Abstract
Keeping honeybees healthy is essential, as bees are not only important for honey production but also cross-pollination of agricultural and horticultural crops; therefore, bees have a significant economic impact worldwide. Recently, the lethal disease, the American foulbrood (AFB), caused great losses of honeybee and decline of global apiculture. Recent studies have focused on using natural insect-derived antibiotics to overcome recently emerged AFB-resistance to conventional antibiotics. In support of these studies, here we investigate the possibility of producing bee-derived anti-AFB antibiotics from an indigenous honeybee, Apis mellifera jemenitica. The immune responses of the third instar stage were first induced against the standards Micrococcus luteus and Escherichia coli compared with the indigenous Paenibacillus larvae (ksuPL5). Data indicated a strong immune response against M. luteus, E. coli and P. larvae 24 h post-P. larvae-injection as revealed by the detection of lysozyme-like, cecropin-like and prophenoloxidase (PO) activities in the plasma of P. larvae-injected third instars. Nodulation activity against injected P. larvae as early as 4 h and peaking 48 h post-P. larvae injection were observed. Potentially active anti-P. larvae immune peptide fractions purified by high-performance liquid chromatography (HPLC) showed significant in vivo therapeutic effects on P. larvae-infected first instars. Mass spectrophotometric analysis and Orbitrap measurements of P. larvae-injected plasma indicated the expression of PO (Mr: 80 kDa), beta-1,3-glucan-binding protein (Mr: 52 kDa) and serine protease 44 isoform X1 (Mr: 46 kDa). This suggests that one or all of these immune peptides contribute to significant survivorship of P. larvae-infected broods, and could be a valuable clue in the search for honeybee-derived anti-AFB natural therapeutic agents. Further molecular characterization and description of the functional roles of these predicted antimicrobial peptides from both broods and adult honeybee may enrich the arsenal of insect-derived antibiotics of therapeutic purposes.
Keywords: Honeybees, American foulbrood, Apis mellifera jemenitica, Immune peptides, Natural antibiotics, Apiaries
1. Introduction
Apiculture is valued worldwide not only for honey production but also for it role in improving crop quality via cross-pollination (Ghramh et al., 2020, Meixner, 2010). Infectious diseases in honeybee are one of the main causes of apiculture decline, which resulted in economic losses worldwide (Genersch, 2010). Among such diseases is the American foulbrood (AFB), which is caused by P. larvae, a Gram-positive, spore-forming bacterium, and is the most devastating disease in bee larvae both globally (Evans, 2003, Genersch, 2010) and locally (Al-Ghamdi et al., 2018, Ansari et al., 2017a). Infection of one larva can lead to an entire colony being infected, after which the apiary will collapse (Ansari et al., 2017a, Ansari et al., 2017b, Genersch, 2010). The infection initially takes place with the spores of the first instar, during the first 36 h after hatching (Genersch et al., 2005). Upon ingestion, the spores begin to germinate and grow rapidly in the larval midgut for several days, then destroy the midgut peritrophic membrane and epithelium (Garcia-Gonzalez and Genersch, 2013). This opens a path for the bacteria to enter the hemocoel (Djukic et al., 2014), resulting in larval death (Yue et al., 2008).
Controlling AFB has long been a global challenge involving two main practical treatment strategies. The first, burning infected bee colonies and hive materials, is the easiest and fastest way to getting rid of the disease. The second, conventional antibiotics such as oxytetracycline, is the main manageable treatment option for infected bee colonies. However, the prolonged use of conventional antibiotics has resulted in bee larvae become resistant to P. larvae (Evans, 2003, Miyagi et al., 2000), and adverse impacts on brood health and development (Thompson et al., 2005). This has attracted the attention of specialists exploring alternative, safe, and natural compounds for controlling AFB. Some recent studies have test the effectiveness of natural products such as essential oils (Erler and Moritz, 2016, Kuzyšinová et al., 2016; Anjum et al., 2018) and develop natural insect-derived antibiotics. Insect antimicrobial peptides (AMPs) offer novel mechanisms that are rarely challenged by bacterial resistance (Rajanbabu and Chen, 2011) and they provide broad-spectrum coverage by working synergistically against pathogens.
Insect’s immune system comprises highly developed cellular and humoral components that can be activated by invasion or exposure to foreign agents, including pathogens (Khan et al., 2020, Sheehan et al., 2020, Tsakas and Marmaras, 2010). The first and fastest component, cellular immunity, is mediated by hemocytes in an immediate and non-specific manner that relies on phagocytosis, nodule formation, and encapsulation of macro-organisms (Dimopoulos et al., 2001, Kotthoff et al., 2011, Ratcliffe et al., 2011). Humoral immunity involves induction of antimicrobial peptides against invading microorganisms (Amaral et al., 2010, Irving et al., 2004). Upon microbial infection, AMPs are rapidly released from fat bodies and selected hemocytes into the hemolymph, where they act against invading microorganisms (Gätschenberger et al., 2013). Insects produce different AMPs depending on the type of invading microorganism (Irving et al., 2004, Tzou et al., 2002). In contrast to solitary insects, such as Drosophila melanogaster, a honeybee colony offers a valuable opportunity to investigate the progression of diseases in a community environment. Because of the high pathogen load of honeybees social lifestyle, it has been assumed that bees can express a wider variety immunity responses than solitary insects. However, bees have been shown to have significantly fewer immune responses compared with Drosophila (Chen et al., 2013, Ratcliffe et al., 2011), possibly to their unique living environments and their clean food and food-sources which limits the chance of being infected with particular pathogens (Mundo et al., 2004). This may explain the high susceptibility of the first instars of bee broods to AFB infection (Bastos et al., 2008).
