Abstract
Insects are important for humanity; play role in crop pollination, and biocontrol of harmful pests. The red palm weevil, Rhynchophorus ferrugineus, is a major pest of date palms and has become a serious threat. Scientists needs ample numbers of insects for bioassays to explore control options. The alga Spirulina platensis, is enriched by protein, natural vitamins, minerals, and amino acids, stimulate the development of organisms that feed on it. I assessed the value of Spirulina as a nutritional supplement for red palm weevil larvae by adding its various percentages to the artificial diet. Once a week, the larvae were removed from the containers, washed with distilled water, dried, weighed using an electronic scale, returned to a new container, and supplied with Spirulina mixed fresh diet. Larvae fed with lower concentrations showed vigorous growth and significant weight gain. Particularly, larvae fed 0.5%, 1%, and 2% Spirulina powder supplementation to their diet were healthier and gained more weight than larvae reared with >5% concentration. Overall 40% mortality was recorded in larvae fed with 10% concentration. Higher concentrations were lethal, and all larvae died within two weeks when fed 20% Spirulina. The present research findings indicate that Spirulina used in concentrations from 0.5% to below 5% had a beneficial effect on red palm weevil larval growth but a detrimental effect and even mortality was recorded when used ≥5%.
Keywords: Spirulina, Nutrition, Entomophagy, Red palm weevil
1. Introduction
Insects are important for the survival of humanity as they play a role in crop pollination, biocontrol of harmful pests, and serve as food for thousands of people worldwide (Van Huise et al., 2013; Liu et al., 2019, Schwarz and Frank, 2019). In contrast, thousands of insect species are harmful to field crops, vegetables, and fruit trees. The red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryopthoridae), is a serious pest of the date palm (Phoenix dactylifera L.) (European and Mediterranean Plant Protection Organization, 2008). The date palm is a prehistoric tree grown globally in warm climates (Chao and Krueger, 2007). The date fruit is nutritious, carbohydrate-rich, and contains minerals and vitamins (Ahmed et al., 2014, Assirey, 2015). Unlike most other fruit crops, date palm cultivation is laborious, costly, and requires significant attention. The R. ferrugineus has become a significant concern for date palm growers worldwide. Plant protection scientists are attempting to overcome this pest problem using several management techniques. Laboratory studies require the red palm weevil individuals for bioassay; however, rearing is difficult and requires significant attention, and several researchers have proposed specific diets for rearing it in the laboratory for experimental purposes (Al-Ayedh, 2011).
There are several beneficial uses of insects other than crop pollination. Such as, entomophagy is well-known in several parts of the world, particularly in African countries, and could help to alleviate malnutrition in developing countries and food security issues (Van Huis, 2015). The increasing world population might lead to food security risks and require alternative sources of protein. There are approximately 1900 species of insects worldwide reported as edible for humans (Van Huise et al., 2013; Chakravorty, 2014, Bernard and Womeni, 2017, Orkusz et al., 2020). The Coleopteran and Lepidopteran insects are mainly entomophagic, however, termites and grasshoppers are also a favorite food of humans in several parts of Africa, Mexico, and Arabian countries (Chakravorty, 2014). Previous studies have confirmed that crickets contain 1562 mg of iron per 100 g of dry matter and it had been suggested that to overcome iron deficiency issue adult human males and females can consume 9.5 and 19.5 mg of dry matter per day, respectively (Christensen et al., 2006).
It has been reported that as an estimate one billion people worldwide consume insects as food, including in Africa, Asia, and Latin America. However, if insects are to become an important resource, they need to be farmed (Van Huis, 2015). Insects are not only eaten for their nutritional value but also their taste (Nonaka, 2009). For example, consumers tried and reported liking to eat insect-based products such as insect burgers (Schouteten et al., 2016). Similarly, a survey-based study reported that 72% and 74% of adults living in America and India, respectively, were willing to eat insects as food (Ruby et al., 2015). Insects have a high nutritional value, and the comparison between the nutritional value of insects and other livestock is relevant in that insects have been shown to have high energy levels (Kcal) per 100 g of edible portion. The palm weevil larvae showed 479 Kcal/100 g, while beef, chicken, and pork have been reported having 169, 152, and 186 Kcal/100 g, respectively (Gere et al., 2017). It has been reported that edible insects are a potential food source because of their high energy and protein content (Rumpold and Schlüter, 2013). Palm weevil larvae contain protein up to 36% and are consumed as food in several parts of the world (Van Huis et al., 2013).
