Abstract
Introduction
The identification of specific molecular signatures and the development of new targeted drugs have changed the paradigm of onco-nephrology, now allowing a multiscale approach of kidney involvement related to hematologic malignancies relying on combined hematologic and molecular assessments. In this study, we aimed to refine the spectrum of kidney disorders associated with chronic myelomonocytic leukemia (CMML) or BCR-ABL–negative myeloproliferative neoplasms (MPNs), 2 very rare conditions scarcely described.
Methods
Case series. Patients with myeloid neoplasms who were referred to Toulouse University Hospital Nephrology Unit and were diagnosed with acute kidney injury (AKI), chronic kidney disease (CKD), or urine abnormalities were retrospectively included.
Results
Eighteen patients (males n=13, CMML n=8, essential thrombocytosis [ET] n=7, polycythemia vera [PV] n=1, and myelofibrosis n=2) developed kidney disease 7.7±2 years after the diagnosis of the malignancy. Twelve patients had AKI at presentation. Eight patients had glomerular presentation (high-range proteinuria 33%, microscopic hematuria 56%). Kidney biopsy (n=14) showed various patterns, including pauci-immune glomerulosclerosis (n=5), extramedullary hematopoiesis (n=6), or tubular atrophy and interstitial fibrosis with polymorphic inflammation (n=8). Immunostaining of CD61 confirmed the infiltration of megakaryocytes within glomeruli or interstitium in 5 of 8 patients. Other pictures of glomerulopathy were identified in 3 patients (IgA nephropathy n=2, AA amyloidosis n=1). Massive kidney infiltration by CMML was identified in 1 patient. After a mean follow-up of 24±6 months, malignancy was considered as stable in 11 patients (61%), but 22% of patients had progressed to end-stage renal failure. The remaining had persistently reduced kidney function. No correlation between the malignancy and the renal presentation and outcomes could be identified.
Conclusions
Kidney complications of CMML/MPN are heterogenous, and kidney biopsy may help to identify new molecular targets to prevent the development of kidney fibrosis.
Keywords: chronic kidney disease, chronic myelomonocytic leukemia, essential thrombocytosis, megakaryocytes, myeloid neoplasms, myeloproliferative neoplasms
The spectrum of kidney disorders in patients with hematologic malignancies is highly heterogenous. Whereas renal complications of B cell– and plasma cell–related malignancies were extensively studied, those associated with myeloid neoplasms are poorly described.
Myeloid neoplasms include myeloid leukemia, myelodysplastic syndromes, and MPNs1 and account for 30% of all newly diagnosed hematologic malignancies.2 BCR-ABL–negative MPN share common pathophysiological processes promoting the proliferation of an abnormal hematopoietic stem cell clone (owing to driver mutations and micro-environmental changes).3,4 Hence, mutations in the JAK2 gene are identified in most patients with PV, and to a lesser extent ET and primary myelofibrosis (PMF). CMML is another rare BCR-ABL–negative myeloid neoplasm with a different molecular mechanism. Besides hematologic complications (cytopenia, thrombosis, splenomegaly, and progression toward acute myeloid leukemia), BCR-ABL–negative MPN can also lead to systemic disorders characterized by poor prognosis.5,6
Among others, renal complications directly impact global prognosis, and renal failure significantly reduces progression-free survival.7 In a retrospective Korean study of 136 MPN patients, the prevalence of CKD was 11%.8 However, the landscape of kidney disorders related to MPN and CMML remains elusive precluding tailored management. Single-case studies reported various kidney disorders, including chronic and/or acute kidney injuries resulting from direct (e.g., myeloid neoplasm infiltration, urine lysozyme, kidney venous thrombosis) and indirect (e.g., vasculitis, infarction, tumor lysis syndrome) mechanisms. Rarely, extramedullary hematopoiesis has been reported in the urinary tract.9 Glomerular injuries were described, but pathologic data are very rare. The largest series, published in 2011, included only 11 patients (PMF n=8, ET n=1, PV n=1, chronic myeloid leukemia n=1). In this series and in anecdotal case reports, focal segmental glomerulosclerosis (FSGS) was the main pathologic finding.10, 11, 12 Renal pathologies in patients with chronic CMML or ET are far less described.12 Furthermore, neither mutation status nor hematologic outcomes were reported in most published cases.
The objectives of the present study were to describe in depth the clinical course of patients with documented MPN or CMML and kidney disorders, and to assess their hematologic and renal outcomes.
Methods
In this monocentric retrospective study, we included all patients with MPN or CMML who were referred to the Department of Nephrology and Organ Transplantation of the University Hospital of Toulouse (France) between January 2010 and December 2020.
