ABSTRACT
Increasing evidence indicates that enhanced peripheral carbonyl stress markers exist in subtype of schizophrenia, although it may not be the primary cause.
This study aimed to investigate whether plasma concentrations of methylglyoxal, 3-deoxy-glucosone, and glyoxal, which are reactive intermediates of protein metabolism in carbonyl stress, are changed in patients with schizophrenia and can function as potential biomarkers for schizophrenia with enhanced carbonyl stress.
Plasma concentrations of these di-carbonyls were simultaneously estimated in 40 patients with schizophrenia and 40 healthy controls.
As a result, no statistically significant differences were observed in mean plasma concentrations of three di-carbonyls between patients and controls. However, a remarkable increase in methylglyoxal concentrations was observed in four patients but not in controls. This increase was not found with regard to 3-deoxyglucosone and glyoxal both of patients and controls.
Our correlation analysis showed that both the plasma methylglyoxal and glyoxal concentrations were significantly correlated with 3-deoxyglucosone concentrations in 40 patients and 40 controls. However, the plasma methylglyoxal concentrations did not show any significant correlation with the glyoxal concentrations in the patients or the controls. In four patients with extremely high methylglyoxal levels, the plasma methylglyoxal and glyoxal concentrations were not correlated to the 3-deoxyglucosone concentrations.
Methylglyoxal is a physiological substrate of the glyoxalase system, and the accelerated accumulation of this compound lowers the glyoxalase I activity.
These results suggested that this increase in four patients with high methylglyoxal levels may indicate the presence of a subtype of chronic schizophrenia that is associated with enhanced carbonyl stress.
Key Words: schizophrenia, carbonyl stress, methylglyoxal, glyoxal, 3-deoxyglucosone
INTRODUCTION
Biomarker analysis has not yet been included in the diagnostic criteria for patients with schizophrenia; however, it is very important to identify potential biomarkers for this disease to avoid its heterologous expression and understand its pathogenesis and molecular basis of each substance implicated.
Recent reports have revealed some interesting evidences indicating that oxidative stress affecting a possible pathophysiological mechanism in schizophrenia1-4 and carbonyl stress affecting the peripheral tissues are involved in the pathophysiology of schizophrenia.5 Especially, increasing evidence indicates that enhanced peripheral carbonyl stress markers exist in subtype of schizophrenia, although it may not be the primary cause.5-8 Previous study reported regarding a missense polymorphism of glyoxalase I (GLO I) in patients with schizophrenia resulting in 50% GLO I dysfunction and a significant increase in the advanced glycation end product (AGE), pentosidine, in the peripheral blood of patients with chronic schizophrenia compared with controls.6 Patients with high pentosidine levels had genetic factors that revealed decreased GLO I activity. Their studies have been conducted to examine the use of pentosidine as a disease biomarker.6,8
