Abstract
Introduction
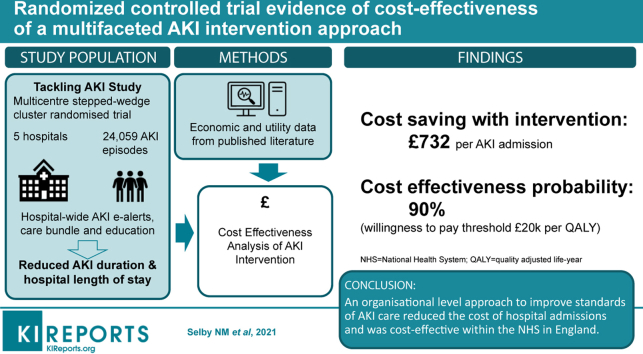
Acute kidney injury (AKI) is associated with increased health care utilization and higher costs. The Tackling AKI study was a multicenter, pragmatic, stepped-wedge cluster randomized trial that demonstrated a reduced hospital length of stay after implementation of a multifaceted AKI intervention (e-alerts, care bundle, and an education program). We tested whether this would result in cost savings.
Methods
A decision-analytic tree model from the payer perspective (National Health Service in the United Kingdom) was generated on which cost-effectiveness analyses were performed using a probabilistic sensitivity analysis, accounting only for direct medical costs. Clinical data from the Tackling AKI study were used as inputs and economic and utility data derived from relevant published literature.
Results
A total of 24,059 AKI episodes occurred during the study period, and in 18,887 admissions the patient was discharged alive. When all AKI stages were considered together, the cost per AKI admission was £5065 in the control arm and £4333 in the intervention arm, representing an incremental cost saving of £732 per admission with the intervention. Similar results were obtained when AKI stages were included as separate variables. Costs per quality-adjusted life year were £61,194 in the control group and £51,161 in the intervention group. At a willingness to pay threshold of £20,000 per quality-adjusted life year, the probability of the intervention being cost-effective compared with standard care was 90%.
Conclusion
An organizational level approach to improve standards of AKI care reduces the cost of hospital admissions and is cost effective within the National Health Service in the United Kingdom.
Keywords: AKI, care bundle, e-alert, health economics, length of stay
Graphical abstract
At least 10% of people admitted to the hospital sustain an acute kidney injury (AKI) as part of their acute illness during treatment or after surgery. In the United Kingdom (UK), there are >500,000 episodes of AKI annually,1 with worldwide estimates of the incidence of AKI extending to millions of affected individuals.2 AKI is associated with significantly increased risks of mortality and longer, more complicated hospital admissions, with a number of studies reporting the ensuing economic burden. UK studies include a patient-level costing approach that reported costs of a hospital admission with AKI to be £3748, rising to £8404 in those who required renal replacement therapy (RRT).3 In a pilot study of 48 patients with AKI, a microcostings exercise calculated the annual costs of an episode of AKI to be £5661 over 12 months.4 When these costs are extrapolated nationally, taking into account the large number of people affected, it becomes clear that the annual cost of AKI-related inpatient care is significant, with estimates of >£1 billion or 1% of the total budget for the National Health Service (NHS) in the UK.5 In Canada, the incremental cost of AKI is >$200 million Canadian dollars per year,6 with corresponding figures in the United States ranging between $5 billion and $24 billion US dollars.7,8
Despite this enormous economic impact, a lack of proven therapies for AKI has limited opportunities for cost-effectiveness studies, so at present it is uncertain whether costs associated with AKI are modifiable.4,9, 10, 11 The absence of therapies has also driven interest in strategies to improve the delivery of basic elements of AKI care. While some previous studies have reported improved patient outcomes with interventions designed to improve standards of clinical care, methodologic concerns, such as a lack of randomization, before and after design, and largely single-center approaches have prevented firm conclusions from being made.12, 13, 14, 15 To address this, the Tackling AKI study was the first multicenter randomized trial that tested the effectiveness of a multifaceted intervention (AKI e-alerts, an AKI care bundle, and an education program) on delivery of AKI care and patient outcomes. Although the intervention did not alter 30-day mortality, significant reductions in hospital length of stay (LoS) and AKI duration were observed in conjunction with improved rates of AKI recognition, medication review, fluid assessment, and urinalysis.16 These results led us to hypothesise that cost savings would be realized from shortened hospital LoS that would be greater than the costs required to deliver the intervention. We sought to test this in a cost-effectiveness analysis.
Methods
We aimed to determine the cost-effectiveness of an intervention designed to improve the systematic delivery of supportive AKI care. To do so, we developed a decision-analytic tree model from the payer (UK NHS) perspective on which cost-effectiveness analyses were performed, accounting only for direct medical costs. Clinical data from the Tackling AKI study database were used as inputs and economic and utility data derived from relevant sources in the published literature.
