Abstract
Introduction
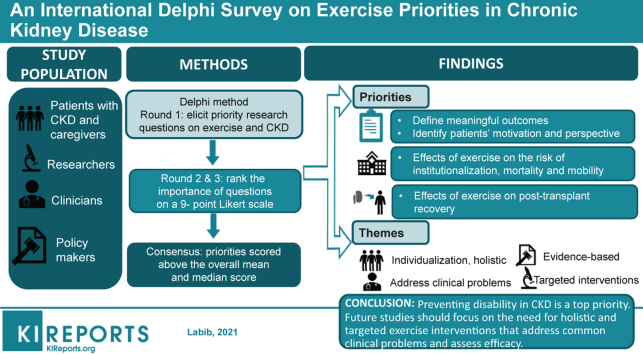
Defining the role of exercise in chronic kidney disease (CKD) is a top research priority for people with CKD. We aimed to achieve consensus on specific research priorities in exercise and CKD among an international panel of stakeholders.
Methods
Using the Delphi method, patients/caregivers, researchers, clinicians, and policymakers submitted their top research priorities in round 1 and ranked their importance in rounds 2 and 3 using a 9-point Likert scale. The mean, median, and proportion of scores ranked 7 to 9 were calculated. Consensus was defined as priorities that scored above the overall mean and median score within each stakeholder panel. Qualitative description was used to understand participants’ rankings.
Results
Seventy participants (78% response) completed round 1: 15 (21.4%) clinicians, 33 (47.1%) researchers, 13 (18.6%) policymakers, and 9 (12.9%) patients; (85.7%) completed round 3. The top research priorities were defining exercise-related outcomes meaningful to patients, identifying patients’ motivation and perspective towards exercise, understanding the effect of exercise on the risk of institutionalization, mortality, and mobility, and understanding the effect of pre- and post-transplant exercise on postoperative recovery. Themes from the qualitative analysis were individualization, personal experience, and holistic approach to exercise (patients), the need to address common clinical problems (clinicians), developing targeted interventions (researchers), and the importance of evidence-based development versus implementation (policymakers).
Conclusions
Preventing physical disability was a common priority. Policymakers emphasized that more efficacy studies were needed. Other panels expressed the need for holistic and targeted exercise interventions and for outcomes that address common clinical problems.
Keywords: chronic kidney disease, Delphi, exercise, outcomes, research priorities, survey
Graphical abstract
Chronic kidney disease (CKD) is associated with high rates of morbidity and mortality.1 Although the factors contributing to poor health outcomes for people with CKD are multifactorial and complex, sedentary behavior and low physical activity are associated with higher risks of all-cause mortality and CKD progression and with a lower quality of life.2 In other chronic conditions, such as cardiovascular and pulmonary disease, exercise has been shown to improve disease-specific health outcomes and has been incorporated into routine clinical care.3 However, the role of exercise in the management of CKD has not been clearly defined and the availability of renal exercise programs remains limited.
Several factors may explain the limited uptake of exercise in CKD care. From systematic reviews of randomized controlled trials, exercise is effective for improving strength, physical fitness, physical function, and quality of life for people with CKD.4 However, knowledge gaps in key areas of CKD management have been identified, such as understanding the effect of exercise on cardiovascular risk, symptom burden (e.g., restless legs, cramping, and fatigue), and the safety of exercise in certain subpopulations.5,6 In addition, exercise counseling by kidney health care professionals is low,7 suggesting either a lack of agreement with the evidence base, failure of evidence translation, or a lack of knowledge and confidence in exercise counseling.
Despite its limited uptake, there is growing interest in the role of exercise in CKD. This interest is in part driven by the research priorities and preferences of patients. In several research priority setting studies, exercise was identified as a top research priority for people with CKD, from the perspectives of understanding the effect of exercise on the health of patients undergoing dialysis and the role of lifestyle factors in delaying the progression of CKD.8,9 However, specific research questions related to exercise have not been prioritized in the CKD population. Therefore, this study sought to identify a range of exercise-related research priorities including but not limited to specific interventions, target populations, outcomes, implementation, and/or scientific understanding. Identifying research priorities has the potential to guide allocation of research resources and support funding initiatives to promote relevant research in this area.
In this study, we aimed to gain consensus on the top research priorities for exercise in people in all stages of CKD (e.g., transplant, dialysis or CKD not requiring dialysis). To increase the relevance of our findings, we included an international panel of stakeholders that included patients and their caregivers, policymakers, researchers, and clinicians.
Methods
The Delphi method is a structured process of iterative surveys or “rounds” that includes providing feedback to participants on their responses to achieve consensus.10 The advantage of the Delphi method is that participants over a broad geographic area can participate and anonymity is maintained, thus minimizing influence of dominant participants.10
Participant Selection, Recruitment, and Survey Distribution
Adults (>18 years of age) were purposively sampled according to geographic location and expertise or experience with CKD to construct 4 stakeholder panels. These panels were patients and caregivers, policymakers, researchers, and clinicians. People with personal experience with CKD self-identified as either patients or caregivers. A policymaker was defined as a member of a national or international CKD guideline committee or granting agency, renal program director, or health system payor. Researchers were defined as those who were involved in exercise research in CKD and were identified through a search of PubMed and Embase. Individuals with >1 publication related to exercise in CKD within the past 5 years and an available e-mail address were invited to participate. Clinicians were defined as nephrologists, CKD nurses, or exercise specialists (e.g., kinesiologists, physiotherapists, and exercise physiologists). The Health Ethics Board at the University of Alberta approved the study (Pro00088558).
We used multiple recruitment strategies: direct invitation via e-mail through existing professional networks (the Global Renal Exercise Network),11 including clinical leads of established exercise programs for people with CKD, advertisements with kidney care organizations, and snowball sampling. Patients and caregivers were recruited by participating investigators at respective sites and by patient advisory boards within kidney research networks in Canada, Australia, and the United States. Clinicians and policymakers were emailed an information letter that contained a hyperlink to the survey. Patients and caregivers were instructed to contact the investigators via email to receive an invitation with the survey link. For all participants, completing the survey implied consent. Participants were also provided with an opt-out link with each survey invitation.