It is therefore reasonable to conclude that AMPs can kill a wide range of microorganisms (Bastos et al., 2008, Hoffmann, 1995). Exploring insect AMPs has provided insights into innate immunity and templates for the design of novel and broad-spectrum insect-derived antibiotics that can overcome resistance to conventional antibiotics. Detailed studies of the putative functional cooperativity of honeybees’ humoral immune peptides may generate useful ideas for new combinations of antimicrobial drug therapy. The present study targeted the indigenous Saudi honeybee A. mellifera jemenitica, a tropical African bee has a tight distribution along countries of Northern coast of Africa (Alqarni et al., 2011), for exploring the immune responses of its third instar broods. Larval immune responses were induced against different types of Gram-negative and positive bacteria, including the local AFB-causing bacterium P. larvae (ksuPL5). Experimentally induced AMP fractions were purified and investigated against isolated AFB P. larvae (ksuPL5) both in vivo and in vitro (in P. larvae-infected broods). This study constitutes an essential step toward the production of natural and safe bee-derived antimicrobial antibiotics for tackling AFB.
2. Materials and methods
2.1. Experimental insect
Broods of A. mellifera jemenitica were collected from natural apiary hives of the Plant Protection Department’s Bee Research Unit of the Faculty of Food Sciences and Agriculture at King Saud University. A Jenter comb box (Hammann, Hassloch-Germany) (Fig. 1) was used as described in Ayaad et al. (2018) to obtain first or third instars (24 or 72 h old, respectively) to conduct in vivo bioassays or explore the immune responses, respectively. Briefly, a Jenter box was fixed in a wax comb frame inside a hive and a queen was added manually for egg laying. After the eggs hatched and larvae reached the third instar stage (as estimated by size), the Jenter box containing the broods was brought to the laboratory and reared in suitable incubator (Binder, Tutlingen, Germany) at 34 °C and 80% RH. During the experiment, larvae were provided with a pre-warmed (34 °C) artificial royal diet prescribed by Ayaad et al. (2018). The amount of the daily diet provided to the broods was determined according to Vandenberg and Shimanuki (1987) (Table 1).
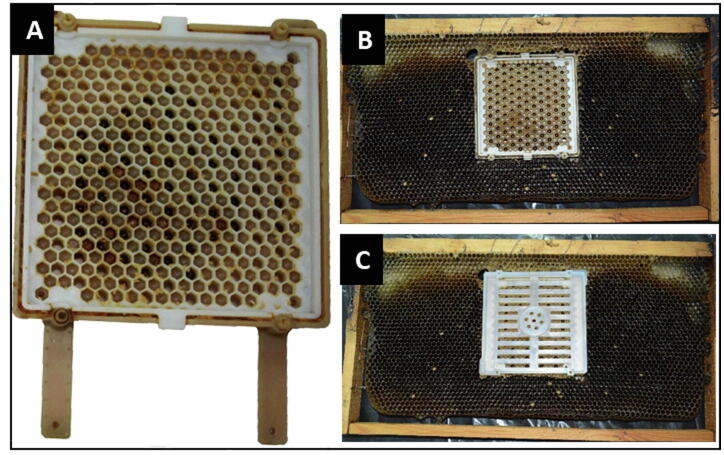
Fig 1.
A Jenter comb system used for collecting broods of similar ages from their natural hives. A: Free un-covered Jenter comb; B and C: Un-covered and covered Jenter, respectively, both fixed within a wax comb. The queen was added to the covered box (C) as the cover openings are smaller than the queen’s body size, while they fit workers’ bodies, facilitating free ingress and egress to serve the queen and eggs. Upon egg laying, the queen was freed from the box. The box was kept in the hive for three days until eggs hatched then moved to the laboratory.
Table 1.
Daily volume of artificial diet supplied to the experimental A. mellifera jemenitica broods.
| Larval feeding period (days) | |||||||
|---|---|---|---|---|---|---|---|
| D-1 | D-2 | D-3 | D-4 | D-5 | D-6 | Total | |
| Diet (µL/larva) | 5 | 10 | 20 | 20 | 30 | 40 | 125 |
2.2. Experimental bacteria
Standard reference bacteria Escherichia coli (ATCC 10536), Micrococcus luteus (National Collection of Type Cultures NCTC 2665; Sigma, UK), Staphylococcus aureus (ATCC 6538), Bacillus subtilus (ATCC 6051), and Pseudomonas aeruginosa (ATCC 9027) were purchased from Sigma, UK. Locally isolated AFB bacteria, P. larvae (ksuPL5) (Ansari et al., 2017a, 2017b), were used for immune induction in the third instar and for in vitro and in vivo immune-verification experiments in the first instar. All bacteria were kept at −20 °C in 20% glycerol (v/v) added brain heart infusion broth. Bacteria were propagated and prepared for experiments according Al-Ghamdi et al. (2020).
2.3. Induction of immune responses
Immune responses of third instar (72 h old) A. mellifera jemenitica were investigated upon challenge with the experimental bacteria. Bacterial injections were carried out as described by Laughton et al. (2011). Briefly, 50 larvae were surface-sterilized with 70% ethanol (v/v) prior to injection with P. larvae (ksuPL5), (1 × 106 CFU/larva each) using a sterilized 20-gauge micro-syringe (Hamilton, USA). A non-injected group of larvae were used as a control in parallel. Injected larvae were maintained for recovery in their standard rearing conditions (34 ± 1 °C and 80 ± 1% RH) with sufficient royal diet during the experiment (Table 1). Active larvae were used to carry out the experimental protocols (see below).