Adequate nutrition is important and vital at all stages of an organism’s growth and development. Like humans and all other organisms, insects require basic nutritional inputs for proper growth and development, and the proportion of nutrients in insect food is important (House, 1969). Unfortunately, few studies have been reported for nutritional requirements of phytophagous insects (Offor, 2010). The Spirulina platensis is an alga containing high levels of protein, vitamins, minerals, and amino acids and could enhance the growth of a wide range of organisms by supplying appropriate quantities of nutrients (Salmeán et al., 2015). Spirulina powder is a promising resource as a supplement for livestock and animal feed (Holman and Malau-Aduli, 2013), and it has been used in bird, poultry, and fish feed to enhance production (Habib, 2008, Swiątkiewicz et al., 2015, Yusuf et al., 2016, Khalila et al., 2018, El-Bahr et al., 2020). In addition, it has been reported that Spirulina has the same nutritional value as pollen and can be used as a dietary supplement for honey bees (Ricigliano and Simone-Finstrom, 2020). It can also reduce the toxic effects of chemicals as it contains antioxidant compounds, and it has been reported that Spirulina supplementation can alleviate the toxic stress of deltamethrin in male mice (Abdel-Daim et al., 2016). Another study assessing the toxicity of Spirulina at high doses (10 and 30 g/kg body weight) in mice reported no abnormality in appearance (Hutadilok-Towatana et al., 2008).
Similarly, rats fed 800 mg/kg showed no mortality and there was no allergic skin reaction when fed 2000 mg/kg body weight (Salmeán et al., 2015); however, toxic effects have been reported in insects. Several studies have reported the effects of fungal isolates as pathogenic to different insects like Diaphorina citri Kuwayama (Hemiptera: Psyllidae), and Trogoderma granarium (Everts) (Coleoptera: Dermestidae) (Qasim et al., 2018, Islam et al., 2020, Qasim et al., 2020a, Qasim et al., 2020). Unfortunately, very limited information is available regarding toxic effects of Spirulina against field crop pests. Aly and Abdou, 2010, Rashwan and Hammad, 2020, have reported that Spirulina can cause 100% mortality in the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae), at 5% concentration. In present study, I evaluated the effects of Spirulina as a nutritional supplement and its toxicity to red palm weevil larvae.
2. Materials and methods
2.1. Rearing of red palm weevil
In this study, a red palm weevil colony was reared at the Economic Entomology Research Unit, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia, at 25 °C ± 2 °C, 65% ± 5% relative humidity, and photoperiod 6:18 h light: dark. The colony was reared on an artificial diet that included chopped date palm fronds, corn, wheat flour, and ascorbic acid as a preservative. Approximately 2-d-old, freshly hatched red palm weevil larvae were collected and used for study.
2.2. Production of red palm weevil larvae
Newly emerged red palm weevil adults were placed in plastic boxes with 10% sugar solution cotton soaked at base of the box to provide the adults with sugar that could boost egg-laying. Female adults laid eggs in the cotton which were collected daily, placed on wet filter paper in a plastic petri dish, and kept in an incubator at 25 °C ± 2 °C, 65% ± 5% relative humidity, and photoperiod 6:18 h light: dark. The eggs hatched between 3 and 4 d; the neonates were gathered, placed in 50 g plastic cups, and supplied with 1 g artificial diet. The larvae were taken off the standard artificial diet after 1-d, weighed, and fed a diet mixed with Spirulina.
2.3. Diet preparation and feeding protocol
I used different percentages of Spirulina powder (Pharma Care Europe Ltd., UK) as a nutritional supplement for the larvae. The six treatments consist of 0.5%, 1%, 2%, 5%, 10%, and 20% Spirulina powder mixed with the artificial feed, whereas the control treatment feed contained no Spirulina powder (Fig. 1). The artificial diet and Spirulina powder for each treatment were measured and mixed with a disposable plastic spoon and the mixed diet was fed to the larvae in the plastic cups. Once a week, the fresh diet was prepared and fed to the larvae.
Fig. 1.
Spirulina powder mixed with the diet displaying all the concentrations.
2.4. Weighing and feeding of larvae
The larvae were weighed using an electronic balance (PGL 3002 Adam Equipment UK). During the first week, larvae were fed 1 g of Spirulina-mixed diet; the quantity was increased every week with larval growth (Fig. 2). In the control treatment, the larvae were fed the standard artificial diet. Once a week, they were removed from the cups, washed with sterilized distilled water for 1–2 s, dried on filter paper and weighed. Dead larvae were removed, and the mortality rate was recorded. After weighing the larvae were transferred to a new 50 g plastic cup, and fresh Spirulina-mixed diet was provided. The cups were transferred to an incubator at 25 °C ± 2 °C, 65% ± 5% relative humidity, and photoperiod 6:18 h light: dark.
Fig. 2.
Amounts of diet given to larvae each week.
2.5. Data collection and statistical analysis
Observations were made weekly until the larvae reach the pre-pupal stage. The weight of each larva was recorded and the mean body weight gain was calculated. Initially, there were ten larvae in each treatment, considering each larva as one replicate. The dead larvae from each treatment were removed, the numbers were recorded, and the mean mortality was calculated. For each of the six treatments and the control, the parameters weight gain and mortality were recorded weekly. The data were analyzed using analysis of variance (ANOVA) and the means were separated using the least significant difference (LSD) test at P < 0.05 (SAS 2009).
3. Results
The present study assessed the nutritional benefits and toxicity of Spirulina to the development of red palm weevil larvae. The findings showed that Spirulina could be used as a dietary supplement for red palm weevil larval growth. In general, the weekly-recorded weight gains of larvae fed the Spirulina-mixed diet were significantly higher than the control.