Sample Size
The Delphi process does not depend on statistical power but rather group dynamics for arriving at consensus. The number of panel participants varies widely, and the optimal panel size is not established.12 We aimed for 15 to 20 participants per panel with a total of 60 to 80 participants.
Data Collection
Three online surveys were administered via Qualtrics (Qualtrics Software, Provo, Utah) between April and October 2019. Each round was open for approximately 4 weeks with 3 reminders sent during each round. Before distribution, the survey was pretested for clarity among the research team and 2 patients.
Round 1
Participants provided demographic information and submitted ≤3 research questions they viewed as a top priority on exercise for people with CKD. To encourage consistency and specificity, respondents were given examples of how to structure the question, i.e., defining the study population (e.g., transplantation, dialysis, or CKD not requiring dialysis) and the outcomes to be studied (e.g., blood pressure, restless legs, or strength) and were asked to explain their selections.
Round 2
Nonrespondents from round 1 could participate in round 2. Participants were presented with the list of condensed questions from round 1 and were asked to rank the questions using the Grading of Recommendations Assessment, Development, and Evaluation process (a 9-point Likert scale).13 A score of 1 to 3 ranks the question as not important, 4 to 6 as important but not critical, and 7 to 9 as critically important. Participants could abstain from scoring a question by selecting “unsure.” Participants were asked to explain their rankings and could submit additional research questions. If a question ranked below the overall median score across all 4 panels, it was not carried forward.
Round 3
Only respondents who completed round 2 were invited to participate in round 3. For each question, participants were presented with their own score, the median score from each of the 4 panels (displayed in a bar graph), the overall score, and comments made from panel members. Participants were asked to re-rank the questions using the same system as described in round 2.
Analysis
Categorizing Research Priorities From Round 1
The research questions submitted by participants in round 1 were independently reviewed by 2 authors (ML and ST). The questions were categorized inductively based on outcome (e.g., cardiovascular, patient-reported) and/or key concept (e.g., safety, counseling) and condensed by omitting redundancies, expanding questions to incorporate concepts with the same meaning, and removing questions outside the scope of exercise in CKD. To ensure credibility, the revised list of questions was compared with the initial list by members of the research team (KW, CB, PB, JM, and SM). Any disagreements were resolved by consensus.
Quantitative Analysis
Descriptive results are reported as counts and percentages. Missing responses were excluded from the calculation of percentages. We calculated the mean, median, and the proportion of scores ranked 7 to 9 (critically important) and 1 to 3 (not important) overall and for each of the 4 panels. Differences in rankings across the 4 panels were evaluated using the Kruskal-Wallis test. P < 0.05 was considered statistically significant. Statistical analysis was done using Stata MP software (version 15.1; StataCorp LP, College Station, TX, www.stata.com).
Definition of Consensus
As the distribution of the rankings was not known a priori and participation within each stakeholder panel varied, we defined consensus for critically important questions as a score above the overall mean and median score for all questions within each of the 4 panels. As a secondary outcome, questions that were critically important were defined as those for which >70% of the scores ranked 7 to 9 and <15% of scores ranked 1 to 3. Questions that were not important were defined as those for which >70% of scores ranked 1 to 3 and <15% scores ranked 7 to 9.
Qualitative Analysis
Comments from participants were imported into a Microsoft Word document for qualitative analysis. To better understand participants’ rankings and perspectives on the research questions, we used qualitative description.14 The data were coded independently by ST using a broad-based coding scheme and grouped into common themes first within and then across panels. The themes were reviewed with the members of the research team (ML, KW, CB, PB, JM, and SM).
Results
Participant Characteristics
The participant characteristics are shown in Table 1. In the first round, 70 people (78% response rate) from 15 countries participated; 15 (21.4%) were clinicians, 33 (47.1%) were researchers, 13 (18.6%) were policymakers, and 9 (12.9%) were patients. In all rounds, most participants were 36 to 65 years of age, with equal participation by gender. Respondents were primarily from North America and Europe (Supplementary Table S1).
Table 1.
Participant characteristics
| Characteristic | Round 1, n (%) | Round 2, n (%) | Round 3, n (%) |
|---|---|---|---|
| Surveys sent, n | 90 | 90 | 68 |
| Total respondents | 70 (77.8) | 68 (75.6) | 60 (88.2) |
| Participant type | |||
| Researchers | 33 (47.1) | 29 (42.6) | 28 (46.7) |
| Expertise | |||
| CKD | 7(21.2) | 7 (24.1) | 7 (25.0) |
| ESRD | 23 (69.7) | 20 (69.0) | 19 (67.9) |
| CKD/ESRD | 3 (9.1) | 2 (6.9) | 2 (7.1) |
| Policymakers | 13 (18.6) | 13 (19.1) | 11 (18.3) |
| Patients | 9 (12.9) | 11 (16.2) | 7 (11.7) |
| Clinicians | 15 (21.4) | 15 (22.1) | 14 (23.3) |
| Exercise specialist | 7 (46.7) | 9 (60.0) | 9 (64.3) |
| Nephrologist | 5 (33.3) | 4 (26.7) | 3 (21.4) |
| Nurse | 2 (13.3) | 2 (13.3) | 2 (14.3) |
| Other health care provider | 1 (6.7) | 0 (0) | 0 (0) |
| Age, yr | |||
| <35 | 12 (17.1) | 12 (17.6) | 12 (20.0) |
| 36–50 | 28 (40.0) | 25 (36.8) | 21 (35.0) |
| 51–65 | 27 (38.6) | 28 (41.2) | 24 (40.0) |
| >65 | 3 (4.3) | 3 (4.4) | 3 (5.0) |
| Gender | |||
| Female | 35 (50.0) | 34 (50.0) | 30 (50.0) |
| Male | 35 (50.0) | 33 (48.5) | 30 (50.0) |
| Declined to answer | 0 (0) | 1 (1.5) | 0 (0) |
| Continent | |||
| Asia | 1 (1.4) | 1 (1.5) | 1 (1.7) |
| Europe | 19 (27.1) | 16 (23.5) | 14 (23.3) |
| North America | 42 (60.0) | 44 (64.7) | 38 (63.3) |
| Oceania | 7 (10.0) | 6 (8.8) | 6 (10.0) |
| South America | 1 (1.4) | 1 (1.5) | 1 (1.7) |
CKD, chronic kidney disease; ESRD, end-stage renal disease.