2.4. Hemolymph collection and total protein estimates
Twenty active third instar A. mellifera jemenitica were surface-sterilized using 70% (v/v) ethanol before hemolymph collection 24 h post-injection with P. larvae (ksuPL5) as described by Laughton et al. (2011). Hemolymph was decanted from larvae directly into microfuge tubes containing equal volumes of anticoagulant [186 mM NaCl, 17 mM EDTA, 98 mM NaOH and 41 mM citric acid (pH: 4.5)] with an EDTA-free protease inhibitor cocktail. To achieve hemocyte-free plasma, 8 µL of pooled hemolymph per replicate for three replicates was spun at 1,500 rpm in a micro centrifuge (Biofugefresco, Heraeus, and D-3752) at 4 °C for 10 min in order to pellet and discard hemocytes. The resulting clear and hemocyte-free plasma was stored at − 80 °C for later use. Total protein concentration of the plasma was estimated following Bradford (1976) using Coomassie Brilliant Blue-G250 (CBB) (ICI Americas, Inc.). Bovine serum albumin was used for standard calibrations according to the manufacturer’s instructions.
2.5. Exploring immune responses
2.5.1. Bacterial growth inhibition assay
A solid growth-inhibition assay was carried out for in vitro investigation of the induced immune peptides in hemocyte-free crude plasma of P. larvae-injected or non-injected (control) 3rd instars against Gram-positive M. luteus (NCTC 2665) and P. larvae (ksuPL5), and Gram-negative E. coli (ATCC 10536) according to Ayaad et al. (2012). Disks of the antibiotic ciprafoxacin (CIP) were used in parallel as a positive control for comparisons in each test (Hultmark, 1998, Mundo et al., 2004). The resulting growth-inhibition zone diameters were measured in triplicate for statistical analysis (n = 3).
2.5.2. Lysozyme-like activity assay
Plasma (50 μL) of non-injected (control) or P. larvae-injected third instars were subjected to a lysozyme-like activity assay against P. larvae (ksuPL5) 24 h post-injection as described by Brogden et al. (2003). In parallel, a similar amount (50 μL) of phosphate buffered saline (PBS) [10 mM Na2HPO4 and 150 mM NaCl (pH: 6.5)] were used as a control for normalization. Experimental plasma or PBS was added to M. luteus cell wall suspension (0.9 mL) dissolved in PBS (12.5 mg/25 mL; pH: 6.5) in cuvettes and incubated at room temperature. The change in optical density (OD) was measured at 450 nm every 5 min (for a period of 30 min). The change in enzyme activity was estimated and lysozyme-like activity was considered as the change in absorbance/mg protein/min. This assay was carried out in triplicate (n = 3) for statistical analysis.
2.5.3. Phenoloxidase activity assay
Phenoloxidase (PO) activity was investigated in 50 μL of plasma of control or P. larvae-injected third instars 24 h post-injection according to Laughton et al. (2011). Hen egg white lysozyme (HEWL) (Sigma-Aldrich, UK) was used in parallel as a positive control for comparison. The OD was measured at 490 nm every 15 min for 1 h. The change in enzyme activity was measured as the linear rate of substrate conversion. PO activity was estimated as the change in absorbance/mg protein/min. This assay was carried out in triplicate (n = 3) for statistical analysis.
2.5.4. Cecropin-like activity assay
Cecropin-like activity was estimated in 50 μL of plasma of control or P. larvae-injected third instars 24 h post-injection against standard E. coli using a growth-inhibition zone assay according to Lee et al. (2000). Cecropin-B (Sigma-Aldrich, UK) was used in parallel as positive control for comparison. Measurements of growth-inhibition zones were carried out in triplicate (n = 3), as described earlier.
2.5.5. Nodulation activity assay
Melanization and nodulation activities were investigated in control or P. larvae-injected third instars 24 h post-injection. Melanization was assessed by removing the dorsal abdominal tergites of treated larvae to count the formed melanized nodules. Larvae were fixed dorsally in PBS for careful cutting the ventral side from the cranial to the caudal end. Resulting larval skins were then turned over and used for counting the formed nodules under a stereomicroscope (Olympus SZX7, Hamburg, Germany). Nodules were counted and categorized based on diameter as small (5–10 µm), medium (10–30 µm), or large (4–80 µm) according to Eleftherianos et al. (2007). Five individual larvae (n = 5) were dissected to count the melanized nodules for statistical analysis.
2.5.6. Potentiality of immune peptides against AFB
2.5.6.1. Purification of immune peptides/proteins
Plasma obtained from control or P. larvae-injected third instars 24 h post-injection was processed for partial purification of immune peptides using Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) according to Venkatasami and Sowa Jr (2010). Briefly, an aliquot (18 µL) was dissolved in 10% acetic acid and partially purified using RP-HPLC on a C18 Bondapak column (1 μm, 10 × 250 mm) using an acetonitrile gradient in water (30%–70% for 15 min) with 0.05% trifluoroacetic acid (TFA). Resulting peptide fractions were further separated by RP-HPLC on an analytical C18 Pepmap column (12 μm, 4.6 × 120 mm) using an acetonitrile gradient in water (30%–70% for 30 min) and 0.05% TFA. The isolated peptide fractions were then hydrolyzed using 6 N-HCl at 100 ℃ for 24 h. Fractions that displayed significant similar peaks were pooled, vacuum-dried, and stored until use. The total protein concentration was determined for each collected fraction as detailed previously. One-dimensional SDS-PAGE (1D-SDS-PAGE) was conducted according to Laemmli (1970).