Definition of Hematologic Disease
Diagnosis criteria for myeloid neoplasms were based on the 2016 revised WHO criteria.1 Myelodysplastic and myeloproliferative neoplasms include CMML and 4 other diseases. MPNs are a subgroup containing 8 diseases: chronic myeloid leukemia, BCR-ABL1 (CML); PV; PMF with 2 stages: proven PMF and precocious PMF (or prefibrotic PMF); ET; chronic neutrophilic leukemia; chronic eosinophilic leukemia, not otherwise specified; systemic mastocytosis; and unclassified MPNs. Cytogenetic analysis was determined using standard procedures and molecular analyses were performed as previously described.13
Definition of Kidney Diseases
According to the National Kidney Foundation’s Kidney Disease Outcome and Quality Initiative (KDOQI) guidelines, CKD was defined as renal damage or estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2 for at least 3 months. CKD is classified into 5 stages. CKD stages 1 and 2 are defined by evidence of kidney damage (proteinuria, hematuria, abnormal imaging, or biopsy) and eGFR >90 and 60 to 89 ml/min per 1.73 m2, respectively. CKD stages 3 to 5 are defined based solely on eGFR: stages 3, 4, and 5 are characterized by eGFRs in the ranges of 30 to 59, 15 to 29, and <15 ml/min per 1.73 m2, respectively.14 eGFR was calculated with the CKD-EPI formula.15 For AKI, the following definition was used: an increase in serum creatinine (SCr) by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume ≤0.5 ml/kg/h for 6 hours. AKI was staged for severity according to the KDIGO criteria.16 eGFR loss was calculated as the difference between eGFR at diagnosis and GFR at last follow-up.
Kidney Biopsies
Processing of kidney biopsies included light microscopy and immunofluorescence. For light microscopy, all cases were stained with hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, and Jones methenamine silver. For immunofluorescence, 0.3-μm cryostat sections were stained with polyclonal antibodies to IgG, IgM, IgA, C3, C1q, kappa, lambda, fibrinogen, and albumin-FITC (rabbit, polyclonal; Agilent, Santa Clara, CA). Immunoperoxidase studies were performed on paraffin sections using antibodies directing against CD61 (mouse antihuman clone 2F2; Roche, Basel, Switzerland), myeloperoxidase (rabbit antihuman polyclonal; Roche), glycophorin C (mouse antihuman clone Ret40f; Agilent), and lysozyme (rabbit polyclonal; Roche).
Clinical Data
Clinical data included demographic profile and routine clinical and laboratory findings that were obtained from medical records.
Statistics
Continuous variables are expressed as means and standard error of the mean and compared with Mann-Whitney U test. Discontinuous variables are expressed as numbers and percentages and compared with the Fisher exact test.
Ethics
This study was conducted according to the Helsinki declaration, as revised in 2004, and fulfilled the recommendations of French law regarding retrospective observational studies. According to the recommendations of the Institutional Review Board of the University Hospital of Toulouse, written informed consent was waived.
Results
Characteristics of the Patients
Over 11 years, 18 consecutive patients (male gender n=13; mean age 70±5 years) fulfilled the inclusion criteria.
Hematologic Profile
As depicted in Table 1, hematologic malignancies were heterogenous: CMML n=8, ET n=7, PV n=1, PMF n=1, secondary myelofibrosis n=1. Karyotype abnormalities and mutations status were available in 16 patients.
Table 1.
Hematologic and renal characteristics of 18 patients with CMML or myeloproliferative neoplasms
| Gender Patient number |
Age, yr | Hematologic |
Delaya | Basal status |
Renal presentation |
Kidney pathology |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignancy (mutation) | GFR | CKDb | AKIb | Hu | uPCr | Diagnosis | Mesangial sclerosis | Extramedullary hematopoiesis (CD61 staining) | Immune deposits | |||
| M1 | 75 | MP-CMML-1 Del (7q) |
4 | 73 | 1 | 0 | Yes | 3 | FSGS; AIN | Mild | Glomeruli and interstitium (+) | none |
| F2 | 69 | MD-CMML-2 N/A |
3 | 55 | 2 | 1 | No | 0.6 | CTIN | none | none (–) | none |
| M3 | 79 | MD-CMML-1 N/A |
1 | 18 | 4 | 3 | No | 3.6 | AIN; T-cells infiltrate |
none | none (NA) | none |
