Abstract
This investigation displayed the good catalytic activity of silver nanoparticles (AgNPs) on the reduction of methylene blue dye. During this work, Honey was chosen for environmentally reducing and stabilizing agents for preparation of silver nanoparticles then characterized these nanoparticles by ultraviolet–visible spectroscopy (UV–Vis), functional biomolecules were confirmed by Fourier transform infrared spectroscopy (FTIR). Via transmission electron microscopy (TEM), the size and shape of silver nanoparticles revealed that the particles are spherical and monodispersed without major agglomeration, the particle size ranging from 5 to 25 nm, in addition, the largest particle density levels are 5–10 nm, ZETA Seizers studied the size distribution of the colloidal solution. UV/Vis spectrophotometer and HPLC were used to study and analyze the degradation performance of silver nanoparticles on methylene blue. The results show that 92% of methylene blue has been degraded after 72 h. additionally, several new peaks have appeared after treatment of the samples by using HPLC.
Keywords: Honey, Methylene blue, Degradation, Silver nanoparticles
1. Introduction
Honey has undergone comprehensive studies all over the world on its ingredients, physicochemical properties, mineral and vitamins, content, and quality control. Due to its high energy, the availability of chemical elements, vitamins, and enzymes, it is stated to aid human longevity. Honey is wealthy in vitamin C and the essential minerals, as well as it includes components that can act as antioxidants which play a significant role in preventing cancer (White, 1978, Singh and Bath, 1997, Terrab et al., 2002, Nanda et al., 2003, Devillers et al., 2004). This is a study on a simple, cost-effective, and environmentally friendly biosynthesis of very tiny AgNPs using normal honey in the water at laboratory conditions with its very active ingredients serving as reducing and protecting agents. It contains glucose and fructose ingredients that have permitted its use in the green biosynthesis of AgNPs as both capping and reducing agents. Besides, it has remarkable qualities that are often due to its acidic pH and hydrogen peroxide. At alkaline pH did the conversion of silver ions into AgNPs using natural honey occur (Haiza et al., 2013). The treatment of pigment effluents is important since many of the colors are poor in biodegradability and extremely carcinogenic before discharge into the receiving water (Brown and De Vito, 1993). It is also important that the dye effluent is processed before it is discharged into the water. Latter technological systems for the movement of contaminants have recently been developed, including chemical systems such as chlorination and ozonation physical methods like adsorption (Dejohn et al., 1976), physical systems like adsorption (Sloker et al., 1998) and biological systems like biodegradation (Moore et al., 1989). Biosynthesis of metal nanoparticles by a green approach has recently appeared. Due to its various advantages over other methods, like non-toxic, environmentally sustainable, and low cost, biosynthesis of metal nanoparticles (NPs). Researchers used biological systems to biosynthesize NP by developing simple protocols involving using metal ion of biological systems as extracellular reductants exporter in reduction processes (Fayaz et al., 2009, Sharma et al., 2009, Kamel et al., 2016). In this study, AgNPs were synthesized using natural honey, with its very active ingredients, and the catalytic activity of the resulting AgNPs was evaluated against methylene blue dye.
2. Materials and methods
2.1. Biosynthesis of silver nanoparticles using natural honey
Natural honey obtained from the Al-Riyadh market and such by AgNO3 (Sigma-Aldrich). In 80 mL of deionized water, 20 g of honey was dissolved, then 15 mL of this honey was applied to 20 mL of aqueous 10−3 M AgNO3 solution and then stirred for 1 min. The pH was changed to 6.5 using NaOH to start the removal of Ag ions. Reduction happens easily, as shown by the solution's golden yellow colour.
2.2. Silver nanoparticle characterization
The silver ion reduction was observed by various wavelengths from 200 to 1000 nm by measuring double-beam UV–Vis spectra (model Victoria, Australia) (Al-Zaban et al., 2019a). The size and shape of AgNPs were performed using transmission electron microscopy (Al-Othman et al., 2017) (JEM 1400 plus model with an acceleration voltage of 100 kV). ZETA Seizers (Malvern Zetasizer ZEN 3600, UK) was used to determine the distribution size of AgNPs of the colloidal solution (Abd El-Aziz and Al-Othman 2019). AgNPs were freeze-dried using a Heto Lyophilizer (Heto-Holten, Denmark) for XRD measurements, then the powdered sample was analyzed by an X'pert PRO PANalytical diffractometer using CuKpha radiation (k = 1.54056 Å) in the range of 20 ≤ 2ɵ ≤ 80 ≤ at 40 keV (Al-Othman et al., 2017). The FTIR a Nicolet 6700 spectrometer (Thermo Electron Corporation, USA) was confirmed functional biomolecules, the region spectrum in the 400–4000 cm−1 (Abd El-Aziz, 2014).