2.5.6.2. Determination of minimal inhibitory concentrations
In this experiment, the micro-titer dilution-plate method was utilized to determine the minimal inhibitory concentration (MIC) of the three RP-HPLC-purified immune peptide/protein fractions (100 µL each) according to Amsterdam (1996) against P. larvae (KsuPL5), Staphylococus aureus (ATCC 6538), Micrococcus luteus (NCTC 2665), Pseudomonas aeruginos (ATCC 9027), Escherichia coli (ATCC 10536), and Bacillus subtilus (ATCC 6051). CIP was used in parallel as a reference. Briefly, bacterial suspensions were prepared and standardized to a turbidity equivalent in density to 0.5 McFarland standards (1.5 × 108 CFU/mL), which ultimately was diluted to 1 × 106 CFU/mL. Upon incubation of each bacterial type with each immune peptide fraction for 24 h, the MIC was evaluated as the lowest concentration that inhibited the bacterial viability. This experiment was conducted in triplicate (n = 3) for performing statistical analysis.
2.5.6.3. In vitro antibacterial activities of immune peptides
The antibacterial activities of the purified immune peptide fractions were determined by growth-inhibition zone assays against P. larvae (ksuPL5) 24 h post-incubation as explained earlier. Upon demonstration of potential anti-P. larvae activities, the strongest immune fraction, “B”, was used to carry out an in vivo bactericidal bioassay against P. larvae (ksuPL5) as detailed below. This experiment was carried out in triplicate (n = 3) for statistical analysis.
2.5.6.4. In vivo verification of anti-AFB therapeutic effect
-
i.
Preparation of P. Larvae (ksuPL5) for oral infection
Spores of the locally isolated P. larvae (ksuPL5) bacteria Ansari et al. (2017a, 2017b) were prepared according to Al-Ghamdi et al. (2020). The resulting spore suspension was counted under a light microscope (40 × ) and adjusted to approximately 1 × 106 CFU/mL to carry out the oral infection assay as detailed below.
-
ii.
Preparation of experimental broods
First instar A. mellifera jemenitica of the same size and age (24 h) were collected from their apiary hives using a Jenter comb box as detailed earlier (Fig. 1). After egg hatching, the Jenter box containing the first instars was brought to the laboratory and kept in an incubator (Binder, Tutlingen, Germany) at 34 °C and 80% RH. Royal diets were prepared and provided to larvae as detailed earlier (Table 1).
-
iii.
Oral infection bioassay
Immune fraction B was used for this bioassay as it demonstrated the strongest effect against P. larvae in an in vitro micro titer dilution-plate assay (64 µg/mL) (see results, Table 3). Four groups of first instars (50 broods each), prepared in a Jenter comb box as detailed earlier were subjected to oral infection bioassays according to Ayaad et al. (2018) with some modifications. As shown in Table 2, group 1 (G1) was provided with a normal diet (ND) as a negative control, group 2 (G2), a positive control, was provided with an ND spiked with the MIC of the immune fraction B, group 3 (G3) was provided with an ND spiked with P. larvae (ksuPL5) (1 × 106 CFU/mL), and group 4 (G4) was provided with an ND spiked with P. larvae (1 × 106 CFU/mL) mixed with the immune peptide fraction B (64 µg/mL). Each group was provided with its allocated diet combination on day 1 of the experiment only. From day 2 onward, larvae of both group G1 and G3 were provided with an ND until the end of the experiment (day 5), while groups G2 and G4 were provided with an ND supplemented with the immune peptide fraction B (64 µg/mL) only. Larvae were supplied with suitable amounts of diet according to Aupinel et al. (2005), as shown in Table 1. Daily larval mortality was calculated in each group throughout the experimental period. This experiment was carried out in triplicate (n = 3) for statistical analysis.
Table 3.
Minimal inhibitory concentrations (µg/mL) of purified immune peptide/protein fractions from A. mellifera jemenitica third instars 24 h post-injection with P. larvae (ksuPL5), against different bacterial strains.
| Purified immune peptide fractions |
||||
|---|---|---|---|---|
| Microorganisms | A | B | C | CIP |
| Staphylococus aureus | 4.20 ± 0.1* | 4.3 ± 0.13* | 0.25 ± 0.01 | 0.25 ± 0.03 |
| Paenibacillus larvae | 128.9 ± 5.8* | 64.1 ± 1.7** | 128.2 ± 7.3* | 4.40 ± 0.20 |
| Micrococcus luteus | 0.25 ± 0.0 | 0.25 ± 0.0 | 0.25 ± 0.0 | 0.25 ± 0.0 |
| Pseudomonas aeruginosa | 64.8 ± 2.3* | 64.3 ± 2.2* | 64.6 ± 1.8* | 0.25 ± 0.0 |
| Escherichia coli | 0.25 ± 0.0 | 0.25 ± 0.0 | 0.25 ± 0.0 | 0.25 ± 0.0 |
| Bacillus subtilus | 4.33 ± 0.2* | 32.3 ± 2.3* | 8.30 ± 0.9* | 0.5 ± 0.0 |
Table 2.