| F4 | 79 | MP-CMML-0 KITD816 |
1 | 69 | 1 | 2 | Yes | 0.4 | AIN | Mild | Interstitial (+) | none |
| M5 | 66 | MP-CMML-0 ASXL1 |
0 | 75 | 1 | 3 | Yes | 1.3 | AIN, massive infiltration by CMML | none | Glomeruli and interstitium (NA) | N/A |
| M6 | 63 | MP-CMML-1 N/A |
0 | 56 | 3 | 1 | Yes | 1 | FSGS | none | Glomeruli (+) | N/A |
| F7 | 75 | MP-CMML-1 X chro. |
3 | 87 | 1 | 3 | Yes | 1 | CTIN | none | none | none |
| M8 | 72 | MP-CMML-0 JAK2 |
0 | 55 | 3 | 3 | Yes | 0.9 | Massive EMH | none | Glomeruli and interstitium (+) | none |
| M9 | 40 | ET CALR |
5 | 96 | 1 | 1 | Yes | 3.2 | IgAN; FSGS | Mild | none | IgAλ |
| M10 | 84 | ET JAK2 |
12 | 55 | 3 | 0 | No | 3.2 | AA amyloidosis; CTIN | none | none | N/A |
| M11 | 86 | ET None |
24 | 43 | 3 | 1 | No | 0.2 | N/A | N/A | N/A | N/A |
| M12 | 56 | ET None |
25 | 128 | 1 | 3 | Yes | 2.7 | IgAN | Severe | none | IgA C3 |
| F13 | 80 | ET JAK2 |
14 | 75 | 2 | 0 | No | 13 | FSGS | Mild | Glomeruli and interstitium (+) | none |
| M14 | 74 | ET MPL, DNMT3A |
0 | 64 | 2 | 0 | No | 1 | CTIN | Mild | none (–) | none |
| M15 | 67 | ET JAK2 |
0 | 96 | 1 | 2 | No | <0.2 | N/A | N/A | N/A | N/A |
| M16 | 59 | PV Tri. 8 |
26 | 23 | 4 | 1 | Yes | 7.7 | N/A | N/A | N/A | N/A |
| M17 | 65 | PMF JAK2 |
1 | 48 | 3 | 0 | No | 0.4 | N/A | N/A | N/A | N/A |
| F18 | 71 | SMF (PV) JAK2 | 11 | 38 | 3 | 0 | Yes | 4.4 | FSGS | Mild | none (–) | IgM, C3, C1q (rare) |
AIN, acute interstitial nephropathy; AKI, acute kidney injury; CKD, chronic kidney disease; CMML, chronic myelomonocytic leukemia; CTIN, chronic tubulointerstitial nephropathy; EMH, extramedullary hematopoiesis; ET, essential thrombocytosis; FSGS, focal and segmental glomerulosclerosis; GFR, glomerular filtration rate; Hu, hematuria; IgAN, IgA nephropathy; MD, myelodysplastic; MP, myeloproliferative; PMF, primary myelofibrosis; PV, polycythemia vera; SMF, secondary myelofibrosis; uPCR, urinary protein-to-creatinine ratio (g/g).
Delay from the diagnosis of myeloid neoplasm to the onset of the kidney disease (in years).
CKD and AKI stage according to the KDIGO classifications.
Among the 8 patients with CMML, disease subtypes were the following: type 1 in 4 patients, type 0 in 3 patients, and type 2 in 1 patient. Observed chromosomal and molecular abnormalities were as follows: deletion in the long arm of chromosome 7 (del(7q); n=1), point mutation of c-KIT (D816 mutation; n=1), JAK2 (V617F mutation; n=1), NRAs (n=1), ASXL1 (n=2), SRSF2 (n=1), and X chromosome anomaly (n=1). Mean white blood cell count at admission to the renal unit was 25±8 G/L. Mean monocytes count was 4.9±2 G/L. No thrombocytosis was noted, even in the patient with JAK2 mutation. A bone marrow biopsy was performed only in 2 patients and revealed no myelofibrosis. A circulating monoclonal IgG kappa was detectable in 3 patients and IgM kappa in 1 CMML patient, leading to the diagnosis of monoclonal gammopathy of unknown significance.
Among the 10 patients with MPN, 5 of the 10 tested patients had the recurrent V617F mutation of JAK2 gene (allelic frequency 10%–88%). Mutations were also identified in DNMT3A and MPL (n=1) or CALR (n=1). No M-spike was identified in MPN patients.
Kidney and Systemic Involvements
Delay between the diagnosis of myeloid neoplasms and the onset of the kidney disease was 7.7±2 years. Data on clinical history are available in Supplementary Table S1. In 5 patients, kidney and hematologic diseases were identified in the same period. At admission to the renal unit, 11 patients were on hydroxyurea treatment, associated with targeted therapy in 1 case. No patient received nephrotoxic drugs. Twelve patients presented with AKI (stage 1 in 5, stage 2 in 3, and stage 3 in 5). Two patients required dialysis at diagnosis. In the 6 patients who did not develop AKI, mean baseline GFR was 59±2 ml/min per 1.73 m2. Hypertension was noted in 13 patients (72%) and peripheral edema in 8 (50%). Autoimmunity was identified in 8 of the 16 tested patients (antinuclear antibodies n=8, anti–RNA polymerase III antibodies n=1, and anticentromere antibodies n=1). Five of them had CMML. Four patients had detectable type 2 (n=3) or type 3 (n=1) cryoglobulinemia, but none had low serum complement C3 or C4 levels.