Study Cohort and Clinical Outcomes
The Tackling AKI study was a multicenter, pragmatic, stepped wedge cluster randomized trial that evaluated a complex intervention for AKI comprised of AKI e-alerts, an AKI care bundle, and an education program. Between December 2014 and February 2017, the intervention was introduced across 5 NHS hospital sites in the UK. Detailed methods, description of the intervention, and results have been published in full elsewhere.16,17 Inclusion criteria were broad, with all patients who were ≥18 years of age, had been hospitalized for ≥1 night during the study period, and who sustained AKI during that admission included in the study. AKI was defined according to serum creatinine according to modified Kidney Disease: Improving Global Outcomes (KDIGO) definitions,18 using the NHS England automated algorithm as previously described.19 Urine output criteria were not used. The only exclusion criterion was chronic dialysis for end-stage kidney disease. Data collection and analysis were conducted independently by researchers at the UK Renal Registry who were not involved in the delivery of the intervention at participating hospitals.
Statistical Analysis
In the primary analysis of the Tackling AKI study, 30-day mortality was analyzed using multilevel logistic regression at the individual patient level with hospital modelled as a random effect, and adjusting for time, patients’ covariables (age, gender, and comorbid conditions), and the effect of seasonality. Time was pooled into quarterly intervals and treated as equally spaced in analytic models. The hospital length of stay (LoS) and AKI duration data were highly skewed, so quantile regression models were fitted to allow comparisons at points across the whole distribution (after adjustment for age, gender, comorbid conditions, time, season, and center) in addition to comparison of average values.20,21 For LoS analyses, only patients who survived to hospital discharge were included. Statistical analyses were conducted using Stata MP12 (StataCorp LLC, College Station, TX) and SAS 9.3 (SAS, Cary, NC).
Costs
All costs were corrected for inflation to 2020 values (British pound) considering the current and historical consumer price inflation rate. The cost of hospital admission in the control group (hospitalized AKI episode receiving standard care) was taken from a UK study that used patient level costing.3 This gave corrected cost per admission for any stage of AKI of £5034 (95% confidence interval [CI] £4628–£5435), equating to a cost per day of £475. Using the same reference source, separate costs for each AKI stage were derived as follows: hospital stay with AKI stage 1 was £4343 (95% CI £3701–£5052, daily cost £418); hospital stay with AKI stage 2 incurred costs of £4306 (95% CI £3664–£4997, daily cost £437); and AKI stage 3 £5758 (95% CI £5173–£6355, daily cost £519).3 A cost of £1084 per day was assumed for AKI (any stage) requiring intensive care unit (ICU) admission, as per the 2019 to 2020 NHS reference price list.22
The cost of the intervention was calculated by estimating per patient costs of providing the education program and individual elements of the AKI care bundle, with incremental costs calculated using delivered activity in control and intervention arms of the study. As such, the incremental cost of the intervention was £11.04 per AKI admission; a breakdown of this calculation is shown in Table 1. Most of the cost of the intervention arose from additional activities of care (£10.07 per AKI episode), with only a small cost due to additional educational activities (£0.97 per AKI episode). Costs were not included for the setup of AKI alerts as these have been mandated in England since 2014 and were in place at all sites before the commencement of the study.23
Table 1.
Breakdown of individual costs that were attributed to the intervention
| Cost per AKI episode | Control period: Proportion of patients receiving element of careb | Control period: Average cost per patient of delivered care | Intervention: Proportion of patients receiving element of careb | Intervention: Average cost per patient of delivered care | Cost differential per AKI episode between control and intervention | ||
|---|---|---|---|---|---|---|---|
| Care bundle elementa | |||||||
| Fluid assessment | £31.72 | 74.4% | £23.60 | 91.2% | £28.93 | £5.33 | |
| Medication review | £31.72 | 60.1% | £19.06 | 71.3% | £22.61 | £3.55 | |
| Urinalysis | £4.35 | 37.4% | £1.63 | 64.7% | £2.82 | £1.19 | |
| Referral/sepsis management | No difference | No difference | |||||
| Subtotals | £67.79 | £44.29 | £54.36 | £10.07 | |||
| Education programme | |||||||
| Hours per year | Cost per year | Cost per AKI episode | |||||
| Extra educational events | 16c | £10,356 | £0.97 | ||||
| Total | £11.04 | ||||||
AKI, acute kidney injury.