The larval weights at the end of week-1 for larvae fed the Spirulina-supplemented treatment diets were significantly higher than the artificial-feed-only control diet (Table 1). There was a maximum weight gain of 0.04 g/larvae in larvae fed the 2% concentration diet, followed by the 0.5% and 10% diets, in both of which the weight gain was 0.031 g/larvae. In contrast, the least weight gain of 0.010 g/larvae was in the control treatment larvae.
Table 1.
Mean weight gain (g ± SE) at the end of week-1 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.010 ± 0.001c | 9 | 6.68 | 6, 62 | < 0.0001 |
| 0.5% Spirulina | 0.031 ± 0.005ab | 9 | |||
| 1% Spirulina | 0.028 ± 0.003b | 9 | |||
| 2% Spirulina | 0.040 ± 0.002a | 9 | |||
| 5% Spirulina | 0.021 ± 0.002b | 9 | |||
| 10% Spirulina | 0.031 ± 0.005ab | 9 | |||
| 20% Spirulina | 0.022 ± 0.003b | 9 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
At the end of week-2, larvae fed with 2% concentration diet gained the most weight of 0.092 g/larva, followed by larvae fed with 0.5% Spirulina diet with 0.078 g/larva weight gain. In the control treatment, larval weight gain was 0.040 g/larva. During week-2 with 20% concentration, larval weight was reduced and there was 100% mortality by the end of week-3 (Table 2).
Table 2.
Mean weight gain (g ± SE) at the end of week-2 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.040 ± 0.007c | 8 | 8.92 | 6, 55 | < 0.0001 |
| 0.5% Spirulina | 0.078 ± 0.014a | 8 | |||
| 1% Spirulina | 0.077 ± 0.011a | 8 | |||
| 2% Spirulina | 0.092 ± 0.006a | 8 | |||
| 5% Spirulina | 0.044 ± 0.008bc | 8 | |||
| 10% Spirulina | 0.072 ± 0.017ab | 8 | |||
| 20% Spirulina | −0.00 ± 0.002d | 8 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
At the end of week-3, the maximum larval weight gains of 0.383 and 0.389 g/larva were recorded in the 0.5% and 2% concentrations, respectively. Larvae fed the 5% and 10% diet did not gain more weight during week-3 and their weight gain was not significant than the larval weight gain in control (Table 3).
Table 3.
Mean weight gain (g ± SE) at the end of week-3 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.097 ± 0.021c | 8 | 17.78 | 5, 47 | < 0.0001 |
| 0.5% Spirulina | 0.383 ± 0.038a | 8 | |||
| 1% Spirulina | 0.281 ± 0.025b | 8 | |||
| 2% Spirulina | 0.389 ± 0.047a | 8 | |||
| 5% Spirulina | 0.111 ± 0.024c | 8 | |||
| 10% Spirulina | 0.103 ± 0.036c | 8 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
At the end of week-4, the maximum weight gain was 0.806 and 0.781 g/larvae for larvae fed the 1% and 2% concentrations, respectively. The least weight gain of 0.068 g/larvae was in the 10% diet. Similar to 3rd week; during the 4th week, larvae fed with 10% diet could not gain more weight. However, during this week, larvae fed in control gain more weight as compared to the 3rd week (Table 4).
Table 4.
Mean weight gain (g ± SE) at the end of week-4 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.247 ± 0.027bc | 6 | 20.99 | 5, 35 | <0.0001 |
| 0.5% Spirulina | 0.389 ± 0.115b | 6 | |||
| 1% Spirulina | 0.806 ± 0.057a | 6 | |||
| 2% Spirulina | 0.781 ± 0.090a | 6 | |||
| 5% Spirulina | 0.216 ± 0.046bc | 6 | |||
| 10% Spirulina | 0.068 ± 0.030c | 6 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
Generally, weight gain increased with time and larval growth. At the end of week-5, larvae fed the 5% concentration gained 0.618 g/larvae. This was the first record of a greater increase in weight with the 5% concentration than the 2% treatment. However, the greatest weight increase was in the 1% concentration treatment in which the larval weight was 0.846 g/larvae, followed by 0.5% concentration where the weight gain was 0.721 g/larva (Table 5).
Table 5.
Mean weight gain (g ± SE) at the end of week-5 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.418 ± 0.038c | 6 | 9.85 | 5, 35 | <0.0001 |
| 0.5% Spirulina | 0.721 ± 0.64ab | 6 | |||
| 1% Spirulina | 0.846 ± 0.056a | 6 | |||
| 2% Spirulina | 0.493 ± 0.140c | 6 | |||
| 5% Spirulina | 0.618 ± 0.046bc | 6 | |||
| 10% Spirulina | 0.190 ± 0.049d | 6 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
At the end of week-6, the larval weight gain was maximum among the treatments as compared to the previous five-week data. During week-6, larvae in almost all treatments gained more weight than in the same treatments within each of the previous 5 weeks. Following the pattern of larval weight gain, during week-6, there were weight gains of 0.953 and 1.12 g/larvae for the 1% and 2% concentrations, respectively. Although the weight gain was more during this week yet there was no significant difference among the treatments except 10% concentration where weight gain was almost 40–50% less than other treatments (Table 6).
