Introduction
Targeted radionuclide therapies (TRNT) are novel cancer treatments that deliver radiation selectively to cancer cells and the immediate tumor microenvironment. These agents consist of radiation-emitting radionuclides conjugated with monoclonal antibodies, peptides, or small molecules, each of which are able to specifically target tumor-associated antigens and hence allow for delivery of radiation directly, and almost exclusively, to tumor cells. The radiation emitted by these radionuclide particles causes DNA damage, triggering cancer cell death.
Prostate-specific membrane antigen (PSMA) is a transmembrane protein expressed at high levels in prostatic cancer, compared with the low levels of expression seen in a variety of tissue, including normal prostate and kidneys.1 Even higher levels are seen in metastatic and castration-resistant disease, allowing for targeted therapy. Radionuclide agents targeting PSMA include alpha (e.g., actinium-225) and beta emitters (e.g., lutetium-177). Alpha particle emissions like actinium-225 (225Ac) are more effective in targeted killing of tumor cells compared with beta emitters, due to greater energy generated, coupled with shorter path length.2 Unfortunately, kidney toxicity may result from tubular nuclide accumulation, intrinsic affinity of actinium for the kidney, and low-level kidney PSMA expression.2,3,S1 As seen in animal models, the multiple alpha particles generated in the decay chain of 225Ac, termed daughter nuclides, can accumulate in the kidney and lead to a progressive nephropathy.4
Promising therapeutic efficacy has been demonstrated with 225Ac-PSMA617 in patients with metastatic castration-resistant prostate cancer (mCRPC).5,S2 There are few data, however, on clinical renal toxicity associated with 225Ac-PSMA617. We report our experience with 225Ac-PSMA617 therapy in 2 patients with mCRPC who received these novel agents and developed progressive kidney disease.
Case Reports
Patient 1 was a 68-year-old man with mCRPC with metastases to the bones, pleura, pelvis, and retroperitoneal lymph nodes. He had chronic bilateral hydronephrosis secondary to his underlying malignancy and had required bilateral nephrostomy tubes since 2016. His initial cancer diagnosis was in 2005 for which he underwent radical prostatectomy. Prior oncological treatments included androgen-deprivation therapy, abiraterone, flutamide, ketoconazole, and multiple chemotherapy lines including docetaxel, carboplatin, and cabazitaxel. Past medical history was significant for impaired glucose tolerance as well as 2 episodes of sepsis with acute kidney injury in the year before initiation of TRNT.
The patient received 3 cycles of 225Ac-PSMA617 in 2-month intervals starting in July 2017. The patient traveled to Heidelberg, Germany, to receive this therapy, as it is not available in Canadian institutions. Baseline serum creatinine was 1.6 mg/dl (estimated glomerular filtration rate [eGFR] 44 ml/min per 1.73 m2) and it increased up to 3.3 mg/dl (eGFR 18 ml/min per 1.73 m2) after 225Ac-PSMA617 therapy. Routine urine analysis showed microscopic hematuria and leukocyturia. Urinary protein-to-creatinine ratio was 204 mg/mmol of creatinine. Computed tomography scan revealed no hydronephrosis with nephrostomies in adequate position. The patient did not receive any other nephrotoxic medication during this period, and was on no concomitant chemotherapy.
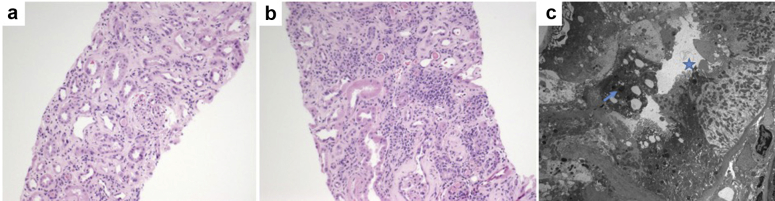
A kidney biopsy was performed (Figure 1). On light microscopy, there were 23 glomeruli, 6 sclerosed, and remaining with no increase in matrix or cellularity, although many were shrunken, including some with periglomerular fibrosis. Nonatrophic tubules showed reactive atypia amidst focal mild inflammation and mild tubulitis (Figure 1a), while diffuse lymphoplasmacytic inflammation was present amidst atrophic tubules (Figure 1b). Interstitial fibrosis/tubular atrophy was severe. An arcuate artery showed severe sclerosis. There was no arterial intimal edema, thrombi, or vasculitis. Staining for immunoglobulin and complement was negative. Two glomeruli were examined by electron microscopy, which showed wrinkled basement membranes, but no subendothelial widening or endothelial cell swelling, mild foot process effacement, a few tubuloreticular inclusions, and no deposits. The tubules showed brush border loss and mottled lysosomes (Figure 1c).