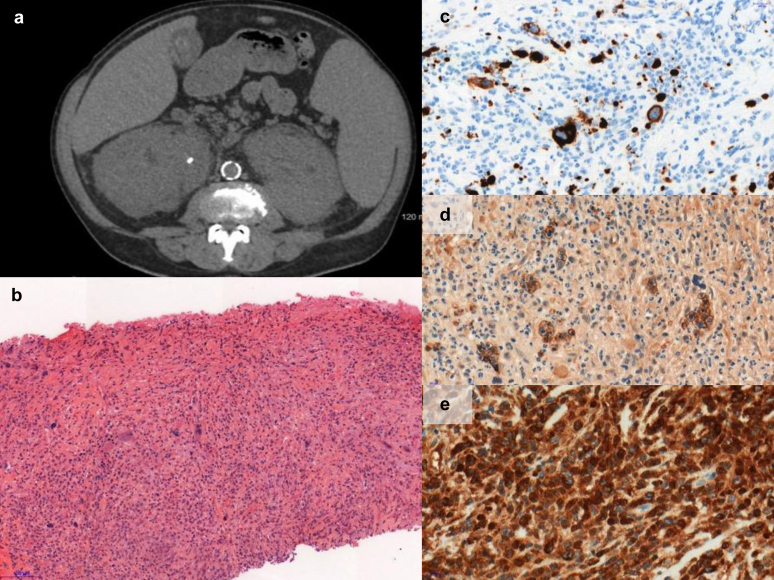
Renal profiles were heterogenous, including high-range proteinuria (urinary protein-to-creatinine ratio ≥ 3 g/g) in 4 patients (22%), full nephrotic syndrome in 4 patients (22%), and microscopic hematuria in 10 patients (56%) (Table 1). In 1 patient, AKI resulted from ureteral obstruction related to both uric acid lithiasis and massive extramedullary hematopoiesis (Figure 1a shows computed tomographic scan findings). In addition, kidney size was increased and kidney biopsy confirmed the specific infiltration. Of note, 2 patients had a history of uric acid lithiasis. Three patients presented with extra-renal signs such as vasculitic manifestations including purpura, arthralgia, or skin eruption.
Figure 1.
Massive infiltration of kidneys and obstructive uric acid lithiasis in a patient with chronic myelomonocytic leukemia requiring dialysis. (a) Abdominal computed tomographic scan. (b) Hematoxylin-eosin (×12). Colony-forming aggregates of erythropoiesis, myelopoiesis, and megakaryopoietic cells were considered to be extramedullary hematopoiesis. (c) Megakaryocytes characterized by positive CD61 staining (×41). (d) Erythroid cells characterized by positive glycophorin C staining (×41). (e) Myeloid white cells characterized by myeloperoxidase (MPO) (×41).
Histopathologic Findings
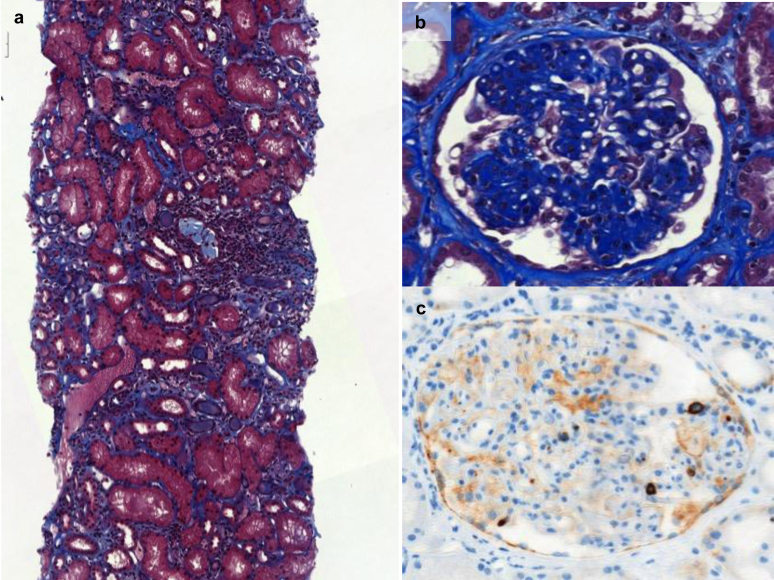
Kidney biopsy was available in 14 patients. As described in Table 1, no frank correlation could be established between myeloid neoplasm subtypes and kidney pathologic findings. Tubulointerstitial injury was the most frequent finding (n=8; 57%) with either chronic (n=4; 29%) or acute (n=4; 29%) lesions. Tubular atrophy and inflammatory fibrosis with polymorphic lymphocytic infiltration were the main features (Figure 2a). Area of interstitial fibrosis ranged from 10% to 50%. The degree of mesangial hypertrophy and proliferation is summarized in the “mesangial sclerosis” column in Supplementary Table S1.
Figure 2.
Kidney biopsies of patients with chronic myelomonocytic leukemia or BCR-ABL–negative myeloproliferative neoplasms. (a) Tubulointerstitial pattern with interstitial fibrosis and tubular atrophy (polymorphic lymphocytic infiltration) (Masson trichrome staining, ×14). (b) Mesangial sclerosis (Masson trichrome staining). (c) Megakaryocytes within glomeruli (CD61 staining; brown).
Glomerular lesions were heterogenous, FSGS being the most frequent (n=5; 36%), including 2 with typical deposition of IgM, C3, and C1q. No thrombotic microangiopathy was reported. The lack of electron microscopy precluded precise characterization of glomerular lesions. Glomerular immunostaining was negative in 7 patients. Two patients had mesangial sclerosis (Figure 2b) with IgA mesangial deposits (polyclonal IgA n=1; monoclonal IgA lambda n=1). One patient had SAA deposits within glomeruli leading to the diagnosis of AA amyloidosis.