2.3. Catalytic activity of AgNPs on reduction of methylene blue
Stock solution of methylene blue dye prepared by 1000 mL of double distilled water and 10 mg of dye. About 10 mg of biosynthesized AgNPs was added to 100 mL of methylene blue dye solution using at different pH levels (1.0, 2.0, 3.0, 4.0, 5.0, 6.0 and 70) (Mettler Toledo Seven Excellence). The suspension of the reaction was mixed for 30 min with magnetic stirred. Then the solution was exposed to the light of the sun from morning until sunset. At specific time intervals, 2–3 mL suspension aliquots were used to estimate the photocatalytic dye degradation at various wavelengths using the UV–Vis spectrophotometer. The dye degradation was measured at 660 nm using the absorbance value. The proportion of dye degradation was calculated according to the following formula:
%Degradation = (1 − At/A0)
At is the absorbance after time t min
Ao is the absorbance at zero time
2.4. HPLC analysis
Degraded intermediates were detected by the HPLC system (PerkinElmer series 200 UV/VIS model) with a C18 column with an internal diameter (300 mm × 3.9 mm, 4 µm) with a mobile phase of methanol (80 percent) and deionized water (20 percent). The HPLC device has been supported by a fluorescence detector and UV with 365 emission wavelengths and 430 nm excitation. Sample filtration was carried out using a 0.45 μl membrane and then analyzed at a flow rate of 1 mL/min and a total run time of 15 min.
3. Results
3.1. Biosynthesis of silver nanoparticles using natural honey
Fig. 1 showed the golden yellow color of the solution of biosynthesis of AgNPs after the addition of honey.
Fig. 1.

Three eppendorf tube containing the aqueous solution of 10−3 M AgNO3 (A), 20% honey (B) and Synthesis of silver nanoparticles after addition honey(C).
3.2. UV–Visible spectral analysis
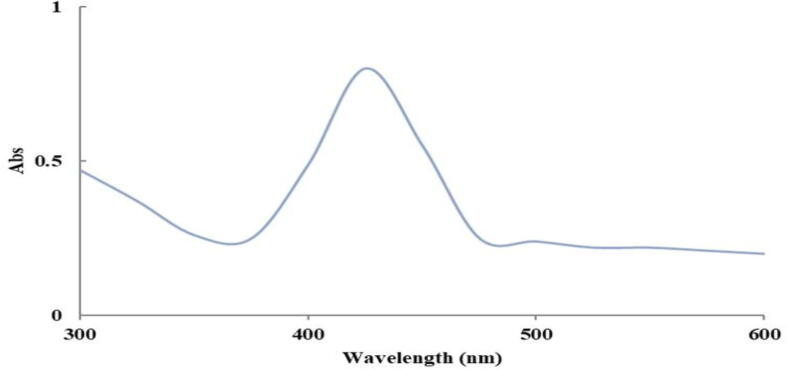
Fig. 2 displayed UV–Vis spectrophotometer of AgNPs. Silver ion reduction contributes to an increase in color intensity. The absorption band that was characteristic of monodispersed spherical AgNPs is observed at 417 nm. UV–Vis spectrophotometer is one of the most notable features of the optical absorption spectrum of AgNPs in the Surface Plasmon Resonance (SPR).
Fig. 2.
UV–vis spectrum of the AgNPs prepared using 20 g of honey using pH = 10.
