Abstract
Purpose
To compare quantitative optical coherence tomography angiography (OCT-A) measurements of the parafoveal microvasculature in retinal capillary plexuses among Behҫet uveitis (BU) patients, non-ocular Behҫet's disease (NOBD) patients, and healthy volunteers (HVs).
Methods
Sixty-eight subjects were enrolled in this prospective observational cross-sectional study. OCT-A imaging was performed using the Heidelberg Engineering Spectralis OCT. A custom algorithm was developed to calculate the vessel density (VD) in three retinal vascular layers: deep capillary plexus, intermediate capillary plexus, and superficial vascular plexus. The foveal avascular zone (FAZ) and acircularity index were calculated for the whole retinal vascular complex.
Results
We analyzed one eye from 21 BU patients (age, 51 ± 10 years), 23 NOBD patients (age, 48 ± 14 years), and 22 HVs (age, 44 ± 13 years). One-way multivariate analysis of covariance showed a statistically significant difference in VD among the three groups when combining the layers after controlling for scan quality (P < 0.001). The VD was lowest in the BU group and highest in the HV group in all layers. The FAZ area was also statistically significant different among the groups (P < 0.005), with the largest FAZ areas in BU patients and smallest FAZ areas in the HV group. However, no statistically significant difference was found for the acircularity index.
Conclusions
The parafoveal microvasculature is affected not only in BU patients but also in NOBD patients. Most deviations in the retinal microcirculation in Behҫet patients were found in the deeper layers of the retina by using the quantitative VD measurement.
Keywords: Behҫet's disease, OCT angiography, vessel density, foveal avascular zone, acircularity index
Behҫet's disease is a systemic autoinflammatory vasculitis affecting multiple organs.1,2 Ocular involvement occurs in approximately 50% to 90% of Behҫet patients.3 Uveitis in Behҫet is characterized by recurrent episodes of intraocular inflammation, most commonly presenting as bilateral panuveitis with retinal vasculitis.1,2,4,5 Retinal vasculitis can cause vascular occlusions leading to visual deterioration and often requires intensive immunosuppressive treatment.2,3,6,7
Fluorescein angiography (FA) is the golden standard for detecting and monitoring retinal vasculitis in Behҫet uveitis (BU)2,5,8; however, FA has some limitations with regard to visualization of the retinal microvascular circulation. First, due to dye leakage, the microvascular circulation is only visible in the early phases.6 Furthermore, FA has a very poor depth resolution and is unable to distinguish the retinal capillary plexuses separately.6,9 The functional extension of conventional optical coherence tomography (OCT), OCT angiography (OCT-A), enables visualization of the various microvascular retinal plexuses without the disturbance of dye leakage.10–12 OCT-A is even considered superior to conventional FA for visualization of microvascular changes in active BU that involve the posterior segment.13
Previous studies have demonstrated that patients suffering from Behҫet's disease with ocular involvement show hypo- or non-perfused areas on OCT-A in the superficial vascular plexus (SVP) and deep capillary plexus (DCP),13,14 in which the DCP seems most severely affected.13,15 Also, in comparison to healthy eyes, reduced vessel density (VD) and an enlarged foveal avascular zone (FAZ) are seen in Behҫet uveitis and are considered indicators for microvascular alterations.6,16,17 Furthermore, it is suggested that FAZ irregularity could be a marker of ocular involvement of Behҫet's disease.18 Because FAZ irregularity has only been assessed subjectively, further exploration for quantitative measurement of FAZ irregularity is warranted.
Because Behҫet's disease is a systemic vasculitis, we hypothesize that preclinical retinal microvascular changes may occur in patients with non-ocular Behҫet's disease (NOBD). Some studies have investigated VD and FAZ area changes in NOBD patients, but have shown inconsistent results.8,18–20 VD was reduced in NOBD patients compared to healthy control subjects in three out of the four studies,8,19,20 and FAZ area was larger in the NOBD than in healthy controls in only one of the four studies.19
Given the limited number of studies and their inconsistent results mainly due to small sample sizes, further exploration of preclinical retinal microvascular changes in NOBD patients compared to BU patients in a large group and a comparison to healthy volunteers (HVs) is warranted. Also, a quantitative measure for FAZ irregularity as a marker of ocular involvement in BD has not been explored. Therefore, the purpose of this study was to compare quantitative measurements of the parafoveal microvasculature among patients with BU, patients with NOBD, and HVs.