Priorities and Consensus
Round 1
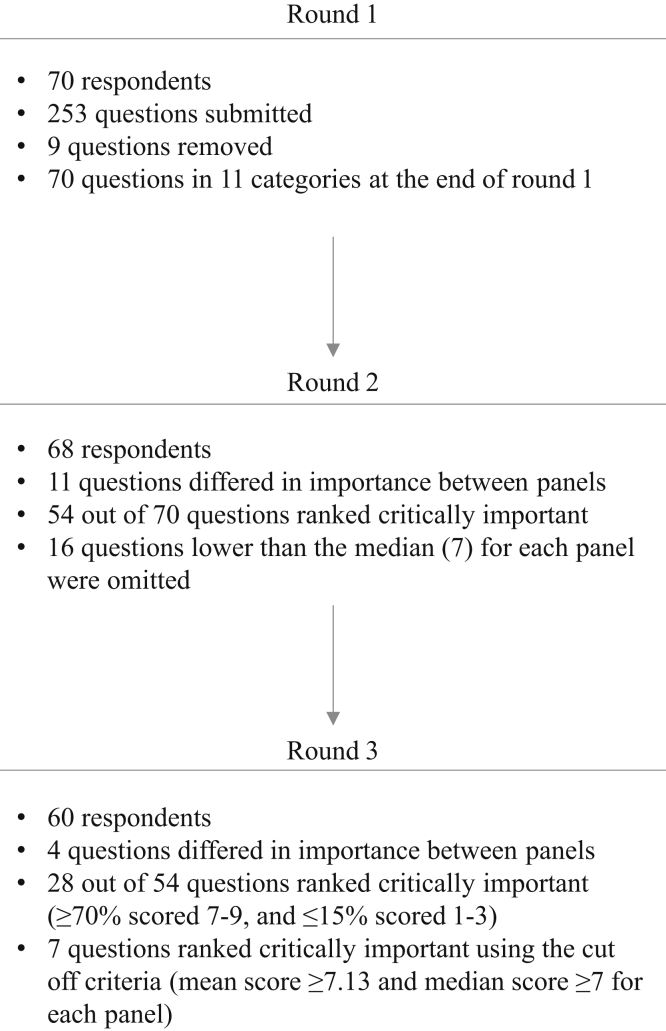
An overview of the Delphi process and results is shown in Figure 1. Respondents submitted 253 research questions, of which 7 questions outside the scope of exercise and CKD were omitted (Supplementary Table S2). The remaining questions were synthesized as described above to yield 70 questions grouped into 11 categories (Supplementary Table S3): patient-reported outcome measures (13%), health outcomes (21%), exercise prescription (10%), cost (3%), implementation (13%), counseling and adherence (13%), safety and risks (4%), nutrition (3%), mechanistic science (4%), dialysis-specific (6%), and transplant-specific (10%).
Figure 1.
Process of developing and prioritizing questions.
Round 2
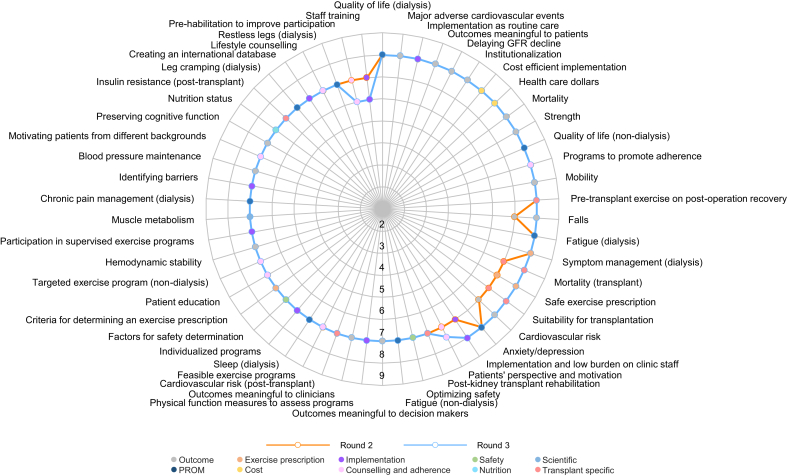
The highest ranked questions per panel are shown in Table 2. Respondents ranked 54 of 70 questions as critically important. The overall median score for round 2 was 7 (interquartile range [IQR] 7–8). Overall, the highest ranked questions in round 2 were: defining exercise-related outcomes that are meaningful to patients (median 8 [IQR 7–9]), understanding the effect of exercise on mobility at all stages of CKD (median 8 [IQR 7–9]), understanding whether exercise decreases fatigue in nondialysis patients (median 8 [IQR 7–9]), and whether exercise improves quality of life in nondialysis (median 8 [IQR 7–9]) and dialysis patients (median 8 [IQR 7–9]). Sixteen questions were not carried forward (median <7). No new questions were suggested by participants.
Table 2.
