Abstract
In this investigation, an alternate green-route based on myco-synthesised silver nanoparticles (Ag NPs) was evaluated to control plant disease to reduce the usage of synthetic chemicals. Here, we described biologically synthesised Ag NPs using the corn grain contaminant, Nigrospora oryzae, and were well-characterised by UV–visible spectrophotometer, X-ray powder diffraction (XRD), transmission electron microscopy (TEM), Energy Dispersive Spectroscopy (EDS) and particle size analyzer. The pathogenic behaviour of the Fusarium spp. were checked on Giza 86 and Giza 90 cultivars under greenhouse conditions. F. moniliforme and F. oxysporum exhibited high pathogenecity against Giza 90 and Giza 86 cultivars respectively. The antifungal activity of biosynthesised Ag NPs was evaluated against eight species of Fusaria causing damping-off of cotton seedlings. In vitro treatments with different concentrations of Ag NPs were achieved on Czapek Dox agar and Potato dextrose agar plates. Fungal growth was drastically retarded from 25 to 200 ppm of Ag NPs interaction. The antifungal activity of Ag NPs against the Fusarium spp. was clearly proven.
Keywords: Biosynthesis, Pathogenic fungi, Silver, Cotton
1. Introduction
In recent years, in the field of nanoscience, there has been increasing interest in the fabrication of antimicrobial nanomaterials (Cheeseman et al., 2020). Metal nanoparticles, in particular silver, copper and zinc, have attracted great attention and have emerged as a novel class of nanomaterials (Ghodake et al., 2020, Pariona et al., 2019, Vishvanath et al., 2018). The conventional chemical synthesis processes are expensive, and both utilize and produce toxic chemicals that are hazardous to the environment. Researchers have therefore, examinedthe ambient biological process that usually occurs in nature. Microbial and plant extract-based processes have resulted from the exploration of natural systems and emerged as an alternative for nanosynthesis. The green synthesis of nanomaterials such as silver nanoparticles (Ag NPs) using microorganisms has generated considerable attention. The biological and physicochemical properties of these NPs nanoparticles are valuable in various applications in many fields including biomedical and agricultural production (Li et al., 2014). Several microorganisms, including different genera of fungi, have successfully been employed for bio-fabrication of Ag NPs (Spagnoletti et al., 2019, Aygün et al., 2020, Schlüter et al., 2014). Moreover, different plant pathogenic fungi have successfully been tested for bio-synthesis of Ag NPs (Yassin et al., 2016). Of these, cereal contaminating fungi such as Aspergillus clavatus, Curvularia pallescens, Penicillium citrinum, and Phoma leveillei have also been used in the green fabrication of Ag NPs (Elgorban et al., 2016, Yassin et al., 2016; Yassin et al., 2017). Utilization of bio-synthesized Ag NPs in the control of other phytopathogenic fungi to eradicate them, or at least minimize their effects, is promising (Abdel-Hadi et al., 2014).
Fusarium species are one of the most serious plant pathogens. Their large host range can cause a variety of damages depending on the susceptibility of the host, the infected regions of the plant, and the virulence of the isolate. Diseases of cotton seedlings caused by Fusarium spp. result in seedling death and root rot of adulate plants in most cotton-producing areas (Palmateer et al., 2004, Costa et al., 2005, El-Samawaty et al., 2008, El-Samawaty et al., 2012). The extent of injuries and the amount of damage in the host plants depends on the virulence of the infecting Fusarium species and the susceptibility of the cotton cultivars (Aly et al., 2000, Palmateer et al., 2004, El-Samawaty et al., 2013). Eradicating or at least minimizing the Fusarial rot disease of cotton seedlings is vital (El-Samawaty et al., 2013).
Chemical fungicides are detrimental to the biological balance and can prompt the development of resistant plant pathogenic fungi (Calhelha et al., 2006). Thus, the identification and use of alternatives are required (Reddy et al., 2010, Yassin et al., 2013). This study investigated the green route for synthesis of Ag NPs using the corn grain contaminant, Nigrospora oryzae. The successful formation of Ag NPs was verified using various analytical techniques UV–vis, XRD, TEM, and EDS measurements. Myco-synthesis Ag NPs was tested for antifungal effect against eight species of Fusaria causing damping-off of cotton seedlings (Elgorban et al., 2017, Yassin et al., 2017a, Yassin et al., 2017b). The study aimed to eradicate pathogenic plant microorganisms without using synthetic chemical.