Table 6.
Mean weight gain (g ± SE) at the end of week-6 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.892 ± 0.101a | 6 | 2.47 | 5, 35 | 0.0548 |
| 0.5% Spirulina | 0.878 ± 0.143a | 6 | |||
| 1% Spirulina | 0.953 ± 0.143a | 6 | |||
| 2% Spirulina | 1.120 ± 0.199a | 6 | |||
| 5% Spirulina | 0.873 ± 0.106a | 6 | |||
| 10% Spirulina | 0.475 ± 0.082b | 6 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
After week-6, larval weight gain started to decrease as the peak larval growth stage had been reached, after which larvae stopped feeding and prepared for pupation. In week-7, there was a decrease in weight in the 1% and 2% Spirulina diets. Surprisingly, the weight gain in the 5% and 10% concentrations was greater during week-7. The weight gain data at the end of week-7 are presented in Table 7.
Table 7.
Mean weight gain (g ± SE) at the end of week-7 in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 0.610 ± 0.060b | 6 | 6.72 | 5, 35 | 0.0003 |
| 0.5% Spirulina | 0.246 ± 0.170c | 6 | |||
| 1% Spirulina | 0.640 ± 0.084b | 6 | |||
| 2% Spirulina | 0.260 ± 0.162c | 6 | |||
| 5% Spirulina | 0.990 ± 0.087a | 6 | |||
| 10% Spirulina | 0.885 ± 0.104ab | 6 | |||
Means followed by the same letters do not differ significantly (at P < 0.05).
The overall weight gain data of the red palm weevil larvae among all treatments were significant (Table 8). These results indicate that the 1% and 2% concentrations supported the growth of the red palm weevil larvae. Although the 5% concentration resulted in greater overall weight gain as compared to the 0% (control) however, to avoid the risk of mortality, it should not be used.
Table 8.
Mean of total weight gain (g) throughout larval span in red palm weevil larvae reared on Spirulina-supplemented artificial diet.
| Treatment | Total weight gain (g) | ANOVA Parameters | |||
|---|---|---|---|---|---|
| N | F | df | P | ||
| 0% Spirulina (control) | 2.33 ± 0.120 cd | 6 | 11.05 | 5, 35 | <0.0001 |
| 0.5% Spirulina | 3.08 ± 0.253ab | 6 | |||
| 1% Spirulina | 3.64 ± 0.165a | 6 | |||
| 2% Spirulina | 3.17 ± 0.177ab | 6 | |||
| 5% Spirulina | 2.87 ± 0.195bc | 6 | |||
| 10% Spirulina | 1.84 ± 0.217d | 6 | |||
Means followed by the same letters do not differ significantly (P < 0.05).
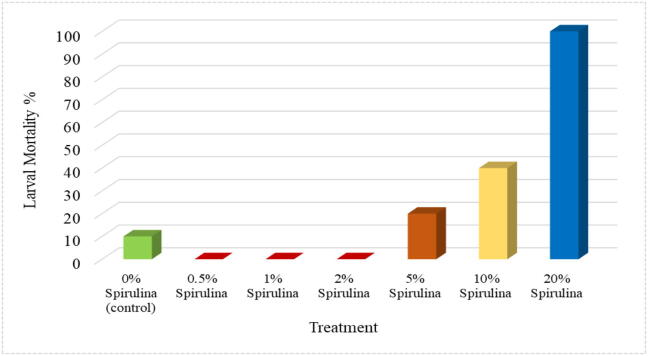
The mean mortality among larvae shows that at the dietary concentration of 20% Spirulina was toxic and resulted in 100% mortality by the end of week-3, while 20% and 40% larval mortality rates were recorded in the 5% and 10% Spirulina treatments, respectively (Fig. 3). However, apart from the control, there was no larval mortality in the rest of Spirulina-mixed diet treatments. In the control treatment, 10% mortality was recorded by the end of week-1.
Fig. 3.
Red palm weevil larval mortality (%) reared on Spirulina-supplemented artificial diet.
4. Discussion
The Spirulina powder is a product with opposing effects on insects. While it exhibits high nutritional value to insects at low concentrations, it has been reported to have significant toxic effects on several insect pests at higher concentrations and could be a potential source of natural pesticides against them (Rashwan and Hammad, 2020). I found a reduction in larval weight and mortality rates at concentrations of ≥ 5% of the diet. I observed an increase in larval weight with Spirulina mixed diet during the first week particularly more weight gain with lower concentrations. It has been previously reported that lower concentrations enhanced larval growth of Spodoptera littoralis, the maximum larval weight was recorded with 0.5% and 1% Spirulina concentrations (Aly and Abdou, 2010). These findings align with our results that indicate maximum larval weight gains of 3.64 and 3.17 g/larva in red palm weevil at the 1% and 2% concentrations, respectively (Table 8).