Highest ranked questions in each panel, round 2 and round 3
| Round 2 |
Round 3 |
||
|---|---|---|---|
| Question | Median (IQR) | Question | Median (IQR) |
| Patients | |||
| Can exercise reduce changes in blood pressure in patients at all stages of CKD, including dialysis? | 8 (7.5–9) | What factors must be taken into consideration when deciding which patients are safe to start and continue an exercise program (e.g., age, frailty, cardiovascular comorbidities, etc.)? | 9 (8–9) |
| Does increased physical activity affect the occurrence of major adverse cardiac events (e.g., heart attacks, strokes) in patients at all stages of CKD, including dialysis? | 9 (7–9) | What is the effect of exercise on mortality among the transplant population? | 8.5 (8–9) |
| What are the patients’ perspectives on exercise and what factors would motivate patients at all stages of CKD to exercise? | 8 (7–9) | What exercise programs are feasible and sustainable for dialysis patients, allowing a high level of adherence in the long term (i.e., the role of guidance and accountability on adherence and patient encouragement)? | 8.5 (7–9) |
| Policymakers | |||
| Does exercise improve quality of life in patients who require dialysis? | 9 (8–9) | Does exercise improve quality of life in patients who require dialysis? | 8 (8–9) |
| What exercise-related outcomes are meaningful to patients? | 8 (8–9) | Does increased physical activity affect the occurrence of major adverse cardiac events (e.g., heart attacks, strokes) in patients at all stages of CKD, including dialysis? | 8 (7–9) |
| Does exercise decrease fatigue in nondialysis CKD patients? | 8 (7.5–9) | How can exercise reduce the risk of institutionalization in patients at all stages of CKD, including dialysis? | 8 (7–9) |
| Researchers | |||
| How can we make exercise a standard component of care for patients at all stages of CKD, including dialysis? | 9 (7–9) | How can we make exercise a standard component of care for patients at all stages of CKD, including dialysis? | 9.0 (8–9) |
| What exercise-related outcomes are meaningful to patients? | 8 (7–9) | Does exercise improve quality of life in patients who require dialysis? | 8.0 (8–9) |
| To what extent can exercise impact the likelihood of falls in patients at all stages of CKD, including dialysis? | 8 (7–9) | How can we implement exercise in a dialysis unit setting in a cost-efficient way (e.g., medical insurance coverage, funding, etc.)? | 8.5 (8–9) |
| Clinicians | |||
| Does regular exercise within the CKD population save health care dollars (e.g., hospital admissions, length of stay, re-admissions, etc.)? | 9 (8–9) | Does regular exercise within the CKD population save health care dollars (e.g., hospital admissions, length of stay, readmissions, etc.)? | 9 (8–9) |
| Can early introduction of exercise in CKD patients delay initiation of dialysis/slow GFR decline? | 9 (7–9) | Can early introduction of exercise in CKD patients delay initiation of dialysis/slow GFR decline? | 9 (8–9) |
| How can we implement exercise in a dialysis unit setting in a cost-efficient way (e.g., medical insurance coverage, funding, etc.)? | 9 (7–9) | How can we implement exercise in a dialysis unit setting in a cost-efficient way (e.g., medical insurance coverage, funding, etc.)? | 9 (7–9) |
CKD, chronic kidney disease; GFR, glomerular filtration rate; IQR, interquartile range.
Round 3
The highest ranked questions per panel are shown in Table 2. The median score and proportion of participants scoring outcomes as 7 to 9 (critically important) are shown in Supplementary Table S4. The overall median score for round 3 was 7 (IQR 7–8).
Consensus
Seven questions met consensus criteria as critically important (Table 3). These were: defining exercise-related outcomes that are meaningful to patients (median 8 [IQR 7–9]), identifying patients’ motivation and perspective toward exercise (median 7.5 [IQR 7–8]), understanding the effect of exercise on the risk of institutionalization (median 8 [IQR 7–9]), mortality (median 8 [IQR 7–9]), and mobility (median 8 [IQR 7–9]), as well as understanding the effect of pretransplant (median 8 [IQR 7–9]) and post-transplant (median 8 [IQR 7–8]) exercise interventions on postoperative recovery. Using the secondary outcome for consensus, 28 questions were deemed critically important (Supplementary Table S5). Three of the top 5 of these priorities were the same as those identified by the primary consensus criteria: understanding the effect of exercise on the risk of institutionalization, defining exercise-related outcomes that are meaningful to patients, and understanding the effect of pretransplant exercise interventions on postoperative recovery. Understanding the effect of exercise on the loss of muscle and strength and adverse cardiac events were 2 additional priorities.
Table 3.
Seven questions as critically important using the primary consensus criteria (overall mean ≥7.13 and median ≥7 within each panel)
| Question | Mean (SD) |
Median (IQR) |
Proportion of scores ranked 7–9 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Researchers | Clinicians | Policymakers | Patients | Overall | Researchers | Clinicians | Policymakers | Patients | Overall | Researchers | Clinicians | Policymakers | Patients | |
| What exercise-related outcomes are meaningful to patients? | 7.7 (1.1) | 7.8 (1.0) | 7.8 (1.2) | 7.6 (1.0) | 7.2 (1.8) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 8 (7–8) | 7 (7–9) | 85.7 | 88.5 | 84.6 | 81.8 | 83.3 |
| How can exercise reduce the risk of institutionalization in patients at all stages of CKD, including dialysis? | 7.6 (1.4) | 7.6 (1.5) | 7.8 (1.1) | 7.9 (1.2) | 7.2 (2.2) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 7.5 (7–9) | 86.2 | 88.9 | 85.7 | 81.8 | 83.3 |
| What are the effects of exercise on mortality in patients at all stages of CKD, including dialysis? | 7.6 (1.5) | 7.7 (1.4) | 7.9 (1.0) | 7.2 (1.8) | 7.2 (2.6) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 7.5 (7–8) | 8.5 (5–9) | 82.5 | 81.5 | 92.9 | 80 | 66.7 |
| How can exercise affect mobility in patients at all stages of CKD, including dialysis? | 7.6 (1.4) | 7.7 (1.2) | 7.4 (1.4) | 7.5 (0.9) | 7.4 (2.6) | 8 (7–9) | 8 (7–9) | 8 (6–9) | 8 (7–8) | 9 (7–9) | 81.7 | 89.3 | 64.3 | 81.8 | 85.7 |
| Can exercise interventions before kidney transplant improve surgical outcomes (e.g., graft function, expedite improvements in GFR) and postoperative recovery? | 7.5 (1.7) | 7.4 (1.5) | 8.1 (1.0) | 7.3 (1.8) | 7.2 (3.5) | 8 (7–9) | 8 (7–8) | 8 (7–9) | 8 (6–8) | 9 (8–9) | 83.9 | 84.6 | 92.9 | 72.7 | 80.0 |
| What is the patients’ perspective on exercise and what factors would motivate patients at all stages of CKD to exercise? | 7.4 (1.2) | 7.3 (1.4) | 7.4 (1.1) | 7.7 (0.9) | 7.6 (1.7) | 7.5 (7–8) | 7 (6–8) | 7 (7–8) | 8 (7–8) | 8 (7–9) | 76.8 | 70.4 | 76.9 | 90.9 | 80.0 |
| What is the effect of an intensive post–kidney transplant rehabilitation program on patients who have been on dialysis for many years? | 7.3 (1.3) | 7.2 (1.2) | 7.6 (1.2) | 7.2 (1.1) | 7.6 (2.6) | 7 (7–8) | 7 (7–8) | 7 (7–9) | 7 (7–7) | 9 (8–9) | 79.2 | 77.8 | 83.3 | 77.8 | 80.0 |
CKD, chronic kidney disease; GFR, glomerular filtration rate; IQR, interquartile range.
