Graphical abstract
Keywords: Nematocyst venom, MTT, SDS, DPPH, Lung, And liver cancer cell lines
Abstract
This study aimed to investigate the antiproliferative and antioxidant properties of crude venom from the nematocyst of Jellyfish Acromitus flagellates on human lung cancer (A549) and liver cancer (HepG2) cell lines. The prepared crude venom was subjected to analyses of the biochemical constituents, protein profiles, antioxidant and anticancer activities by standard methods. The extracted venom was pale-yellow in color and viscous/sticky. The biochemical composition such as, protein (1.547 mg/ml), lipid (0.039 mg/ml) and carbohydrate (0.028 mg/ml) was estimated. Protein profiles were determined by SDS PAGE, the result revealed that the molecular weight range from 205 − 3.5 kDa. The free radical scavenging activity was analyzed by the reducing potential (56.36%), DPPH (72.47%), hydroxyl (68.50%), superoxide anion (65.75%), and nitric oxide (33.04%). The cell viability was observed by using different concentrations (20 to 100 µg/ml) of crude venom on A549 and HepG2 cancer cell lines and the IC50 values were recorded in (60 μg/ml and 40 μg/ml) respectively, while it had none cytotoxic effects on Vero cell line up to the concentration of 90 μg/ml. These results suggest that crude venom from nematocyst of A. flagellatus possesses anti-cancer activity and able to develop novel drugs on marine-derived compounds.
1. Introduction
Cancer is a term used for diseases in which abnormal cells divide without control and are able to invade other tissues. It, despite the all out efforts from developed countries still causes one in five deaths (Kanwal et al., 2015). The estimated numbers of new cancer cases and deaths in 2020 will be an estimated 1.8 million new cancer cases diagnosed and 606,520 cancer deaths in the United States (Siegel et al., 2020). The prognosis of lung cancer is very poor and the survival rate is only about 16%, which has not significantly altered in the past several decades (Bowtell et al., 2015). Nearly 85% of lung cancer is caused by tobacco users, affection both active and passive smokers (Rao et al., 2002, Gray et al., 2005). Proof of 24 years examination by the Indian Council of Medical Research (ICMR) assessed that lung cancer cases per one lakh, in that male populace has expanded by around 60% in Chennai, 100% in Bangalore, and 40% in Delhi during this time of (1988–2005) (Rapiti et al., 1999). Hepatocellular carcinoma (HCC) is the 6th most normal disease and the third driving reason for mortality around the world (Alves et al., 2016). HCC is the most common and lethal malignancies in the human population, with approximately 5,50,000 new cancer cases and almost death has been increased every year (Parkin, 2006). The 5th most frequently diagnosed carcinoma worldwide in men is liver cancer and the second most frequent probabilities of mortality. In women, the seventh most commonly diagnosed cancer and the sixth leading cause of cancer death. In both developing (20%) and developed countries (33%) estimated 748,300 new liver cancer cases have been reported (Schottenfeld and Fraumeni, 2006).
The marine creatures are likely wellsprings of fundamental and novel naturally dynamic substances for the improvement of therapeutics. Specifically, marine proteins and peptides have a lot of consideration because of their likely impacts on embracing wellbeing and forestalling diseases (Kang et al., 2015). A marine organism, which produces a substance has defined ecological functions and some of these molecules also exhibit pharmacological properties such as anticancer and antibacterial (Simmons et al., 2005), antifungal (Wattanadilok et al., 2007), cytotoxic (Mancini et al., 2007), antioxidant (Friedman et al., 2008), and antiviral (Utkina et al., 2010). Development in marine pharmacology was demonstrated to invent several new compounds in pre-clinical evaluation (da Rosa Guimarães et al., 2013). The venom and toxins from an animal source are newly recognized as a major source of bioactive molecules that lead to the development of novel drugs.
Venom is a secretion produced in specialized glands in an animal and delivered to a target animal through prey prediction. This secretion contains unique active molecules that disrupt normal physiological processes (Moore et al., 2011). Venomous animals produce assorted synthetic compounds that are utilized for contender prevention, protection, prey catch, and absorptions (Fry et al., 2006). Distinctive toxins are a helpful natural asset, containing a few pharmacologically dynamic segments that could be an exceptionally strong remedial worth. For as long as barely any many years, parts in toxin have become the focal point of analysts and have been broadly studied for their different anticancer properties. Approximately half the number of marine organisms comprise a vast source of discovered therapeutics and new anticancer drugs. The present study, to evaluate the anticancer and antioxidant properties of nematocyst crude venom from jellyfish A. flagellates on human cancer cell lines.