Core elements that were included in care bundles at each of the Tackling AKI study sites. Costs for fluid assessment and medication review were based on staff time (general physician, £127 per hour,35 15 minutes per assessment). Urinalysis cost was taken from the National Clinical Guideline Centre document.36
The proportion of patients receiving each element of the care bundle was taken from the Tackling AKI study (process measures assessed in 1048 patients, comparing control and intervention periods).16
Comprised of departmental teaching, nursing/pharmacy/advanced practitioner teaching, and ad hoc ward-based teaching that were additional to activities that were already in place in control period (hospital grand rounds, postgraduate medical teaching, and induction training). Staff costs were estimated based on 50% of teaching delivered by senior clinician (£147 per hour) and 50% by AKI nurse specialist (£48 per hour) that were taken from Curtis and Burns.35
Cost-Effectiveness Analysis
As Tackling AKI was a pragmatic study, questionnaires to collect individual quality of life data were not possible. Utility data (quality-adjusted life years [QALYs]) were estimated using EQ-5D scores derived from a meta-analysis and transformed into utility per day,24 as described previously.5 We assumed that there was no difference in health utility between control and intervention arms, nor any differences between different AKI stages (i.e., QALYs were the same across these comparator groups), but total utility accrued could be influenced by number of days in hospital.25 For each quantile of hospital LoS, the QALY per quantile during a predefined and constant period of 36 cycles (days) were calculated taking into consideration the probability of being in that quantile. QALYs per arm (intervention vs. control) were then calculated using the sum of all QALYs per quantile. The incremental cost-effectiveness ratio was then calculated as follows:
| Cost of intervention − Cost of control |
| Utility of intervention − Utility of control |
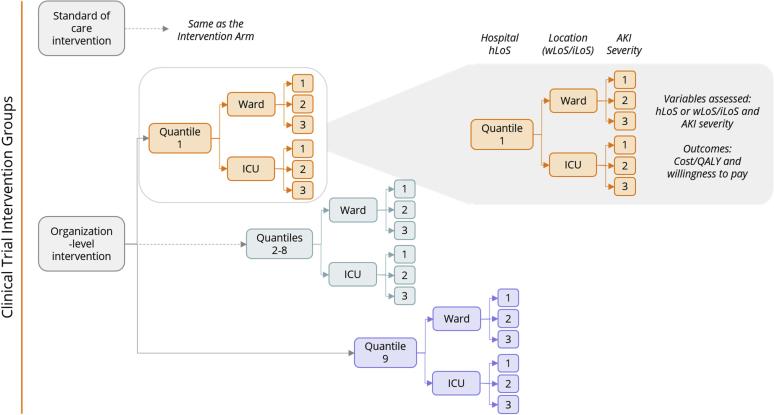
A decision-analytic tree model was constructed that included the intervention groups (standard of care intervention defined hereafter as control and organization-level intervention defined hereafter as the intervention), AKI stage and quantiles (1–10) for hospital LoS, although quantile 10 was excluded due to high variability. This is summarized in Figure 1. Cost-effectiveness was assessed using a probabilistic sensitivity analysis (PSA), which is typically conducted using a simulation approach as follows: for each of a sequence of iterations (s = 1,…, S), a value h(s) (e.g., length of duration) is simulated from the probability distribution. The decision analysis was then conducted using that specific value as if this were the value realised. The lognormal distribution was used for LoS data (parameters: mu/mean and sigma/standard deviation); a beta distribution for probabilities and a gamma distribution for the costs and utilities were used to propagate uncertainty (parameters: alpha and beta). The gamma distribution was used for variables that were continuous, always positive, and that had skewed data. By means of this procedure, it is possible to produce a sample from the distribution of utility, incremental cost, or any other related random quantity.26 We used this approach to generate probability, utility, and cost trees per study arm and quantile following the model tree structure using the clinical data from the Tackling AKI study database (duration from summary statistics on the LoS). Using the calculated CIs for the probability, duration, utility, and cost values, we generated 5000 iterations from these distributions. Models were also constructed with AKI separated into stages of severity (1–3) and with and without ICU admission. Models that did not include ICU admission were used as primary analysis, as ICU admission data from 2 hospitals in the study were not considered accurate (but considering the costs associated with ICU admission, ICU analyses are included as sensitivity analyses). A cost-effectiveness scatterplot was generated from the iterations from the PSA by plotting incremental costs (£) per incremental QALY. A cost-effectiveness acceptability curve for each model was generated by representing the probability of the intervention of being cost-effective for every willingness to pay (WTP). The WTP threshold for cost-effectiveness was taken as £20,000 per QALY, which is the lower end of the range used by the National Institute for Health and Care Excellence.27
Figure 1.
The decision-analytic tree model. AKI, acute kidney injury; ICU, intensive care unit; LoS, length of stay; QALY, quality-adjusted life year.
Results
Summary of Main Findings of the Clinical Study
During the study period, there were a total of 24,059 AKI episodes that occurred in 20,179 patients, giving a crude incidence of 7.6 AKI episodes per 100 admissions. There were 14,042 episodes of AKI (58.4%) in the control period and 10,017 (41.6%) in the intervention period. The characteristics of the study population were typical of a general hospitalized AKI cohort as summarized in Table 2.
Table 2.