In aquaculture, several research findings have reported Spirulina as the best protein dietary supplement to improve the immune system and boost reproduction (James et al., 2006, Watanuki et al., 2006, Güroy et al., 2012, Jana et al., 2014, Mosha, 2019). Previous studies have reported Spirulina to have a beneficial effect on guppy growth. Guppies fed fishmeal with 40% Spirulina diets displayed improved growth and weight (Dernekbasi et al., 2010). In the present study, I assessed the weight gain in red palm weevil larvae every week and found a continuous increase in larval weight gain in larvae fed on the Spirulina-mixed diet. In contrast to previous results, our results showed the toxic effects of Spirulina on red palm larvae resulted in 100% and 40% mortality at 20% and 10% concentrations, after week-2 and 6, respectively. Similarly, Spirulina fed as a dietary supplement to green tiger shrimps resulted in a higher survival rate of shrimp larvae than the regular diet (Ghaeni et al., 2011). Similar advantages of Spirulina dietary supplementation, such as increased specific growth and increased live weight gain, have been documented in Nile tilapia (Abu-Elala et al., 2016).
In red palm weevil larvae, the maximum weight gain of 0.953 and 1.120 g/larva was recorded during 6-week of larval span for 1% and 2% concentration, respectively. The larval stage is usually completed in 8–9 weeks on the standard artificial diet. During week-6, the larvae fed actively and were provided with the highest quantity of feed (20 g/larva). At week-7, the average larval weight gain started to decrease as compared to the previous week because the larvae had stopped feeding to prepare for the pupation stage. During the observation it was noticed that all the larvae fed with lower concentrations of Spirulina supplemented diet were active and healthier as the Spirulina has acted like a supertonic for them. In literature, medicinal values of Spirulina also have been reported in many animals, including chickens, rats, mice, and even in humans. Spirulina possesses significant nutritional value and contains vitamins to support the growth and development of living organisms and protect them from several diseases (Belay, 2002, Colla et al., 2008, Ghaeni and Roomiani, 2016). It has been reported that S. platensis has the highest source of Vitamin B12 and β-carotene; it is good for human health and could improve the body’s defense system (Sindhumole, 2015).
Spirulina has partially replaced soybean and corn as a protein source for chickens. Several studies have documented significant improvements in chicken body weight, growth performance, immunity, fatty acid profile, and biological traits (Kaoud, 2012, Bonos et al., 2016, Zeweil et al., 2016, Neumann et al., 2018, Sugiharto et al., 2018, Velten et al., 2018, Sharmin et al., 2020). Additionally, several studies have concluded that microalgae show significant advantages as an economical and eco-friendly food source in the poultry industry.
In the animal production and poultry industries, and aquaculture, Spirulina has become an important dietary supplement with no acute toxic effects. In contrast, toxic effects on insects have been documented in the literature. At low concentrations, Spirulina supplementation resulted in positive effects on insect growth and performance, whereas at higher concentrations, it caused mortality. These findings agree with this study in which I report 100% mortality of red palm weevil larvae at 20% concentration (Fig. 3). In the black cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae), 80% mortality was reported in the 2nd stage larval instars when fed with castor bean leaf discs dipped in different microalgae strains solutions (Abdel-Rahim and Hamed, 2013a). Similar findings have been documented in 4th instar larvae of S. litoralis, showing 100% mortality when fed with a 5% concentration of Spirulina (Aly and Abdou, 2010). Similarly, when S. litoralis 2nd instar larvae were fed with water and phenolic based extracts of 7% Spirulina, 19% mortality was documented (Rashwan and Hammad, 2020). Similar to our findings, several studies have reported the toxic effects of different algae against insects (Abdel-Rahim and Hamed, 2013b, Saber et al., 2018). There is a need for further studies on the effectiveness of Spirulina as a toxicant at different developmental stages of the red palm weevil to pave the way for its field use as an eco-friendly bio-pesticide against the weevil.
5. Conclusions
The present research findings indicated that Spirulina, used in lower concentrations, has a beneficial effect on the growth of red palm weevil larvae. However, when used ≥5% dietary concentration, it has detrimental effects and can cause mortality. Further studies could explore the effects of Spirulina as a dietary supplement on other biological parameters of the red palm weevil and investigate its potential as an eco-friendly bio-pesticide.
Funding
This work was funded by the Researchers Supporting Project Number (RSP-2020/293) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The author declared that there is no conflict of interest.