2. Materials and methods
2.1. Preparations and extraction of nematocysts venom
Jellyfish A. flagellates were collected from brackish water in Muttukadu
Chennai Tamilnadu, India and it was identified by the scientist from the Zoological Survey of India (ZSI). Chennai (Fig. 1). The marginal filaments and oral arms were removed from the sub-umbrella region of jellyfish. After that, it was stored at −20 °C then the further process was carried out. The frozen marginal filaments and oral arms were placed in 5 volumes of fresh seawater at 4 °C and incubated for four days of autolysis. The supernatant was decanted and the settled material was resuspended once every day. The resulting suspension was filtered with a 54 μm sieve filter. Then the final suspension of nematocysts was allowed to settle down. Finally, sediments were collected separately and washed several times with 0.9% NaCl solution by centrifugation at 10, 000 rpm at 4 °C for 30 min. Then washed sediments that contain nematocysts were stored at −20 °C (Blois, 1958). The isolated nematocysts were homogenized with PBS buffer (20 mM pH 7.2). The homogenized samples were centrifuged at 4 °C for 15 min at 13,000 g. The supernatant containing nematocyst venom was collected and stored at −20 °C (Feng et al., 2010)
Fig. 1.

Jellyfish Acromitues flagellates.
2.2. Biochemical analyses
The total protein content of the extracted nematocyst crude venom was estimated according to the method of Bradford, (1976), lipid content followed by the method of Barnes and Blackstock, (1973), and carbohydrate described by Roe, (1955).
2.3. Analysis of protein profile
The molecular weight of proteins from the crude venom was analyzed by SDS-PAGE described by the Laemmli, (1970). The resolving gel was made up of 10% polyacrylamide and stacking gel with 5% porosity. The sample was resuspended in SDS-PAGE sample buffer (Tris-HCl pH 6.8, 10% glycerol, 1% SDS, 0.01% bromophenol) and incubated 95 °C for 2 min. The denatured sample was electrophoresed at 70 V.
2.4. Antioxidant activities
The free radical scavenging activity of crude venom was analyzed by Reducing
potential (Barros et al., 2007). The antioxidant activity of the crude venom was determined in terms of the ability to donate hydrogen or radical scavenging, using the constant radical DPPH, (Blois, 1958). Measurement of superoxide anion scavenging activity of crude venom was followed by Panda et al., (2011). Hydroxyl radical scavenging activity was measured by examining the competition between deoxyribose and the test compound (proteins) of hydroxyl radical generated by Fe3+- Ascorbate-EDTA-H2O2 system (Fenton reaction) according to the method described by Dubey and Batra, (2009). The nitric oxide radical inhibition activities of crude venom were measured by Balamurugan and Menon, (2009).
2.5. Cell culture and chemicals
The Vero cells (green monkey kidney cell), Human hepatoma (HepG2), and lung cancer (A549) cell lines were obtained from the National Centre for Cell Science (NCSS), Pune, India, and was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 37 °C and 5% CO2 atmosphere. DMEM, Trypsin-EDTA, Fetal Bovine Serum (FBS), 3-(4,5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT), sodium bicarbonate, Dimethyl sulphoxide (DMSO), an antibiotic solution were purchased from Hi-Media Laboratories, Mumbai, India. 6 well plates, 96 well plates, Centrifuge tubes (15 and 50 ml), and Tissue culture flasks (25 and 75 mm) were purchased from Tarsons Products Pvt, Kolkata, India. Chemicals used in the present study were of the highest quality available locally.
2.6. MTT assay
The cytotoxicity and cell viability of normal and cancer cells were measured by the MTT method by Mosmann, (1983). Briefly, normal cells were seeded on 96-well culture plates at adversity and treated with crude venom at a concentration (30–150 µg/ml) for 24 h. The A549 and HepG2 cells were seeded in 96 well plates at a density of 5 × 104 cells per well and incubated overnight to get attached to the substratum. Then the cells were exposed to different concentrations of crude venom (20–100 µg/ml) at different time intervals of 24 and 48 h, after the completion of the incubation period, 20 µl of MTT stock solution (5 mg/ml) was added to each well. Subsequently 4 hrs of the incubation period, the purple color precipitates are visible seen, the supernatant was aspirated and the formazan precipitates were solubilized by the addition of 100 µl of DMSO per well. Following 1 h of incubation in a darkroom and absorbance was taken at 570 nm using an ELISA microplate reader.