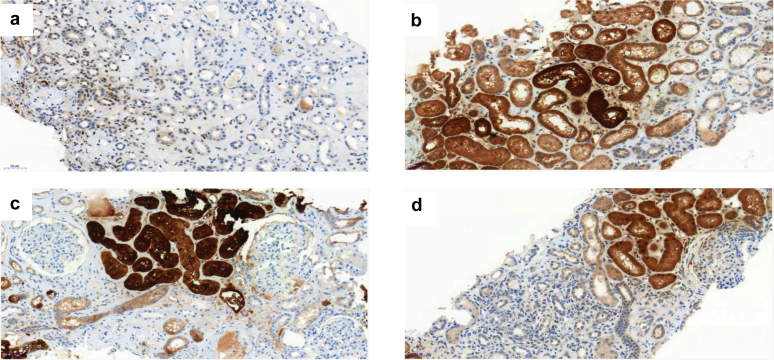
The presence of megakaryocytes (positive CD61 marker) was identified in 5 of the 8 tested patients (CMML n=5; primary ET n=2, secondary myelofibrosis n=1) with acute tubulointerstitial injury, FSGS, or both. Megakaryocytes were identified in glomerular and interstitial compartments (Figure 2c). Extramedullary hematopoiesis was observed on kidney biopsy of 5 patients (36%), all positive for CD61 staining. Lysozyme staining was positive in 4 of the 8 tested biopsies (ET n=3 and secondary myelofibrosis n=1; Figure 3). As expected,17 staining was restricted to proximal tubules. In 2 patients, lysozyme was also detected within the urine (details in Supplementary Table S1).
Figure 3.
Lysozyme staining of 4 representative kidney sections. (a) Patient with negative staining (patient 7). (b-d) Positive staining corresponding respectively to patients 9, 14, and 18 (Table 1).
One patient with CMML had massive extramedullary hematopoiesis precluding glomerular analysis (Figure 1b), as confirmed by CD61 (megakaryocytes; Figure 1c), glycophorin C (erythroid precursor cells; Figure 1d), and myeloperoxidase (myeloid white cells; Figure 1e).
Outcomes
Outcomes are summarized in Table 2. Following the identification of myeloid neoplasm–associated kidney disease, treatment was started or modified in 11 patients (61%) and consisted of the introduction or a switch from hydroxyurea to anagrelide (n=1), azacytidine (n=4), decitabine (n=1), or ruxolitinib (n=1). Four patients received oral steroids.
Table 2.
Outcomes of 18 patients with CMML or BCR-ABL–negative myeloproliferative syndromes and renal involvement
| Malignancy | Hematologic Treatments |
Follow-up (mo) | Outcomes |
||||
|---|---|---|---|---|---|---|---|
| At diagnosis of the kidney disease | After | eGFR at last follow-up | GFR change | Malignancy | Overall | ||
| MP-CMML-1 | HU | Decitabine | 9.5 | 101 | 28 | Acute myeloid leukemia | Death |
| MD-CMML-2 | No | Azacytidine | 92 | 53 | –2 | Stable | Death |
| MD-CMML-1 | No | Steroids, azacytidine | 19 | 15 | –3 | Stable | Alive |
| MP-CMML-0 | No | HU | 21 | 18 | –51 | Mastocytosis | Death |
| MP-CMML-0 | HU | HU, steroids, azacytidine | 10 | 50 | –25 | Stable | Alive |
| MP-CMML-1 | No | Azacytidine | 2 | 29 | –27 | Stable | Alive |
| MP-CMML-1 | HU | HU, steroids | 1 | 19 | –68 | Stable | Death |
| MP-CMML-0 | No | HU, ruxolitinib | 1 | Dialysis | Dialysis | Stable | Alive |
| ET | No | No | 84 | 58 | –38 | Stable | Alive |
| ET | HU | No | 50 | 54 | –1 | Stable | Death |
| ET | HU | Anagrelide | 2 | 28 | –15 | Stable | Alive |
| ET | HU | No | 48 | Dialysis | Dialysis | Stable | Death |
| ET | HU | Ruxolitinib | 17 | 40 | –35 | Myelofibrosis | Death |
| ET | No | HU, steroids | 21 | 46 | –18 | Stable | Alive |
| ET | HU | No | 17 | Dialysis | Dialysis | Myelofibrosis | Death |
| PV | HU | No | 21 | Dialysis | Dialysis | Acute myeloid leukemia | Death |
| PMF | Steroids, HU | No | 11 | 34 | –14 | Stable | Death |
| SMF (PV) | HU, ruxolitinib, pipobroman | Ruxolitinib | 3 | 36 | –2 | Stable | Alive |
CMML, chronic myelomonocytic leukemia; eGFR, estimated glomerular filtration rate; ET, essential thrombocytosis; GFR, glomerular filtration rate; HU, hydroxyurea; MD, myelodysplastic; MP, myeloproliferative; PMF, primary myelofibrosis; PV, polycythemia vera; SMF, secondary myelofibrosis.