Acknowledgments
This work was funded by the Researchers Supporting Project Number (RSP-2020/293) King Saud University, Riyadh, Saudi Arabia. Author thanks the Research Support Service Unit (RSSU) at King Saud University, for their technical support. The author would also like to express his gratitude to Dr. Mureed Husain for his assistance with the practical part of this project.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Daim M., El-Bialy B.E., Rahman H.G.A., Radi A.M., Hefny H.A., Hassan A.M. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed. Pharmacother. 2016;77:79–85. doi: 10.1016/j.biopha.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahim E.F.M., Hamed S.M. Comparative toxic activity of four algae, against the 2nd and 4th larval instars of black cutworm, Agrotis ipsilon. Hufnagel) Egypt J. Agric. Res. 2013;91:1303–1318. [Google Scholar]
- Abdel-Rahim E.F., Hamed S.M. Efficacy of Anabaena flos aquae alga against Larvae of the Cotton Leaf Worm, Spodoptera littoralis (Boisd.) Egypt. J. Biol. Pest Control. 2013;23:1–7. [Google Scholar]
- Abu-Elala N.M., Galal M.K., Abd-Elsalam R.M., Mohey-Elsaeed O., Ragaa N.M. Effects of dietary supplementation of Spirulina platensis and garlic on the growth performance and expression levels of immune-related genes in Nile tilapia (Oreochromis niloticus) J. Aquac Res. Dev. 2016;7:2. doi: 10.4172/2155-9546.1000433. [DOI] [Google Scholar]
- Ahmed J., Al-Jasass F.M., Siddiq M. Wiley Blackwell; Chichester: 2014. Date Fruit Composition and Nutrition. Dates: Postharvest Science, Processing Technology and Health Benefits; pp. 261–283. [Google Scholar]
- Al-Ayedh H.Y. Evaluating a semi-synthetic diet for rearing the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Int. J. Trop. Insect Sci. 2011;31:20–28. doi: 10.1017/S1742758411000063. [DOI] [Google Scholar]
- Aly M.S., Abdou W.L. The effect of native Spirulina platensis on the developmental biology of Spodoptera littoralis. J. Gen. Eng. Biotech. 2010;8:65–70. [Google Scholar]
- Assirey E.A.R. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. J. Taibah Univ. Sci. 2015;9:75–79. doi: 10.1016/j.jtusci.2014.07.002. [DOI] [Google Scholar]
- Belay A. The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. J. Am Nutraceutical Assoc. 2002;5:27–48. [Google Scholar]
- Bernard T., Womeni H.M. Insect physiology and ecology. InTech; Rijeka, Croatia: 2017. Entomophagy: insects as food; pp. 233–254. [Google Scholar]
- Bonos E., Kasapidou E., Kargopoulos A., Karampampas A., Christaki E., Florou-Paneri P., Nikolakakis I. Spirulina as a functional ingredient in broiler chicken diets. SA J. An. Sci. 2016;46:94–102. doi: 10.4314/sajas.v46i1.12. [DOI] [Google Scholar]
- Chakravorty J. Diversity of edible insects and practices of entomophagy in India: an overview. J. Biodivers. Biopros Dev. 2014;1:3. doi: 10.4172/2376-0214.1000124. [DOI] [Google Scholar]
- Chao C.T., Krueger R.R. The date palm (Phoenix dactylifera L.): overview of biology, uses, and cultivation. Horts. 2007;42:1077–1082. doi: 10.21273/HORTSCI.42.5.1077. [DOI] [Google Scholar]
- Christensen D.L., Orech F.O., Mungai M.N., Larsen T., Friis H., Aagaard-Hansen J. Entomophagy among the Luo of Kenya: a potential mineral source? Int. J. Food Sci. Nutr. 2006;57:198–203. doi: 10.1080/09637480600738252. [DOI] [PubMed] [Google Scholar]
- Colla L.M., Muccillo-Baisch A.L., Costa J.A.V. Spirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbits fed with a hypercholesterolemic diet. Braz. Arch. Biol. Technol. 2008;51:405–411. doi: 10.1590/S1516-89132008000200022. [DOI] [Google Scholar]
- Dernekbasi S., Una H., Karayucel I., Aral O. Effect of dietary supplementation of different rates of Spirulina (Spirulina platensis) on growth and feed conversion in guppy (Poecilia reticulata Peters, 1860) J. Anim. Vet. Adv. 2010;9:1395–1399. doi: 10.3923/javaa.2010.1395.1399. [DOI] [Google Scholar]
- El-Bahr S., Shousha S., Shehab A., Khattab W., Ahmed-Farid O., Sabike I., El-Garhy O., Albokhadaim I., Albosadah K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals. 2020;10(5):761. doi: 10.3390/ani10050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO Rhynchophorus ferrugineus. EPPO Bull. 2008;38:55–59. doi: 10.1111/j.1365-2338.2008.01195.x. [DOI] [Google Scholar]
- Gere A., Zemel R., Radványi D., Moskowitz H. Insect based foods a nutritional point of view. Nutr. Food Sci. Int. J. 2017;4(3) doi: 10.19080/NFSIJ.2017.04.555638. [DOI] [Google Scholar]