2.7. General morphology
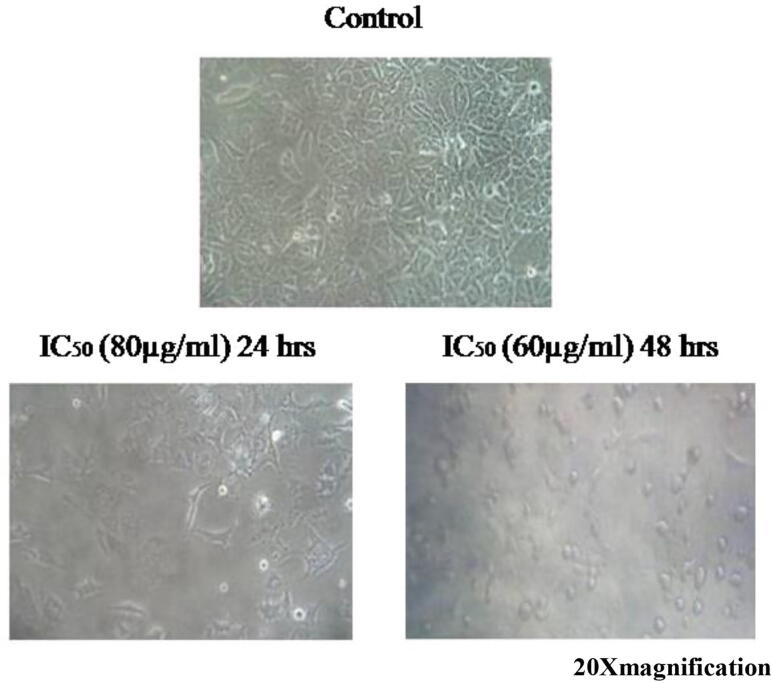
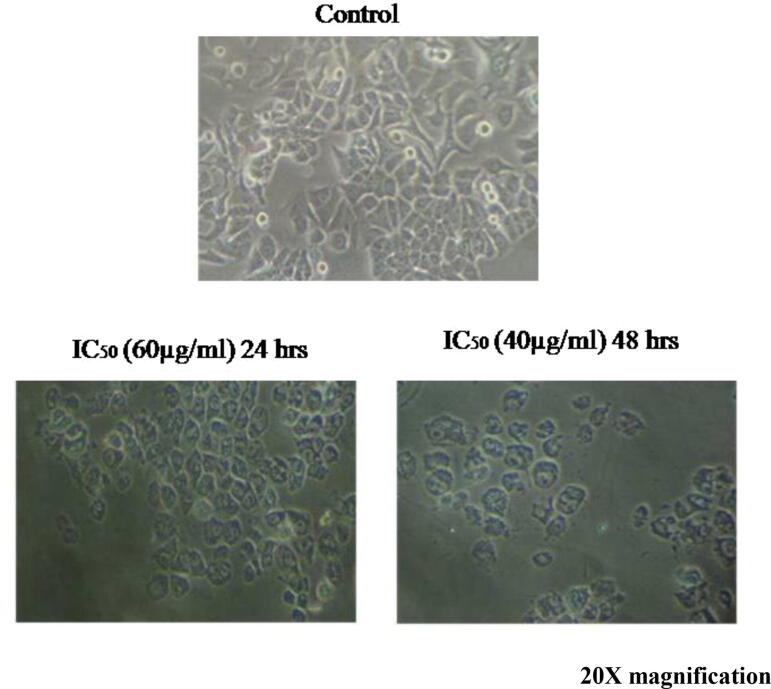
Human cancer cells (A549 and HepG2) were trypsinized and plated in the 6 well plates, the cells were incubated for 24 and 48 hrs for attachment with DMEM medium containing 10% FBS. A crude venom sample was added to the respective cell lines. In that control and IC50 concentrations of 80 and 60 µg/ml of 24 and 48 hrs incubation against lung cancer line, whereas 60 and 40 µg/ml of 24 and 48 hrs incubation on liver cancer cells. Control cells were maintained with serum less medium, then completion of 24 h incubation the cells were observed under an inverted microscope (Eno et al., 2008).
2.8. Statistical analysis
The results are expressed as a mean ± standard deviation (S.D). Turkey test was used to assess the significance of differences between two mean values. P < 0.05 was considered as statistically significant.
3. Results
3.1. Biochemical composition and protein profiles of nematocyst crude venom
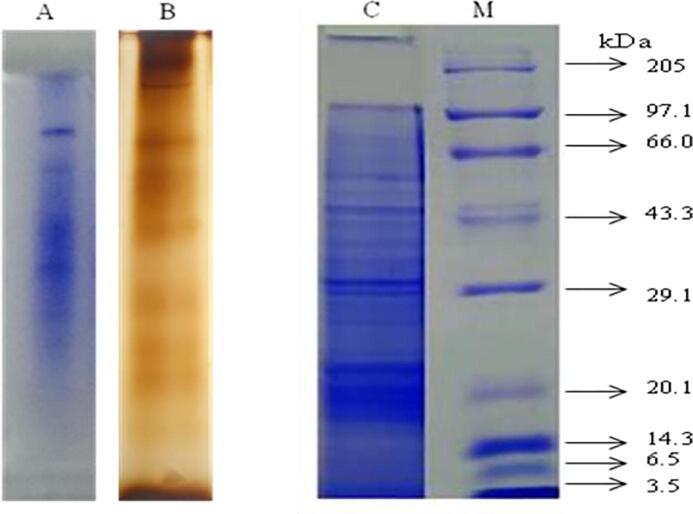
The biochemical composition of protein, lipid, and carbohydrate from crude venom of jellyfish A. flagellates was found to be 1.547, 0.039, and 0.028 µg/ml respectively. The protein profile of the crude venom was analyzed by Native and SDS-PAGE. The crude proteins were resolved into many polypeptide fractions under denaturing conditions in which ten major bands appeared very clearly in CBB staining as shown in Fig. 2. The protein sample showed bands of molecular weight ranging from 205.0 kDa to 3.5 kDa. Thus the crude sample showed both low and high molecular weight proteins.