GLO I and certain aldehyde reductase and dehydrogenase isozymes detoxify reactive carbonyls and α-oxoaldehyde glycating compounds (methylglyoxal, glyoxal, and 3-deoxyglucosone and others).9,10 GLO I also represents a part of the enzymatic defense against glycation.11,12 Accordingly, decreased GLO I activity results in insufficient detoxification of the reactive di-carbonyls, the intermediates of AGE formation, with accelerated accumulation of di-carbonyls and AGE formation in vivo,12 and further influence the clinical features in schizophrenia.13
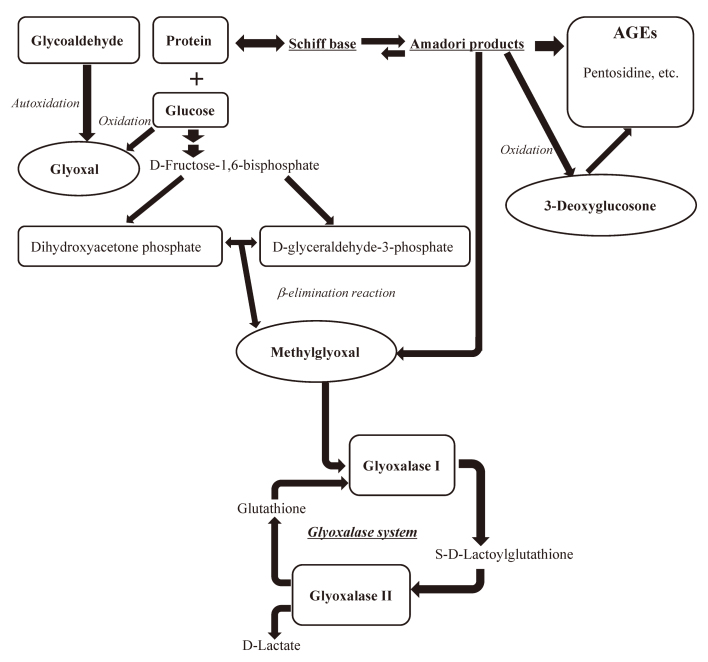
Methylglyoxal (MGO), Glyoxal (GO), and 3-Deoxyglucosone (3-DG) are normal metabolic products and are well-known reactive α, β-di-carbonyl intermediates in AGE formation. MGO, GO, and 3-DG react with proteins, nucleotides, and lipids through various pathways,12 forming N-carboxyalkyl amino acids, imidazolones, and imidazolium salts.14-16 As shown in Fig. 1, AGEs, including pentosidine, can be formed via GO generated from metal-catalyzed autoxidation of glucose, MGO generated from non-enzymatic fragmentation of triose phosphates11 and Amadori product. 3-DG induces AGE formation in the early stage of Maillard reaction. MGO is a physiological substrate of GLO I and is mainly metabolized to the unreactive D-lactate by the glyoxalase system (GLO I and GLO II) in the presence of glutathione, thus, its detoxification is reflected by GLO I dysfunction12 (Fig. 1).
Fig. 1.
Flow chart showing the production pathway of 3 di-carbonyl compounds
These di-carbonyls lead to the generation of advanced glycation end products (AGEs).
These di-carbonyl compounds are vital reactive intermediates that induce AGE formation; however, because of their instability in the atmosphere, there are no reports of their use as biomarkers for schizophrenia with enhanced carbonyl stress.
In the present study, we determined the estimated plasma concentrations of MGO, GO, and 3-DG, substrates of protein metabolism in carbonyl stress, simultaneously using a highly selective and specific assay, electrospray ionization liquid chromatography-mass spectrometry. We investigated whether the plasma concentrations of these parameters were changed in patients with schizophrenia and if these 3 di-carbonyls could function as potential biomarkers for chronic schizophrenia that enhanced carbonyl stress.
MATERIALS AND METHODS
Patients
Fresh plasma samples were obtained from 40 Japanese patients with chronic schizophrenia (all patients’ duration of illness were over 20 years and all patients were hospitalized in Higashiowari National hospital, mean age; 49.2 years, SD; 8.8 years) and 40 healthy Japanese controls (mean age; 41.2 years, SD, 8.7 years, Table 1). The patient group comprised 28 male (mean age, 49.9 years; SD, 8.9 years) and 12 female (mean age, 47.5 years; SD, 8.8 years) subjects. The control group comprised 23 male (mean age, 42.8 years; SD, 8.2 years) and 17 female (mean age, 40.0 years; SD, 9.6 years) subjects.
Table 1.
Clinical characteristics and plasma 3 di-carbonyl compound levels in patients with schizophrenia and control subjects
| Schizophrenic patients | Healthy Controls | |
| Number of subjects | 40 | 40 |
| Gender (Male/Female) | 28/12 | 23/17 |
| Age (years) | 49.2 ± 8.8 | 41.2 ± 8.7 |
| Disease duration | over 20 years | none |
| Family history | 12/40 | none |
|
MGO (ng/ml)
(Minimum – Maximum) (ng/ml) |
118.3 ± 58.2
(46.6 – 266.8) |
120.2 ± 42.7
(34.9 – 198.5) |
|
GO (ng/ml)
(Minimum – Maximum) (ng/ml) |
42.7 ± 16.4
(16.2 – 90.4) |
51.7 ± 27.7
(18.2 – 112.3) |
|
3-DG (ng/ml)
(Minimum – Maximum) (ng/ml) |
48.0 ± 10.8
(24.4 – 82.1) |
48.6 ± 17.0
(17.9 – 91.4) |
MGO: Methylglyoxal, GO: Glyoxal, 3-DG: 3-Deoxyglucosone.