3.3. Transmission electron microscopy (TEM)
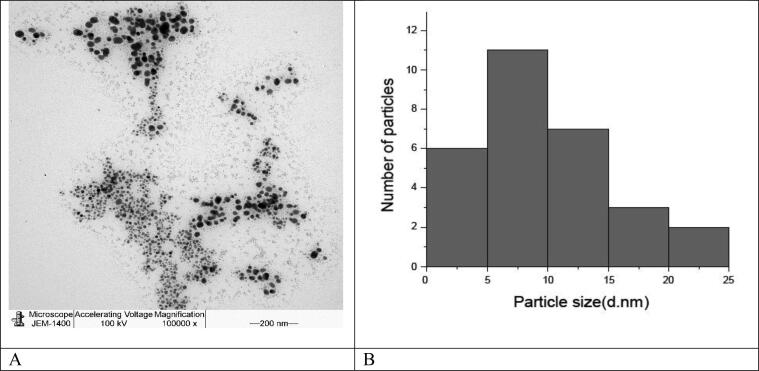
The TEM image in Fig. 3 shown the AgNPs sizes were decreased when the pH is increased and almost archived spherical AgNPs of average size 5–25 nm are obtained.
Fig. 3.
TEM image of the AgNPs prepared using 20 g of honey of 120,000× (a) and the histogram of the particle size distribution of the AgNPs (b).
3.4. X-ray analysis
Intense XRD peaks corresponding to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes were observed at 2θ angles of 36.45, 44.31°, 65.41°, and 76.25°, respectively (Fig. 4).
Fig. 4.
XRD pattern of dried powder of silver nanoparticles prepared using 20 g of honey.
3.5. Fourier Transform-Infrared spectral analysis (FTIR)
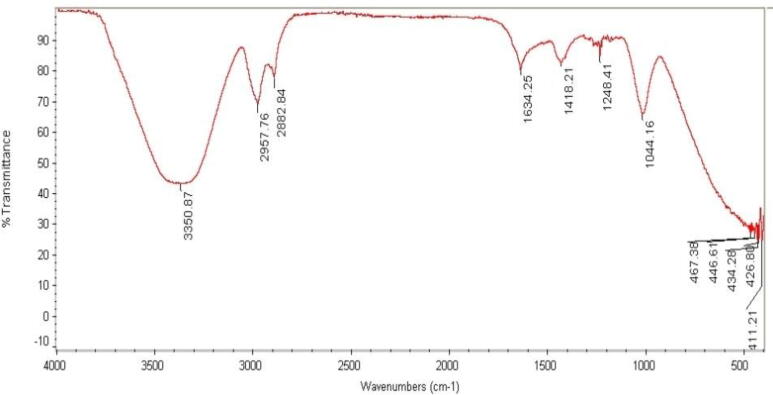
The green synthesis of AgNPs was utilizing natural honey has been examined using Fourier Transform Infrared spectroscopy (FTIR) based on Fig. 5. Glucose, fructose, sucrose, fats, vitamins, and minerals are essential ingredients in honey (Al-Zaban et al., 2019b, Al-Othman et al., 2017, Abd El-Aziz, 2014).
Fig. 5.
FTIR spectrum of AgNPs prepared using 20 g of honey.
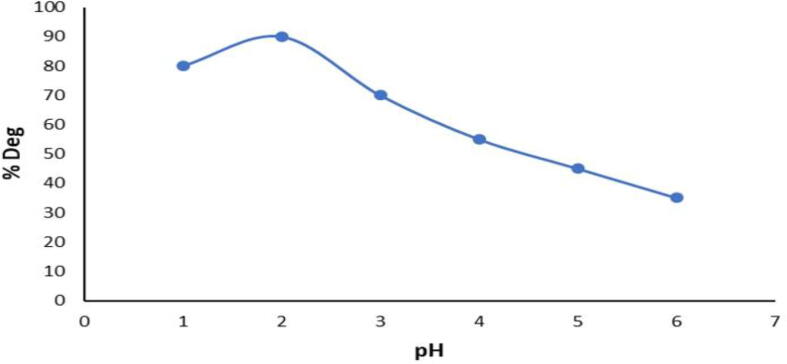
3.6. Effects of the pH levels on the methylene blue degradation
Acidic state leads to creating more reactive sites and contributing to greater forms of pollutant degradation. The results on reaction rates of various pH values (1.0, 2.0, 3.0, 4.0, 5.0, 6.0 and 7.0) have been studied (Fig. 6). The degradation efficiency of Methylene blue was observed to more than 90% within 24 h, at a pH of 2.0.0. At pH 2.0, which can be due to the release of metal oxides on the surfaces of the nanoparticles at an acidic pH, the highest rate of degradation was observed. The removal of methylene blue was limited at pH 4, 5, 6, and 7 due to the low H + concentration at which the removal of methylene blue is not favorable.