Patient demographics in control and intervention periods
| Control | Intervention | |
|---|---|---|
| AKI episodes, n | 14,042 | 10,017 |
| Male, % | 50.3 | 48.1 |
| Age group, yrs, % | ||
| 18–59 | 23.1 | 20.3 |
| 60–69 | 15.7 | 15.3 |
| 70–79 | 23.7 | 23.5 |
| 80–89 | 27.2 | 29.8 |
| ≥90 | 10.3 | 11.1 |
| Median age, yrs | 75.4 | 76.6 |
| Charlson comorbidity score, % | ||
| 0 | 16.4 | 18.8 |
| 1 | 20.3 | 21.0 |
| 2 | 20.2 | 19.4 |
| ≥3 | 43.1 | 40.8 |
| Individual comorbidities, % | ||
| Previous myocardial infarction | 15.1 | 14.4 |
| Heart failure | 23.0 | 22.6 |
| Previous stroke | 7.0 | 6.9 |
| Diabetes mellitus | 27.3 | 28.1 |
| Chronic kidney disease | 22.0 | 23.5 |
| Chronic liver disease | 8.8 | 7.0 |
| Ethnicity, % | ||
| Afro-Caribbean | 1.4 | 0.8 |
| South Asian | 5.5 | 5.9 |
| Other | 2.8 | 2.8 |
| White | 86.1 | 85.3 |
| Missing | 4.2 | 5.2 |
| Social deprivation score,a % | ||
| 1 (least deprived) | 23.6 | 36.4 |
| 2 | 17.8 | 16.7 |
| 3 | 16.0 | 15.8 |
| 4 | 15.7 | 13.3 |
| 5 (most deprived) | 26.8 | 17.6 |
| Missing | 0.1 | 0.2 |
| Peak AKI stage, % per stage | ||
| 1 | 60.6 | 64.5 |
| 2 | 21.4 | 19.8 |
| 3 | 18.0 | 15.7 |
| Hospital-acquired AKI,b % | 53.8 | 49.4 |
| 30-day crude mortality, % | 25.2 | 23.9 |
| Median hospital LoS, days (IQR) | 10 (5–20) | 9 (4–18) |
AKI, acute kidney injury; Ashford, Ashford and St Peter’s Hospital; Bradford, Bradford Royal Infirmary; Frimley, Frimley Park Hospital; IQR, interquartile range; LGI, Leeds General Infirmary; LSJ, Leeds St James’ Hospital.
Data shown are unadjusted and because of the stepped wedge study design, centers contributed differing amounts of data to control and intervention periods. Unadjusted comparisons between control and intervention periods are therefore not valid.
Social deprivation scores show the proportion of patients in each quintile of the index of multiple deprivation.
Hospital-acquired AKI defined as AKI onset >24 hours after hospital admission.
Overall 30-day mortality was 24.5%, with no difference between control and intervention periods (OR 1.04 [95% CI 0.91–1.21]). A significant reduction in hospital LoS with the intervention was observed in the 18,887 admissions in which the patient was discharged alive. At the 0.5 quantile, the effect size was a reduced LoS of −0.7days, extending to −1.3 days at the 0.7 quantile. Overall, this represented a 6.6% (95% CI 1.3–11.6%) reduction in LoS (negative binomial regression). Similar results were seen in a sensitivity analysis that included all admissions, regardless of whether the patient was alive at discharge (reduced LoS of −0.8 days at the 0.5 quantile and −1.1 days at the 0.7 quantile). A reduction in AKI duration (defined as number of days between first and last serum creatinine results that met the definition of AKI) was also observed (from 0.7 quantile onwards with effect size in the range of −0.3 to −1.0 days). Only 2.6% of participants received acute RRT and this was no different between study arms (odds ratio 1.1 [95% CI 0.8–1.6]).
Cost Effectiveness Model
In a scenario that considered all AKI stages together and did not include ICU admission data, the cost per AKI admission was calculated to be £5065 in the control arm and £4333 in the intervention arm, representing an incremental cost saving of £732 per admission with the intervention. When AKI was separated into 3 stages of severity, the results were similar: cost per admission of £5016 in control group; £4287 in intervention group; and incremental cost saving with the intervention was £729. A scenario that considered ICU admission also showed a cost saving with the intervention, although this was reduced when AKI stages were considered separately (Supplementary Material).