Data are expressed as means ± standard deviations (range).
No significant differences were found in the mean values and distribution of gender and age between patients and controls. Schizophrenia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition with consensus from experienced psychiatrists. Furthermore, available medical records and family information were considered. All patients included in the study had a severe clinical condition, with no significant differences in physical factors, disease duration, or pharmacological treatment.
Control subjects were recruited from the hospital staff after confirming the absence of mental illness based on a short interview conducted by experienced psychiatrists.
No patient or control subject had diabetes mellitus or renal dysfunction (estimated glomerular filtration rate > 60 ml/min).
All participants provided written informed consent to participate, and study protocol was approved by the ethics committee of all participating institutions (Nagoya University Research Center of Health, Physical Fitness and Sports, Aichi Medical University, and National Hospital Organization Higashiowari National Hospital).
All the plasma samples were separated within 15 minutes after blood collection to prevent the autoxidation of these di-carbonyls, sealed with nitrogen and stored at −40°C prior to analysis.
Measurement of MGO, GO, and 3-DG
The plasma levels of these compounds were simultaneously measured in 40 Japanese patients and 40 healthy Japanese controls using an electrospray ionization liquid chromatography-mass spectrometry (ESI/LC/MS). For the mass spectrometric analysis, the formation of MGO, GO, and 3-DG derivatives on reaction with 2, 3-diaminonaphthalene was performed as per a previously reported method.17
In this study, these derivatization reactions including incubations, extractions and evaporations were performed at 4°C under nitrogen atmosphere.
All the derivatized di-carbonyl compounds were injected into a reversed-phase high-performance liquid chromatography and analyzed with ESI/LC/MS using a LTQ-Velos mass spectrometer (Thermo fisher, USA).
To identify and confirm the structures of each di-carbonyl derivative, daughter ions (positive ions) were trapped and monitored with an electrospray/mass spectrometry/mass spectrometry (ESI/MS/MS) system.
Quantitative analysis of the three di-carbonyl compounds was performed according to each protonated molecular ion peak area ratio obtained by the selected ion monitoring mode spectra. The correlation coefficient between the added MGO standard concentration and peak area (m/z: 195) ratio was 0.99 (regression equation: y = 1079.7x + 5503.9, with standard concentration range from 10 ng/ml to 500 ng/ml ), between the GO standard concentration and peak area ratio (m/z: 181) was 0.98 (regression equation: y = 398.5x + 1558.8, with standard concentration range from 5 ng/ml to 500 ng/ml ), and between the 3-DG standard concentration and peak area ratio (m/z: 285) was 0.99 (regression equation: y = 1240.0x + 5204, with standard concentration range from 5 ng/ml to 500 ng/ml).
Statistical Analysis
Mean and SD were estimated and data were compared using unpaired t-test or the Mann–Whitney U-test (both 2-tailed).
Plasma concentrations of the three compounds were compared between the 40 patients and 40 controls using the Mann–Whitney U-test.
RESULTS
Table 1 showed the characteristics of patients with schizophrenia and healthy controls.
No significant differences were found between groups in the mean values or distribution of gender and age. No correlation was found among plasma levels of the three di-carbonyls and age in patients (MGO: r = 0.139, P < 0.05; 3-DG: r = −0.018, P < 0.05; GO: r = −0.179, P < 0.05) or controls (MGO: r = 0.051, P < 0.05; 3-DG: r = 0.182, P < 0.05; GO: r = 0.076, P < 0.05).