After a mean follow-up of 24±6 months, 2 ET patients progressed toward secondary myelofibrosis. A diagnosis of atypical mastocytosis was ultimately established in a formerly diagnosed CMML. The only patient with PV developed acute myeloid leukemia after 26 years of follow-up. Hematologic malignancy was considered as stable in 11 patients (61%), but only 8 patients were alive at last follow-up (survival rate 44%). In all patients except one, renal function worsened with time, with a mean decline of –19±6 ml/min per 1.73 m2. eGFR loss was not different between the CMML and ET groups (–21 vs –18 ml/min per 1.73 m2, P > 0.05).
Discussion
Owing to the development of new targeted therapies with their own toxicities, onconephrology emerged as a major research field in nephrology.18 For instance, the accurate characterization of kidney diseases associated with hematologic malignancies allowed the individualization of treatments in patients with monoclonal gammopathy of renal significance and other B cell–related renal diseases.19,20 In contrast, descriptions of kidney disorders associated with myeloid neoplasms are very scarce, especially in patients with CMML, precluding individualized treatment strategies.21, 22, 23
Although the development of a kidney disease was mostly delayed (7.7 years in our series and 7.2 years in the cohort reported by Said et al.12), 5 patients had concomitant kidney involvement at diagnosis of hematologic malignancy, suggesting that kidney pathology result from diverse mechanisms. Myeloid neoplasms clones may develop additional genetic abnormalities leading to the production of molecules driving renal fibrosis. Interestingly, several studies showed that MPN cells can secrete large amounts of the soluble form of the urokinase plasminogen activator receptor (suPAR).24 suPAR is a signaling glycoprotein involved in the pathogenesis of kidney diseases. High levels of circulating suPAR are associated with the progression of CKD and may prevent renal recovery in patients with AKI.25,26 Other studies also reported that megakaryocytes can drive bone marrow and spleen fibrosis through the secretion of transforming growth factor-β27. Using CD61 immunostaining on kidney biopsies, we could demonstrate that megakaryocytes can infiltrate the kidneys of a subset of patients with myeloid neoplasm–related kidney diseases, thus suggesting that lesions developed when myeloid neoplasm megakaryocytes acquire the potential to migrate within kidneys. The development of new TGF-ββ inhibitors in myelofibrosis and myelodysplastic syndromes28 now paves the way to test these molecules in patients with renal complications of MPN and proven kidney infiltration by megakaryocytes. Moreover, these two mechanisms are not exclusive and may be additive, CD61 (or integrin-β3) being the receptor of suPAR. In line with our findings, CD61–/– mice are protected from glomerulosclerosis induced by overproduction of a suPAR isoform.29
According to these hypotheses, the main glomerular pathologic finding in our patients and those previously reported was mesangial sclerosis and hypercellularity.12 In a subset of patients, glomerular injuries culminate in overt FSGS, as previously described in patients with ET or MPN.30 Intracapillary hematopoietic cells were also identified in 23% to 36% of patients,12 but we did not observe signs of chronic thrombopathic microangiopathy, contrasting with findings reported by Said et al.12 In the latter cohort, MPN was PMF in 8 of 11 (73%) versus ET in 7 of 10 (70%) in ours. In further studies, comparison between secretome of PMF and ET clonal cells may help to identify new players in the pathogenesis of the myeloid neoplasm–related glomerular capillary lesions but also thrombotic microangiopathy.
Even if a significant association has been established between CMML and autoimmunity (both systemic vasculitis31 and autoantibodies32), we did not observe a high prevalence of either extrarenal manifestations or renal vasculitis. Indeed, polymorphic interstitial fibrosis with tubular atrophy was the main renal pathologic change (75%), followed by FSGS (25%) in CMML patients. Interestingly, overt infiltration by the CMML was observed in only 1 patient and is thus not the main cause of renal failure. Extramedullary hematopoiesis was massive in 1 patient also presenting with obstructive AKI related to uric acid lithiasis.
Monocytic and myelomonocytic neoplasms, most notably CMML, are associated with overproduction of lysozyme, a low-molecular-weight protein freely filtered by the glomerulus, and can be associated with nephrotic-range lysozymuria. Lysozyme accumulates in proximal tubular cells, and there is a threshold at which this accumulation is associated with toxic proximal tubular injury and AKI.17 In our series, of the 4 patients with a positive staining for lysozyme, only 1 had a tubulointerstitial kidney injury pattern, whereas the others had FSGS. There was no correlation between lysozyme staining and underlying hematologic malignancy. Thus, lysozyme kidney staining suggests a multifactorial pathogenesis in myeloid neoplasm–induced kidney diseases.