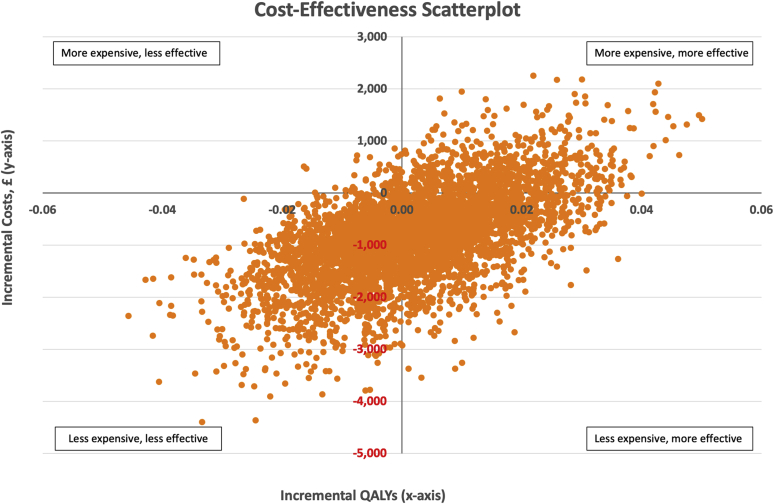
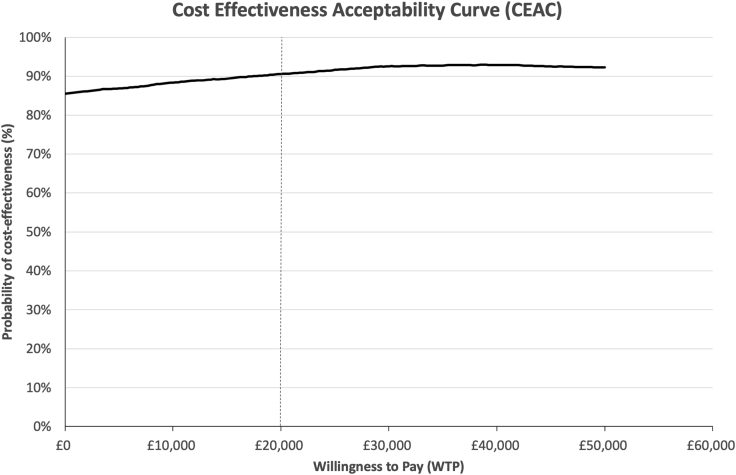
In the first scenario (AKI stage not included in model), QALYs were estimated at 0.08 in control and intervention arms, which resulted in costs per QALY of £61,194 and £52,161, respectively. The cost-effectiveness scatterplot generated from the PSA is shown in Figure 2. Of the 5000 iterations, 44.7% suggested the intervention was more effective and less expensive, 13.3% more effective but more expensive, 40.8% that the intervention was both less effective and less expensive, and only 1.2% that the intervention was less effective but more expensive. At the WTP threshold of £20,000 per QALY, the probability of the intervention being cost-effective as compared with standard care was 90.1%. Even at a WTP threshold of £0 per QALY, the probability of cost-effectiveness was 85.5%. Figure 3 shows these results as a cost-effectiveness acceptability curve.
Figure 2.
Cost-effectiveness scatterplot showing the 5000 iterations from the probabilistic sensitivity analysis for incremental costs (£) and incremental quality-adjusted life years (QALYs).
Figure 3.
Cost effectiveness acceptability curve, representing the probability of the intervention of being cost effective for every willingness to pay (WTP) value up to a maximum WTP of £50,000. The WTP threshold was taken as £20,000 per quality-adjusted life year.37
The second scenario, when AKI stages were considered separately, generated similar results. Costs per QALY were £60,607 in the control group versus £51,608 with the intervention, and at the WTP threshold of £20,000 per QALY there was a 96.7% probability of the intervention being cost-effective. The cost-effectiveness scatterplot and cost-effectiveness acceptability curve for this scenario are shown in Supplementary Figure S4. The models including ICU admission should be interpreted with caution but are also included as Supplementary Table S3 and Supplementary Figure S5—probability of cost-effectiveness in these models ranges between 82.9% and 86.7%.
Extrapolation of Findings
Since 2014, the UK Renal Registry has collected nationwide data on AKI in England, using the same approach as the Tackling AKI study to define AKI episodes. The recently published report from the UKRR (using 2018 data) described 313,932 hospital admissions with an AKI episode, who had similar patient demographics and AKI characteristics as those in the Tackling AKI study, and a median hospital LoS of 12 days (IQR 6–24 days).1 Of these, 221,894 admissions met standards for data reliability and survived to hospital discharge. A crude extrapolation of the £732 incremental cost saving from our model across these admissions would suggest a cost-saving in the range of £162.4 to £229.8 million per annum to NHS England, assuming that the Tackling AKI intervention was rolled out to all hospitals and had the same costs and effect in all. As a reference, total NHS England spending (Department of Health and Social Care) in 2018 and 2019 was £130 billion,28 so £162.4 million represents 0.12% of this amount. Alternatively, this would represent a 16% cost savings against the estimated annual costs of £1.02 billion for AKI-related inpatient care in England.5
Discussion
The Tackling AKI study demonstrated that an organizational level intervention aimed at improving consistency of delivered AKI care resulted in reduced hospital LoS. In this study, we show that this leads to an incremental cost saving and that the intervention, consisting of AKI e-alerts, an AKI care bundle, and an education program, is cost effective in the setting of the NHS in England.