Methods
Study Design and Population
This prospective observational cross-sectional study was approved by the internal review board of the Rotterdam Eye Hospital and the Medical Research Ethical Committee of the Erasmus University Hospital (Rotterdam, The Netherlands; MEC-2018-050), and it adhered to the tenets of the Declaration of Helsinki. All subjects signed an informed consent prior to participation. Subjects were recruited between July 2018 and June 2019. BU patients were recruited at the uveitis service of the Rotterdam Eye Hospital and patients with NOBD were recruited at the immunology department of Erasmus Medical Center. Behҫet's disease was diagnosed based on the diagnostic criteria of the International Study Group for Behҫet's disease.21 With unilateral involvement, the non-uveitis eye was excluded for analysis. The NOBD group consisted of patients that have never suffered from any form of uveitis. The healthy volunteers were recruited among employees from the Rotterdam Eye Hospital and among the partners and family members of participants in the Behҫet's disease groups. They were gender and age matched to Behҫet's disease participants. The exclusion criterion for all subjects was a refractive error higher than 4 diopters (D).
Study Measurements
All subjects underwent a complete ophthalmic examination, including best-corrected visual acuity (BCVA) measurement, dilated fundus examination, enhanced depth imaging OCT, OCT-A, and split-lamp examination performed by the ophthalmologist. All participants were checked for signs of active uveitis at the time of the study visit, and non-uveitis participants were also checked for signs of past inflammation (posterior synechiae, old vitreous cells, vascular sheathing, chorioretinal scars22).
OCT-A Acquisition and Processing
OCT-A images were acquired with a Spectralis OCT2 (Heidelberg Engineering, Heidelberg, Germany), which has a wavelength of 840 nm and operates at a 40-kHz A-scan rate. The distance between B-scans was 6 µm, and the pattern size (width × height) was 10° × 10° (± 3.0 mm × 3.0 mm), resulting in 512 B-scans per OCT-A image. Scanning was performed by a single operator. Images of both eyes were acquired, but only one eye per participant was included for analysis. The study eye was chosen based on the highest scan quality or, when applicable, on the involvement of uveitis. In case of unilateral involvement in a BU patient, the affected eye was included irrespective of scan quality.
The automated segmentation by the Heidelberg software (version 6.9) of the DCP, ICP, and SVP was manually verified for each OCT-A scan and manually adjusted when needed. The DCP included the outer plexiform layer and the outer half of the inner nuclear layer. The ICP consisted of the inner half of the inner nuclear layer and the outer half of the inner plexiform layer. The SVP included the inner half of the inner plexiform layer and the whole ganglion cell layer.23 The resulting en face images of the DCP, ICP, and SVP and the whole retinal vascular complex (i.e., including the whole ganglion cell layer until the whole outer plexiform layer) were exported for analysis of OCT-A features.
A custom-made algorithm (MATLAB, The MathWorks, Inc., Natick, MA, USA) was used for calculation of VD and the FAZ. VD was the percentage of vessels within the parafoveal area (between concentric circles with a diameter of 1 mm and 2.5 mm centered on the fovea), and the FAZ area was calculated in square millimeters. VD was calculated for the DCP, ICP, and SVP, whereas the FAZ was calculated for the whole retinal vascular complex. Furthermore, we determined irregularity of the FAZ both qualitatively and quantitatively. Qualitative assessment of contour irregularity of the FAZ was performed on all images in a random order by one researcher, who looked at the image and compared the FAZ to an imaginary perfect round circle. The FAZ contour scores could be either regular or irregular. For the quantitative approach, we used the acircularity index,24 which was defined as the ratio of the perimeter (in mm) of the demarcated FAZ to the perimeter of a circle with an area equal to that of the FAZ.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 24 (IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant. Normality of the distribution of the data was evaluated with the Kolmogorov–Smirnov and Shapiro–Wilk tests. A transformation of data was necessary for both VD and FAZ area to attain a reasonably normal distribution. For analysis, transformed data were used; however, to aid in clinical interpretation, the data were back transformed for comprehensive presentation in the figures. Differences in VD among the BU, NOBD, and HV groups were analyzed using a one-way multivariate analysis of covariance (MANCOVA), with scan quality as a covariate. Differences in FAZ area and acircularity index among the groups were separately analyzed using a one-way ANOVA. The qualitative score for irregularity of the FAZ area was compared among the groups using a Kruskall–Wallis test. Spearman's ρ was used to analyze whether BCVA was correlated with the vascular parameters in the BU group, and a partial correlation with age as a covariate was used to determine a correlation between disease duration and the vascular parameters in both BU and NOBD groups. Continuous data were expressed as mean ± SD, and categorical data were expressed as count and percentage.