Experimental groups of A. mellifera jemenitica first instars and their relevant diet components as designed for investigation of the in vivo susceptibility of P. larvae (ksuPL5) toward purified immune peptides.
| Experimental groups | Diet components |
|---|---|
| G1 | ND only (negative control) |
| G2 | ND + IPF-B (64 µg/mL−1) (positive control) |
| G3 | ND + P. larvae spores (1 × 106 CFU/mL) |
| G4 | ND + P. larvae spores (1 × 106 CFU/mL) + IPF-B (64 µg/mL−1) |
Experimental groups G1, G2, G3 and G4 (50 larvae each); ND: Normal diet (artificial royal diet); IPF-B: Immune peptides of fraction B.
2.5.7. Identification of antibacterial immune peptides/proteins
Purification of further peptides was carried out from another aliquot of crude plasma corresponding to 24 h post-P larvae injection. Plasma was immediately frozen in liquid nitrogen prior to mass spectrometry (MS) analysis according to Jin and Manabe (2005). Briefly, nitrogen-stored plasma were processed for extraction in three repeats of 18 µL each and separated via 1D-SDS-PAGE. The resulting bands were removed, homogenized, and centrifuged at 10,000 rpm. Proteins in the supernatant were processed for cleavage with trypsin, and quantifications via spectral counting and measured by Orbitrap according to procedures described by Michalski et al. (2011) and their sequences were matched against those in the NCBI database for Apis mellifera and Paenibacillus larvae as identification references.
2.5.8. Statistical analysis
In each experiment, three replicates (n = 3) were processed for statistical analysis using MINITAB software (MINITAB, State College, PA, v: 18.0, 2018). Basic statistical analysis was performed to determine the means and standard errors. Anderson–Darling normality tests were carried out on each dataset (Morrison, 2002) and homogeneity tests were run to determine variances prior to further analysis. As the results of the normality tests showed all data were normally distributed, One-way ANOVA or Student’s t-tests were used to compare differences between means in each experiment.
3. Results
3.1. Total protein content and SDS-PAGE
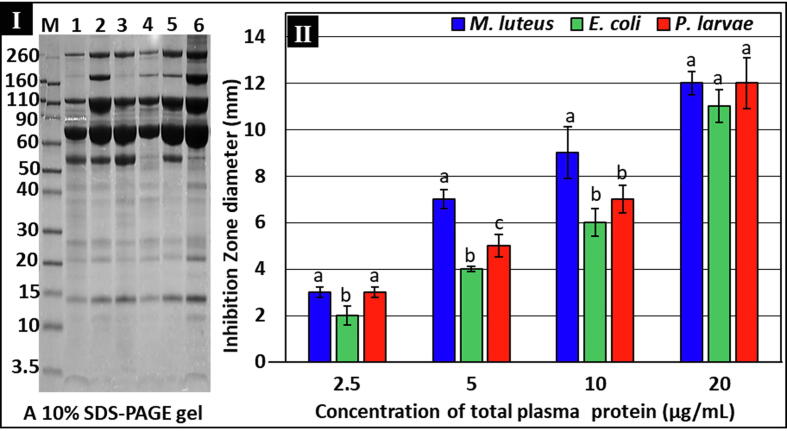
Crude hemocyte-free plasma of third instars (72 h old) showed significantly higher total protein contents 24 h post-injection with P. larvae compared with non-injected controls (2.7 ± 0.12 vs 1.2 ± 0.03 OD units, respectively) (P ≤ 0.05; Student’s t-test; n = 3). This may indicate immune peptide-enriched plasma in P. larvae-injected broods compared with the non-induced controls. SDS-PAGE showed clearly visible bands that indicated a number of proteins of various molecular weights (≈ 3.5 to 260 kDa) (Fig. 2-I). These proteins showed overexpression at 24 h post-injection with P. larvae, which may indicate that these are P. larvae injection–related proteins/peptides, (Fig. 2-I, lane 6).
Fig 2.
Total protein content and antibacterial activities of crude plasma from A. mellifera jemenitica third larval instars 24 h post-injection with P. larvae (ksuPL5) (1 × 106 CFU/mL). I: One-dimensional SDS-PAGE of CBB-G250 plasma; each lane was loaded with 2.5 µL of plasma (pooled from five different individual larvae); M: Standard marker reference proteins (kDa). Bands showing different proteins’ expression in a range of ~ 3.5–260 kDa. Lanes 1, 3, and 5 were loaded with plasma of control (non-injected) larvae, while lanes 2, 4, and 6 were loaded with plasma from P. larvae-injected broods. II: Antibacterial activities of different concentrations of crude plasma against various bacterial strains. Bars marked with similar letters, at each concentration represent non-significant differences (P > 0.05); while different letters indicate significant differences (P ≤ 0.05; One-way ANOVA). Error bars represent standard errors of means.
3.2. Humoral immune responses
3.2.1. Growth-inhibition activity
Data from growth-inhibition zone assays revealed growth-inhibition activity toward all tested bacteria at the lowest protein concentration (2.5 µg/mL) (Fig. 2-II). However, similar growth-inhibition patterns were observed against all tested bacteria at the lowest and highest concentrations (2.5 and 20 µg/mL, respectively) (P > 0.05; One-way ANOVA). Further, protein concentrations of both 5 and 10 µg/mL produced growth-inhibition activities that were significantly higher against M. luteus and lower against E. coli compared with that of P. larvae (P ≤ 0.05; One-way ANOVA). Finally, the highest observed antibacterial activity was toward M. luteus and P. larvae (zone diameter of 12.0 ± 0.88 mm) followed by E. coli (11.0 ± 0.88 mm) at the highest concentration (20 µg/mL). Overall, growth-inhibition activity against each of examined bacterium increased with plasma protein concentrations (Fig. 2-II).