- Ghaeni M., Matinfar A., Soltani M., Rabbani M., Vosoughi A. Comparative effects of pure Spirulina powder and other diets on larval growth and survival of green tiger shrimp, Peneaus semisulcatus. Iran. J. Fish. Sci. 2011;10:208–217. [Google Scholar]
- Ghaeni M., Roomiani L. Review for application and medicine effects of Spirulina, platensis microalgae. Adv. Agric. Technol. 2016;3:114–117. doi: 10.18178/joaat.3.2.114-117. [DOI] [Google Scholar]
- Güroy B., Şahin İ., Mantoğlu S., Kayalı S. Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid Pseudotropheus acei. Aquacult. Int. 2012;20:869–878. doi: 10.1007/s10499-012-9512-x. [DOI] [Google Scholar]
- Habib M.A.B. Food and agriculture organization of the united nations; Rome: 2008. Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. [Google Scholar]
- Holman B.W.B., Malau-Aduli A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013;97:615–623. doi: 10.1111/j.1439-0396.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- House H.L. Effects of different proportions of nutrients on insects. Entomol. Exp. Appl. 1969;12:651–669. doi: 10.1111/j.1570-7458.1969.tb02560.x. [DOI] [Google Scholar]
- Hutadilok-Towatana N., Reanmongkol W., Satitit S., Panichayupakaranant P., Ritthisunthorn P. A subchronic toxicity study of Spirulina platensis. Food Sci. Technol. Res. 2008;14:351–358. doi: 10.3136/fstr.14.351. [DOI] [Google Scholar]
- Islam W. Phyto-derivatives: an efficient eco-friendly way to manage Trogoderma granarium (Everts) (Coleoptera: Dermestidae) Int. J. Trop. Insect Sci. 2020 doi: 10.1007/s42690-020-00370-x. [DOI] [Google Scholar]
- James R., Sampath K., Thangarathinam R., Vasudevan I. Effect of Dietary Spirulina Level on Growth, Fertility, Coloration and Leucocyte Count in Red Swordtail. Xiphophorus helleri. 2006 [Google Scholar]
- Jana A., Saroch J.D., Borana K. Effect of Spirulina as a feed supplement on survival and growth of Pangasius sutchi. Int. J. Fish. Aquat. Stud. 2014;1:77–79. [Google Scholar]
- Kaoud H.A. Effect of Spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Sci. J. Appl. Res. 2012;1:44–48. [Google Scholar]
- Khalila H.S., Fayed W.M., Mansour A.T., Srour T.M., Omar E.A., Darwish S.I., Moussa Nour A.A.M. Dietary supplementation of Spirulina, Arthrospira platensis, With plant protein sources and their effects on growth, feed utilization and histological changes in Nile tilapia, Oreochromis niloticus. J. Aquac Res. Dev. 2018;9:2. doi: 10.4172/2155-9546.1000549. [DOI] [Google Scholar]
- Liu Q., Xu P., Yan K., Guo Y. Pollination Services from Insects in Home gardens in the Chengdu Plain will be Confronted with Crises. Sustainability. 2019;11(7):2169. doi: 10.3390/su11072169. [DOI] [Google Scholar]
- Mosha S.S. The significance of Spirulina meal on fishmeal replacement in aquaculture. A review. J. Fish AQUA Dev.: JFAD. 2019;145 [Google Scholar]
- Neumann C., Velten S., Liebert F. The graded inclusion of algae (Spirulina platensis) or insect (Hermetia illucens) meal as a soybean meal substitute in meat type chicken diets impacts on growth, nutrient deposition and dietary protein quality depending on the extent of amino acid supplementation. Open J. Anim. Sci. 2018;8:163–183. doi: 10.4236/ojas.2018.82012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka K. Feasting on insects. Entomol. Res. 2009;39:304–312. doi: 10.1111/j.1748-5967.2009.00240.x. [DOI] [Google Scholar]
- Offor E. The nutritional requirements of phytophagous insects: why do insects feed on plants? Available at SSRN. 2010;1535274 doi: 10.2139/ssrn.1535274. [DOI] [Google Scholar]
- Orkusz A., Wolańska W., Harasym J., Piwowar A., Kapelko M. Consumers’ attitudes facing entomophagy: polish case perspectives. Int. J. Environ. Res. Public Health. 2020;17:2427. doi: 10.3390/ijerph17072427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim M., Islam S.U., Islam W., Noman A., Khan K.A., Hafeez M., Hussain D., Dash C.K., Bamisile B.S., Akutse K.S., Rizwan M. B. Characterization of mycotoxins from entomopathogenic fungi (Cordyceps fumosorosea) and their toxic effects to the development of Asian citrus psyllid reared on healthy and diseased citrus plants. Toxicon. 2020;188:39–47. doi: 10.1016/j.toxicon.2020.10.012. [DOI] [PubMed] [Google Scholar]
- Qasim M., Jiang R., Waqar I., Habib A., Khalid A.K., Chandra K.D., Zakia A.J., Liande W. Comparative pathogenicity of four entomopathogenic fungal species against nymphs and adults of citrus red mite on the citrus plantation. Int. J. Trop. Insect Sci. 2020 doi: 10.1007/s42690-020-00263-z. [DOI] [Google Scholar]
