi: 10.3892/ol.2017.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manickam A., Sivanandham M., Tourkova IL. Immunological Role of Dendritic Cells in Cervical Cancer. Adv. Exp. Med. Biol. 2007;601:155–162. doi: 10.1007/978-0-387-72005-0_16. [DOI] [PubMed] [Google Scholar]
- 140.Machado F.A., Abdalla D.R., Montes L., Etchebehere R.M., Michelin M.A., Murta E.F. An evaluation of immune system cell infiltrate in the cervical stroma of patients with grade III cervical intraepithelial neoplasia after treatment with intralesional alpha-2B interferon. Eur. J. Gynaecol. Oncol. 2014;35(1):20–25. [PubMed] [Google Scholar]
- 141.Zhen J.Lu, Liu Y.H., Chen W., Li X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2019;27:1–11. doi: 10.1038/s41417-019-0131-9. [DOI] [PubMed] [Google Scholar]
- 142.Yang W., Song Y., Lu Y.L., Sun J.Z., Wang H.W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu S., Pu D., Xia X., Guo B., Zhang C. CTLA-4 rs5742909 polymorphism and cervical cancer risk: a meta-analysis. Medicine (Baltimore) 2020;99(11):e19433. doi: 10.1097/MD.0000000000019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karpathiou G., Chauleur C., Mobarki M., Peoc'h M. The immune checkpoints CTLA-4 and PD-L1 in carcinomas of the uterine cervix. Pathol. Res. Pract. 2020;216(1) doi: 10.1016/j.prp.2019.152782. [DOI] [PubMed] [Google Scholar]
- 145.Heeren A.M., Rotman J., Stam A.G.M., Pocorni N., Gassama A.A., Samuels S., Bleeker M.C.G., Mom C.H., Zijlmans H.J.M.A.A., Kenter G.G., Jordanova E.S., de Gruijl T.D. Efficacy of PD-1 blockade in cervical cancer is related to a CD8(+)FoxP3(+)CD25(+) T-cell subset with operational effector functions despite high immune checkpoint levels. J. Immunothe.r Cancer. 2019;7(1):43. doi: 10.1186/s40425-019-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dorta-Estremera S., Hegde V.L., Slay R.B., Sun R., Yanamandra A.V., Nicholas C., Nookala S., Sierra G., Curran M.l A., Sastry K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD- 1 immunotherapy in preclinical model of HPV(+) oral cancer. J. Immunother. Cancer. 2019;7(1):252. doi: 10.1186/s40425-019-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McCormack S.E., Cruz C.R.Y., Wright K.E., Powel l.A.B., Lang H., Trimble C., Keller M.D., Fuchs E., Bollard C.M. Human papilloma virus-specific T cells can be generated from naive T cells for use as an immunotherapeutic strategy for immunocompromised patients. Cytotherapy. 2018;20(3):385–393. doi: 10.1016/j.jcyt.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He Y., Li X.M., Yin C.H., Wu Y.M. Killing cervical cancer cells by specific chimeric antigen receptor-modified T cells. J. Reprod. Immunol. 2020;139 doi: 10.1016/j.jri.2020.103115. [DOI] [PubMed] [Google Scholar]
- 149.Li N., Tian Y.W., Xu Y., Meng D.D., Gao L., Shen W.J. Combined treatment with autologous CIK cells, radiotherapy and chemotherapy in advanced cervical cancer. Pathol. Oncol. Res. 2019;25(2):691–696. doi: 10.1007/s12253-018-0541-2. [DOI] [PubMed] [Google Scholar]
- 150.Lagunas-Cruz M.D.C., Valle-Mendiola A., Trejo-Huerta J., Rocha-Zavaleta L., Mora-García M.L., Gutiérrez-Hoya A., Weiss-Steider B., Soto-Cruz I. IL-2 induces transient arrest in the G1 phase to protect cervical cancer cells from entering apoptosis. J. Oncol. 2019;2019 doi: 10.1155/2019/7475295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morgan E.L., Macdonald A., Galloway D.A. Autocrine STAT3 activation in HPV positive cervical cancer through a virus-driven Rac1-NFκB-IL-6 signalling axis. PLoS Pathog. 2019;15(6) doi: 10.1371/journal.ppat.1007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Du G.H., Wang J.K., Richards J.R., Wang J.J. Genetic polymorphisms in tumor necrosis factor alpha and interleukin-10 are associated with an increased risk of cervical cancer. Int. Immunopharmacol. 2019;66:154–161. doi: 10.1016/j.intimp.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grunwitz C., Salomon N., Vascotto F., Selmic A., Bukurc T., Diken M., Kreiter S., Türeci Ö., Sahin U. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology. 2019;8(9) doi: 10.1080/2162402X.2019.1629259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahimi S.B., Mohebbi A., Vakilzadeh G., Biglari P., Jahromi S.R., Mohebi S.R. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018;163(3):587–597. doi: 10.1007/s00705-017-3647-z. [DOI] [PubMed] [Google Scholar]
- 155.Vici P., Pizzuti L., Mariani L., Zampa G., Santini D., Di Lauro L., Gamucci T., Natoli C., Marchetti P., Barba M., Maugeri-Saccà M., Sergi D., Tomao F., Vizza E., Di Filippo S., Paolini F., Curzio G., Corrado G., Michelotti A., Sanguineti G., Giordano A., De Maria R., Venuti A. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert. Rev. Vaccines. 2016;15(10):1327–1336. doi: 10.1080/14760584.2016.1176533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.