2. Materials and methods
2.1. Biosynthesis of Ag NPs
Ag NPs were biosynthesized by N. oryzae isolate. Synthetic autoclaved broth was used for the biomass production that was described previously by Yassin et al. (Yassin et al., 2016). After obtaining biomass, the fungal cells were incubated in deionized water for 72 h at 140 rpm in an orbital shaker. Then the cell-free filtrate was collected by washing (Elgorban et al., 2016). The filtrate and 1.0 mM silver nitrate suspension was mixed in 1:1 ratio. The suspension was incubated at 200 rpm in a rotary shaker at 28 ± 2 °C in the dark to allow complete reaction and development of the yellowish colour for 2 days. The solution was then centrifuged at 10,000 rpm for 14 min. The pellet was dried at 60 °C for 24 h.
2.2. Characterisation of Ag NPs
Dried pellet was used to analyze energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD) patterns. XRD was applied to evaluate metallic nature of biosynthesized Ag-NPs. X-pert pro diffractometer was used for XRD analysis with Cu-Kα radiation at 40 KV and 40 mA. The dried powder was dispersed in 1 ml double distilled water prior to measurement using an ultraviolet–visible (UV–VIS) spectrophotometer and was sonicated for 1 h prior to transmission electron microscopy (TEM) using 740 and 740X Ultrasounds Sonicator TEM and the Ag NPs. EDS is clubbed with TEM; used to enumerate the compositional analysis of NP.
2.3. Source and behaviour of Fusarium spp.
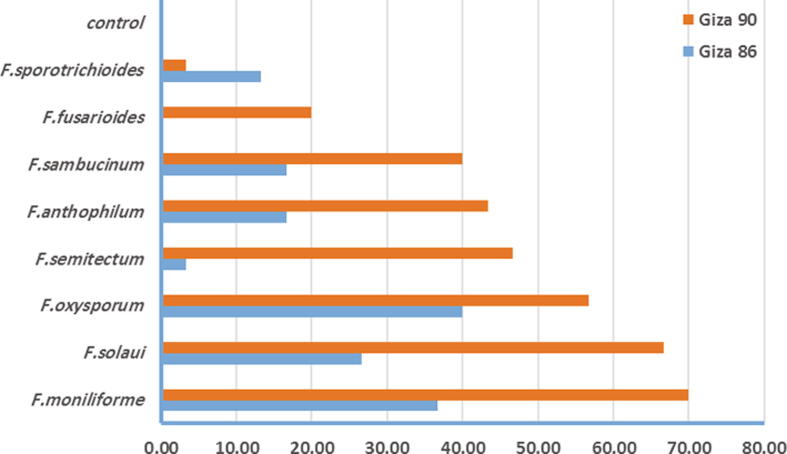
Fusarium spp. isolates used in this study were provided by Cotton and Fiber Disease Research Department, Plant Pathology Research Institute, Agricultural Research Center, Giza, Egypt (Table 1). To determine the pathogenicity of these isolates the soil infestation technique was used. Briefly large amounts of mycelia was obtained by transferring a small piece of PDA containing the growing fungus to 250 ml Erlenmeyer flasks containing 100 ml yeast broth and held at 25 °C for 10–12 days in the dark. After this period, the mycelial mat was taken from the Erlenmeyer flasks, washed with tap water, and 0.3 g was blended for 1 min with 100 ml tap water. In general, 100 ml of the blended mycelia was used to infest 3 kg of soil, After adding the fungal suspension, the soil was hand mixed to obtain uniform distribution of the propagules. The experiment was performed under greenhouse conditions. The seedling damping-off was monitored on Giza-86 and Giza-90 cotton cultivars. Seedling damping-off was recorded 15–45 days after planting (Fig. 1). Table 2.Table 3.