3.2.2. Lysozyme-like activity
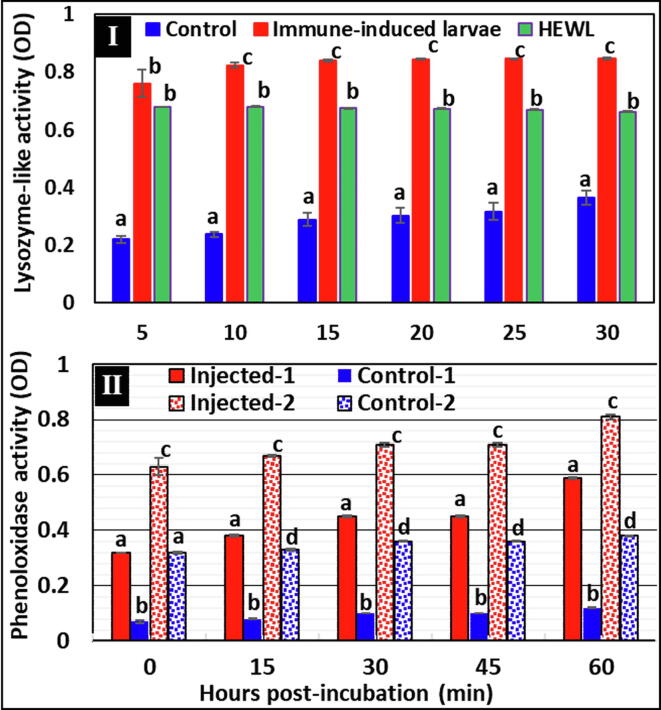
Plasma of P. larvae-injected broods expressed a significant increase in lysozyme-like activity compared with standard HEWL controls at each incubation time point (P ≤ 0.05; One-way ANOVA) (Fig. 3-I). Significantly high activity for all was clearly noticeable in the P. larvae-injected plasma compared with those of non-injected or standard HEWL controls. This may indicate induction of this type of humoral antibacterial activity against P. larvae.
Fig 3.
Humoral activities of crude plasma proteins of the third instar A. mellifera jemenitica 24 h post-injection with P. larvae (ksuPL5) (1 × 106 CFU/larva) or non-injected controls. I: Lysozyme-like activity (OD/mg protein/min) at different times post-incubation (minutes) in plasma of control, P. larvae-injected A. mellifera jemenitica third instars and hen egg white lysozyme used as standard. II: Prophenoloxidase activity (OD/mg protein/min) at different times post-incubation (minutes) without tyrosine (injected-1 and control-1) or with tyrosine (injected-2 and control-2). Bars marked with the same letters at each time point represent no significant differences (P > 0.05), however, those marked with different letters represent significant differences (P ≤ 0.05; One-way ANOVA). Error bars represent standard errors of means of three replicates in each case (n = 3).
3.2.3. Phenoloxidase activity
Data for PO activity assay showed significantly high activity in P. larvae-injected plasma compared with controls when both were added to tyrosine-mixed media throughout all time points of incubation (P ≤ 0.05; One-way ANOVA) (Fig. 3-II). Plasma from P. larvae-injected larvae showed also significantly higher PO activity compared with that of controls, when both were added to media with no added tyrosine over all time points of incubation. Plasma of P. larvae-injected larvae showed significantly higher PO activity when incubated in tyrosine-mixed media compared with non-tyrosine-mixed media (P ≤ 0.05; One-way ANOVA) (Fig. 3-II). In comparison to control plasma in tyrosine-mixed media, plasma of P. larvae-injected larvae in non-tyrosine media showed similar PO activity at the 0 time point of incubation (P > 0.05; one-way ANOVA) and began to significantly increase at 15 min onward until the end of the experiment. Finally, P. larvae-injected plasma in both tyrosine- and non-tyrosine media showed steady elevation in PO activity over the incubation period (60 min) compared with controls.
3.2.4. Cecropin-like activity
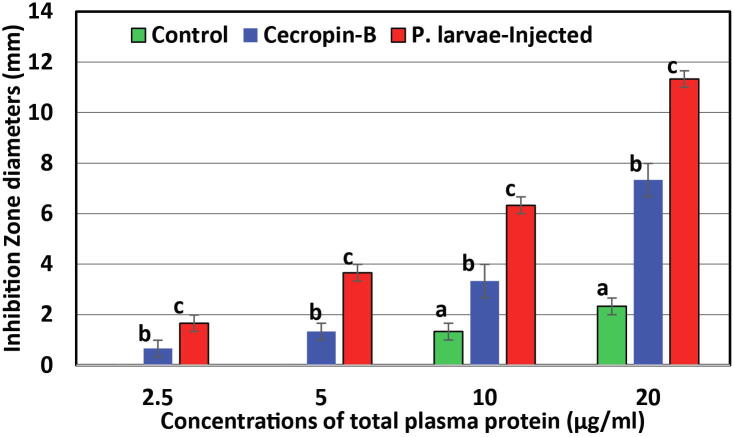
Growth-inhibition zone assays showed significantly higher growth-inhibition against Gram-negative E. coli in plasma protein with a concentration as low as 2.5 µg/mL compared with that of non-injected controls, as well as to cecropin-B (P ≤ 0.05; one-way ANOVA) (Fig. 4). Control plasma showed significant lower activities throughout all time points compared with both P. larvae-injected broods and cecropin-B references (P ≤ 0.05; One-way ANOVA), and was un-detectable at the lower concentrations (2.5 and 5 µg/mL). These data suggest that cecropin-like activity was induced upon P. larvae injection.