Results
Demographic Data
Sixty-eight subjects were enrolled in the study, 46 patients with Behҫet's disease and 22 control subjects (HVs). The patients with Behҫet's disease included 23 patients without ocular involvement (NOBD) and 23 patients diagnosed with concomitant uveitis (BU). Two BU patients were excluded for the study due to an inability to acquire OCT-A images. One eye per subject was included for analysis. None of the participants had active ocular inflammation at the time of investigation, and none of the NOBD patients or HVs had any sign of past inflammation. Demographic data are presented in Table 1, and additional information on immunosuppressive medication and ophthalmological features in the BU group is presented in Supplementary Tables S1 and S2. Examples of the OCT-A en face images of BU, NOBD, and HV subjects are shown in Figure 1.
Table 1.
Demographic Data for BU Patients, NOBD Patients, and HV Control Group
| BU (n = 23) | NOBD (n = 23) | HV Control Group (n = 22) | P | |
|---|---|---|---|---|
| Age (y), mean ± SD (range) | 51 ± 10 (27–72) | 48 ± 14 (23–71) | 44 ± 13 (27–74) | 0.25† |
| Gender, n (%) | 0.68‡ | |||
| Male | 12 (52) | 12 (52) | 14 (64) | |
| Female | 11 (48) | 11 (48) | 8 (36) | |
| Disease duration (y), mean ± SD (range) | 17 ± 9 (2–36) | 11 ± 8 (1–31) | NA | 0.070§ |
| BCVA study eye (letters), mean ± SD (range) | 43 ± 9 (1–63) | 59 ± 3 (51–64) | 61 ± 5 (47–70) | <0.001*,† |
| Spherical equivalent, mean ± SD (range) | 0.2 ± 1.0 (–1.8 to 2.5) | –0.3 ± 1.3 (–3.9 to 2.0) | –0.3 ± 1.4 (–3.3 to 1.4) | 0.36† |
| Current immunosuppressive treatment, n (%) | 0.82‡ | |||
| No medication (%) | 3 (13) | 4 (17) | NA | |
| One drug (%) | 9 () | 10 (44) | NA | |
| Two drugs (%) | 7 () | 5 (22) | NA | |
| Three or more drugs (%) | 4 (17) | 4 (17) | NA | |
| Concomitant diseases, n (%) | 0.066‡ | |||
| Hypertension | 4 (17) | 1 (4) | 0 (0) | |
| Diabetes mellitus | 2 (9) | 1 (4) | 0 (0) |
NA, not applicable.
Statistically significant.
One-way ANOVA.
χ2 test.
Independent samples t-test.
Figure 1.
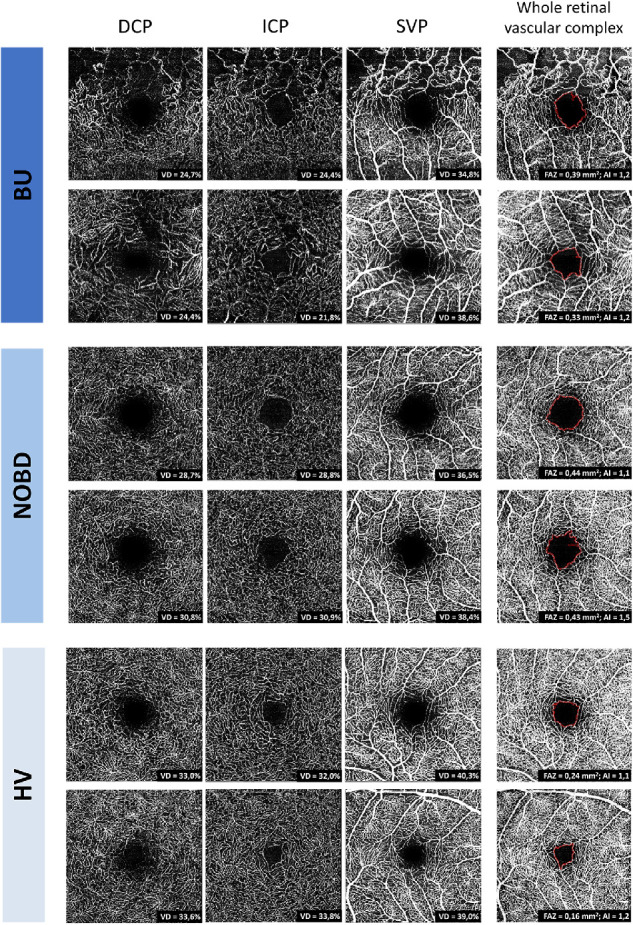
OCT-A en face images of two BU patients (top), two NOBD patients (middle), and two HVs (bottom). The subjects’ ages, from top to bottom, were 53 years and 35 years (BU); 54 years and 32 years (NOBD); and 54 years and 37 years (HVs). Shown from left to right are the DCP, ICP, SVP, and those retinal vascular layers together (whole retinal vascular complex). The corresponding metrics are presented in the images; the VD (%) was calculated in the DCP, ICP, and SVP, and the FAZ area (mm2) and acircularity index (AI) were calculated in the whole retinal vascular complex.