Figure 1.
Kidney biopsy of patient 1. (a) Nonatrophic tubules showed reactive atypia amidst focal mild inflammation and mild tubulitis. Hematoxylin and eosin (H&E) stain, original magnification ×100. (b) Diffuse lymphoplasmacytic inflammation was present amidst atrophic tubules. H&E stain, original magnification ×200. (c) Electron microscopy showing tubular brush border loss (star) and mottled lysosomes (arrow). Original magnification ×3000.
A trial of corticosteroids (daily prednisone 1 mg/kg) was attempted but there was no improvement in kidney function and this therapy was progressively tapered 4 weeks after its introduction. The 225Ac-PSMA617 therapy was discontinued because of related kidney failure. Serum creatinine was 3.0 mg/dl (eGFR 20 ml/min per 1.73 m2) on last follow-up in December 2018. Patient 1 died from cancer progression and stroke complications in January 2019.
Patient 2 was a 67-year-old man with mCRPC with brain, hepatic, skeletal, and pulmonary metastasis as well as retroperitoneal lymphadenopathy and involvement of the left kidney. His cancer was initially diagnosed in 2004, for which the patient underwent radical prostatectomy followed by radiation therapy. Prior oncological treatment included androgen-deprivation therapy, abiraterone, enzalutamide, docetaxel, cabazitaxel, PI3K inhibitor as a part of a clinical trial (AZD8186), and mammalian target of rapamycin inhibitor (AZD2014) as part of a clinical trial. Comorbidities included chronic hypertension, well controlled with perindopril and amlodipine, and dyslipidemia. It should be noted that the patient had a biopsy-proven infiltrative metastatic left kidney lesion that extended into the collecting system since 2016. In February 2017, mild progression of left hydronephrosis was noted, but remained stable since and throughout TRNT therapy.
In May 2017, he initiated TRNT therapy with 177Lu-PSMA617. In April 2018, due to treatment resistance, the patient was started on tandem therapy with 177Lu-PSMA617 and 225Ac-PSMA617 in 2-month intervals. This patient also received treatment externally in Heidelberg, Germany, similar to patient 1. The patient received 5 cycles of 225Ac-PSMA617 in total, the last round being complicated with grade 3 cytopenias leading to cessation of treatment. Baseline serum creatinine at initiation of TRNT was 1.2 mg/dl (eGFR 64 ml/min per 1.73 m2). The patient subsequently developed progressive chronic kidney disease (CKD) and serum creatinine was 1.9 mg/dl (eGFR 35 ml/min per 1.73 m2) on last follow-up in December 2019. Urinary protein-to-creatinine ratio was 32 mg/mmol of creatinine. Urine analysis showed no hematuria and no leukocyturia. The patient remained on angiotensin-converting enzyme inhibitor medication (with adequate blood pressure control) during the course of TRNT therapy, but was not exposed to any other potential nephrotoxic medication. Because targeted alpha therapy had already been stopped and CKD was not severe, no kidney biopsy was performed for this patient. Patient 2 died due to cancer progression in January 2020.