Table 1.
Geographic origin, host plant, and previous crop of Fusarium isolates.
| Fusarium species | Geographic origin | Host | Previous Crop | |
|---|---|---|---|---|
| F1 | F. sambucinum | Minya | Giza 90 | Onions |
| F2 | F. semitectum | Sohag | Giza 90 | Egyptian clover |
| F3 | F. sporotrichioides | Sohag | Giza 83 | Egyptian clover |
| F4 | F. anthophilium | Assuit | Giza 90 | Egyptian clover |
| F5 | F. oxysporum | Sohag | Giza 90 | Egyptian clover |
| F6 | F. moniliforme | Sohag | Giza 90 | Egyptian clover |
| F7 | F. fusarioids | Sohag | Giza 90 | Egyptian clover |
| F8 | F. solani | Assuit | Giza 90 | Wheat |
Fig. 1.
Pathogenicity of Fusarium species on Giza-86 and Giza-90 cotton cultivars under greenhouse conditions.
Table 2.
Effect of Ag NPs on the growth of Fusarium spp. on Czapek Dox medium.
| Fusarium spp. | Concentration (ppm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | 25 | 50 | 75 | 100 | 150 | 200 | Mean | |
| F. sambucinum | 90.00 | 90.00 | 84.00 | 72.25 | 70.25 | 64.75 | 54.67 | 75.13 |
| F. semitectum | 90.00 | 76.50 | 58.50 | 42.50 | 37.00 | 35.00 | 33.38 | 53.27 |
| F. sporotrichioides | 90.00 | 83.50 | 58.25 | 43.75 | 38.25 | 31.75 | 27.57 | 53.29 |
| F. anthophilium | 90.00 | 79.25 | 64.50 | 49.50 | 43.00 | 41.75 | 40.13 | 58.30 |
| F. oxysporum | 90.00 | 80.25 | 57.75 | 46.00 | 36.50 | 32.25 | 30.17 | 53.27 |
| F. moniliforme | 90.00 | 84.75 | 76.50 | 64.50 | 57.00 | 51.25 | 50.63 | 67.80 |
| F. fusarioids | 90.00 | 73.75 | 62.00 | 55.50 | 40.75 | 30.00 | 25.07 | 53.86 |
| F. solani | 90.00 | 85.50 | 79.75 | 67.25 | 52.75 | 52.00 | 42.38 | 67.09 |
| Mean | 90.00 | 81.69 | 67.65 | 55.15 | 46.94 | 42.34 | 38 | |
LSD (P ≤ 0.05) for fusarium (F) = 1.10.
LSD (P ≤ 0.05) for concentration (C) = 0.52.
LSD (P ≤ 0.05) for interaction F × C = 2.91.
Table 3.
Effect of Ag NPs on the growth of Fusarium spp. on Czapek Dox medium.
| Fusarium spp. | Concentration (ppm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | 25 | 50 | 75 | 100 | 150 | 200 | Mean | |
| F. sambucinum | 90.00 | 90.00 | 75.50 | 60.50 | 51.00 | 40.75 | 34.61 | 63.19 |
| F. semitectum | 90.00 | 78.00 | 53.50 | 45.00 | 40.50 | 38.75 | 28.36 | 53.44 |
| F. sporotrichioides | 90.00 | 87.50 | 69.75 | 58.25 | 45.25 | 31.50 | 30.28 | 58.93 |
| F. anthophilium | 90.00 | 79.00 | 64.25 | 46.00 | 41.25 | 33.50 | 27.24 | 54.53 |
| F. oxysporum | 90.00 | 89.00 | 75.75 | 66.00 | 53.50 | 43.75 | 31.11 | 64.16 |
| F. moniliforme | 90.00 | 77.50 | 61.75 | 56.00 | 44.75 | 31.75 | 26.36 | 55.44 |
| F. fusarioids | 90.00 | 90.00 | 84.25 | 60.75 | 50.00 | 37.75 | 32.28 | 63.58 |
| F. solani | 90.00 | 72.25 | 65.25 | 51.50 | 42.50 | 37.25 | 31.74 | 55.78 |
| Mean | 90.00 | 82.97 | 68.75 | 55.50 | 46.09 | 36.87 | 30.25 | |
LSD (P ≤ 0.05) for fusarium (F) = 1.02.