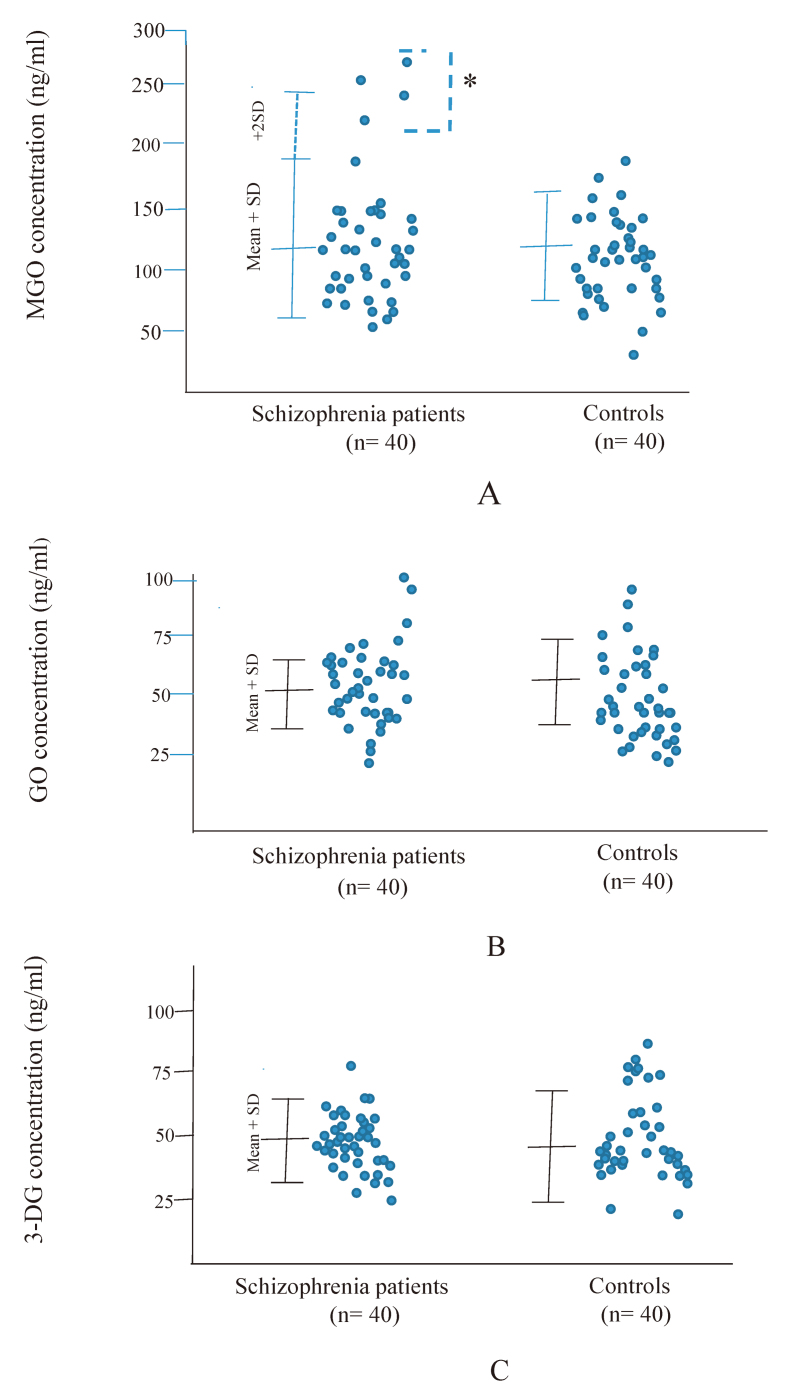
Plasma accumulation of MGO, GO and 3-DG in patients and controls was depicted in Fig. 2, respectively.
Fig. 2.
