Although kidney transplant (KT) success has largely been attributed to improvements in 1-year allograft survival, improvements in long-term survival have lagged behind,1 making allograft failure the fourth most common cause of kidney failure in the USA.2
Post-KT glomerulonephritis (GN), typically classified under nonalloimmune etiologies, is a significant cause of allograft failure.3 It can be classified as recurrent, de novo, and very rarely donor derived. Despite the presumed high incidence of de novo GN,4 its natural history and pathogenesis remain poorly understood. Some studies have demonstrated that a proportion of allograft biopsies with de novo GN have concurrent T cell–mediated rejection or antibody-mediated rejection (AMR).5, 6, 7, 8, 9 We conducted this study to understand the clinicopathologic correlates of de novo GN with a particular focus on its possible association with alloimmunity. We retrospectively studied 46 cases of de novo GN and compared them with 77 cases of recurrent GN, which served as post-KT GN controls.
Results
Patient, Clinical, Laboratory, and Transplant Characteristics
De Novo GN
Eighty percent of the patients were male,11% were African American, and 52% received a deceased donor transplant. Patients had a median age of 52 years (interquartile range 33, 66) at the time of transplantation (Table 1). The median time to development of de novo GN after transplantation was 4.3 years. Eleven percent had a simultaneous kidney and other solid organ transplant, and 35% had a prior organ transplant (Table 1). As demonstrated in Table 2, 55% of patients received anti-thymocyte globulin for induction immunosuppression, whereas 34% received an interleukin-2 receptor antagonist. Fourteen percent had positive donor-specific antibodies (DSAs) at the time of transplantation, and 37% had positive DSAs at the time of de novo GN diagnosis. Seventeen percent of biopsies at de novo GN diagnosis were diagnostic of AMR (Table 3).
Table 1.
Demographic characteristics of the study population
| Characteristics of the Study Population | De novo GN (n = 46) | Recurrent GN (n = 77) | P value |
|---|---|---|---|
| Male | 37/46 (80.4) | 55/77 (71.4) | 0.3 |
| African American | 5/46 (10.9) | 9/77 (11.7) | >0.9 |
| Deceased donor | 24/46 (52.2) | 24/77 (31.2) | 0.02 |
| Age at the time of transplantationa | 52.3 (33.4, 65.5) | 37.5 (32.9, 50.6) | 0.003 |
| Years from transplantation to GNa | 4.3 (2.2, 7.4) | 2.7 (0.2, 8.2) | 0.06 |
| Simultaneous organ transplant | 5/46 (10.9) | 0/77 (0) | 0.006 |
| Prior solid organ transplant | 16/46 (34.7) | 14/77 (18.2) | 0.05 |
| Kidney Transplant | 10/46 (21.7) | 14/77 (18.2) | 0.8 |
| Nonkidney solid organ transplant | 6/46 (13.0) | 0/77 (0) | 0.002 |
GN, glomerulonephritis.
Unless otherwise noted, values are n (%).
Values are median (25th, 75th percentile).
Table 2.
Clinical and pathologic characteristics of the study population
| Clinical and Pathologic Characteristics | De novo GN (n = 46) | Recurrent GN (n = 77) | P value |
|---|---|---|---|
| Induction agenta | |||
| Antithymocyte globulin | 24/44 (54.6) | 46/68 (67.7) | 0.2 |
| Alemtuzumab | 5/44 (11.4) | 11/68 (16.2) | 0.6 |
| Interleukin 2 receptor antagonists | 15/44 (34.1) | 10/68 (14.7) | 0.02 |
| Total HLA mismatch (0–6)b,c | 4 (2, 5) | 3 (2, 5) | 0.2 |
| DSAd | |||
| Pre-KT DSAs | 5/36 (13.9) | 8/64 (12.5) | >0.9 |
| DSAs at time of GN discovery | 15/41 (36.6) | 10/59 (17.0) | 0.03 |
| Glomerulonephritis at index biopsy | |||
| Anti-GBM GN | 0/46 (0) | 1/77 (1) | >0.9 |
| C3 glomerulopathy | 0/46 (0) | 9/77 (12) | 0.03 |
| IgA nephropathy | 11/46 (24) | 46/77 (60) | 0.0002 |
| Membranous nephropathy | 8/46 (17) | 14/77 (18) | >0.9 |
| HCV-related GN | 4/46 (9) | 0/77 (0) | 0.02 |
| Infection related GN | 2/46 (4) | 0/77 (0) | 0.1 |
| ICGN-NOS | 21/46 (46) | 7/77 (9) | <0.0001 |
| Graft outcomes | |||
| Graft failure | 11/46 (23.9) | 21/77 (27.3) | 0.8 |
| Time from KT to graft failure, yrc | 7.9 (4.5, 12.7) | 6.8 (4.9, 12.2) | 0.9 |
| Time from GN discovery to graft failure, yrc | 1.4 (0.2, 3.5) | 1.2 (0.7, 2.6) | 0.7 |
| Last creatinine if graft has not failedc | 1.8 (1.3, 2.5) | 1.7 (1.4 2.6) | 0.8 |
Anti-GBM, anti–glomerular basement membrane; DSA, donor-specific antibody; GN, glomerulonephritis; HCV, hepatitis C virus; HLA, human leukocyte antigen; IGCN-NOS, immune complex–mediated glomerulonephritis not otherwise specified; KT, kidney transplant.