To date, the largest available cohort on MPN patients (not including myelodysplastic neoplasms) reported 11 patients and focused on glomerular disorders, but follow-up was shorter.12 Two patients died (month 3 and 62) and 4 reached end-stage renal failure. In our series, the mortality rate was high (56%) despite stable hematologic malignancy in 61% of patients. Renal function worsened in all except 1 patient. Four patients (22%) required long-term dialysis. Patients requiring dialysis did not receive any therapeutic changes, except 1 who received hydroxyurea initiation. The lack of overt renal improvement following cytoreductive therapy in our series contrasts with the results of a previous cohort that included 136 patients with BCR-ABL–negative MPN (ET, PV, and PMF).8 In this study, most patients had slowly progressive CKD, whereas patients included in ours frequently had AKI that could be triggered by different pathophysiological mechanisms. Thus, which patients with renal complications of MPN may benefit from specific drugs (eg, ruxolitinib for PMF) remains to be determined.33 Furthermore, azacytidine (n=4) and decitabine (n=1) were used as second-line treatment after the identification of kidney injury with closed monitoring of potential additional renal impairment, as they are known to be sometimes nephrotoxic, especially for tubules.34,35
Limitations of this work first and foremost relied on its retrospective design and the small size of the cohort even though this is the largest published study in the field. That is why it is not possible to completely rule out the hypothesis of a fortuitous association of renal disease with hematologic disorder. For example, 1 patient presented with amyloidosis AA and suffered from ankylosing spondylitis for 28 years and from ET for 12 years. The chronic tubulointerstitial pattern observed on the kidney biopsy can be attributed to both ET and chronic lesions related to amyloidosis. Second, we could not perform exhaustive immunostaining, including CD61 and CALR staining,30 in all biopsies, but our recent results highlighted the need to better understand how myeloid clonal cells can trigger renal fibrosis. It has been shown that the presence of clonal hematopoiesis in peripheral-blood cells was associated with nearly a doubling in the risk of coronary heart disease in humans and with accelerated atherosclerosis in mice,36,37 suggesting clonal monocytes could also participate in kidney lesions. Third, electron microscopy was not available. Fourth, a diagnosis of atypical mastocytosis was ultimately established in a formerly diagnosed CMML. Fifth, our study was not designed to assess the incidence of AKI and CKD in patients with myeloid neoplasms but showed that (i) clinically significant renal involvement is uncommon: with 18 cases over 11 years the yearly incidence rate reached 1.6, which is very low; and (ii) it should be regularly searched for in patients with these peculiar forms of myeloid malignancies. Last, the only patient with PMF and renal involvement in our study did not have a kidney biopsy. But, as stated among, pathology of PMF patients was already described by Said et al.12,38,39
In summary, we show that renal complications of CMML and MPN are rare but characterized by poor renal and global prognosis. Glomerulosclerosis and interstitial fibrosis and tubular atrophy are the main kidney lesions and may be driven by megakaryocyte infiltration within the kidneys, opening a new therapeutic window. Regular screening for proteinuria and renal failure should be proposed to all patients with myeloid neoplasms in order to detect the kidney disease early and adapt the treatment of the malignancy.
Disclosures
All the authors declared no competing interests.
Author Contributions
SF and JB designed the study; CK collected the data; SF and JB analyzed and interpreted the data; MC performed pathologic analyses; IL and VD performed the bone marrow and molecular analyses; ST, DR, AH, DC, SF, JB, and OB-R followed the patients; JB and SF wrote the manuscript. All the authors read and approved the manuscript.
Footnotes
Table S1. Detailed clinical history and kidney pathology data, including lysozyme staining.
Supplementary Material
Table S1. Detailed clinical history and kidney pathology data, including lysozyme staining.
References
- 1.Arber D.A., Orazi A., Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Roman E., Smith A., Appleton S. Myeloid malignancies in the real-world: Occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 2004-15. Cancer Epidemiol. 2016;42:186–198. doi: 10.1016/j.canep.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoda R.C., Duek A., Grisouard J. Pathogenesis of myeloproliferative neoplasms. Exp Hematol. 2015;43:599–608. doi: 10.1016/j.exphem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Benton C.B., Nazha A., Pemmaraju N., Garcia-Manero G. Chronic myelomonocytic leukemia: Forefront of the field in 2015. Crit Rev Oncol Hematol. 2015;95:222–242. doi: 10.1016/j.critrevonc.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimran E., Hoffman R., Kremyanskaya M. Current approaches to challenging scenarios in myeloproliferative neoplasms. Expert Rev Anticancer Ther. 2018;18:567–578. doi: 10.1080/14737140.2018.1457441. [DOI] [PubMed] [Google Scholar]
- 6.Murphy I.G., Mitchell E.L., Raso-Barnett L. Imaging features of myeloproliferative neoplasms. Clin Radiol. 2017;72:801–809. doi: 10.1016/j.crad.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Strati P., Abdelrahim M., Selamet U. Ruxolitinib therapy is associated with improved renal function in patients with primary myelofibrosis. Ann Hematol. 2019;98:1611–1616. doi: 10.1007/s00277-019-03708-9. [DOI] [PubMed] [Google Scholar]