Fig. 2.
Protein profile of nematocysts crude venom from A. flagellatus by Native and SDS PAGE.
3.2. Antioxidant activities
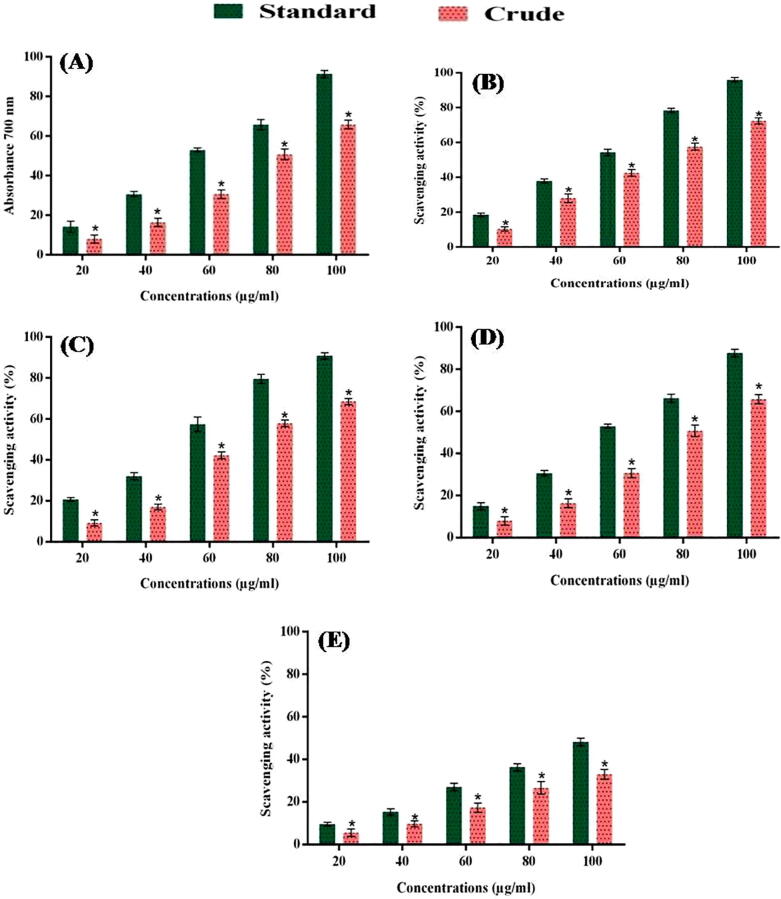
The DPPH test consequences of A. flagellates nematocyst proteins displayed incredible DPPH revolutionary scavenging activity. The absorbance values for free radical scavenging activity of crude venom was compare to ascorbic acid (standard), as follows reducing power potential (7.03 to 56.36%) and 11.31 to 88.96% (Fig. 3A); DPPH with (10.38 to 72.47%) and 18.51 to 95.94% (Fig. 3B); hydroxyl with 9.29 to 68.50% and 20.70 to 90.83% (Fig. 3C); superoxide anion amide 8.03 to 65.75% and 15.02 to 87.73% (Fig. 3D) and nitric oxide through 5.59 to 33.04% and 9.56 to 48.26% (Fig. 3E) at concentrations of 20 to 100 µg/ml , respectively.
Fig. 3.
Free radical scavenging effect of nematocysts crude venom from A. flagellatus (A) Reducing power, (B) DPPH, (C) Hydroxyl, (D) Superoxide ion and (E) Nitric oxide.