LSD (P ≤ 0.05) for concentration(C) = 0.48.
LSD (P ≤ 0.05) for interaction F × C = 2.68.
2.4. Antifungal activity of Ag NPs
Czapek Dox and PDA media containing 50, 100, 150, and 200 ppm Ag NP were used. Four-millimeter diameter plugs of each Fusarium spp. isolate was cultured in 90 mm Petri plates containing the Ag NP supplemented media. Four replicate plates per treatment along with untreated control plates were used, and all of these plates were incubated at 28 ± 2 °C. Colony diameters were measured daily and the collected data were used for efficacy calculations.
3. Results
3.1. Ag NP biosynthesis
The reaction of silver nitrate with the culture supernatant and the gradual colour change to the yellowish colour was visually observed after 2 days of incubation.
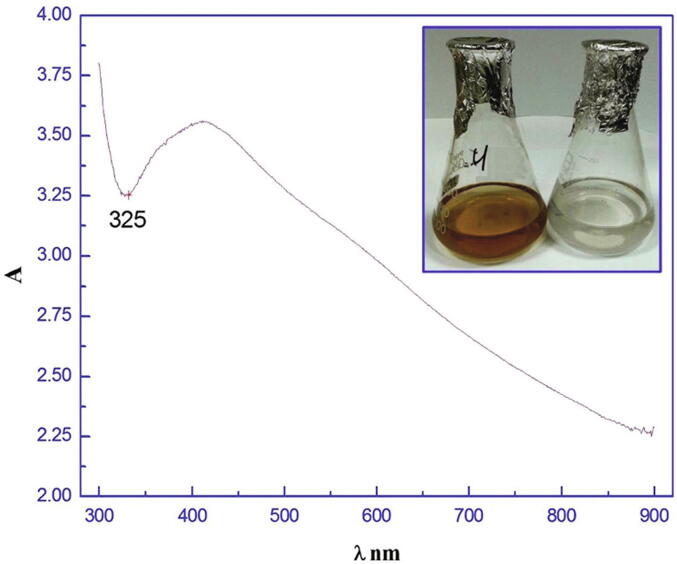
The preliminarily characterization of prepared biosynthezied Ag NPs was done with UV–vis spectrophotometer and it gave an absorbance peak at 420 nm (Fig. 2). The excitation of NPs was due to strong localized SPR property. The yellow colour appearance and the absorbance peak around 400 nm represents the Ag NPs formation.
Fig. 2.
Reaction solution showing the colour change in the culture filtrate with silver ions (b) compared with the control (a).
3.2. Ag NP characterisation
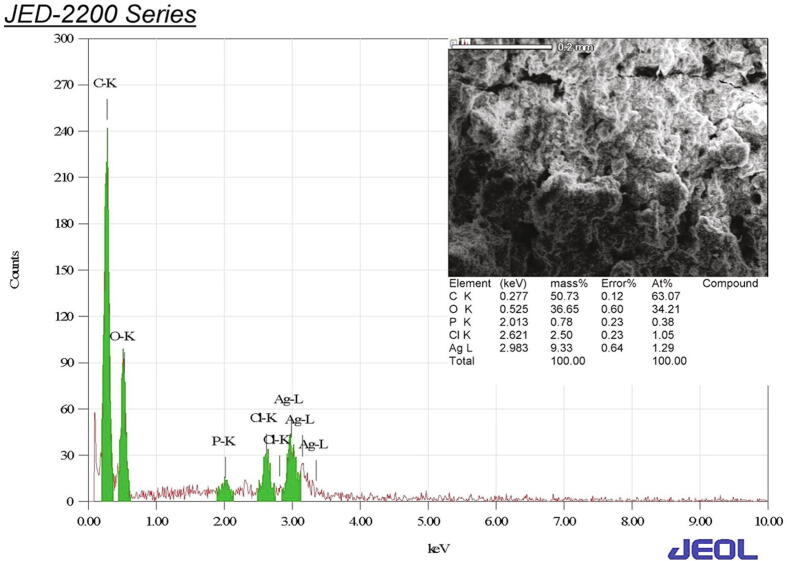
Ag presence were confirmed by EDS analysis (Fig. 3). The presence of other elements in the EDS spectra were from biomolecules in the reaction mixture.