Fig. 6.
Effect of different pH at methylene blue degradation.
3.7. Photocatalytic degradation of methylene blue dye
Degradation of the dye was initially observed by color shift. Initially, after 24 h of incubation with AgNPs, the color of the dye changed from dark blue to light blue while exposed to solar radiation (Fig. 7). Consequently, light blue was transformed into light green. Finally, the degradation process was completed after 72 h.
Fig. 7.
Color change methylene blue dye indicates degradation at different time)initial time (A), 24 h, 48 h and 72 h(. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.8. UV–Vis spectrophotometer
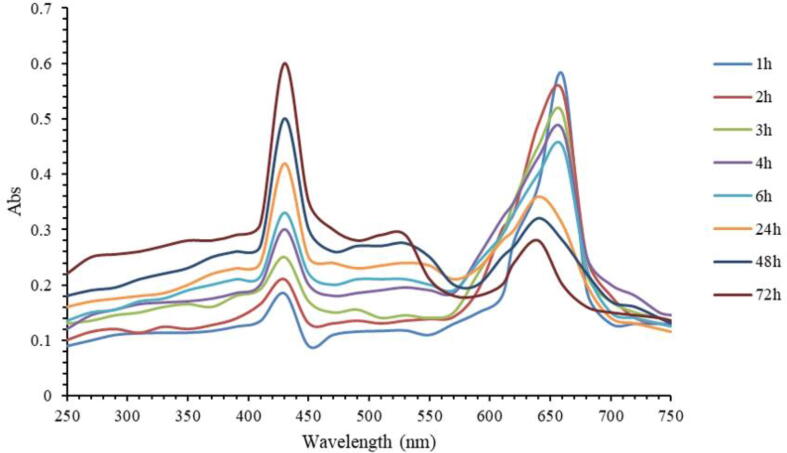
Fig. 9 showed the exposure time for complex dye AgNPs at solar radiation increased, the percentage of oxidation increased. The peak absorption of methylene blue dye was decreased and the absorption of AgNPs at 417 nm was improved. Due to the incremental decrease in the absorption value of dye approaching the baseline and the increased peak for AgNPs, the completion of the photocatalytic degradation of dye was occurring.
Fig. 9.
Absorption spectra of methylene blue dye treated with 10 mg/L of AgNPs synthesized using natural honey at different time points.
3.9. HPLC
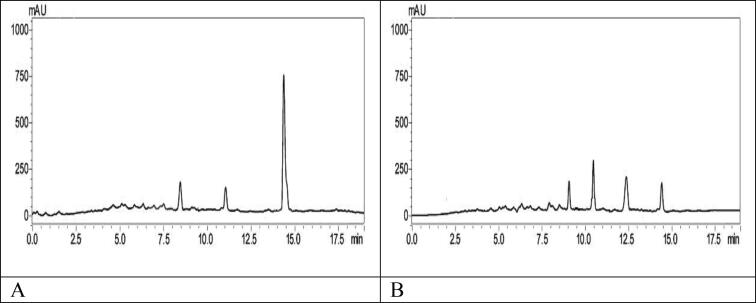
The degradation of the dye was studied by HPLC before and after photocatalytic degradation. Fig. 10 (A) indicated that the peak of methylene blue dye occurred at retention time (Rt) 14.5 min, while Fig. 10 (B) showed that in addition to the peak of methylene blue dye and other compounds, several peaks of varying intensities were found at Rt 12.2, 11.8, 10.5, and 9 and 14.5 min (methylene blue dye), these addition peaks included many compounds difference in the structure and concentration and related to the degradation products.
Fig. 10.
HPLC analysis of methylene blue (A) and (B) after dye degradation.
4. Discussion
The reducing agents of natural honey like fructose, glucose, and vitamin C reduction of Ag ions occurs with NaOH added. Metal ions allowed the oxidization of glucose to gluconic acid at the base level, and proteins and/or enzymes can also perform a role in oxidation recaption (Raveendran et al., 2016). SPR was due to collective electron oscillation around the surface mode of the NPs (Ahmad et al., 2010), the formation of SPR is due to the particle shape and size (Muthukrishnan et al., 2015). The possibility of adding more NaOH leads to produce more gluconic acid, resulting in a fast reduction of Ag ions and a large amount of very tiny nanoparticles, resulting in sharp and intense SPR (Philip, 2010).