3.3. Cell viability and morphological observation of human cancer cell lines
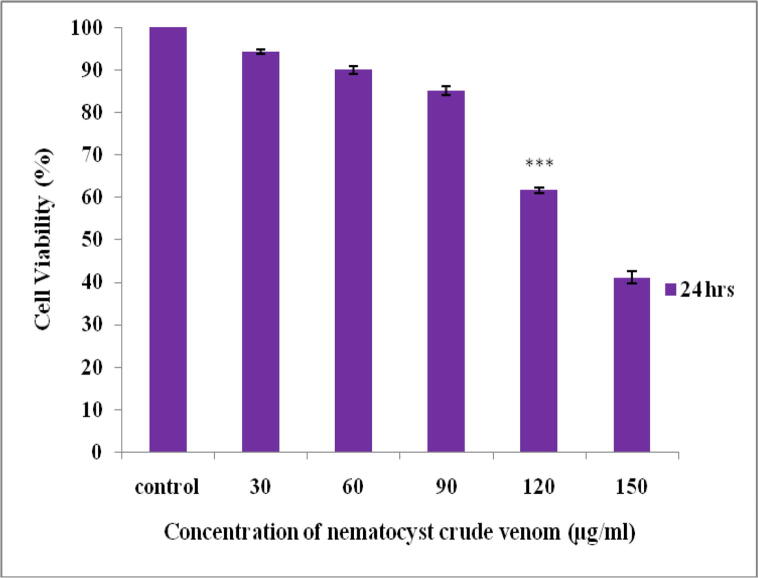
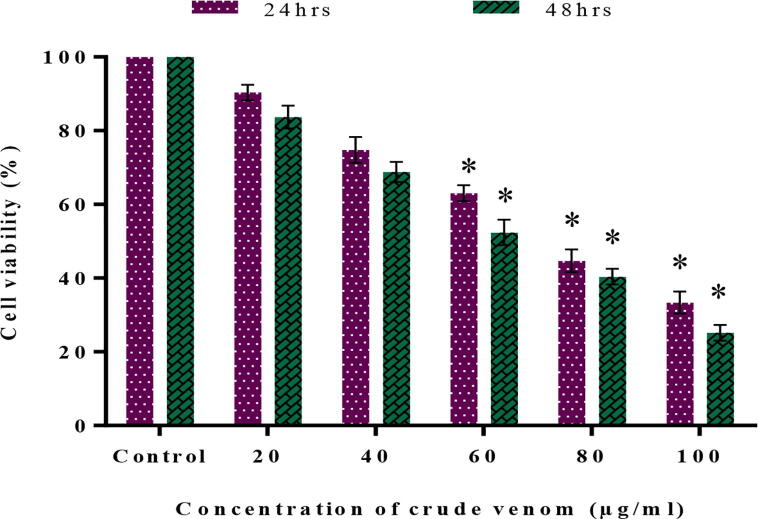
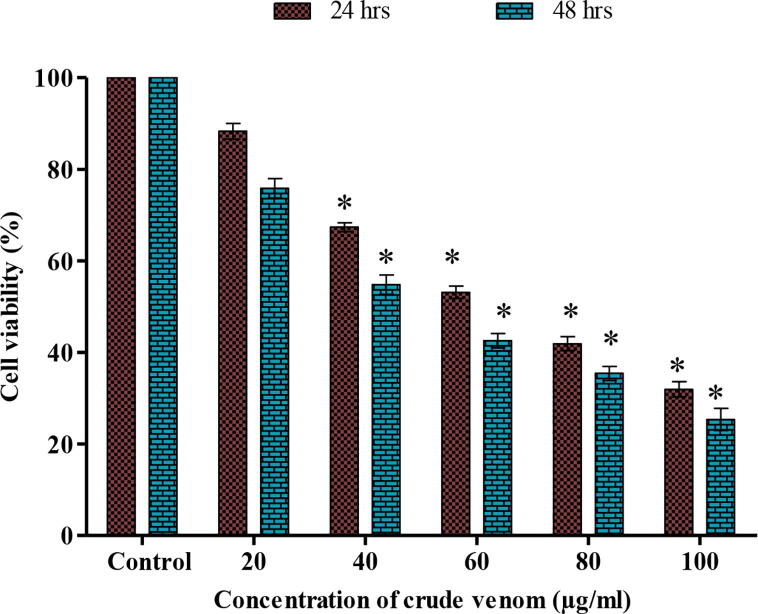
The cell viability and cytotoxic effect of nematocyst crude venom of A. flagellatus were tested by the MTT method using Vero and cancer cell lines (A549 and Hep G2). Cytotoxicity of Vero cells exhibits the 85% of cells were viable up to 90 µg/ml (Fig. 4). The treatment of crude venom inhibits the growth of cancer cell lines at different concentrations in a time and dose-dependent manner. When there is a decrease in the viability of cells, the concentration of crude venom was increased vice versa at 24 and 48hrs treatment which inhibits the lung cancer (A549) cells with an IC50 value of 80 and 60 µg/ml respectively (Fig. 5) and the HepG2 cells were inhibited with an IC50 value of 60 and 40 µg/ml respectively (Fig. 6).
Fig. 4.
Cytotoxicity effect of different concentration of nematocyst crude venom on Vero cell line at 24 h incubation.
Fig. 5.
Cell viability of human Lung cancer cells (A549) treated with different concentration of nematocyst crude venom at 24 and 48 hrs incubation.
Fig. 6.
Cell viability of (Hep G2) cells treated with different concentration of nematocyst crude venom at 24 and 48 h incubation.
Morphological variations were observed in A549 and HepG2 cells both control and treated cells were examined through light microscopy. The treated cells at 24 h of incubation, the polygonal cells begin to shrink and distribution of cell to cell contact was observed. After 48 h of incubation, most cells are completely shrunk and turn into the shape of spherical (Fig. 7). The morphological observations of HepG2 cells in both control and IC50 concentration are visualized and the control cells of HepG2 show triangular epithelial with polygonal shape as well as a clear nucleus at the center of the cell. Upon treatment of crude venom, polygonal cells get shrunk and are seen as oval at the inhibitory concentrations of 60 and 40 µg/ml (Fig. 8).
Fig.7.
Morphological observation of A549 cancer cells treated with nematocyst crude venom.
Fig. 8.
Morphological observation of control and HepG2 cancer cells treated with nematocyst crude venom.