Fig 4.
Cecropin-like activity of crude plasma proteins of A. mellifera jementica third instars 24 h post-injection with P. larvae (ksuPL5) against E. coli using cecropin B as a reference. Bars marked with the same letters represent no significant differences (P > 0.05); while those marked with different letters at each concentration represent significant differences (P ≤ 0.05; One-way ANOVA). Error bars represent standard errors of means of three replicates in each case (n = 3).
3.2.5. Cellular immune responses
3.2.5.1. Nodulation response
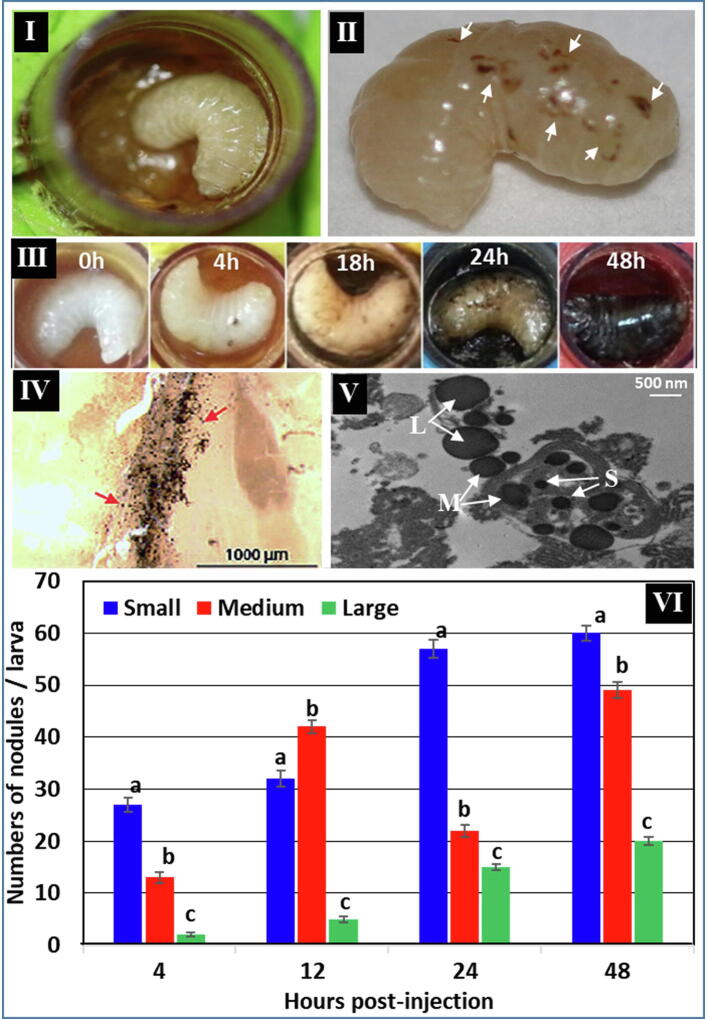
Melanization and nodule formation increased gradually until complete melanization 48 h post-P. larvae injection (Fig. 5-III). The number of differently sized nodules also increased in response to P. larvae injection over the experimental time points (Fig. 5-IV, V, and VI). The significantly highest number was recorded in small nodules at each time point of the experiment (51% of the total), followed by the medium-sized (36%) and larger nodules (12%) (P ≤ 0.05; One-way ANOVA) (Fig. 5-VI). A gradual increase in number over time was recorded in all three sizes of nodules, and most surrounded the dorsal blood vessel 24 h after injection (Fig. 5-IV). These data suggest that nodulation is an effective cellular immune response against injected P. larvae bacterium.
Fig 5.
Melanization/nodulation cellular immune response of A. mellifera jementica third instars upon injection with P. larvae (ksuPL5). I: Intact control larva in a rearing well. II: P. larvae-injected brood showing early formed nodules 4 h post-injection (arrows). III: A panel of treated broods showing gradual increase of melanization/nodulation at different hours post-injection. IV: Dissected larval cuticle under a light microscope 24 h post-P. larvae injection showing large numbers of nodules of different sizes. V: Transmission electron microscopy ultra-thin sections showing large (L: 40–80 µm), medium (M: 10–30 µm), and small (S: 5–10 µm) nodules. VI: A histogram of the numbers of formed nodules at different times post-P. larvae injection. Bars marked with different letters at each time point represent significant differences (P ≤ 0.05; One-way ANOVA). Error bars represent standard errors of means of three replicates in each case.
3.2.5.2. In vivo verification against P. Larvae
Data from RP-HPLC showed three different peptide/protein fraction peaks (A, B, and C). Three replicates of each fraction were pooled, separately and confirmed results from the MIC assay showed variant MIC of these individual fraction pools depending on differential bacterial potency against different types of bacterial strains. As shown in Table 3, each fraction pool showed strong antibacterial potency compared with that of the reference CIP against E. coli and M. luteus. In comparison, MIC of the different fractions against P. larvae (ksuPL5), followed by P. aeruginosa, were significantly higher (P ≤ 0.05; One-way ANOVA). However, peptides/proteins fraction B showed the lowest MIC compared with the other two fractions (A and C) against P. larvae (ksuPL5) (MIC: 64 µg/mL) (Table 3).