Health care costs associated with AKI are substantial. In the UK, these have been estimated at £1.02 billion per year,5 with corresponding figures in the United States of $5 billion to $24 billion.7,8 Increased resource utilization arises from longer hospital stays, the requirement for ICU care and RRT, as well as costs attributable to long-term sequelae, such as chronic kidney disease, end-stage kidney disease, and cardiovascular disease. Of these, RRT is particularly expensive on an individual patient basis but is required in only a small percentage of patients. At a population level, excess bed days are a major driver of additional costs. In the study by Kerr et al.,5 81% (£825 million) of the annual £1.02 billion cost of inpatient AKI care in England was incurred by excess bed days. Similarly, a US study found the incremental cost of an episode of AKI after coronary intervention to be $9448, of which half was due to increased length of hospital stay.29 While the economic impact of AKI has been well described,8 there are few examples of studies that have reported cost-effectiveness analyses, making it challenging to determine whether cost savings can be realized through improved management of AKI. Mistry et al.4 performed a micro-costings exercise in a pilot study of an AKI outreach service as compared with standard care. Only 48 patients were studied, which limited statistical power, although trends toward shorter LoS and costs were suggested.4 In a study that tested an AKI alerting and decision-making application, Connell et al.10 reported a reduction in inpatient costs of £2123 (possibly due to shorter LoS) but did not consider the costs of providing the intervention, so cost-effectiveness analyses were precluded. In a randomized trial, a biomarker-guided care bundle after major abdominal surgery led to a lower incidence of severe AKI (stages 2–3), shorter duration of ICU stay, and reduced hospital LoS, which was estimated to result in a cost savings of €2301 per admission.30 Other than this, studies have either focused on critical care, choice of RRT modality, or theoretical models.9,31
Our study is consistent with these results and adds to observations that costs of AKI admissions can be reduced by organization-wide interventions that reduce hospital LoS. Our study has been performed using data from a rigorously performed randomized trial and shows that an approach focused on improving delivered AKI care is cost effective, with a high probability of cost effectiveness across the range of WTP thresholds. These findings were robust in PSA analyses with 5000 model iterations and across different clinical scenarios. The dominant effect is facilitated by the low cost of the intervention. In our assumptions, we were deliberately conservative by including additional senior clinician time for delivery of care and for the educational activities. In practice, it is likely that some of these actions can be carried out by existing staff, which would incur lower expenditure for implementation. Conversely, we did not include initial expenditure for installation of the AKI alerts as this has been mandated in England since 2014 and was in place at participating centers in the Tackling AKI study. However, a one-off cost for software purchase is unlikely to change the cost per AKI episode of the intervention significantly due to the high incidence of AKI. This is shown as the cost for delivering the education program (>£10,000 per year) changed the cost per AKI episode by <£1. It is also possible that additional unmeasured expense could have resulted if there were changes in practice with the intervention that were not included in the modelling (e.g., additional laboratory testing), although it should be noted that there were no differences observed in rates of specialist referral, renal imaging, and urinary catheterization between the 2 arms.
The relevance of these results to organizations, commissioners, and for those involved in health care reimbursement is that wider adoption of similar strategies could result in significant systemwide cost savings. Extrapolating results beyond the scope of the Tacking AKI study should be regarded with a degree of caution, because it is unlikely that costs and clinical effects of the Tackling AKI intervention would be identical across different hospitals, it is possible that the intervention may require tailoring for local context to maintain effectiveness, and some hospitals may now have AKI initiatives in place that were not in place when the Tackling AKI study was conceived, including responses to the Think Kidneys Programme.32 However, comparison with the UKRR dataset allows a nationwide view on the potential health care savings to the NHS in England, which could amount to >£100 million if AKI care was the focus of systemwide improvement. While crude, these extrapolations are premised on identical detection of AKI episodes using the same creatinine-based algorithm in both the UKRR dataset and the Tackling AKI study, and that the sociodemographic details of the patient cohorts are comparable. Although reductions in hospital LoS are one of the most consistently reported effects of AKI quality improvement initiatives, these estimations should be tempered by the range of effect sizes reported in the published literature (with some studies reporting zero effect).12,13,33,34 It should also be noted that our findings may not be generalizable outside of the NHS, although there are reports from outside of the UK that suggest similar effects, for example a US study that reported a similar magnitude of reduced hospital LoS after introduction of a clinical decision support system for AKI.15
There are several limitations of this study. Because of the pragmatic design of the Tackling AKI study, there was no available information on quality of life (utility data), nor detailed costings for an AKI admission during the control period, so values were taken from the published literature. However, we aimed to make conservative assumptions so assigned the same utility data values (QALYs) to control and intervention arms and to different stages of AKI. We also used UK data sources. ICU admissions were not accurately coded in the Tackling AKI dataset, so although the effect of ICU admission was included in a sensitivity analysis, results of models containing ICU admission should be regarded with caution. Inclusion of ICU admission did however confirm that the main findings of the cost-effectiveness analysis were not significantly altered. We considered only direct health care costs and did not have access to long-term clinical or health utility outcomes, including costs associated with hospital readmission. We did not include the cost of implementing e-alerts, and although this may have altered the cost per AKI episode in the intervention arm, a “one-off” cost is unlikely to have changed the results significantly. However, this should be considered if implementation in a different organization did incur such a cost. A further limitation is that while clinician time was included as part of the cost of the intervention, it was not possible to estimate whether this led to effects elsewhere in the system due to redistribution of clinician time. Finally, extrapolations of potential cost savings beyond the Tackling AKI dataset are hypothesis-generating only.