Differences Between Stakeholder Groups
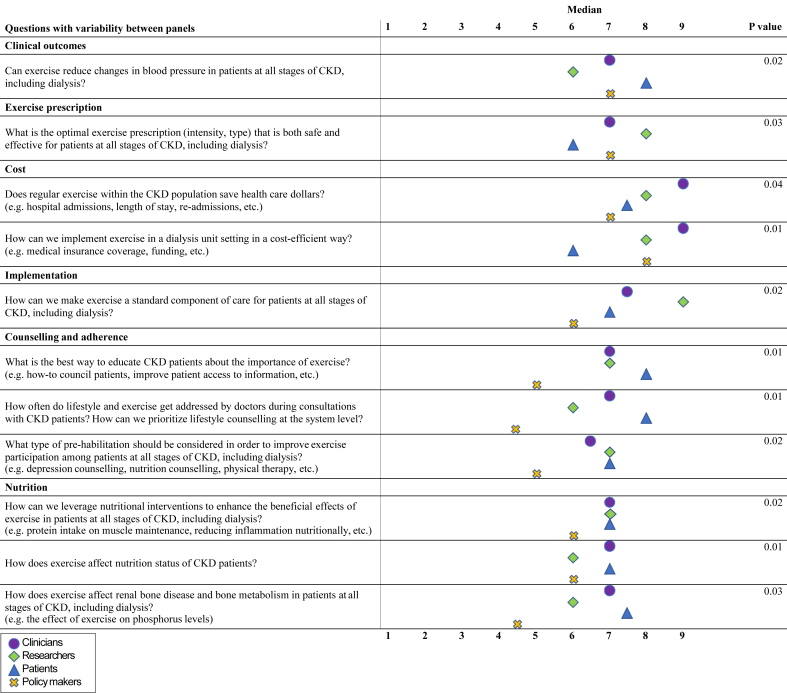
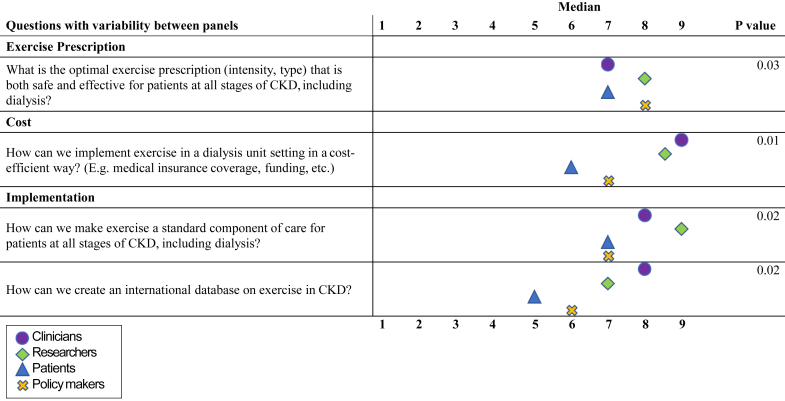
Differences in median scores between all stakeholder groups are shown in Figure 2 (round 2) and Figure 3 (round 3). In round 2, 11 questions differed significantly in ranking between the stakeholder groups. Patients ranked questions regarding health outcomes as well as counseling and adherence higher than the other panels. Questions related to the cost effectiveness of exercise were ranked highest by researchers and clinicians. In general, policymakers ranked the importance of the questions lower than the other panels, most notably regarding implementation, patient counseling, and adherence. In round 3, 4 questions differed significantly in ranking between the stakeholder groups. Similar to round 2, cost and implementation questions were scored highest by clinicians and researchers, while exercise prescription was scored highest by researchers.
Figure 2.
Differences in median scores between stakeholder groups in round 2.
Figure 3.
Differences in median scores between stakeholder groups in round 3.
Changes in Scores From Rounds 2 to 3
Changes in the overall median scores between rounds 2 and 3 are shown in Figure 4. Of the 7 research questions that fulfilled the primary consensus criteria, 6 questions remained the same and only the median score for identifying patients’ perspective and motivation to exercise increased. Changes in median scores within the stakeholder groups for all questions are shown in Supplementary Figures S1–S5.
Figure 4.
Change in overall median scores between rounds 2 and 3.
Qualitative Findings
Overall, 24 participants provided comments in the survey in round 2. Themes identified from the comments are shown in Box 1. Three themes emerged from the patient/caregiver panel comments: individualization, personal experience, and holistic integration of exercise. With respect to individualization, participants expressed that differences in their physical abilities, medical conditions, and preferences necessitated an individualized approach to exercise prescription, and that this may differ from a conventional “exercise” prescription. Several patients also related their rankings to their own experience, particularly with respect to the loss of muscle and strength. The need for holistic approaches to exercise addressed the importance of also addressing nutrition and including other team members, such as dieticians, nurses, and physicians in the promotion of exercise.