4. Discussion
Many investigations have been carried out on the biological molecules in various species of Cnidarian. The venom of such animals is the strategy to survive in a specific environment so that it exhibits very potent biological effects. Among the various classes of cnidarians, the proteinaceous venom was isolated and characterized. Jellyfish venom is varying from one species to another, thus biochemical composition and activity are differing from each other. The protein content of the sea anemone Bunodosoma cavernata sample was recorded as 39.4% (McClintock et al., 1991). In the present study, jellyfish A. flagellatus crude venom contains protein 1.547 µg/ml, and the lipid content was 0.039 µg/ml. The lower concentration of lipid content was recorded for some other species of poriferans and cnidarians; probably it was related to the presence of great amounts of inorganic substances in their animal bodies. Among the mollusks, the octopus E. massyae was the species with the lowest concentration of lipid. In general carbohydrate concentration on invertebrates consider as very less than 7.5% dry weight. A similar result was observed in jellyfish crude venom carbohydrate content of 0.028 µg/ml. Nagai et al., (2002) reports that an Antarctic species Cnemidocarpa verrucosa, found much lower concentrations of carbohydrate ranging from 0.5% to 1.3% dry weight was seen on various organs of the ascidian.
The venom of Chiropsalmus quadrigatus has a hemolytic toxin of 44 kDa, and Chironex fleckeri nematocysts which contain a 20 kDa protein. Two other venom proteins (43 and 45 kDa) were isolated from the box jellyfish C. fleckeri. Ayed, et al., (2011) reported that a protein with hemolytic activity in box jellyfish (Carybdea alata) had a molecular weight of 42 kDa. The major part of the proteins somewhere in the range of 10 and 50 kDa and there were likewise some minor protein groups more than 50 kDa. Particularly, the molecules of 20–40 kDa and 10–15 kDa gave off an impression of being the significant protein components of the toxin. Fragments of protein from Nemopilema nomurai jellyfish venom was separated by using SDS-PAGE, and two major molecular weights 20–40 kDa and 10–15 kDa seen approximately. Analysis of Pelagia noctiluca venom on SDS-PAGE revealed at least 15 protein bands ranging in molecular weights from 4 to 120 kDa (Soare, et al., 1997). In our study, the protein profile of the crude venom sample was analyzed using Native and SDS-PAGE. The venom contains numerous proteins with various sizes of molecular weight resolved into many polypeptides under denaturing conditions in which ten major bands appeared very clearly along with the resolving gel upon CBB staining. The electrophoretic pattern of crude venom protein subunits upon electrophoresis has shown in the molecular weight ranging between 205 kDa and 3.5 kDa regarding the standard protein marker
The DPPH radical has been widely used to test the ability of compounds as free radical scavengers or donors of hydrogen. The data shows, that smaller peptides have a higher level of radical scavenging activity than larger proteins, Free radicals are involved in initiating and propagating lipid oxidation, and hence, food antioxidants would play an important role in scavenging these radicals (Theodore et al., 2008). DPPH free radicals (stable) and monetarily accessible organic nitrogen radicals which can recognize an electron or H + to turn into a steady molecule. DPPH has an absorbance at 517 nm which vanishes upon decrease by an antiradical compound. Lower absorbance of the response mixture showed a higher DPPH radical scavenging movement (Moosmann and Behl, 2002). In our investigations, the free radical scavenging maximum activity has shown up on hydroxyl when compare to other scavenging activities. The reducing power potential and scavenging activity (DPPH, hydroxyl, superoxide anion, and nitric oxide) of nematocysts crude venom of A. flagellatus.
In the present study, the cytotoxic effect of nematocysts crude venom from jellyfish A. flagellatus was evaluated against the Vero cell line by MTT colorimetric method. The maximum cytotoxic effect was observed at 120 µg/ml in Vero cells. Similarly, the P. noctiluca venom has been described to have a cytotoxic effect on normal cells where it promotes apoptotic cell death (Ayed et al., 2012). To assess the inhibitory effect of jellyfish crude venom on cell growth of A549 and HepG2 cells were analyzed by cell viability assay. The cells were treated with different concentrations of jellyfish crude venom (20–100 µg/ml) for 24 and 48 h of incubation. The jellyfish crude venom was inhibiting the proliferation of lung cancer cells in a dose and time-dependent manner. The cytotoxicity study of jellyfish venom has not been widely used, where previous literature demonstrates on IC50 values of jellyfish venom from C. quinquecirrha on CCL-13 hepatocytes were compare to A.flagellatus seems to be lower (IC50 < 1 µg/ml) (Oršolić, 2012). The anticancer potential of bee venom has proven the antiproliferative activity in in vitro studies and the ability to reduce tumor growth in-vivo (Wang and ji, 2005). Consequently, the cancer prevention agent action of nematocyst crude venom concentrates may add to its cytotoxic activity. Numerous chemo-preventive specialists seemed to target flagging intermediates in apoptosis actuating pathways and a significant number of them are accounted for to have favorable to antioxidant or cell reinforcement movement (Sun et al., 2004).