Vessel Density
Figure 2 shows the VD for each group in all three layers. One-way MANCOVA, with DCP, ICP, and SVP VD as dependent variables and OCT-A scan quality as the covariate, showed a statistically significant difference among the groups (P < 0.001). The VD was also statistically significantly different among the groups for each of the retinal vascular layers separately (Table 2). Bonferroni post hoc results are presented in Figure 2. A positive correlation between BCVA and VD in DCP, ICP, and SVP was found in the BU group (Table 3). In the BU and NOBD groups, no correlation was found between disease duration and VD in any of the layers (Table 4).
Figure 2.
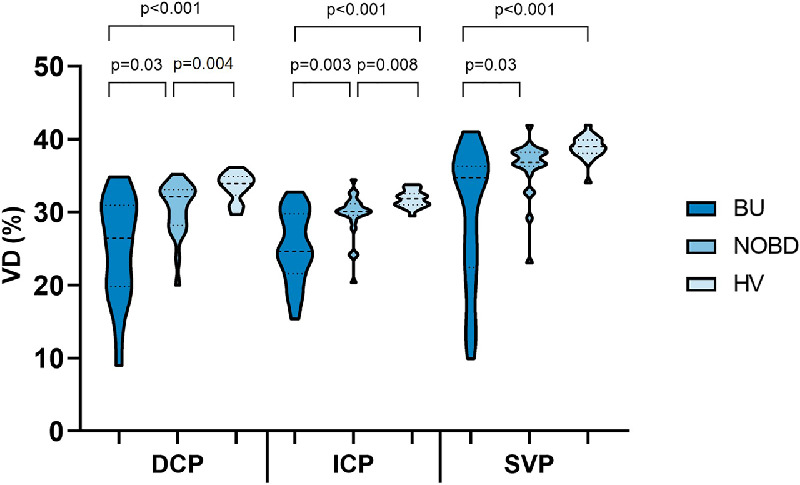
The distribution of VD (%) in the three groups (BU, NOBD, and HV) in the DCP, ICP, and SVP. The dotted lines represent the median and quartiles.
Table 2.
Quantitative OCT-A Features for VD, FAZ Area, and Acircularity Index for BU Patients, NOBD Patients, and HV Control Group
| Mean ± SD | ||||
|---|---|---|---|---|
| BU | NOBD | HVs | P | |
| VD | ||||
| DCP | 25 ± 7 | 30 ± 4 | 33.5 ± 1.9 | <0.001* |
| ICP | 25 ± 5 | 30 ± 3 | 31.8 ± 1.1 | <0.001* |
| SVP | 30 ± 9 | 36 ± 4 | 38.9 ± 1.6 | <0.001* |
| FAZ area (mm2) | 0.5 ± 0.3 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.003* |
| Acircularity index | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.27 |
Statistically significant.
Table 3.
Spearman's ρ Between BCVA and Vascular Parameters in BU Patients
| ρ | P | |
|---|---|---|
| VD | ||
| DCP | 0.76 | <0.001* |
| ICP | 0.80 | <0.001* |
| SVP | 0.52 | 0.02* |
| FAZ area | –0.37 | 0.10 |
| Acircularity index | –0.31 | 0.18 |
| Qualitative FAZ irregularity | –0.62 | 0.003* |
Statistically significant.
Table 4.