Box 1. Themes and selected exemplar quotes.
|
Patients/caregivers Individualization “I think there will need to be a broad gradient from severely deconditioned people to the more active people. So ‘exercise’ could be more functional-based, so the person is standing, dressing, sitting on the edge of the bed, trying shoes, etc., which would differ from an actual exercise program. I also feel that the patient experience is essential to really hearing how activity affects recovery, health, and wellness. Input from support persons can also provide more than a survey could about the benefits of activity and exercise.” “I think the key to having CKD patients want to adhere to exercise ‘programs’ is to individualize the ‘program’ for each patient. The patient must identify what would make them want to exercise/move for the rest of their lives in some form. If it is fun and enjoyable, I think people are more likely to keep it going.” “One cannot force an exercise on a patient. Often patients are fatigued due to dialysis and have metabolic issues; one has to leave it to the patient.” Personal experience “Exercise is very important part of dealing with CKD. Although, I did not have the energy to exercise and now I am struggling as I am in remission and trying to gain strength and muscle back…so hard as dealing with CKD is hard enough.” “Anything that would maintain or build muscle back up without many adverse drug reactions would be great (growth hormones, testosterone).” “For many elderly people on dialysis, they would definitely benefit from basic exercise programs individualized to build muscle mass and function to the point of having the ability to carry out their activities of daily living and better their mobility and strength. Keeping fit by any manner (e.g., weights, resistance bands, machines, etc.) promotes health.” “Dialysis erodes the muscles; it is a crusade to maintain them. Build muscles and dialysis erodes them!” Holistic approaches “Not sure how to leverage nutritional interventions as many supplement or healthy optimal foods are not covered and are costly for many. In order to track the impact of these interventions everyone would need access to the same nutritional interventions/supplements. How much does Replavite do for oxidative stress and inflammation? Exercise for me makes my appetite better and I feel well enough to plan optimal meals.” “I think it is important to ensure the patient understands that “exercise" can mean many things and that movement is the main theme. The biggest sell I see, is promoting it early and constantly as something that is just as important as their dialysis treatments, that maintains their independence." “This is more about teamwork...including the physicians and having the staff needed to support.” “I’d like to see dietitians involved as this is about the whole person.” “It has been my experience that RNs usually talk about exercise, not the doctors. Prioritizing exercising/lifestyle should ideally be part of the intake assessment and history. Probing the patient’s knowledge and understanding of the benefits of exercise to their quality of life is important.” Clinicians Priority populations “Predialysis patient research is limited but translation of known information for the general population may be applied up to a point in the progression of CKD.” “Kidney transplant should be a separate survey.” Common clinical problems “Exercise-related outcomes are important to all exercise specialists, patients, and caregivers e.g., improvement in overall function, increased energy and overall feeling of well-being.” “Regarding ‘Does exercise reduce leg cramping in patients who require dialysis?,’ it would be interesting to know the minimum intradialytic exercise dose and resistance required to elicit a reduction in restless legs.” “Does exercise reduce complications such as intradialytic hypotension, falls, and amputations?” Researchers What is already known “I have rated the barriers as lower because several studies have assessed barriers already.” “Motivators to exercise is already well described. The key is how we can target these motivators to influence outcomes.” “The questions about barriers to exercise participation has been exhausted and there are multiple qualitative and quantitative studies that adequately address this issue already.” “It is important to try and get doctors on board but having results showing outcomes should be a higher initial priority. This will help motivate doctors.” Targeted interventions “I rated the question about the optimal exercise prescription very low because it seems to imply to me that one size fits all. I think we do need to figure out what is best, but it may be for different populations or targeting different outcomes.” “We need to focus not only on the safety of specific populations of patients but tailoring to the patient group rather than mode of therapy delivery.” Policymakers Evidence base development “The knowledge creation questions should take priority over the knowledge translation and implementation science questions.” “The reason implementation is of lower priority is that there needs to be evidence that exercise is effective and safe before learning how to implement.” |
Clinicians identified priority populations and common clinical problems as key themes. Several clinicians indicated that knowledge from the general population may be transferable to those at earlier stages of kidney disease, suggesting that priorities for this group may have been of lower priority for some Delphi participants. Although clinician respondents prioritized a range of outcomes, the comments emphasized addressing common clinical problems, frequently those reported by patients, such as cramping and well-being.
The theme from the researcher panel of “what is already known” explained the view that particular topics, such as barriers and facilitators to exercise in CKD, were known or “established” and could explain why these questions were ranked lower. A few participants expressed the importance of addressing knowledge gaps on “outcomes” because of the perception that these types of data were necessary to persuade physicians to use exercise in clinical care. Targeted interventions related to the need to design an intervention according to the population and the desired outcome. One researcher suggested that safety profiles for exercise should be based on population characteristics rather than modality alone.
The development of a strong evidence base was a key theme for policymakers. Several policymakers expressed the need for future studies to address knowledge gaps on the safety and efficacy of exercise before research on its implementation is considered.
Discussion
This is the first study to report consensus on research priorities for exercise in CKD among all major stakeholder groups, notably patients, policymakers, researchers, and clinicians and the first study to elicit patients’ opinions in priority setting within this topic. The 7 research priorities included defining exercise-related outcomes that are meaningful to patients, understanding patients’ perspectives on exercise and identifying factors to increase motivation, examining the effect of exercise on the risk of institutionalization, mortality, and mobility for patients at all stages of CKD, and understanding the effect of pre– and post–kidney transplant exercise interventions on postoperative recovery. Questions regarding transplant patients were top priorities, presumably because this group is at a higher risk for low physical function15 and may experience differential effects.