Morphological variations were observed in A549 cells both control and IC50 concentration. We show here that both of those properties are available in the crude nematocyst venom from A. flagellatus, which has a significant antiproliferative effect and an incredible cancer prevention agent action. The outcomes announced here are promising pointers for the possible use of this animal bioactive protein in the form of venom for preventive and remedial purposes. Hence these morphological changes and growth inhibitory effects are due to the effect of crude venom on human lung and liver cancer cell lines.
5. Conclusion
In the present study, the biochemical analysis of crude venom from nematocyst of jellyfish A. flagellate results revealed that the amount of protein was rich when compared to other constituents. Moreover, it possesses higher antioxidant and anticancer activities on A549 and HepG2 cells. These results show that the venom has bio-pharmacological properties due to the protein nature of the venom and able to develop novel drugs on marine-derived compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to University Grants Commission-Basic Scientific Research (UGC-BSR), Delhi, India, for providing the meritorious fellowship.
Footnotes
Peer review under responsibility of King Saud University.
References
- Kanwal, R., Gupta, K., Gupta, S., 2015. Cancer epigenetics: an introduction 2015. In: Cancer Epigenetics. Humana Press, New York, NY, pp. 3–25. [DOI] [PubMed]
- Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020;70(3):145–164. doi: 10.3322/caac.v70.310.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- Bowtell D.D., Böhm S., Ahmed A.A., Aspuria P.J., Bast R.C., Jr, Beral V., Berek J.S., Birrer M.J., Blagden S., Bookman M.A., Brenton J.D. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer. 2015;15(11):668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Henningfield J.E., Benowitz N.L., Connolly G.N., Dresler C., Fagerstrom K., Jarvis M.J., Boyle P. Toward a comprehensive long term nicotine policy. Tobacco Control. 2005;14(3):161–165. doi: 10.1136/tc.2004.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D.N., Ganesh B., Dinshaw K.A., Mohandas K.M. A case-control study of stomach cancer in Mumbai, India. Int. J. Cancer. 2002;99(5):727–731. doi: 10.1002/ijc.10339. [DOI] [PubMed] [Google Scholar]
- Rapiti E., Jindal S.K., Gupta D., Boffetta P. Passive smoking and lung cancer in Chandigarh, India. Lung Cancer. 1999;23(3):183–189. doi: 10.1016/s0169-5002(99)00013-6. [DOI] [PubMed] [Google Scholar]
- Alves R.C.P., Alves D., Guz B., Matos C., Viana M., Harriz M., Terrabuio D., Kondo M., Gampel O., Polletti P. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann. Hepatol. 2016;10(1):21–27. [PubMed] [Google Scholar]
- Parkin D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D., Fraumeni J.F., editors. Cancer epidemiology and prevention. Oxford University Press; 2006. [Google Scholar]
- Kang H.K., Seo C.H., Park Y. Marine peptides and their anti-infective activities. Marine Drugs. 2015;13(1):618–654. doi: 10.3390/md13010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons T.L., Andrianasolo E., McPhail K., Flatt P., Gerwick W.H. Marine natural products as anticancer drugs. Mol. Cancer Therapeut. 2005;4(2):333–342. [PubMed] [Google Scholar]
- Wattanadilok R., Sawangwong P., Rodrigues C., Cidade H., Pinto M., Pinto E., Silva A., Kijjoa A. Antifungal activity evaluation of the constituents of Haliclona baeri and Haliclona cymaeformis, collected from the Gulf of Thailand. Marine Drugs. 2007;5(2):40–51. doi: 10.3390/md502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini I., Defant A., Guella G. Recent synthesis of marine natural products with antibacterial activities. Anti-Infective Agents Med. Chem. (Formerly Current Medicinal Chemistry-Anti-Infective Agents) 2007;6(1):17–48. [Google Scholar]
- Friedman M.A., Fleming L.E., Fernandez M., Bienfang P., Schrank K., Dickey R., Bottein M.Y., Backer L., Ayyar R., Weisman R., Watkins S. Ciguatera fish poisoning: treatment, prevention and management. Marine Drugs. 2008;6(3):456–479. doi: 10.3390/md20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utkina N.K., Denisenko V.A., Krasokhin V.B. Sesquiterpenoid aminoquinones from the marine sponge Dysidea sp. J. Nat. Products. 2010;73(4):788–791. doi: 10.1021/np1000285. [DOI] [PubMed] [Google Scholar]
- da Rosa Guimarães T., Quiroz C.G., Rigotto C., De Oliveira S.Q., De Almeida M.T.R., Bianco É.M., Moritz M.I.G., Carraro J.L., Palermo J.A., Cabrera G., Schenkel E.P. Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae) Marine Drugs. 2013;11(11):4176–4192. doi: 10.3390/md11114176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.C., Southekal S., Park M.A., McQuaid S.J., Kijewski M.F., Muller S.P. Improved regional activity quantitation in nuclear medicine using a new approach to correct for tissue partial volume and spillover effects. IEEE Trans. Med. Imaging. 2011;31(2):405–416. doi: 10.1109/TMI.2011.2169981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry B.G., Vidal N., Norman J.A., Vonk F.J., Scheib H., Ramjan S.R., Kuruppu S., Fung K., Hedges S.B., Richardson M.K., Hodgson W.C. Early evolution of the venom system in lizards and snakes. Nature. 2006;439(7076):584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. [Google Scholar]