Partial Correlation Between Disease Duration and Vascular Parameters With a Correction for Age in BU and NOBD Patients
| BU | NOBD | |||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| VD | ||||
| DCP | –0.05 | 0.85 | –0.40 | 0.06 |
| ICP | –0.03 | 0.91 | –0.28 | 0.20 |
| SVP | 0.02 | 0.92 | –0.23 | 0.30 |
| FAZ area | 0.13 | 0.60 | 0.27 | 0.22 |
| Acircularity index | 0.23 | 0.33 | 0.02 | 0.93 |
| Qualitative FAZ irregularity | 0.18 | 0.46 | 0.17 | 0.46 |
Foveal Avascular Zone
Figure 3 shows the FAZ area and the acircularity index for all three groups. The area of the FAZ was statistically significantly different among the groups (Table 2). A Bonferroni post hoc test showed that there were statistically significant differences in FAZ area between BU and NOBD and between NOBD and HVs (Fig. 3). No statistically significant difference between any of the groups was found for the quantitative FAZ irregularity measurement (i.e., the acircularity index of the FAZ) (Table 2). In contrast, the qualitative FAZ irregularity was significantly different among the groups (P = 0.02). Pairwise comparisons showed that BU differed significantly from HVs (P = 0.004) and from NOBD (P = 0.011). BCVA was negatively correlated with the qualitative FAZ irregularity in BU patients (Table 3), but no significant correlation was found with the acircularity index nor the FAZ area. No correlation was observed between disease duration and both qualitative and quantitative FAZ measurements (Table 4).
Figure 3.
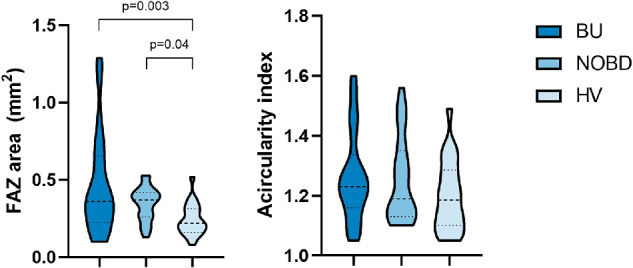
The violin plot on the left shows the distribution of the FAZ area (mm2) of the three groups (BU, NOBD, and HV) in all of the vascular layers combined. The violin plot on the right shows the distribution of the acircularity index of the FAZ for each group. The dotted lines represent the median and quartiles.
Discussion
In this study, quantitative measurements of the parafoveal microvasculature demonstrated that the parafoveal microvasculature is most eminently affected in BU patients, but deviations were also observed in the eyes of NOBD patients. Most alterations in parafoveal microvasculature of Behҫet's disease patients were found using the quantitative vessel density measurement. Also, vessel density was significantly correlated with BCVA in BU patients.
The vessel density was significantly lower in the BU group than in the control group in all three retinal layers, which is in line with results of vessel density or hypoperfusion area measurements in previous studies.6,13,15,17,18 The VD of NOBD patients was significantly different from both the vessel density in BU patients in all three layers and from the VD in healthy control subjects in the deeper retinal layers (DCP and ICP). This suggests that the deeper layers of the retina in NOBD patients deviate more from a normal microcirculation than does the SVP. Previous studies indicate that the DCP is more involved than the SVP in BU patients,13–15 which also suggests that microcirculation in the DCP is more affected by the disease. The DCP and ICP are both thin layers of capillaries, supplied by vertical anastomoses from the SVP, which consists of a mixture of large and small vessels. Because the vasculitis in BD involves all vessel types,2,25 it is less likely that the inflammation itself explains the more extended involvement of retinal capillaries. One possible explanation is that, due to their smaller diameter, capillaries might be more vulnerable to ischemia and subsequent drop-out. On the other hand, as blood flow velocity in normal capillaries is slower than in larger vessels,26 blood flow velocity in diseased capillaries may be decreased toward a level under the minimal detectable blood flow velocity of OCT-A.