- 8.Baek S.W., Moon J.Y., Ryu H. Chronic kidney disease in the BCR-ABLl-negative myeloproliferative neoplasm: A single-center retrospective study. Korean J Intern Med. 2018;33:790–797. doi: 10.3904/kjim.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyams E.S., Gupta R., Melamed J. Renal involvement by chronic myelomonocytic leukemia requiring nephroureterectomy. Rev Urol. 2009;11:33–37. [PMC free article] [PubMed] [Google Scholar]
- 10.Bardy A., Tiple A., Rabant M. Les glomérulopathies associées aux néoplasies myéloprolifératives [Myeloproliferative neoplasms related glomerulopathy] Rev Med Interne. 2014;35:222–230. doi: 10.1016/j.revmed.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Perazella M.A., Buller G.K. Nephrotic syndrome associated with agnogenic myeloid metaplasia. Am J Nephrol. 1994;14:223–225. doi: 10.1159/000168720. [DOI] [PubMed] [Google Scholar]
- 12.Said S.M., Leung N., Sethi S. Myeloproliferative neoplasms cause glomerulopathy. Kidney Int. 2011;80:753–759. doi: 10.1038/ki.2011.147. [DOI] [PubMed] [Google Scholar]
- 13.Ugo V., Tondeur S., Menot M.L. Interlaboratory development and validation of a HRM method applied to the detection of JAK2 exon 12 mutations in polycythemia vera patients. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Coresh J., Balk E. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139 doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 16.Section 2: AKI Definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoriello D., Andal L.M., Cox R. Lysozyme-induced nephropathy. Kidney Int Rep. 2017;2:84–88. doi: 10.1016/j.ekir.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porta C., Cosmai L., Gallieni M. Renal effects of targeted anticancer therapies. Nat Rev Nephrol. 2015;11:354–370. doi: 10.1038/nrneph.2015.15. [DOI] [PubMed] [Google Scholar]
- 19.Bridoux F., Leung N., Hutchison C.A. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 20.Ribes D., Hachem H.E.L., Oberic L. Bendamustine plus rituximab for indolent B-cell lymphoma of renal significance. Am J Hematol. 2018;93:356–362. doi: 10.1002/ajh.24984. [DOI] [PubMed] [Google Scholar]
- 21.Patel T.V., Rennke H.G., Sloan J.M. A forgotten cause of kidney injury in chronic myelomonocytic leukemia. Am J Kidney Dis. 2009;54:159–164. doi: 10.1053/j.ajkd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh T.L., Carpenter L., Ly E. Lysozyme nephropathy in haematologically stable chronic myelomonocytic leukaemia. Nephrology. 2018;23:377. doi: 10.1111/nep.13056. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K., Yokote T., Tsuji M. Renal infiltration associated with chronic myelomonocytic leukaemia: images in haematology. Br J Haematol. 2009;147:414. doi: 10.1111/j.1365-2141.2009.07785.x. [DOI] [PubMed] [Google Scholar]
- 24.Hahm E., Wei C., Fernandez I. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23:100–106. doi: 10.1038/nm.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayek S.S., Sever S., Ko Y.A. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayek S.S., Leaf D.E., Tahhan A.S. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416–426. doi: 10.1056/NEJMoa1911481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeremy Wen Q., Yang Q., Goldenson B. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med. 2015;21:1473–1480. doi: 10.1038/nm.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenaux P., Kiladjian J.J., Platzbecker U. Luspatercept for the treatment of anemia in myelodysplastic syndromes and primary myelofibrosis. Blood. 2019;133:790–794. doi: 10.1182/blood-2018-11-876888. [DOI] [PubMed] [Google Scholar]
- 29.Wei C., Li J., Adair B.D. UPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest. 2019;129:1946–1959. doi: 10.1172/JCI124793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruyama K., Nakagawa N., Suzuki A. Novel detection of CALR-mutated cells in myeloproliferative neoplasm-related glomerulopathy with interstitial extramedullary hematopoiesis: a case report. Am J Kidney Dis. 2019;74:844–848. doi: 10.1053/j.ajkd.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Mekinian A., Grignano E., Braun T. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology. 2016;55:291–300. doi: 10.1093/rheumatology/kev294. [DOI] [PubMed] [Google Scholar]
- 32.Fraison J.B., Grignano E., Braun T. Autoantibodies in myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2019;60:2594–2596. doi: 10.1080/10428194.2019.1599114. [DOI] [PubMed] [Google Scholar]
- 33.Rajasekaran A., Ngo T.T., Abdelrahim M. Primary myelofibrosis associated glomerulopathy: significant improvement after therapy with ruxolitinib. BMC Nephrol. 2015;16 doi: 10.1186/s12882-015-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson B.A., Collins A.J., Vogelzang N.J., Bloomfield C.D. 5-Azacytidine and renal tubular dysfunction. Blood. 1981;57:182–185. [PubMed] [Google Scholar]
- 35.Guo C., Pei L., Xiao X. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 2017;92:1194–1205. doi: 10.1016/j.kint.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuster J.J., MacLauchlan S., Zuriaga M.A. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohra G.K., Meena D.S., Bajpai N., Purohit A. Focal segmental glomerulosclerosis in a patient with prefibrotic primary myelofibrosis. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philipponnet C., Ronco P., Aniort J. Membranous nephropathy and intrarenal extramedullary hematopoiesis in a patient with myelofibrosis. Am J Kidney Dis. 2017;70:874–877. doi: 10.1053/j.ajkd.2017.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.