Unless otherwise noted, values are n (%).
HLA mismatch is calculated based on A, B, and DR antigens.
Induction agent is unknown in 2 subjects in the de novo group and in 9 subjects in the recurrent group. One patient in the recurrent group did not receive any induction therapy as the transplant was from a twin sibling.
HLA typing is missing in 2 patients in the de novo group and in 7 patients in the recurrent group.
Values are median (25th, 75th percentile).
DSAs at the time of transplant is missing in 10 patients in the de novo group and in 13 patients in the recurrent group. DSAs at the time of index biopsy is missing in 5 patients in the de novo group and in 18 patients in the recurrent group.
Table 3.
The association between GN and rejection
| Rejection Type |
De novo GN,n(%) (n = 46) |
Recurrent GN, n (%) (n = 77) |
P value |
|---|---|---|---|
| Concurrent AMR | 8/46 (17.4) | 2/77 (2.6) | 0.006 |
| Concurrent TCMR or borderline | 11/46 (23.9) | 20/77 (26.0) | 0.8 |
| Previous AMR | 3/46 (6.5) | 2/77 (2.6) | 0.4 |
| Previous TCMR or borderline | 11/46 (23.9) | 11/77 (14.3) | 0.2 |
AMR, antibody-mediated rejection; GN, glomerulonephritis; TCMR, T cell–mediated rejection.
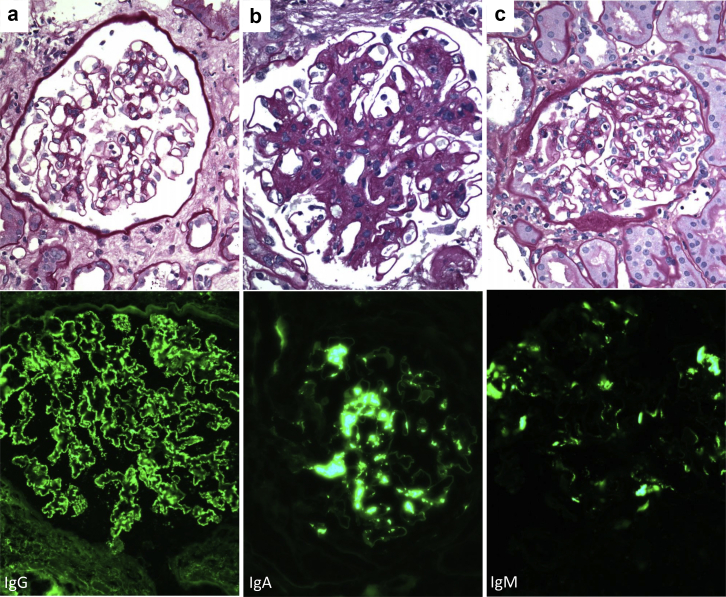
The different GN types diagnosed in allograft biopsies are reported in Table 2. Twenty-one patients in the de novo group had immune complex–mediated glomerulonephritis (ICGN) that did not fit a specific GN, which was the most common form of de novo GN. These ICGN, which included large numbers of membranoproliferative glomerulonephritis of unknown etiology, were classified as ICGN not otherwise specified (ICGN-NOS) (Figure 1). Others included IgA nephropathy (n = 11) and membranous nephropathy (n = 8) (Figure 1). De novo GN also included 4 cases of hepatitis C virus–related GN and 2 infection-related GN (Table 2).
Figure 1.
Representative photomicrographs of de novo GN of the kidney allograft. (a) De novo membranous nephropathy from a patient showing C4d-positive antibody-mediated rejection: from top to bottom, a normocellular glomerulus with unremarkable basement membranes but mild prominence of visceral epithelial cells and a few marginating leukocytes in the capillary Lumina (periodic acid–Schiff, original magnification × 400). This was associated with global granular staining for IgG along glomerular basement membranes in a subepithelial distribution (immunofluorescence, original magnification × 400). (b) De novo IgA nephropathy in a patient without features of antibody-mediated rejection who developed native kidney failure secondary to Alport’s syndrome: from top to bottom, a glomerulus showing mesangial expansion and proliferation (periodic acid–Schiff, original magnification × 600). This was associated with global granular to confluent staining for IgA in the mesangium (immunofluorescence, original magnification × 400). (c) De novo IgM-dominant immune complex–mediated glomerulonephritis not otherwise specified from a patient showing C4d-positive antibody-mediated rejection: from top to bottom, a glomerulus showing mesangial proliferation and scattered leukocytes within the glomerular capillary lumina (periodic acid–Schiff, original magnification × 400). This was associated with global granular to confluent staining for IgM in the mesangium (immunofluorescence, original magnification × 400). GN, glomerulonephritis.