We demonstrated that the FAZ area was significantly larger in both uveitic and non-uveitic Behҫet's disease patients compared to the FAZ area of healthy volunteers. Our results are consistent with previous studies that compared FAZ area between BU patients and HVs.13,17 For the comparison of FAZ area between NOBD patients and HVs, other studies have reported inconsistent results. Similar to our results, Goker et al.19 found FAZ enlargement, whereas three other studies8,18,20 did not find a significant difference in FAZ area between NOBD patients and HVs. These inconsistent reports on FAZ area differences between NOBD eyes and healthy eyes could be explained by the high between-subject variability of FAZ area measurements in healthy eyes.27–30 Another explanation for the discrepancies in FAZ area findings is the difference in FAZ definition. Raafat et al.20 only analyzed the FAZ area in the SVP, and Comez et al.8 looked at the FAZ area in the superficial and deep plexus separately. However, it is more logical to analyze the FAZ area including all retinal vascular plexuses together, as the three retinal vascular layers merge at the edge of the FAZ and therefore define the borders of the FAZ together.23,31 Another bias can be found in the FAZ data reported by Koca et al.,18 who included 94 eyes from 49 Behҫet's disease patients, of which 43 eyes had ocular involvement, and one eye of 53 healthy controls. The fellow eyes of uveitic patients were included in their NOBD group. It may be assumed that microvasculature in the fellow eyes of unilateral BU patients will be affected more severely despite the lack of clinical inflammation, compared to the eyes of NOBD subjects. Hence, these fellow eyes cannot be classified in the NOBD group. To present the most unbiased data as possible, we chose to increase the number of patients per group, included only one eye per subject in all three groups, and combined all retinal capillary layers to define the FAZ.
Koca et al.18 suggested exploring the irregularity of the FAZ, as it might be a more valuable marker than FAZ area to indicate ocular involvement. They found a significant difference in FAZ irregularity between BU patients and healthy control subjects and found FAZ irregularity to be correlated with a lower BCVA in BU patients. Our qualitative analysis of FAZ irregularity showed similar outcomes. We also performed a quantitative measurement, the acircularity index, which quantifies the deviation of the FAZ perimeter from a perfectly round circle,24 but this did not differ significantly among the three groups. Furthermore, this acircularity index was not significantly correlated with BCVA in BU patients, whereas the qualitative FAZ irregularity measurement was (Table 3). Therefore, we still consider FAZ irregularity to be a potentially valuable marker for ocular involvement, but this irregularity is not quantified adequately by this acircularity index. Table 3 shows that BCVA in BU patients is also significantly correlated with vessel density in DCP, ICP, and SVP. Therefore, this quantitative marker is superior to quantitative FAZ measurements as an indicator of ocular involvement.
Several hypotheses can be proposed to explain the occurrence of preclinical retinal vascular alterations in NOBD patients. First, most clinical manifestations in Behҫet's disease are attributed to vascular involvement, although sensitivity is in general low for detecting those vascular abnormalities.20,32 Uveitis is in many patients one of the presenting symptoms of Behҫet's disease; thus, we suspect that subclinical retinal vascular damage can also occur at the onset of the disease without symptomatic ophthalmic inflammation.21 With OCT-A, we were indeed able to detect subclinical deviations in the retinal microcirculation of NOBD patients. Second, we hypothesized that duration of disease activity in NOBD patients could correlate with progressive worsening of the retinal vasculature abnormalities, as this was also described in previous literature for BU patients.17 After correcting for age, however, we did not find a correlation between disease duration and any of the OCT-A variables in either uveitic or non-uveitic Behҫet patients (Table 4). Because vessel density is known to decrease with age,33 this may be an important and overlooked bias in previous reports. Another possibility is that preclinical retinal microvascular alteration in NOBD patients predict future development of uveitis; however, this hypothesis can only be tested with a longitudinal cohort study in NOBD patients to evaluate whether a first uveitis event occurs.
Because the diagnosis of Behҫet's disease currently relies on clinical signs and symptoms, it remains uncertain in many cases. In addition, diagnosis may be delayed because clinical manifestations can present asynchronously.21,25 Quantitative measurements to confirm diagnosis would possibly eliminate or confirm some of those suspected Behҫet's disease cases. Furthermore, because not all Behҫet's disease patients have ocular involvement, quantitative measurements could perhaps be of help in predicting future ocular events. OCT-A, through quantitative parameters such as VD estimations, has the potential to fulfill a role as an additional diagnostic tool in the future. However, to get to this point, robust longitudinal follow-up studies are needed to build a reliable Behҫet's disease database so that we can better define which OCT-A quantitative measurements are most sensitive for the detection of Behҫet's disease and which factors (e.g., age, scan quality) most influence these quantitative outcomes measurements.