- Feng J., Yu H., Li C., Xing R., Liu S., Wang L., Cai S., Li P. Isolation and characterization of lethal proteins in nematocyst venom of the jellyfish Cyanea nozakii Kishinouye. Toxicon. 2010;55(1):118–125. doi: 10.1016/j.toxicon.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976;72(1–2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Barnes H., Blackstock J. Estimation of lipids in marine animals and tissues: detailed investigation of the sulphophosphovanilun method for ‘total’lipids. J. Exper. Marine Biol. Ecol. 1973;12(1):103–118. [Google Scholar]
- Roe J.H. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Bio. Chem. 1955;212:335–343. [PubMed] [Google Scholar]
- Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature, 227, 5259, p. 680–85. [DOI] [PubMed]
- Barros L., Ferreira M.J., Queiros B., Ferreira I.C., Baptista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food chemistry. 2007;103(2):413–419. [Google Scholar]
- Panda B.R., Mohanta S.R., Manna A.K., Si S. In vitro antioxidant activity on the aerial parts of Cocculus hirsutus Diels. J. Adv. Pharmaceut. Res. 2011;2(1):18–23. [Google Scholar]
- Dubey S.K., Batra A. Antioxidant activities of Thuja occidentalis Linn. Asian J. Pharm. Clin. Res. 2009;2(1):73–76. [Google Scholar]
- Balamurugan E., Menon V.P. In vitro radical scavanging activities of Chrysaora quinquecirrha nematocyst venom. Drug Discover. Therapeut. 2009;3(2) [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunolog. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Eno A.E., Konya R.S., Ofem O.E., Itam E.I. Chemical and biological characterization of a crude venom extract from the sea anemone-Bunodosoma cavernata. Port Harcourt Med. J. 2008;3(1):15–26. [Google Scholar]
- McClintock J.B., Heine J., Slattery M., Weston J. Biochemical and energetic composition, population biology, and chemical defense of the antarctic ascidian Cnemidocarpa verrucosa Lesson. J. Exper. Marine Biol. Ecol. 1991;147(2):163–175. [Google Scholar]
- Nagai, H., Takuwa-Kuroda, K., Nakao, M., OSHIRO, N., Iwanaga, S., Nakajima, T., 2002. A novel protein toxin from the deadly box jellyfish (sea wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci., Biotechnol., Biochem., 66, 1, pp. 97–102. [DOI] [PubMed]
- Ayed Y., Boussabbeh M., Zakhama W., Bouaziz C., Abid S., Bacha H. Induction of cytotoxicity of Pelagia noctiluca venom causes reactive oxygen species generation, lipid peroxydation induction and DNA damage in human colon cancer cells. Lipids Health Dis. 2011;10(1):232. doi: 10.1186/1476-511X-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soare J.R., Dinis T.C., Cunha A.P., Almeida L. Antioxidant activities of some extracts of Thymus zygis. Free Rad. Res. 1997;26(5):469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Moosmann B., Behl C. Secretory peptide hormones are biochemical antioxidants: structure-activity relationship. Mol. Pharmacol. 2002;61(2):260–268. doi: 10.1124/mol.61.2.260. [DOI] [PubMed] [Google Scholar]
- Ayed Y., Bousabbeh M., Mabrouk H.B., Morjen M., Marrakchi N., Bacha H. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by the Mediterranean jellyfish Pelagia noctiluca venom and its fractions in cultured glioblastoma cells. Lipids Health Dis. 2012;11(1):1–9. doi: 10.1186/1476-511X-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.X., Ji Y.H. Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J. Neuro-oncol.ogy. 2005;73(1):1–7. doi: 10.1007/s11060-004-4205-6. [DOI] [PubMed] [Google Scholar]
- Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31(1–2):173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- Theodore A.E., Raghavan S., Kristinsson H.G. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J. Agric. Food Chem. 2008;56(16):7459–7466. doi: 10.1021/jf800185f. [DOI] [PubMed] [Google Scholar]
- Sun S.Y., Hail N., Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004;96(9):662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
Further Reading
- Alberg A.J., Brock M.V., Ford J.G., Samet J.M., Spivack S.D. Epidemiology of lung cancer: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., He Q., Guo Y., Zhang J., Nie F., Li Y., Ye X., Zhang L. Cyanea capillata tentacle-only extract as a potential alternative of nematocyst venom: its cardiovascular toxicity and tolerance to isolation and purification procedures. Toxicon. 2009;53(1):146–152. doi: 10.1016/j.toxicon.2008.10.023. [DOI] [PubMed] [Google Scholar]