To identify the possible biomolecules responsible for the capping and effective stabilization of AgNPs synthesized with honey, FTIR measurement was performed. Fig. 5 showed FTIR bands were observed the powerful and wide peak at 3350.87 cm−1 detecting the presence of phenolic compounds. This also is ascribed to the hydrogen-bonded O-H stretch. The 2957.76 and 2882.84 cm−1 area bands resulting from C-H aromatic compound stretching have been reported. Other than that, the functional group of C = C stretching which is alkene occurred in the AgNPs based on the noticeable strong peak at 1634.25 cm−1. The bands were also assigned to the N-H stretching vibration of the proteins at 1418.21 cm−1. In addition, this band could designate the vibrational frequencies corresponding to amide protein, peaks assigned to C-O single bond at 1248.41 cm−1 and 1044.16 cm−1 will range anywhere from 1000 cm−1 to 1300 cm-1 based on what type of compound (Ahmad et al., 2003, Ankamwar et al., 2005, Huang et al., 2007).
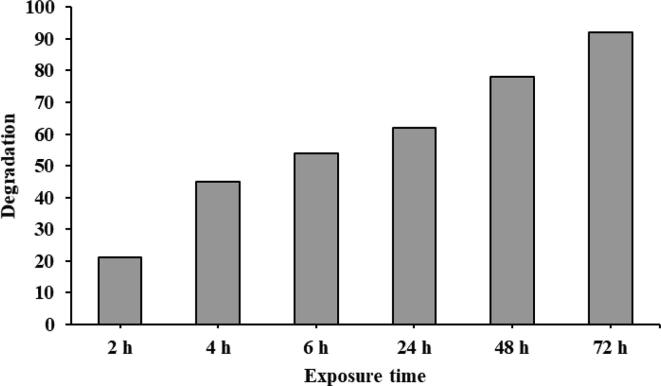
PH is a major parameter that may influence the process of deterioration. The bulk of the oxidation cycle is focused on pH and acidic conditions that support the reaction (Bokare et al., 2009, Liu et al., 2011). Increasing the pH to 7.0 can lead to decreased nanoparticle activity and decreased methylene blue degradation (Babuponnusami et al., 2012). Furthermore, the deposition of metal hydroxide on the surface of AgNPs can impede the generation of reactive oxygen species and limit the removal of blue methylene (Cheng et al., 2015). The process of oxidation was completed and was detected by modifying the color of the reaction mixture to colorless (Kumar et al., 2019, Vanaja et al., 2014). Data in Table 1 and Fig. 8 indicate that the percentage of degradation of methylene blue by 10 mg/L of synthesized AgNPs using natural honey at various time points achieved the highest percentage of dye degradation (92.0%) after 72 h. The absorption peak for methylene blue dye was displayed at 660 nm which is in good agreement with the prior data (Zhao et al., 2010). Finally, the peak was vanished while the reaction time increased, which means that the dye had been degraded.
Table 1.
Degradation of methyl blue dye (%) by synthesized silver nanopartilces (10 mg).
| Exposure time (h) | Amount of degradation of dye (%) |
|---|---|
| 2 | 21.0 ± 0.32 |
| 4 | 45.0 ± 0.28 |
| 6 | 54.0 ± 0.23 |
| 24 | 62.0 ± 0.37 |
| 48 | 78.0 ± 0.29 |
| 72 | 92.0 ± 0.37 |
±Standard deviation.
Fig. 8.
Percentage of methylene blue degradation by 10 mg/L of synthesized AgNPs using natural honey at different time points.
This conclusion was in close alignment with an earlier study in which adsorption, catalytic degradation, or oxidation by manganese oxides could result in methylene blue decoloration since manganese can occur in various oxidation states (Chen et al., 2012). The dominated mechanism depends on forms of manganese oxide (Suib et al., 2008) and the presence of the surface sites (Trasatti et al., 1991).