Plasma concentrations of 3 dicarbonyl compounds in 40 patients with chronic schizophrenia and 40 healthy controls
Fig. 2A: Plasma concentrations of Methylglyoxal (MGO)
Fig. 2B: Glyoxal (GO)
Fig. 2C: 3-Deoxyglucosone (3-DG)
Four patients with extremely high MGO concentrations (> 2 SD higher than the mean in controls) were indicated with * (n = 4). Values were compared with the 2-tailed Mann-Whitney U test. Error bars indicate mean and SD.
From our results, no statistically significant differences were observed in the mean plasma levels of the three di-carbonyls between patients and controls using Mann -Whitney’s U test (Fig. 2A to C). No correlation was found among plasma accumulations of these carbonyls and the disease duration or family history.
However, a remarkable increase in MGO concentrations (higher than mean + 2 SD) was observed in four patients with schizophrenia (10%) but not in controls (Fig. 2A).
As shown in Fig. 2B and 2C, this di-carbonyl increase was only found in MGO levels of the patient group, whereas this was not observed with regard to 3-DG and GO both of patients and controls.
The correlation analysis showed that the plasma MGO and GO concentrations were both significantly correlated with the plasma 3-DG concentrations in 40 patients (MGO: r = 0.450, p < 0.05; GO: r = 0.491, p < 0.05) and in 40 controls (MGO: r = 0.456, p < 0.05; GO: r = 0.484, p < 0.05). In contrast, plasma MGO concentrations did not show any significant positive correlations with the GO concentrations in the patient (n = 40, r = 0.191) or control (n = 40, r = 0.350) group.
In four patients with extremely high MGO levels (> 2 SD higher than that in controls), there was no significant correlation of the plasma MGO, and GO concentrations with the 3-DG concentrations.
These four patients showed severe clinical conditions similar to those in the other 36 patients in this study; however, each psychological symptom was different in the two groups.
Clinically, there was no significant difference in the average total scores of the positive and negative syndrome scale (PANSS) in the four patients with extremely high MGO levels (average total PANSS 111.3, mean age 51.0 ± 9.1 years, and mean MGO concentration 242.1 ± 27.1 ng/mL) and eight randomly selected patients (average total PANSS 105.8, mean age 48.3 ± 8.0 years, mean MGO concentration 76.4 ± 30.4 ng/mL). We confirmed that the number statistically required for comparison with the four patients with high MGO levels was doubled, and then we determined their PANSS scores for eight randomly selected cases. However, the scores for each psychological category were different between the two groups. Although there were no differences in the positive symptoms of the PANSS, unpaired t-test showed significant differences in the negative symptom such as emotional withdrawal (p < 0.05) and tension (p < 0.01) (Table 2).
Table 2.
Plasma concentrations of 3 di-carbonyl compounds and total PANSS scores in the four patients with extremely high MGO concentrations (Patient No. A, B, C, D) and eight randomly selected patients (Patient No. from E to L).
| Patient No. | A | B | C | D | Mean | E | F | G | H | I | J | K | L | Mean |
| Gender | M | M | F | M | M3/F1 | M | M | F | F | F | M | F | M | M4/F4 |
| Age (Years) | 37 | 49 | 59 | 59 | 51 | 48 | 64 | 51 | 35 | 44 | 44 | 46 | 54 | 48.3 |
| MGO (ng/ml) | 248.3 | 266.8 | 240.6 | 212.5 | 242.1 | 87.3 | 128.0 | 62.1 | 122.2 | 46.6 | 50.4 | 58.2 | 56.3 | 76.4 |
| GO (ng/ml) | 90.4 | 69.5 | 39.1 | 40.5 | 59.9 | 22.3 | 38.3 | 34.6 | 47.5 | 35.1 | 53.3 | 27.2 | 9.2 | 33.4 |
| 3-DG (ng/ml) | 38.9 | 57.7 | 44.9 | 61.1 | 50.7 | 55.7 | 49.7 | 60.8 | 47.1 | 43.5 | 67.0 | 48.3 | 28.1 | 50.0 |
| T. PANSS | 115 | 109 | 98 | 123 | 111.3 | 84 | 97 | 109 | 98 | 100 | 107 | 134 | 117 | 105.8 |
| Emotional withdrawal† | 4 | 6 | 4 | 4 | 4.5 | 1 | 4 | 4 | 3 | 3 | 3 | 3 | 4 | 3.1 |
| Tension§ | 3 | 4 | 2 | 4 | 3.25 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2.1 |
T. PANSS, Total Positive and Negative Symptom Scale scores. M, Male. F, Female. Mean values are in bold.
†and §shows the symptoms that have significant differences estimated using unpaired t-test; †p < 0.05, §p < 0.01.