In conclusion, an organizational level approach to improve standards of AKI care comprising of e-alerts, a care bundle, and health care provider education reduces the cost of hospital admissions with AKI and is cost-effective within the NHS in England.
Disclosures
Supported by a grant from the Health Foundation (award number 1502-Derby-Selby-SUI). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no other competing interests.
Author Contributions
NMS designed and led the study, AC collected and analyzed clinical data, LKG, DRA, KDB, DS, CR, and GB were responsible for the cost-effectiveness analyses, NVK helped with UK economic and utility data, and all authors have been involved in the drafting and final approval of this article and agree to be accountable for the aspects of the work to which they contributed.
Footnotes
Table S1. Cost and cost-effectiveness analyses from models that included ICU admission.
Figure S1. Cost-effectiveness scatterplot and cost effectiveness acceptability curve for model that included AKI stages 1–3 separately (ICU admission not considered)
Figure S2. Cost-effectiveness scatterplots and cost effectiveness acceptability curves for models that included ICU admission
Supplementary Material
Table S1. Cost and cost-effectiveness analyses from models that included ICU admission.
Figure S1. Cost-effectiveness scatterplot and cost effectiveness acceptability curve for model that included AKI stages 1–3 separately (ICU admission not considered)
Figure S2. Cost-effectiveness scatterplots and cost effectiveness acceptability curves for models that included ICU admission
References
- 1.The Renal Association website Acute kidney injury (AKI) in England – a report on the nationwide collection of AKI warning test scores from 2018. https://renal.org/audit-research/publications-presentations/report/acute-kidney-injury-aki-england-report-nationwide Available at:
- 2.Hoste E.A.J., Kellum J.A., Selby N.M. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607–625. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 3.Kolhe N.V., Eldehni M.T., Selby N.M. The reimbursement and cost of acute kidney injury: a UK hospital perspective. Nephron Clin Pract. 2014;126:51–56. doi: 10.1159/000358435. [DOI] [PubMed] [Google Scholar]
- 4.Mistry H., Abdelaziz T.S., Thomas M. A prospective micro-costing pilot study of the health economic costs of acute kidney injury. Kidney Int Rep. 2018;3:1285–1293. doi: 10.1016/j.ekir.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr M., Bedford M., Matthews B. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29:1362–1368. doi: 10.1093/ndt/gfu016. [DOI] [PubMed] [Google Scholar]
- 6.Collister D., Pannu N., Ye F. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12:1733–1743. doi: 10.2215/CJN.00950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver S.A., Chertow G.M. The economic consequences of acute kidney injury. Nephron. 2017;137:297–301. doi: 10.1159/000475607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasta J.F., Kane-Gill S. Review of the literature on the costs associated with acute kidney injury. J Pharm Pract. 2019;32:292–302. doi: 10.1177/0897190019852556. [DOI] [PubMed] [Google Scholar]
- 9.Berdugo M.A., Kirson N.Y., Zimmer L. Economic and clinical benefits of early identification of acute kidney injury using a urinary biomarker. J Med Econ. 2019;22:1281–1289. doi: 10.1080/13696998.2019.1636053. [DOI] [PubMed] [Google Scholar]
- 10.Connell A., Raine R., Martin P. Implementation of a digitally enabled care pathway (part 1): impact on clinical outcomes and associated health care costs. J Med Internet Res. 2019;21:e13147. doi: 10.2196/13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Francesco M., Ronco C., Wacinski P.J. Economic impact of contrast-induced acute kidney injury associated with invasive cardiology: role of iso-osmolar contrast media in Germany, Italy, Poland, and Spain. J Med Econ. 2016;19:158–168. doi: 10.3111/13696998.2015.1105809. [DOI] [PubMed] [Google Scholar]
- 12.Ebah L., Hanumapura P., Waring D. A multifaceted quality improvement programme to improve acute kidney injury care and outcomes in a large teaching hospital. BMJ Qual Improv Rep. 2017;6 doi: 10.1136/bmjquality.u219176.w7476. u219176.w7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekar T., Sharma A., Tennent L. A whole system approach to improving mortality associated with acute kidney injury. QJM. 2017;110:657–666. doi: 10.1093/qjmed/hcx101. [DOI] [PubMed] [Google Scholar]