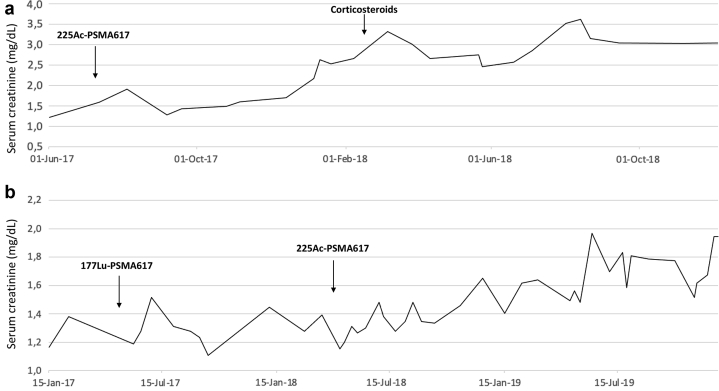
Figure 2 displays serum creatinine concentrations longitudinally over the treatment courses for patient 1 (Figure 2a) and patient 2 (Figure 2b). Laboratory data for each patient are summarized in Table 1.
Figure 2.
Evolution of serum creatinine (mg/dl) over time with actinium-225 (225Ac)–prostate-specific membrane antigen (PSMA)617 therapy for patient 1 (a) and patient 2 (b).
Table 1.
Summary of laboratory data
| Patient 1 | Patient 2 | |
|---|---|---|
| Age (y) | 68 | 67 |
| Hypertension (y/n) | n | y |
| Diabetes (y/n) | y | n |
| RAS blockade (y/n) | n | y |
| Prior oncological treatment | 7 lines | 8 lines |
| Past platinum chemotherapy exposure (y/n) | y | n |
| Baseline Scr (mg/dl) | 1.6 | 1.2 |
| Baseline eGFR (ml/min per 1.73 m2) | 44 | 64 |
| Scr at cessation of 225Ac-PSMA617 | 2.7 | 1.8 |
| Scr at last follow-up | 3.0 | 1.9 |
| eGFR at last follow-up (ml/min per 1.73 m2) | 20 | 35 |
eGFR, estimated glomerular filtration rate; PSMA, prostate-specific membrane antigen; RAS, renin-angiotensin system; Scr, serum creatinine; y/n, yes/no; 225Ac, actinium-225.
Discussion
We present 2 cases of progressive CKD in patients with mCRPC under 225Ac-PSMA617 therapy. Targeted alpha therapy in this setting is a recent therapeutic avenue, and little is known regarding radionuclide therapy–associated nephropathy. Radiolabeled peptides and antibody fragments first undergo glomerular filtration, followed by tubular reabsorption and subsequent lysosomal degradation in the proximal tubular cells.6 Radiolabeled 225Ac is large enough to escape glomerular filtration; however, the radioactive alpha particle-emitting “daughters” can accumulate in the tubular cells and irradiate the kidneys, leading to renal injury.4,6, 7, 8 This mechanism is enhanced by the known intrinsic affinity of actinium for the kidney, and of the daughter nuclide, bismuth, for the proximal tubular cells.2 Moreover, the 10-day half-life of 225Ac and prolonged generation of alpha-emitting daughters may lead to a more significant accumulation of toxic nuclides.2 Animal models have demonstrated severe progressive tubular injury associated with significant reduction in renal function in mice exposed to targeted therapy with 225Ac.4 In this model, early tubular cell changes were observed 10 weeks after injection, progressing to advanced scarring by 40 weeks.
One of our patients had a kidney biopsy 34 weeks after first exposure to 225Ac-PSMA617. The biopsy showed ongoing tubular injury on a background of significant tubular atrophy, and no primary glomerular abnormality, consistent with kidney damage from tubular toxicity, presumably related to radionuclide therapy, as seen in animal models. There was no renal response to corticosteroid therapy, either because the changes were quite advanced and/or because the inflammatory infiltrate was secondary to tubular damage and different from classic tubulointerstitial nephritis.