This study has several strengths. As mentioned before, we aimed to present the most unbiased data as possible by including a high number of subjects and only one eye per subject per group, in addition to combining all retinal capillary layers to define the FAZ. Previous studies have already shown that the deep vascular complex is affected in BU patients,13,14 but our data indicate that microvascular alterations occur in both the DCP and ICP separately in BU and NOBD patients. Our effort to analyze all capillary plexuses was strengthened by the findings of Hirano et al.34 suggesting the presence of distinct vascular morphologies in the DCP and ICP. Furthermore, the study by Koca et al.18 is the only other one that has included BU, NOBD, and HV groups, but, as mentioned earlier, their findings are potentially biased.
A limitation of our study is that axial length was not measured. Axial length variation in subjects may induce image size magnification of the OCT-A en face slabs and could therefore affect the VD and FAZ measurements.35 Because the spherical equivalent, which is correlated with axial length,36 was not significantly different between our groups (Table 1), we feel that this omission has not affected our measurements.
In conclusion, parafoveal microvasculature damage as a result of concurrent subclinical vasculitis occurs in all Behҫet's disease patients, even in those without clinical signs of uveitis. Most deviations in the retinal microcirculation in Behҫet's disease patients were found in the deeper layers of the retina by using the quantitative vessel density measurement.
Supplementary Material
Acknowledgments
Supported by Stichting Wetenschappelijk Onderzoek Oogziekenhuis, Rotterdam, The Netherlands (2017S08).
Disclosure: L.M. Smid, None; K.A. Vermeer, None; T.O.A.R. Missotten, None; J.A.M. van Laar, None; M.E.J. van Velthoven, None
References
- 1. Unoki N, Nishijima K, Kita M, Hayashi R, Yoshimura N. Structural changes of fovea during remission of Behçet's disease as imaged by spectral domain optical coherence tomography. Eye (Lond) . 2010; 24(6): 969–975. [DOI] [PubMed] [Google Scholar]
- 2. Tugal-Tutkun I, Ozdal PC, Oray M, Onal S. Review for diagnostics of the year: multimodal imaging in Behçet uveitis. Ocul Immunol Inflamm . 2017; 25(1): 7–19. [DOI] [PubMed] [Google Scholar]
- 3. Kappen J, van Dijk E, Baak-Dijkstra M, et al.. Behçet's disease, hospital-based prevalence and manifestations in the Rotterdam area. Neth J Med . 2015; 73(10): 471–477. [PubMed] [Google Scholar]
- 4. Kim M, Kim H, Kwon HJ, Kim SS, Koh HJ, Lee SC. Choroidal thickness in Behçet's uveitis: an enhanced depth imaging-optical coherence tomography and its association with angiographic changes. Invest Ophthalmol Vis Sci . 2013; 54(9): 6033–6039. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham ET Jr, Tugal-Tutkun I, Khairallah M, Okada AA, Bodaghi B, Zierhut M. Behçet uveitis. Ocul Immunol Inflamm . 2017; 25(1): 2–6. [DOI] [PubMed] [Google Scholar]
- 6. Emre S, Güven-Yılmaz S, Ulusoy MO, Ateş H. Optical coherence tomography angiography findings in Behçet patients. Int Ophthalmol . 2019; 39(10): 2391–2399. [DOI] [PubMed] [Google Scholar]
- 7. Van Laar J, Missotten T, Van Daele P, Jamnitski A, Baarsma G, Van Hagen P. Adalimumab: a new modality for Behçet's disease? Ann Rheum Dis . 2007; 66(4): 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Comez A, Beyoglu A, Karakucuk Y. Quantitative analysis of retinal microcirculation in optical coherence tomography angiography in cases with Behçet's disease without ocular involvement. Int Ophthalmol . 2019; 39(10): 2213–2221. [DOI] [PubMed] [Google Scholar]
- 9. Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol . 2015; 133(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 10. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res . 2018; 64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Oliveira PR, Berger AR, Chow DR. Optical coherence tomography angiography in chorioretinal disorders. Can J Ophthalmol . 2017; 52(1): 125–136. [DOI] [PubMed] [Google Scholar]
- 12. de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous . 2015; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khairallah M, Abroug N, Khochtali S, et al.. Optical coherence tomography angiography in patients with Behçet uveitis. Retina . 2017; 37(9): 1678–1691. [DOI] [PubMed] [Google Scholar]