Fig. 3.
EDS micrograph of biosynthesised Ag NPs.
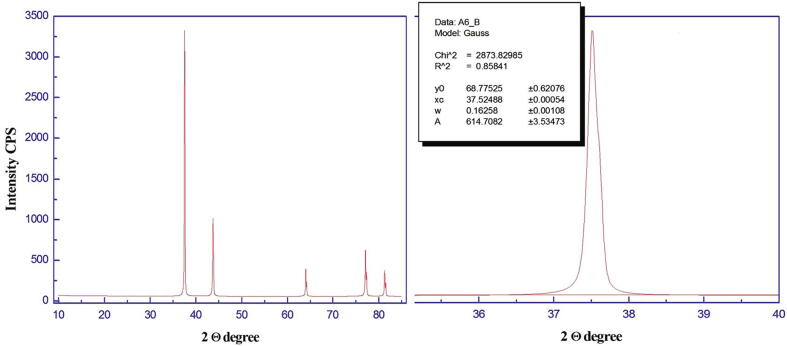
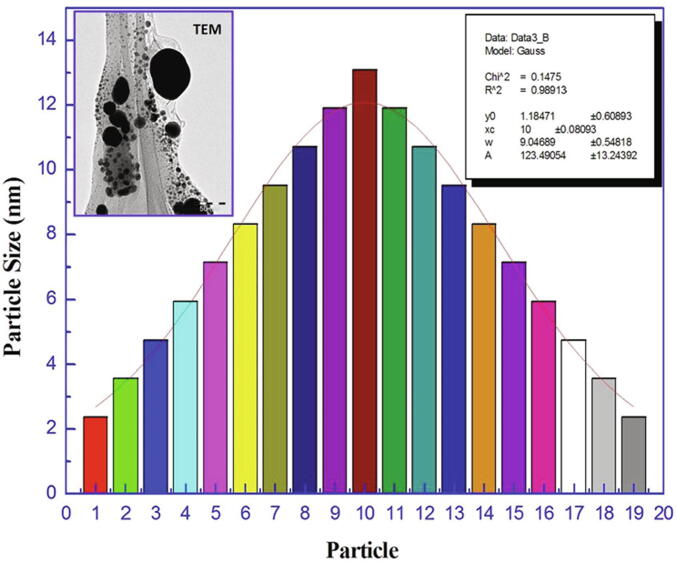
The crystalline nature of the obtained Ag NPs was determined by XRD analysis, which revealed the presence of pure Ag metal with a polycrystalline nature (Fig. 4). An average grain size (D), dislocation density (δ), and strain (ε) for Ag NPs were 46.4 nm, 4.64 × 10–4 nm-2, and 7.3 × 10–3, respectively, as calculated by TEM revealed mostly spherical nanoparticles with a size range from 3 to 13 nm size, with good Gaussian variation (Fig. 5).
Fig. 4.
XRD micrograph of Ag NPs.
Fig. 5.
TEM micrograph and Gaussian distribution of biosynthesised Ag NPs.