DISCUSSION
This study aimed to investigate whether three di-carbonyl compounds can function as better potential biomarkers for chronic schizophrenia with enhanced carbonyl stress than the previously reported AGE biomarkers.18-21
Previous studies regarding schizophrenia with enhanced carbonyl stress have been conducted to examine the use of pentosidine, one of the well-known peripheral blood AGEs, as a disease biomarker.6-8,11-13
It is very important to reveal the exact impact of an increase in plasma concentrations of these reactive di-carbonyl compounds with decreased GLO I activity. First, this is because GLO I is responsible for the detoxification of MGO and other reactive 2-oxoaldehydes, and second, it impacts the inhibition of the formation of some AGEs, including pentosidine.
As per research, 3-DG is rapidly reduced by 3-DG reductases to form 3-deoxyfructose, which is normally excreted via the urine.22 GO is produced by the lipid peroxidation system23 and induces mutation via glyoxal-DNA adducts.24 MGO is formed via the triose phosphate isomerase-catalyzed elimination reactions of dihydroxyacetone phosphate in the intracellular glycolysis system. An unusual increase in triose phosphate levels may not only lead to the aberrant production of cytotoxic MGO but also to the occurrence of various metabolic disorders in vivo.
These reactive di-carbonyls are very unstable in the atmosphere and decompose due to the degradation of derivatization agents during sample processing for the estimation of their concentrations. Therefore, a reliable estimation of their concentrations in physiological systems is difficult. To our knowledge, there are no reports on their potential use as biomarkers for diagnosing schizophrenia with enhanced carbonyl stress.
To identify and determine the plasma concentrations of three di-carbonyls, we performed the most advanced methods, employing derivatization with 2, 3-diaminonaphthalene19 and detection using the ESI/LC/MS system with high selectivity and accuracy. These reactions were performed quickly and quantitatively in cooling condition, and each derivative sample was stable under aerobic conditions during the ESI/LC/MS measurement. In this method, the samples can be identified and quantified simultaneously in the selected ion monitoring mode in a single measurement within 20 minutes at low temperature (8°C).
Accordingly, this study is the first to report the exact plasma concentrations of the di-carbonyl compounds in patients with chronic schizophrenia.
Thus, although there was no significant difference in the mean plasma concentrations of the three di-carbonyl compounds between the patients and controls, we found a remarkable increase only in the MGO concentrations in four patients with chronic schizophrenia (> 2SD, n = 4, 10%) as shown in Fig. 2A.
The correlation analysis showed that both MGO and GO concentrations were significantly correlated to 3-DG concentrations in 40 patients and 40 controls; however MGO concentrations showed no significant correlation with GO concentrations in patients or controls.
In four patients with extremely high MGO concentrations, the MGO and GO concentrations were not significantly correlated with 3-DG concentrations.
In fact, these four patients did not have high 3-DG levels.
Thus, in our study patients with extremely high MGO accumulation may have had different metabolic pathways, including dysfunction of GLO I activity as compared to those (n = 36) with chronic schizophrenia. Furthermore, the increase in plasma MGO concentrations indicated the presence of a specific subtype of schizophrenia associated with enhanced carbonyl stress.