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University, through the Research Groups Program Grant No. (RGP-1441-0031).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Aziz A.R. Eco-friendly biosynthesis of silver nanoparticles by Aspergillus Parasiticus. Dig. J. Nanomater. Bios. 2014;9:1485–1492. [Google Scholar]
- Abd El-Aziz, A.R.M, Al-Othman, M.R., 2019. Gold nanoparticles biosynthesis using zingiber officinale and their impact on the growth and chemical composition of lentil (lens culinaris medic.). Pak. J. Bot. 51, 443-450, 2019. https://doi.org/10.30848/PJB2019-2(21).
- Ahmad A., Senapathi S., Khan M.I., Kumar R., Sastry M. Extracellular Biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir. 2003;19:3550–3553. doi: 10.1021/la026772l. [DOI] [Google Scholar]
- Ahmad N., Sharma S., Alam M.K., Singh V.N., Shamsi S.F., Mehta B.R., Fatma A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf. B. 2010;81–86 doi: 10.1016/j.colsurfb.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Al-Othman M.R., Abd El-Aziz A.R., Mahmoud M.A., Hatamleh A.A. Green biosynthesis of silver nanoparticles using Pomegranate peel and inhibitory effects of the nanoparticles on aflatoxin production. Pak. J. Bot. 2017;55:751–756. [Google Scholar]
- Al-Zaban M.I., Abdel Azim N.S., Abd El-Aziz A.R.M. Antifungal and anti-aflatoxin efficacy of biogenic silver nanoparticles produced by Aspergillus species: Molecular study. Pak. J. Pharm. Sci. 2019;32:2509–2526. https://doi.org/10.36721/PJPS.2019.32.5.SP.2509-2526.1. [PubMed] [Google Scholar]
- Al-Zaban M.I., Abd El-Aziz A.R.M., Abdelazim N.S. Antifungal and anti-aflatoxin efficacy of mycosynthesis nanosilver particles produced by Fusarium species: a physicocultural and molecular study. Dig. J. Nanomater. Bios. 2019;14:943–961. [Google Scholar]
- Ankamwar B., Choudhary M., Sastry M. Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth. React. Inorg. Metal Org. Nanometal Chem. 2005;19(19–26) doi: 10.1081/SIM-200047527. [DOI] [Google Scholar]
- Babuponnusami A., Muthukumar K. Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep. Purif. Technol. 2012;98:130–135. doi: 10.1016/j.seppur.2012.04.034. [DOI] [Google Scholar]
- Bokare A.D., Choi W. Zero-valent aluminum for oxidative degradation of aqueous organic pollutants. Environ. Sci. Technol. 2009;43:7130–7135. doi: 10.1021/es9013823. [DOI] [PubMed] [Google Scholar]
- Brown M.A., De Vito S.C. Crit. Rev. Environ. Sci. Technol. 1993;23:249. [Google Scholar]
- Chen Y., Chen H., Zheng X., Mu H. The impacts of silver nanoparticles and silver ions on wastewater biological phosphorous removal and the mechanisms. J. Hazard. Mater. 2012;239:88–94. doi: 10.1016/j.jhazmat.2012.07.049. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Fu F., Pang Y., Tang B., Lu J. Removal of phenol by acid-washed zero-valent aluminium in the presence of H2O2. Chem. Eng. J. 2015;260:284–290. doi: 10.1016/j.cej.2014.09.012. [DOI] [Google Scholar]
- Dejohn P.B., Paschal B., Hutchins R.A. Treatment of Dye Wastes with Granular Activated Carbon. Tex. Chem. Color. 1976;8:34–37. [Google Scholar]
- Devillers J., Moriot M., Delegue M.H.P., Dore J.C. Classification of monoflora honeys based on their quality control data. Food Chem. 2004;86:305–312. doi: 10.1016/j.foodchem.2003.09.029. [DOI] [Google Scholar]
- Fayaz A.M., Balaji K., Kalaichelvan P., Venkatesan R. Fungal based synthesis of silver nanoparticles - an effect of temperature on the size of particles. Colloids Surf. B: Biointerfaces. 2009;74:123–126. doi: 10.1016/j.colsurfb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Haiza H., Azizan A., Mohidin A.H., Halin D.S. Green synthesis of silver nanoparticles using local honey. Nano Hybrids. 2013;4(87–98) doi: 10.4028/www.scientific.net/NH.4.87. [DOI] [Google Scholar]
- Huang] J., Li, Q., Sun, D., Lu, Y., Su, Y., Yang, X., Wang, H., Wang, Y., Shao, W., He, N., Hong, J., Chen, C., 2007. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology, 18:105104 18:1-11. 18, 105104.