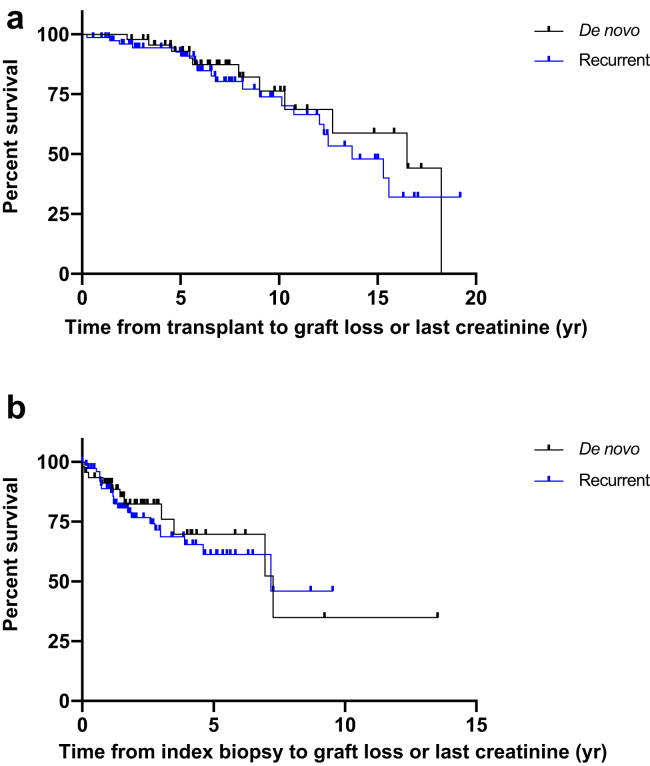
Graft failure occurred in 24% of patients at a median of 7.9 years from transplantation (interquartile range 4.5, 12.7), and at a median of 1.4 years (0.2, 3.5) from de novo GN diagnosis (Table 2, Figure 2). The cause of graft failure was attributed to the de novo GN in 5 of 11 (46%) of patients, and to concomitant rejection and de novo GN in 2 of 11 (18%) of patients. Two patients lost their allograft due to diabetic nephropathy and 1 due to nonresolving acute tubular necrosis.
Figure 2.
(a) Kaplan-Meier curve estimating the survival probability from transplant to graft loss or last follow up. Comparison between de novo and recurrent groups shows a hazard ratio (HR) equal to 0.8, 95% confidence interval (CI): 0.4–1.6. (b) Kaplan-Meier curve estimating the survival probability from index biopsy to graft loss or last follow-up. Comparison between de novo and recurrent groups shows an HR equal to 0.8, 95% CI: 0.4–1.7.
Recurrent GN and Comparison Between Groups
Given the relatively increased frequency of patients with DSAs, we decided to further explore the potential association of de novo GN and alloimmunity by comparing de novo GN to a group of 77 patients with recurrent GN. The different recurrent GN types are reported in Table 2. Demographic and clinical characteristics of recurrent GN are presented in Tables 1 and 2.
A higher percentage of patients with de novo GN received allograft from deceased donors (52% vs. 31%, odds ratio [OR] = 2.41, P = 0.02) and interleukin-2 receptor antagonist for induction immunosuppression (34% vs. 15%, OR = 3.0, P = 0.02). Patients with de novo GN were more likely to have a history of prior solid organ transplants (35% vs. 18%, OR = 2.4, P = 0.05). There was no difference between groups in rates of pretransplant DSAs, T cell–mediated rejection, or AMR preceding the index biopsy, nor in T cell–mediated rejection at the time of index biopsy. However, at the time of GN diagnosis, de novo GN showed increased incidence of AMR (17.4% vs. 2.6%, OR = 7.9, P = 0.006) (Table 3) as well as concurrent DSAs (37% vs. 17%, OR = 2.8, P = 0.03) (Table 2). The latter became more obvious when patients with known cause of de novo GN, namely, infection-related (n = 2) and hepatitis C virus–associated (n = 4), were excluded from the analysis (14/28 [50%] vs. 10/59 [17%], OR = 4.9, P = 0.002). Finally, there was no difference in post-transplant or postbiopsy allograft survival between these 2 groups (Figure 2).