- 14.Kolhe N.V., Reilly T., Leung J. A simple care bundle for use in acute kidney injury: a propensity score matched cohort study. Nephrol Dial Transplant. 2016;31:1846–1854. doi: 10.1093/ndt/gfw087. [DOI] [PubMed] [Google Scholar]
- 15.Al-Jaghbeer M., Dealmeida D., Bilderback A. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. 2018;29:654–660. doi: 10.1681/ASN.2017070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby N.M., Casula A., Lamming L. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. 2019;30:505–515. doi: 10.1681/ASN.2018090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamming L., McDonach E., Mohammed M.A. Barriers and enablers to the implementation of a complex quality improvement intervention for acute kidney injury: a qualitative evaluation of stakeholder perceptions of the Tackling AKI study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0222444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 19.Holmes J., Rainer T., Geen J. Acute kidney injury in the era of the AKI e-alert. Clin J Am Soc Nephrol. 2016;11:2123–2131. doi: 10.2215/CJN.05170516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen C.S., Clark A.E., Thomas A.M. Comparing least-squares and quantile regression approaches to analyzing median hospital charges. Acad Emerg Med. 2012;19:866–875. doi: 10.1111/j.1553-2712.2012.01388.x. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y., Carroll R.J. Quantile regression with measurement error. J Am Stat Assoc. 2009;104:1129–1143. doi: 10.1198/jasa.2009.tm08420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Service England 2019/20 National Tariff Payment System. https://www.england.nhs.uk/pay-syst/national-tariff/2019-20-payment-reform-proposals/ Available at:
- 23.Selby N.M., Hill R., Fluck R.J. Standardizing the early identification of acute kidney injury: the NHS England National Patient Safety Alert. Nephron. 2015;131:113–117. doi: 10.1159/000439146. [DOI] [PubMed] [Google Scholar]
- 24.Wyld M., Morton R.L., Hayen A. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soliman I.W., Frencken J.F., Peelen L.M. The predictive value of early acute kidney injury for long-term survival and quality of life of critically ill patients. Crit Care. 2016;20:242. doi: 10.1186/s13054-016-1416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baio G., Dawid A.P. Probabilistic sensitivity analysis in health economics. Stat Methods Med Res. 2015;24:615–634. doi: 10.1177/0962280211419832. [DOI] [PubMed] [Google Scholar]
- 27.Paulden M. Recent amendments to NICE’s value-based assessment of health technologies: implicitly inequitable? Expert Rev Pharmacoecon Outcomes Res. 2017;17:239–242. doi: 10.1080/14737167.2017.1330152. [DOI] [PubMed] [Google Scholar]
- 28.UK Government, Department of Health and Social Care DHSC annual report and accounts: 2018 to 2019. https://www.gov.uk/government/publications/dhsc-annual-report-and-accounts-2018-to-2019 Available at:
- 29.Amin A.P., McNeely C., Spertus J.A. Incremental cost of acute kidney injury after percutaneous coronary intervention in the United States. Am J Cardiol. 2020;125:29–33. doi: 10.1016/j.amjcard.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Gocze I., Jauch D., Gotz M. Biomarker-guided intervention to prevent acute kidney injury after major surgery: The prospective randomized BigpAK study. Ann Surg. 2018;267:1013–1020. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 31.De Smedt D.M., Elseviers M.M., Lins R.L. Economic evaluation of different treatment modalities in acute kidney injury. Nephrol Dial Transplant. 2012;27:4095–4101. doi: 10.1093/ndt/gfs410. [DOI] [PubMed] [Google Scholar]
- 32.National Health Service England Think kidneys. www.thinkkidneys.nhs.uk Available at:
- 33.Sykes L., Sinha S., Hegarty J. Reducing acute kidney injury incidence and progression in a large teaching hospital. BMJ Open Qual. 2018;7 doi: 10.1136/bmjoq-2017-000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logan R., Davey P., Davie A. Care bundles for acute kidney injury: a balanced accounting of the impact of implementation in an acute medical unit. BMJ Open Qual. 2018;7 doi: 10.1136/bmjoq-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis L., Burns A. Unit Costs of Health and Social Care 2019, Personal Social Services Research Unit, University of Kent, Canterbury. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ Available at:
- 36.National Institute for Health and Care Excellence Preoperative tests. Appendix M: economic considerations for Delphi. https://www.ncbi.nlm.nih.gov/books/NBK367888/ Available at:
- 37.Devlin N., Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–452. doi: 10.1002/hec.864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.