- Kamel Z., Mahmoud S., El Namoury N. Biosynthesis, characterization, and antimicrobial activity of silver nanoparticles from actinomycetes. Res. J. Pharmaceut. Bio Chem. Sci. 2016;2016(7):119–127. [Google Scholar]
- Kumar M.S., Suprajaa N., David E. Photocatalytic activity of methylene blue using zinc nanoparticles synthesized from gymnema sylvestre and antimicrobial assay. Adv. Bioequival. Bioavailab. 2019;2:161–167. https://doi.org/10.31031/ABB.2019.02.000542. [Google Scholar]
- Liu, W.P., Zhang, H.H., Cao, B.P., Lin, K., Gan, J., 2011. Oxidative removal of bisphenol A using zero valent aluminum-acid system. Water Res., 45, 45, 1872, https://doi.org/10.1016/j.watres.2010.12.004. [DOI] [PubMed]
- Moore A.T., Vira A., Fogel S. Biodegradation of trans-1,2-dichloroethylene by methane-utilizing bacteria in an aquifer simulator. Environ. Sci. Technol. 1989;23:403–406. doi: 10.1021/es00181a003. [DOI] [Google Scholar]
- Muthukrishnan S., Bhakya S., Kumar T.S., Rao M.V. Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegia thwaitesii – An endemic species. Ind. Crops Prod. 2015;63:119–124. doi: 10.1016/j.indcrop.2014.10.022. [DOI] [Google Scholar]
- Nanda V., Sarkar B.C., Sharma H.K., Bawa A.S. Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J. Food Comp. Anal. 2003;16:613–619. doi: 10.1016/S0889-1575(03)00062-0. [DOI] [Google Scholar]
- Philip D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta Part A. 2010;75:1078–1081. doi: 10.1016/j.saa.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Raveendran P., Fu J., Wallen S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2016;8:34–38. doi: 10.1039/B512540E. [DOI] [Google Scholar]
- Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Singh N., Bath P.K. Quality evaluation of different types of Indian honey. Food Chem. 1997;58:129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Sloker Y.M., Le Marechal A.M. Methods of Decoloration of Textile Wastewaters”. Dyes Pigments. 1998;37:335–356. doi: 10.1016/S0143-7208(97)00075-2. [DOI] [Google Scholar]
- Suib S.L. Cheminform Abstract: Mesoporous Manganese Oxide Catalyzed Aerobic Oxidative Coupling of Anilines to Aromatic Azo Compounds. Acc Chem. Res. 2008;41:479–491. doi: 10.1002/chin.201623087. [DOI] [PubMed] [Google Scholar]
- Terrab A., Diez M.J., Heredia F.J. Characterization of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002;79:373–379. doi: 10.1016/S0308-8146(02)00189-9. [DOI] [Google Scholar]
- Trasatti S. Physical electrochemistry of ceramic oxides. Electrochim. Acta. 1991;36:225–241. doi: 10.1016/0013-4686(91)85244-2. [DOI] [Google Scholar]
- Vanaja, M., Paulkumar, K., Baburaja, M., Rajeshkumar, S., Gnanajobitha, G., Malarkodi, C., Sivakavinesan, M., Annadurai, G., 22014. Degradation of Methylene Blue Using Biologically Synthesized Silver Nanoparticles Bioinorganic Chemistry and Applications, 2014, Article ID 742346, https://doi.org/10.1155/2014/742346. [DOI] [PMC free article] [PubMed]
- White J.W. Honey Adv. Food Res. 1978;24:287–374. doi: 10.1016/S0065-2628(08)60160-3. [DOI] [PubMed] [Google Scholar]
- Zhao X., Xu S., Wang L. Exchange-biased NiFe2O4/NiO nanocomposites derived from NiFe-layered double hydroxides as a single precursor. Nano Res. 2010;3:200–210. doi: 10.1007/s12274-010-1023-3. [DOI] [Google Scholar]