- 14. Somkijrungroj T, Vongkulsiri S, Kongwattananon W, Chotcomwongse P, Luangpitakchumpol S, Jaisuekul K. Assessment of vascular change using swept-source optical coherence tomography angiography: a new theory explains central visual loss in Behçet's disease. J Ophthalmol . 2017; 2017: 2180723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Accorinti M, Gilardi M, De Geronimo D, Iannetti L, Giannini D, Parravano M. Optical coherence tomography angiography findings in active and inactive ocular Behçet disease. Ocul Immunol Inflamm . 2020; 28(4): 589–600. [DOI] [PubMed] [Google Scholar]
- 16. Cheng D, Shen M, Zhuang X, et al.. Inner retinal microvasculature damage correlates with outer retinal disruption during remission in Behçet's posterior uveitis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci . 2018; 59(3): 1295–1304. [DOI] [PubMed] [Google Scholar]
- 17. Turkcu FM, Sahin A, Karaalp U, et al.. Automated quantification of foveal avascular zone and vascular density in Behçet's disease. Ir J Med Sci . 2020; 189(1): 349–354. [DOI] [PubMed] [Google Scholar]
- 18. Koca S, Onan D, Kalayci D, Alli N. Comparison of optical coherence tomography angiography findings in patients with Behçet's disease and healthy controls. Ocul Immunol Inflamm . 2020; 28(5): 806–813. [DOI] [PubMed] [Google Scholar]
- 19. Goker YS, Yılmaz S, Kızıltoprak H, Tekin K, Demir G. Quantitative analysis of optical coherence tomography angiography features in patients with nonocular Behçet's disease. Curr Eye Res . 2018; 44(2): 212–218. [DOI] [PubMed] [Google Scholar]
- 20. Raafat KA, Allam RSHM, Medhat BM. Optical coherence tomography angiography findings in patients with Behçet disease. Retina . 2019; 39(8): 1607–1612. [DOI] [PubMed] [Google Scholar]
- 21. Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet . 1990; 335(8697): 1078–1080. [PubMed] [Google Scholar]
- 22. van Laar JAM, van Velthoven MEJ, Missotten T, Kuijpers RWAM, van Hagen PM, Rothova A. Diagnosis and treatment of uveitis; not restricted to the ophthalmologist. Ned Tijdschr Geneeskd . 2013; 157(38): A5703. [PubMed] [Google Scholar]
- 23. Campbell JP, Zhang M, Hwang TS, et al.. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep . 2017; 7: 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krawitz BD, Mo S, Geyman LS, et al.. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res . 2017; 139: 177–186. [DOI] [PubMed] [Google Scholar]
- 25. Evereklioglu C. Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol . 2005; 50(4): 297–350. [DOI] [PubMed] [Google Scholar]
- 26. Lee PJ, Peyman GA. Visualization of the retinal and choroidal microvasculature by fluorescent liposomes. Methods Enzymol . 2003:373: 214–233. [DOI] [PubMed] [Google Scholar]
- 27. Odabas ÖY, Demirel S, Özmert E, Batioglu F. Repeatability of automated vessel density and superficial and deep foveal avascular zone area measurements using optical coherence tomography angiography: diurnal findings. Retina . 2018; 38(6): 1238–1245. [DOI] [PubMed] [Google Scholar]
- 28. Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol . 2016; 100(5): 671–676. [DOI] [PubMed] [Google Scholar]
- 29. Samara WA, Say EA, Khoo CT, et al.. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina . 2015; 35(11): 2188–2195. [DOI] [PubMed] [Google Scholar]
- 30. Lupidi M, Coscas F, Cagini C, et al.. Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am J Ophthalmol . 2016; 169: 9–23. [DOI] [PubMed] [Google Scholar]
- 31. Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci . 2017; 58(12): 5548–5555. [DOI] [PubMed] [Google Scholar]
- 32. Owlia MB, Mehrpoor G. Behçet's disease: new concepts in cardiovascular involvements and future direction for treatment. ISRN Pharmacol . 2012; 2012: 760484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin Y, Jiang H, Liu Y, et al.. Age-related alterations in retinal tissue perfusion and volumetric vessel density. Invest Ophthalmol Vis Sci . 2019; 60(2): 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirano T, Chanwimol K, Weichsel J, Tepelus T, Sadda S. Distinct retinal capillary plexuses in normal eyes as observed in optical coherence tomography angiography axial profile analysis. Sci Rep . 2018; 8(1): 9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sampson DM, Gong P, An D, et al.. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci . 2017; 58(7): 3065–3072. [DOI] [PubMed] [Google Scholar]
- 36. Kato K, Kondo M, Takeuchi M, Hirano K.. Refractive error and biometrics of anterior segment of eyes of healthy young university students in Japan. Sci Rep . 2019; 9(1): 15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