There are very few reported clinical cases of 225Ac therapy–associated kidney disease. Early case series for 225Ac-PSMA617 treatment in patients with mCRPC (n = 14) reported no change in serum creatinine.S2 In another report of 17 patients, 1 patient with a solitary kidney and prior CKD developed grade 4 CKD after treatment.5 Our 2 cases both had prior CKD and we may hypothesize that patients with impaired baseline renal function seem to be at greater risk of developing worsening CKD with 225Ac targeted therapy. This is also consistent with prior study with other TRNT 177Lu-PSMA617, which reported a higher risk of kidney toxicity in patients with baseline kidney function impairment.9 In this study, of the 55 patients with mCRPC who received at least 3 cycles of 177Lu-PSMA617, 16 (29%) showed a decrease in glomerular filtration rate (grade 1/2 nephrotoxicity) after the last cycle of therapy. This study also demonstrated a significant correlation between preexisting kidney disease and therapy-induced nephrotoxicity, although the correlation coefficient was modest (r = 0.41).
Last, one of the main challenges highlighted by our cases is that it is difficult to diagnose TRNT-related kidney injury due to the presence of various confounders such as obstructive nephropathy related to cancer progression, prior exposition to nephrotoxic chemotherapy, and other medical comorbidities. The length of time between exposure and significant decline in kidney function make this diagnosis challenging. However, temporal relationship between the onset of worsening kidney function and administration of 225Ac-PSMA617 in our 2 patients is very suggestive of a drug association. Moreover, the absence of other precipitant for patient 1’s biopsy findings and their similarity to animal models with this type of renal injury is strongly suggestive of therapy-related toxicity. Concomitant obstructive nephropathy also remained stable during 225Ac-PSMA617 therapy for both patients. Taken together, these observations are strongly suggestive of therapy-related nephrotoxicity (Table 2).
Table 2.
Key teaching points
|
|
|
For the moment, the high cost and limited supply still pose considerable limitations for clinical application of these treatment approaches. However, promising clinical results obtained for patients with mCRPC who have exhausted other treatment modalities may change clinical practice in the near future. Our cases emphasize the need for careful assessment and long-term monitoring of kidney function in these patients, especially if preexisting kidney impairment is present.
In the future, it would be beneficial to investigate preventive pharmacologic strategies to attenuate TRNT-induced renal damage. In animal models, renin-angiotensin system blockade agents have been shown to prevent kidney damage associated with external beam radiation, and diuretics have been shown to decrease tubular reabsorption and accumulation of toxic nuclides.9 Therefore, similar interventions may be useful in reducing the toxicity of TRNT in clinical settings but require further assessment in human studies. Overall, as with many newer promising cancer therapies, unforeseen kidney risks may be present, and practitioners in both nephrology and oncology should familiarize themselves with potential adverse effects.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References
Supplementary Material
Supplementary File (PDF)
Supplementary References
References
- 1.De Vincentis G., Gerritsen W., Gschwend J.E. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30:1728–1739. doi: 10.1093/annonc/mdz270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roscher M., Bakos G., Benesova M. Atomic nanogenerators in targeted alpha therapies: Curie's legacy in modern cancer management. Pharmaceuticals (Basel) 2020;13:76. doi: 10.3390/ph13040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis I.A., Glowienka K.A., Boll R.A. Comparison of 225actinium chelates: tissue distribution and radiotoxicity. Nucl Med Biol. 1999;26:581–589. doi: 10.1016/s0969-8051(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Jaggi J.S., Seshan S.V., McDevitt M.R. Renal tubulointerstitial changes after internal irradiation with alpha-particle-emitting actinium daughters. J Am Soc Nephrol. 2005;16:2677–2689. doi: 10.1681/ASN.2004110945. [DOI] [PubMed] [Google Scholar]
- 5.Sathekge M., Bruchertseifer F., Knoesen O. (225)Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behr T.M., Goldenberg D.M., Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–212. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 7.Jaggi J.S., Kappel B.J., McDevitt M.R. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65:4888–4895. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 8.Lambert B., Cybulla M., Weiner S.M. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161:607–611. doi: 10.1667/rr3105. [DOI] [PubMed] [Google Scholar]
- 9.Yordanova A., Becker A., Eppard E. The impact of repeated cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–1479. doi: 10.1007/s00259-017-3681-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.