A recent study reported significant differences between mean serum pentosidine levels in chronic schizophrenia patients and controls, and it showed some patients with extremely high pentosidine levels (11.8%) had a severe clinical condition.7 However, Katsuta et al. showed that mean serum pentosidine levels were not significantly altered in patients with acute-stage schizophrenia compared with controls and did not change according to clinical course. Furthermore, their study results showed extremely high pentosidine levels were only shown in patients with schizophrenia (> 2 SD, n = 14, 10.2 %) but not in controls.8 In our present study, the four cases with high MGO levels were found to have carbonyl stress, and they showed similar severe clinical conditions to the other 36 patients, although each psychological symptom was different among them.
These results are consistent with the study by Katsuta et al,8 although they used serum pentosidine levels as a peripheral carbonyl stress marker in schizophrenia patients.
The mechanism responsible for high MGO levels in patients with schizophrenia could be partly attributed to GLO 1 dysfunction; however, additional unknown factors may be involved.
From the psychological aspect, there was no significant difference in the average total scores in the PANSS in the four patients with extremely high MGO concentrations and another eight randomly selected patients in this study, as shown in Table 2.
A comparison of the above mentioned two groups showed no significant differences in the positive symptoms of PANSS; however, the following tendency with respect to the negative symptoms was believed to be attributable to the significant differences observed in the PANSS in the four patients with extremely high MGO concentration: high scores for emotional withdrawal (p < 0.05) and tension (p < 0.01) can make confusion about social communication with healthy individuals challenging for these patients.
CONCLUSION
In conclusion, extremely elevated plasma MGO concentrations in patients with chronic schizophrenia indicate the presence of a disease subtype associated with enhanced carbonyl stress.
ACKNOWLEDGEMENTS
We are greatly appreciate to Prof. Kanemoto (Aichi Medical University, Department of Psychiatry) for giving us the research support facilities. We thank Mr. Fukayama (Aichi Medical University, Institute of Comprehensive Medical Research, Division of Advanced Research Promotion for the assistance of measurements of plasma di-carbonyl compounds in mass spectrometric analysis.
The authors would like to thank ENAGO (www.enago.jp) for the English language review.
CONFLICT OF INTEREST
The authors declared that there is no conflict of interests. This study was not funded by anywhere.
Abbreviations
- MGO
Methylglyoxal
- GO
Glyoxal
- 3-DG
3-Deoxyglucosone
- SD
Standard deviation
- r
Correlation coefficient
REFERENCES
- 1.Marchbanks RM, Ryan M, Day IN, Owen M, McGuffin P, Whatley SA. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65(1):33–38. doi: 10.1016/s0920-9964(03)00011-2. [DOI] [PubMed]
- 2.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9(7):684–697, 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed]
- 3.Yao JK, Reddy RD, van Kammen DP. Oxidative damage and schizophrenia; an overview of the evidence and its therapeutic implications. CNS drugs. 2001;15(4):287–310. doi: 10.2165/00023210-200115040-00004. [DOI] [PubMed]
- 4.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed]
- 5.Itokawa M, Miyashita M, Arai M, Miyata T. Carbonyl stress in schizophrenia. Biochem Soc Trans. 2014;42(2):468–472. doi: 10.1042/BST20140044. [DOI] [PubMed]
- 6.Arai M, Yuzawa H, Nohara I, et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry. 2010;67(6):589–597. doi:10.1001/archgenpsychiatry. 2010.62. [DOI] [PubMed]
- 7.Miyashita M, Arai M, Yuzawa H, et al. Replication of enhanced carbonyl stress in a subpopulation of schizophrenia. Psychiatry Clin Neurosci. 2014;68(1):83–84. doi: 10.1111/pcn.12081. [DOI] [PubMed]