- Qasim M., Yongwen L., Chandra K.D., Bamisope S.B., Keppanan R., Saif Ul I., Habib A., Fangfei W., Liande W. Temperature-dependent development of Asian citrus psyllid on various hosts, and mortality by two strains of Isaria. Microb. Pathog. 2018;119:109–118. doi: 10.1016/j.micpath.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Rashwan R.S., Hammad D.M. Toxic Effect of Spirulina platensis and Sargassum Vulgar as Natural Pesticides on Survival and Biological Characteristics of Cotton Leaf Worm Spodoptera littoralis. Scientific African. 2020;e00323 doi: 10.1016/j.sciaf.2020.e00323. [DOI] [Google Scholar]
- Ricigliano V.A., Simone-Finstrom M. Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (Spirulina) in honey bees. Apidologie. 2020;1–13 doi: 10.1007/s13592-020-00770-5. [DOI] [Google Scholar]
- Ruby M.B., Rozin P., Chan C. Determinants of willingness to eat insects in the USA and India. J. Insects Food Feed. 2015;1:215–225. doi: 10.3920/JIFF2015.0029. [DOI] [Google Scholar]
- Rumpold B.A., Schlüter O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- Saber A.A., Hamed S.M., Abdel-Rahim E.F.M., Cantonati M. Insecticidal prospects of algal and cyanobacterial extracts against the cotton leaf worm Spodoptera littoralis. Vie Milieu. 2018;68:199–212. [Google Scholar]
- Salmeán G.G., Castillo L.H.F., Chamorro-Cevallos G. Nutritional and toxicological aspects of Spirulina (Arthrospira) Nutr. Hosp. 2015;32:34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- Institute S.A.S. SAS Institute 9.2. Users guide; Cary, NC: 2009. SAS. Stat. [Google Scholar]
- Schouteten J.J., De Steur H., De Pelsmaeker S., Lagast S., Juvinal J.G., De Bourdeaudhuij I., Verbeke W., Gellynck X. Emotional and sensory profiling of insect-, plant- and meat-based burgers under blind, expected and informed conditions. Food Qual. 2016;52:27–31. doi: 10.1016/j.foodqual.2016.03.011. [DOI] [Google Scholar]
- Schwarz T., Frank T. Aphid feeding by lady beetles: higher consumption at higher temperature. Biocontrol. 2019;64:323–332. doi: 10.1007/s10526-019-09931-7. [DOI] [Google Scholar]
- Sharmin F., Sarker N.R., Sarker M.S.K. Effect of using Moringa oleifera and Spirulina platensis as feed additives on performance, meat composition and oxidative stability and fatty acid profiles in broiler chicken. 2020;10:772. doi: 10.35248/2155-9600.20.10.772. [DOI] [Google Scholar]
- Sindhumole P. Spirulina –The super food. Sci. India. 2015:32–35. [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E. Effect of feeding duration of Spirulina platensis on growth performance, haematological parameters, intestinal microbial population and carcass traits of broiler chicks. S. Afr. J. Anim. Sci. 2018;48:98–107. doi: 10.4314/sajas.v48i1.12. [DOI] [Google Scholar]
- Swiątkiewicz S., Arczewska-Włosek A., Józefiak D. Application of microalgae biomass in poultry nutrition. Worlds Poult. Sci. J. 2015;71:663–672. doi: 10.1017/S0043933915002457. [DOI] [Google Scholar]
- Van Huis A. Edible insects contributing to food security? Agric. Food Sec. 2015;4:20. doi: 10.1186/s40066-015-0041-5. [DOI] [Google Scholar]
- Van Huis A., Van Itterbeeck J., Klunder H., Mertens E., Halloran A., Muir G., Vantomme P. Edible Insects: Future Prospects for Food and Feed Security. Food and Agriculture Organization of the United Nations; Rome: 2013. Nutritional Value of Insects for Human Consumption; pp. 67–80. [Google Scholar]
- Velten S., Neumann C., Bleyer M., Gruber-Dujardin E., Hanuszewska M., Przybylska-Gornowicz B., Liebert F. Effects of 50 percent substitution of soybean meal by alternative proteins from Hermetia illucens or Spirulina platensis in meat-type chicken diets with graded amino acid supply. Open J. Anim. Sci. 2018;8:119–136. doi: 10.4236/ojas.2018.82009. [DOI] [Google Scholar]
- Watanuki H., Ota K., Tassakka A.C.M.A.R., Kato T., Sakai M. Immunostimulant effects of dietary Spirulina platensis on carp, Cyprinus carpio. Aquaculture. 2006;258:157–163. doi: 10.1016/j.aquaculture.2006.05.003. [DOI] [Google Scholar]
- Yusuf M.S., Hassan M.A., Abdel-Daim M.M., El Nabtiti A.S., Ahmed A.M., Moawed S.A., El-Sayed A.K., Cui H. Value added by Spirulina platensis in two different diets on growth performance, gut microbiota, and meat quality of Japanese quails. Vet. World. 2016;9:1287–1293. doi: 10.14202/vetworld.2016.1287-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeweil H., Abaza I.M., Zahran S.M., Ahmed M.H., AboulEla H.M., Saad A.A. Effect of Spirulina platensis as dietary supplement on some biological traits for chickens under heat stress condition. Asian Biomed Pharm Sci. 2016;6:8–12. [Google Scholar]