Several of the research priorities elicited reflect the importance of preventing physical disability (e.g., institutionalization and improving mobility and rehabilitation post-transplantation). Although CKD is an independent risk factor for disability, and the fear of dependence and the anticipation of further disability with disease progression has been reported by other studies,16, 17, 18 the role of exercise to address this issue has not been previously identified in other priority-setting studies.19, 20, 21 There are no established interventions to mitigate functional decline in this population, but evidence suggests that regular exercise has beneficial effects on health-related quality of life, walking capacity and self-reported physical functioning,22,23 and lowers mortality risk.23
Although understanding the effects of exercise on transplant outcomes is a priority, published exercise studies in this population remains inadequate. The prioritization of mortality and graft function is consistent with the findings of another Delphi survey of patients/caregivers and health care providers on priority outcomes in kidney transplantation trials.24 We extend these findings by identifying exercise, specifically pre- and postoperative rehabilitation, as a therapeutic strategy of interest to modify these outcomes. So far, studies have demonstrated that pretransplant rehabilitation is a potential intervention for increasing physical activity preoperatively and may also decrease post-transplant length of stay.25 Rehabilitation post-transplantation has been associated with improved quality of life, anxiety, and markers of cardiovascular risk.26
Similar to a Canadian survey of patients undergoing dialysis on preferred outcomes in exercise trials, patient participants in our Delphi survey discussed their personal experience with CKD as it related to changes in strength and muscle mass.27 However, in contrast to other studies aimed at identifying research priorities in CKD, symptom management and specifically fatigue did not reach consensus as critically important in our study.28 Although understanding the effect of exercise on fatigue was a priority for policymakers, it was not prioritized beyond the second round because of the low rankings by the patient panel. Rather than implying that addressing fatigue is not important to patients, we recognize that fatigue is a commonly cited barrier to exercise across the spectrum of CKD.29, 30, 31 Therefore, its use as an intervention may be perceived as counterintuitive by patients.32 Furthermore, patient priorities are partly influenced by modality or severity of CKD. For example, in a previous survey, longevity was a more important exercise-related outcome among home hemodialysis patients than for those dialyzing in-center.27 Along these same lines, the patient panel was the only stakeholder group that did not prioritize any question pertaining to quality of life but were the only group that prioritized a question related to examining the effect of exercise on mortality.
Comments from patient participants suggest that incorporating an individualized and holistic approach to promote exercise participation and enjoyment are critical components to include in exercise-related research. This concept of individualization is also consistent with comments received from the researcher panel. However, researchers framed these questions as “targeting” exercise interventions to suit characteristics of the population with the purpose of enhancing efficacy. Although researchers and policymakers both prioritized the role of exercise on quality of life in round 3, these questions did not meet the consensus criteria because of its relatively lower ranking among clinicians. The reasons for this are unclear, because comments from clinicians suggest that well-being was viewed as an important issue. However, given that most clinician respondents were exercise specialists/kinesiologists, it is likely that they viewed exercise as an efficacious intervention for improving certain outcomes and therefore prioritized the funding and implementation aspects of research within this field. In contrast, policymakers did not prioritize any questions relating to implementation or cost and emphasized the need to first demonstrate the efficacy of exercise. This finding underscores the need to not only address remaining knowledge gaps in this unique population but also to include effective knowledge translation strategies such that the evidence is accessible to all main stakeholder groups.
Our study has several strengths. This is the first study to further define research priorities on exercise in CKD including all key stakeholders and representation from 15 countries. In addition, the retention rate between rounds was high and we used qualitative data to help explain our findings. However, our study also has limitations. The patient panel was relatively smaller than the other stakeholder groups. To address this imbalance, we applied a consensus criterion that weighed each panel’s response equally. In addition, we did not identify the specific modality or stage of CKD of the patient panel, which could have been used to help explain the panel’s rankings. In addition, the other 3 panels provided comparatively fewer comments than the patient panel, which would have enhanced the richness of the qualitative data. Although we aimed to include members from a broad geographic location, respondents were primarily from North America and Europe and therefore we are unable to conclude whether rankings may have been influenced by geography as we acknowledge that patients from different geographic locations and socioeconomic backgrounds may have different values and priorities. Finally, the survey was administered online in English, which may have influenced the results.
In conclusion, we reached consensus on research priorities for exercise in CKD relating to preventing disability, understanding patients’ perspectives on exercise-related outcomes and motivation, and examining the role of exercise on improving kidney transplant outcomes. These findings have implications for future research on exercise and CKD. Participants’ desire for individualized or targeted interventions suggests that pragmatic and adaptive designs as well as more granular approaches to characterizing the sample population are also needed to design efficacious interventions. While the importance of patient participation in research has been increasingly recognized, mechanisms to facilitate the integration of this perspective more uniformly in the design of exercise studies are required. Future studies with a large sample size of patients that specify their stages of CKD are needed to ensure the priorities identified are representative of the population and to draw conclusions on the priorities of unique subpopulations. Finally, in addition to addressing knowledge gaps aligned with the priorities of policymakers, ongoing engagement across all stakeholder groups is needed to design effective knowledge translation strategies.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank Sophanny Tiv, BSc, for figure design.
Author Contributions
ML and ST were responsible for research design, data collection, data analysis, and drafting the manuscript. CB, JMM, PNB, KRW, MM-D, MJ, and SM were responsible for design, data analysis, and manuscript writing.
Footnotes
Table S1. Geographic location of the respondents.
Table S2. Questions received in round 1 that were eliminated.
Table S3. Questions in categories.
Table S4. Round 3, mean and median scores, proportion of participants scoring outcomes as 7–9, and missing responses.
Table S5. 28 questions as critically important using the secondary outcome (>70% of the scores ranked 7–9 and <15% of scores ranked 1–3).
Figure S1. Changes in median scores overall between rounds 2–3.
Figure S2. Changes in median scores for patients between rounds 2–3.
Figure S3. Changes in median scores for policymakers between rounds 2–3.
Figure S4. Changes in median scores for researchers between rounds 2–3.
Figure S5. Changes in median scores for clinicians between rounds 2–3.
Contributor Information
Stephanie Thompson, Email: th11@ualberta.ca.
Global Renal EXercise Network:
Mary Labib, Clara Bohm, Jennifer M. MacRae, Paul N. Bennett, Kenneth R. Wilund, Mara McAdams-DeMarco, Manisha Jhamb, Stefan Mustata, and Stephanie Thompson
Appendix
Global Renal EXercise Network
Mary Labib, Clara Bohm, Jennifer M. MacRae, Paul N. Bennett, Kenneth R. Wilund, Mara McAdams-DeMarco, Manisha Jhamb, Stefan Mustata, and Stephanie Thompson.