3.3. Antifungal evaluation of Ag NPs
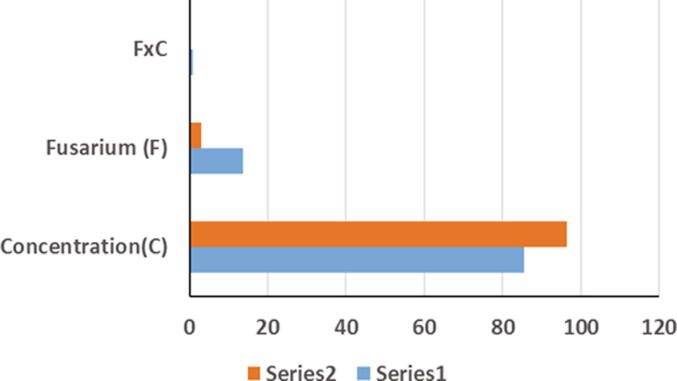
Antifungal activity of Ag NPs against Fusarium spp. was clearly proven in this study. The analysis of variance (ANOVA) showed that Fusaria, concentration of Ag NPs, and their interaction were highly significant sources of variation in the fungal growth on PDA and Czapek Dox media. Relative contribution indicated that the concentration of Ag NPs was the most important source of variation, while the (F × C) interaction was the least important topic (Fig. 6). Regardless of the culture media, all Ag Np concentrations were capable of inhibiting the fungal growth. All tested species were variably inhibited when grown on PDA and Czapek Dox media. Inhibition generally increased as the Ag NP concentration increase. Fungal growth was drastically retarded at a concentration of 200 ppm.
Fig. 6.
Relative contribution of Ag NP concentration, Fusarium spp., and their interaction on the growth of Fusarium spp.
4. Discussion
4.1. Ag NP biosynthesis and characterisation
The development of a yellowish colour was visually observed after 2 days. This colour change is considered the first indicator of the biological synthesis of Ag NPs (Sadowski et al., 2008). The reduction of silver ions that occurs during the formation of Ag NPs is responsible for the colour changes that might occur due to the activity of extracellular fungal enzymes such as nitrate reductase (Naveen et al., 2010).
XRD analysis revealed pure polycrystalline Ag metal. A similar crystalline and metallic nature with face centered-cubic structures of Ag NPs has been described (Sadowski et al., 2008, Gade et al., 2014, Nanda et al., 2015). EDS analysis results suggested that Ag was the major element based on the very intense signal observed at 3 K eV in the EDS profile (Gade et al., 2014, Mallikarjuna et al., 2014). TEM indicated diverse morphology that nevertheless mostly consisted of spherical nanoparticles 3 to 13 nm in size with good Gaussian profile (Elgorban et al., 2016, Goswami et al., 2013).
UV–VIS near infrared spectroscopy indicated the extreme permeability of the reactive solution at 420 nm, suggesting the formation of Ag NPs. The results were similar to the previous description (Gade et al., 2014, Birla et al., 2009) that the absorbance peak of bio-synthesised Ag NPs was approximately 420 to 440 nm and the dependent particle size was 2.237 nm (Shivaraj et al., 2014).
The antifungal activity of Ag NPs was investigated using Fusarium spp. All the species growth was suppressed when exposing to 25 to 200 ppm of biosynthesized Ag NPs. The growth inhibition varied to great extents when grown on the two different media. In general, growth inhibition increased as the Ag NP concentration increased. Fungal growth was drastically retarded at a concentration of 200 ppm. Higher concentrations of Ag NPs in solution might be capable of saturating and adhering to fungal hyphae and disrupting the fungal cells. Such an inhibitory effect can be attributed to Ag + that primarily affects the function of membrane-associated enzymes such as those found in the respiratory chain. Ag + may also affect the expression of some microbial proteins and enzymes. Disruption of DNA replication has also been documented. Further examination for field applications is needed to ensure the antifungal activity of Ag NPs.