Discussion
GN, both recurrent and de novo, is a significant cause of allograft failure.2 The incidence of de novo post-KT GN is much higher than that of native kidney GN.4 In most forms of GN, injury occurs secondary to the formation of antigen-antibody immune complexes formed by ligation of an immunoglobulin to an in situ or circulating antigen, often triggering complement activation and leukocyte influx.S1 In the field of KT, most AMR is mediated by DSAs, which bind to kidney donor alloantigens and can trigger complement fixation, leukocyte influx, and rejection. Moreover, following stem cell transplantation, alloimmunity in the form of graft-versus-host disease can manifest as ICGN that can mimic autoimmune GN both in human and in animal models.S2–S4 Based on the limited but interesting data in the literature, we hypothesized that a portion of de novo GN in the allograft is associated with alloimmunity.
The findings of ICGN and rejection have been described in a rat model of AMR. Kidneys harvested at 9 days and at 6 weeks after AMR had similar histopathologic findings to human kidney allograft AMR findings whereas those harvested at 26 weeks showed ICGN.S5 Giannico et al.S6 described the clinical and histopathologic features of 28 allograft biopsies with mesangial immune complex deposits. They found the biopsies to be associated with concurrent acute T cell–mediated rejection (P = 0.023) as compared to transplant controls without immune complex deposition. Interestingly, 54% of their patients with ICGN had detectable DSAs. Lloyd et al.8 also identified 32 patients with de novo ICGN in the allograft. Of those, 37% had ICGN-NOS. This group had a high frequency of DSA positivity (88%) and a high incidence of concurrent AMR (67%). Recently, Chin et al.9 identified 28 patients with allograft biopsies showing ICGN-NOS. Fifty-seven percent of those patients met criteria for definite or possible allograft rejection, including 43% with features of humoral alloimmunity, and 63% had detectable DSAs.
Our study is the largest to date to examine the association between de novo GN and alloimmunity and the first to use recurrent disease as post-transplant GN controls. Although the incidence of AMR and DSAs in our study was lower than the 2 aforementioned studies (17% and 37%, respectively), when we compared our 46 patients with post-KT de novo GN to 77 patients with recurrent GN, we found data suggestive of an alloimmune mechanism underlying the development of de novo GN, including a higher concurrent rate of AMR, higher DSAs at the time of diagnosis, a higher number of previous solid organ transplants, a higher frequency of allografts from deceased donors, and a less potent induction therapy.
Our study has a number of limitations, including the retrospective nature of the study and missing laboratory studies. Our center does not perform yearly protocol biopsies, which may underestimate the true incidence of both de novo and recurrent GN.
Our results support that a significant proportion of de novo GN may be related to humoral alloimmunity. This relationship deserves further investigation in order to elucidate the underlying pathophysiology and associated risk factors in order to develop strategies to prolong allograft survival.
Acknowledgments
IB is supported by a grant from the American Society of Transplantation (AST) Research Network.
Disclosure
The authors declared no competing interests.
Author Contributions
OK and IB participated in the research design, data collection, data analysis and writing of the manuscript. JK, GS, and RV participated in data collection. RJC participated in the writing of the manuscript. GKD participated in the research design and writing of the manuscript.
Footnotes
Supplementary Material
Supplementary Materials and Methods
Supplementary References
References
- 1.Meier-Kriesche H.U., Schold J.D., Srinivas T.R., Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C. US Renal Data System 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(Suppl 1):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen P.J., Chadban S.J., Craig J.C. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92:461–469. doi: 10.1016/j.kint.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Lim W.H., Shingde M., Wong G. Recurrent and de novo glomerulonephritis after kidney transplantation. Front Immunol. 2019;10:1944. doi: 10.3389/fimmu.2019.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel K., Hirsch J., Beck L., Herlitz L., Radhakrishnan J. De novo membranous nephropathy in renal allograft associated with antibody-mediated rejection and review of the literature. Transplant Proc. 2013;45:3424–3428. doi: 10.1016/j.transproceed.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Honda K., Horita S., Toki D. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011;25:191–200. doi: 10.1111/j.1399-0012.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 7.Batal I., Vasilescu E.R., Dadhania D.M. Association of HLA typing and alloimmunity with posttransplantation membranous nephropathy: a multicenter case series. Am J Kidney Dis. 2020;76:374–383. doi: 10.1053/j.ajkd.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd I.E., Ahmed F., Revelo M.P., Khalighi M.A. De novo immune complex deposition in kidney allografts: a series of 32 patients. Hum Pathol. 2018;71:109–116. doi: 10.1016/j.humpath.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Chin K.K., Charu V., O’Shaughnessy M.M. Histologic case definition of an atypical glomerular immune-complex deposition following kidney transplantation. Kidney Int Rep. 2020;5:632–642. doi: 10.1016/j.ekir.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.