Supplementary Material
Table S1. Geographic location of the respondents.
Table S2. Questions received in round 1 that were eliminated.
Table S3. Questions in categories.
Table S4. Round 3, mean and median scores, proportion of participants scoring outcomes as 7–9, and missing responses.
Table S5. 28 questions as critically important using the secondary outcome (>70% of the scores ranked 7–9 and <15% of scores ranked 1–3).
Figure S1. Changes in median scores overall between rounds 2–3.
Figure S2. Changes in median scores for patients between rounds 2–3.
Figure S3. Changes in median scores for policymakers between rounds 2–3.
Figure S4. Changes in median scores for researchers between rounds 2–3.
Figure S5. Changes in median scores for clinicians between rounds 2–3.
References
- 1.Bikbov B., Purcell C.A., Levey A.S. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricardo A.C., Anderson C.A., Yang W. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CIRC) Study. Am J Kidney Dis. 2015;60:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkman D.L., Edwards D.G., Lennon-Edwards S. Exercise as an adjunct therapy in chronic kidney disease. Renal Nutr Forum. 2014;33:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen K.L., Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59:126–134. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March D.S., Graham-Brown M., Young H.M. There is nothing more deceptive than an obvious fact: more evidence for the prescription of exercise during haemodialysis (intradialytic exercise) is still required. Br J Sports Med. 2017;51:1379. doi: 10.1136/bjsports-2017-097542. [DOI] [PubMed] [Google Scholar]
- 6.Bohm C., Shick- Makaroff K., MacRae J. The role of exercise in improving patient-reported outcomes in individuals on dialysis: a scoping review. Semin Dial. 2019;32:336–350. doi: 10.1111/sdi.12806. [DOI] [PubMed] [Google Scholar]
- 7.Johansen K., Sakkas G.K., Doyle J. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis. 2003;41:171–178. doi: 10.1053/ajkd.2003.50001. [DOI] [PubMed] [Google Scholar]
- 8.Hemmelgarn B., Pannu N., Ahmed S. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplantation. 2017;32:847–854. doi: 10.1093/ndt/gfw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manns B., Hemmelgarn B., Lillie E. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813–1821. doi: 10.2215/CJN.01610214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shariff N.J. Utilizing the Delphi survey approach: a review. J Nurs Care. 2015;4:246–251. [Google Scholar]
- 11.Global Renal Exercise website. http://grexercise.kch.illinois.edu/organizing-committee Available at:
- 12.Santaguida P., Dolovich L., Oliver D. Protocol for a Delphi consensus exercise to identify a core set of criteria for selecting health related outcome measures (HROM) to be used in primary health care. BMC Fam Pract. 2018;19:152. doi: 10.1186/s12875-018-0831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston C.C., Colman A.M. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol (Amst) 2000;104:1–15. doi: 10.1016/s0001-6918(99)00050-5. [DOI] [PubMed] [Google Scholar]
- 14.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 200;23:334–340. [DOI] [PubMed]
- 15.Van Loon I.N., Bots M.L., Boereboom F.T.J. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol. 2017;18:217. doi: 10.1186/s12882-017-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahaf R., Ilali Sadat, Peyrovi H. Uncertainty, the overbearing lived experience of the elderly people undergoing hemodialysis: a qualitative study. Int J Community Based Nurs Midwifery. 2017;5:13–21. [PMC free article] [PubMed] [Google Scholar]
- 17.McGee J., Jackson N.R., Slakey D.P. Disability and kidney transplantation in the United States. Clin Transplant. 2012;26:377–381. doi: 10.1111/j.1399-0012.2012.01612.x. [DOI] [PubMed] [Google Scholar]
- 18.Han E., Shiraz F., Haldane V. Biopsychosocial experiences and coping stratefies of elderly ESRD patients: a qualitative study to inform the development of more holistic and person centered health servies in Singapore. BMC Public Health. 2019;19:1107. doi: 10.1186/s12889-019-7433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand S., Johansen K.L., Tamura M.J. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69:315–322. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarnak M.J., Katz R., Fried L.F. Cystatin C and aging success. Arch Intern Med. 2008;168:147–153. doi: 10.1001/archinternmed.2007.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight E.L., Ofsthun N., Teng M. The assocaition between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 22.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Tentori F., Elder S.J., Thumma J. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25:3050–3062. doi: 10.1093/ndt/gfq138. [DOI] [PubMed] [Google Scholar]
- 24.Sautenet B., Tong A., Manera K.E. Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational Delphi survey with patients, caregivers, and health professionals. Transplantation. 2017;101:1875–1886. doi: 10.1097/TP.0000000000001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco M.A., Ying H., Van Pilsum Rasmussen S. Prehabilitation prior to kidney transplantation: results from a pilot study. Clin Transplant. 2019;33 doi: 10.1111/ctr.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano G., Lorenzon E., Montanaro D. Effects of exercise in renal transplant recipients. World J Transplant. 2012;2:46–50. doi: 10.5500/wjt.v2.i4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moorman D., Suri R., Hiremath S. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol. 2019;14:268–276. doi: 10.2215/CJN.09700818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flythe J.E., Hilliard T., Castillo G. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol. 2018;13:735–745. doi: 10.2215/CJN.10850917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke A.L., Young H.M.L., Hull K.L. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30:1885–1892. doi: 10.1093/ndt/gfv208. [DOI] [PubMed] [Google Scholar]
- 30.Delgado C., Johansen K.L. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27:1152–1157. doi: 10.1093/ndt/gfr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Adrichem E.J., Van de Zande S.C., Dekker R. Perceived barriers to and facilitators of physical activity in recipients of solid organ transplantation, a qualitative study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick J., Ritchie M., Andrews E. Exercise in individuals with CKD: a focus group study exploring patient attitudes, motivations, and barriers to exercise. Kidney Med. 2019;1:131–138. doi: 10.1016/j.xkme.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.