5. Conclusion
Ag NPs were bioengineered using the corn grain contaminant Nigrospora oryzae. These NPs displayed strong antifungal activity against Fusarium spp. plant pathogenic fungi. It is expected that the application of biosynthesised Ag NPs nanoparticles at low concentrations will be eco-friendly and decrease farm management costs. It will be used as a alternate solution for controlling fungal pathogens affecting plants growth instead of using synthetic chemical.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Research Group No (RGP-298).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Hadi A.M., Awad M.F., Abo-Dahab N.F. Extracellular synthesis of silver nanoparticles by Aspergillus terreus: biosynthesis, characterization and biological activity. Biosci, Biotechnol Res Asia. 2014;11:1179–1186. [Google Scholar]
- Aly A.A., Hussien E.M., Allam A.D.A. Pathological studies on fungi involved in damping -off of cotton seedlings and root rot of adult plants in Upper Egypt governorates. J Agric Sci. 2000;25:4015–4034. [Google Scholar]
- Aygün A., Özdemir S., Gülcan M., Cellat K., Şen F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J Pharmaceut Biomed. 2020;178 doi: 10.1016/j.jpba.2019.112970. [DOI] [PubMed] [Google Scholar]
- Birla S.S., Tiwari V.V., Gade A.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48:173–179. doi: 10.1111/j.1472-765X.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- Calhelha, R.C., Andrade, J.V., Ferreira, I.C. and et al. Toxicity effects of fungicide residues on the wine-producing process. Food Microbiol. 23:393-398. [DOI] [PubMed]
- Cheeseman, S., Christofferson, A. J., Kariuki, R., Cozzolino, D., Daeneke, T., 2020. Crawford, R. J., Truong, V. K., Chapman, J., Elbourne, A., Antimicrobial Metal Nanomaterials: From Passive to Stimuli‐Activated Applications. Adv. Sci. 7;1902913. [DOI] [PMC free article] [PubMed]
- Costa M.L.N., Dhingra O.D., Da-Silva J.L. Influence of internal seed bore Fusarium semitectum on cotton seedlings. Fitopatol Bras. 2005;30:183–186. [Google Scholar]
- Elgorban A.M., El-Samawaty A.M.A., Abd-Elkader O.H. Bioengineered silver nanoparticles using Curvularia pallescens and its fungicidal activity against Cladosporium fulvum. Saudi J Biol Sci. 2017;24:1522–1528. doi: 10.1016/j.sjbs.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgorban A.M., El-Samawaty A.M., Yassin M.A. Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotechnol Biotechnol Equip. 2016;30:56–62. [Google Scholar]
- El-Samawaty A.M.A., Yassin M.A., Moslem M.A. Effectiveness of some plant extracts against Fusarium spp. causing cotton seedlings damping-off. Life Sci J. 2013;10:510–515. [Google Scholar]
- El-Samawaty A.M.A., Omar M.R., El-Naggar M.A. Pathological assessment of seed borne fungi involved in cotton seedlings damping-off. Plant Sci. 2012;7:85–95. [Google Scholar]
- El-Samawaty A.M.A., Abdel-Reheem M.A.T., Abd-Esalam K.A. Use of random amplified polymorphic DNA (RAPD) to differentiate among isolates of Fusarium spp. pathogenic on cotton. J Biol Chem Environ Sci. 2008;3:811–827. [Google Scholar]
- Gade A., Gaikwad S., Duran N. Green synthesis of silver nanoparticles by Phoma glomerata. Micron. 2014;59:52–59. doi: 10.1016/j.micron.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Ghodake G., Shinde S., Saratale R.G., Kadam A., Saratale G.D., Syed A., Marraiki N., Elgorban A.M., Kim D.-Y. Silver nanoparticle probe for colorimetric detection of aminoglycoside antibiotics: picomolar-level sensitivity toward streptomycin in water, serum, and milk samples. J Sci Food Agric. 2020;100:874–884. doi: 10.1002/jsfa.10129. [DOI] [PubMed] [Google Scholar]
- Goswami A.M., Sarkar T.S., Ghosh S. An ecofriendly synthesis of silver nano-bioconjugates by Penicillium citrinum (MTCC9999) and its antimicrobial effect. A M B Express. 2013;3:16–21. doi: 10.1186/2191-0855-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xia H., Ding W. Synthesis of monodisperse, quasi-spherical silver nanoparticles with sizes defined by the nature of silver precursors. Langmuir. 2014;30:2498–2504. doi: 10.1021/la4047148. [DOI] [PubMed] [Google Scholar]