- 8.Katsuta N, Ohnuma T, Maeshima H, et al. Significance of measurement of peripheral carbonyl stress markers in a cross-sectional and longitudinal study in patients with acute- stage schizophrenia. Schizophr Bull. 2014;40(6):1366–1373. doi: 10.1093/schbul/sbt234. [DOI] [PMC free article] [PubMed]
- 9.Shinohara M, Thornalley PJ, Giardino I, et al. Overexpression of glyoxalase I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglyceamia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101(5):1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed]
- 10.Suzuki K, Koh YH, Mizuno H, Hamaoko R, Taniguchi N. Overexpression of aldehyde reductase protects PC12 cells from the cytotoxicity of methylglyoxal or 3-deoxy-glucosone. J Biochem. 1998;123(2):353–357.doi: 10.1093/oxfordjournals.jbchem.a021944. [DOI] [PubMed]
- 11.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269(1):1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed]
- 12.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and disease. Drug Metabol Drug Interact. 2008;23(1–2):125–150. doi: 10.1515/dmdi.200823.1-2.125. [DOI] [PMC free article] [PubMed]
- 13.Miyashita M, Arai M, Kobori A, et al. Clinical features of schizophrenia with enhanced carbonyl stress. Schizophr Bull. 2014;40(5):1040–1046. doi:10.1093/ schbul/sbt129. [DOI] [PMC free article] [PubMed]
- 14.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism ofautoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34(11):3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed]
- 15.Glomb M, Monnier VM. Mechanism of protein modification by glyoxal and glycol-aldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270(17):10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed]
- 16.Ahmed MU, Brinkmann-Frye E, Degenhardt TP, Thorpe SR, Baynes JW. Nε-Carboxy-ethyl) lysine, a product of the chemical modification of methylglyoxal, increasing with age in human lens proteins. Biochem J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed]
- 17.Odani H, Shinzato T, Matsumoto Y, Usami J, Maeda K. Increase in three α, β-di-carbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in Advanced Maillard reaction. Biochem Biophs Res Commun. 1999;256(1):89–93. doi: 10.1006/bbrc.1999.0221. [DOI] [PubMed]
- 18.Arai M, Koike S, Oshima N, et al. Idiopathic carbonyl stress in a drug-native case of at -risk mental state. Psychiatry Clin Neurosci. 2011;65(6):606–607. doi: 10.1111/j.1440-1819.2011.02261.x. [DOI] [PubMed]
- 19.Arai M, Miyashita M, Kobori A, Toriumi K, Horiuchi Y, Itokawa M. Carbonyl stress and schizophrenia. Psychiatry Clin Neurosci. 2014;68(9):655–665. doi:10.1111/pcn. 12216. [DOI] [PubMed]
- 20.Arai M , Nihonmatsu-Kikuchi N, Itokawa M, Rabbani N, Thornalley PJ. Measurement of glyoxalase activities. Biochem Soc Trans. 2014;42(2):491–494. doi: 10.1042/BST20140010. [DOI] [PubMed]
- 21.Koike S, Kayama T, Arai M, et al. Characterization of modified proteins in plasma from asubtype of schizophrenia based on carbonyl stress: Protein carbonyl is a possible biomarker of psychiatric disorders. Biochem Biophys Res Commun. 2015;467(2):361–366. doi: 10.1016/j.bbrc.2015.09.152. [DOI] [PubMed]
- 22.Wells-Knecht KJ, Lyons TJ, MacCance DR, Thorpe SR, Feather MS, Baynes JW. 3-Deoxyfructose concentrations are increased in human plasma and urine in diabetes. Diabetes. 1994;43(9):1152–1156. doi: 10.2337/diab.43.9.1152. [DOI] [PubMed]
- 23.Nishiyama T, Hagiwara Y, Hagiwara H, Shibamoto T. Inhibitory effect of 2’’-O-glycosyl isovitexin and α-tocopherol on genotoxic glyoxal formation in a lipid peroxidation system. Food Chem Toxicol. 1994;32(11):1047–1051. doi: 10.1016/0278-6915(94)90145-7. [DOI] [PubMed]
- 24.Murata-Kamiya N, Kamiya H, Kaji H, Kasai H. Nucleotide excision repair proteins may be involved in the fixation of glyoxal-induced mutagenesis in Escherichia coli. Biochem Biophys Res Commun. 1998;248(2):412–417. doi: 10.1006/bbrc.1998.8973. [DOI] [PubMed]