- Mallikarjuna K., Sushma N.J., Narasimha G. Phytochemical fabrication and characterization of silver nanoparticles by using pepper leaf broth. Arabian J Chem. 2014;7:1099–1103. [Google Scholar]
- Nanda A., Majeed S., Abdullah M.S. Efficacy of nano silver from soil fungus enhancing the antiseptic activity of Ciprofloxacin against pathogenic bacteria. Der Pharma Chemica. 2015;7:141–146. [Google Scholar]
- Naveen H.K.S., Kumar G., Karthik L. Extracellular biosynthesis of silver nanoparticles using filamentous fungus Penicillium sp. Arch Appl Sci Res. 2010;2:161–167. [Google Scholar]
- Palmateer A.J., McLean K.S., Morgan-Jones G. Frequency and diversity of fungi colonizing tissues of upland cotton. Mycopathologia. 2004;157:303–316. doi: 10.1023/b:myco.0000024184.56337.f5. [DOI] [PubMed] [Google Scholar]
- Pariona Nicolaza, Martinez Arturo, Sánchez-Rangel D., Carrión Gloria, Paraguay-Delgado F., Rosas-Saito Greta. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Advances. 2019;9:18835–18843. doi: 10.1039/c9ra03110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K.R.N., Nurdijati S.B., Salleh B. An overview of plant-derived products on control of mycotoxigenic fungi and mycotoxins. Asian J Plant Sci. 2010;9:126–133. [Google Scholar]
- Sadowski Z., Maliszewska I.H., Grochowalska B. Synthesis of silver nanoparticles using microorganisms. Mater Sci-Pol. 2008;26:419–424. [Google Scholar]
- Schlüter S., Sheppard A., Brown K., Wildenschild D. Image processing of multiphase images obtained via X-ray microtomography: a review. Water Resour. Res. 2014;50:3615–3639. [Google Scholar]
- Shivaraj N., Vandana R., Dattu S. Characterization and biosynthesis of Silver nanoparticles using a fungus Aspergillus niger. Int. lett. Nat. Sci. 2014;15:49–57. [Google Scholar]
- Spagnoletti F.N., Spedalieri C., Kronberg F., Giacometti R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manage. 2019;231:457–466. doi: 10.1016/j.jenvman.2018.10.081. [DOI] [PubMed] [Google Scholar]
- Vishvanath Tiwari, Neha Mishra, GadaniKeval Solanki P.S., Shah N.A., Monalisa Tiwari. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacterbaumannii. Frontiers in Microbiology. 2018;9:1218. doi: 10.3389/fmicb.2018.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin M.A., El-Samawaty A., Dawoud T.M. Characterization and anti-Aspergillus flavus impact of nanoparticles synthesized by Penicillium citrinum. Saudi J Biol Sci. 2017;24:1243–1248. doi: 10.1016/j.sjbs.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin M.A., El-Samawaty A.M.A., Abdelkader O.H. Bio-synthesis of antifungal active silver nanoparticles using sorghum grain contaminant Phoma leveillei. Fresenius Environ Bull. 2017;26:1447–1452. [Google Scholar]
- Yassin M.A., Elgorban A.M., El-Samawaty A.M.A. An eco-friendly approach for fabrication of silver nanoparticles using Aspergillus clavatus. Fresenius Environ Bull. 2016;25:5929–5934. [Google Scholar]
- Yassin M.A., Moslem M.A., El-Samawaty A.M.A. Effectiveness of Allium sativum in controlling sorghum grain molding fungi. J Pure Appl Microbiol. 2013;7:101–107. [Google Scholar]
Further Reading
- Aly A.A., Hussien E.M., Mostafa M.A. Distribution, identification, and pathogenicity of Fusarium spp. isolated from some Egyptian cottons. Minufiya J Agric Res. 1996;21:819–836. [Google Scholar]
- Kumar A.S., Abyaneh M.K., Gosavi S.W. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 2007;29:439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- Prema, P. 2010. Chemical mediated synthesis of silver nanoparticles and its potential antibacterial application, progress in molecular and environmental bioengineering. In: Angelo Carpi, editor. Analysis and modeling to technology applications; 2010. ISBN: 978-953-307-268-5; p. 151-166.
- Singh D., Rathod V., Ninganagouda S. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioin Chem Appl. 2014;21:1–8. doi: 10.1155/2014/408021. [DOI] [PMC free article] [PubMed] [Google Scholar]