Upon determining the MICs, the anti-P. larvae activities of the three immune peptides/proteins fractions (A, B, and C) were carried out against P. larvae (ksuPL5) in vitro via the growth-inhibition zone assay. Data revealed a significant increase in the growth-inhibition zone relevant to fraction B compared with the other two fractions (A and C) (P ≤ 0.05; One-way ANOVA) (Table 4). These data suggest that peptide/protein fraction B showed the highest anti-P. larvae activity, and fraction B was therefore used for in vivo bioassays of P. larvae-infected broods.
Table 4.
Growth-inhibition zone diameters of P. larvae (ksuPL5) upon treatment with the purified immune peptides of A. mellifera jemenitica third instars 24 h post-injection with P. larvae (ksuPL5) compared with ciprofloxacin antibiotic.
| Purified immune peptides (25 µg/mL) | ||||
|---|---|---|---|---|
| A | B | C | CIP | |
| Growth inhibition zone diameter (mm) (Mean ± SE) | 17.5 ± 0.71a | 20.8 ± 0.71b | 19.5 ± 0.71b | 26.5 ± 0.71c |
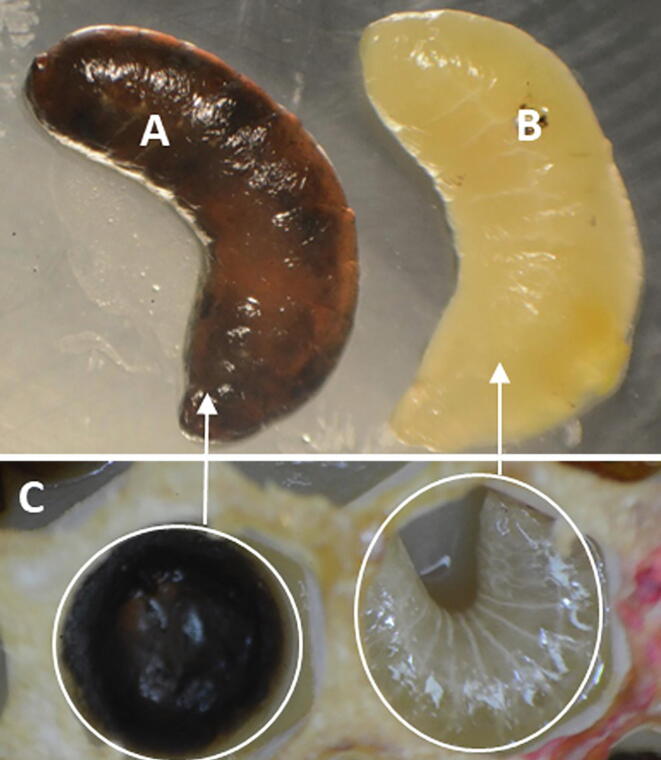
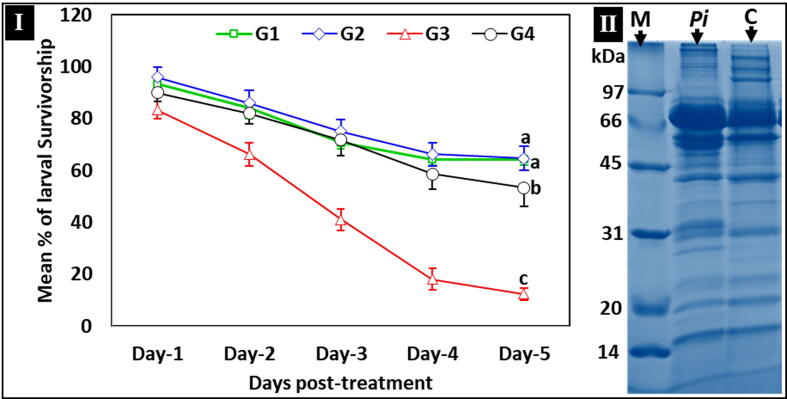
To verify anti-P. larvae activity, the most potentially active peptide/protein fraction B (MIC: 64 µg/mL) was added to a P. larvae-infected diet prior to feeding the first instar (24 h old). Upon P. larvae infection, the larvae turned completely black and died prior to pupation (Fig. 6). Daily mortality data showed a significant decrease in survivorship of the P. larvae-infected group (G3) fed an ND compared with those of the negative and positive controls (G1 and G2, respectively) or those fed an ND with protein fraction B (G4) (P ≤ 0.05; One-way ANOVA) (Table 2 and Fig. 7-I). Larvae fed a P. larvae-infective diet mixed with immune protein fraction B achieved a significantly lower mortality rate compared with those of infected controls, and their survival was similar to that of control groups G1 and G2 (fed non-infective diets) (Table 2 and Fig. 7-I).
Fig 6.
Late thirdlarvalinstars showing P. larvae-infected (A) and healthy (B) broods after removal from their rearing wells in a Jenter comb box (C). Infected larvae appear dark brown and as partially decayed dead cadavers. Photos were taken using Nikon D-810 Digital camera; 105 mm macro-lens by A. M. Ahmed).
Fig 7.
In vivo verification of immune peptide/proteins of fraction B against P. larvae infection. I: Survivorship of control and treated larval groups (50 larvae each) throughout a larval duration period (five days). G1: negative control group fed on a normal diet (ND), G2: positive control group fed on an ND + peptides of fraction B, G3: infected group fed on an ND + P. larvae spores, and G4: treated group fed on an ND + P. larvae spores + peptides of fraction B. Mortality rates were calculated daily in triplicate (n = 3). Lines marked with similar letters represent no significant differences (P > 0.05); While different letters represent significant differences (P ≤ 0.05; One-way ANOVA). II: One-dimensional SDS-PAGE of plasma from P. larvae-injected (Pi) and non-injected control (C) larvae. Each lane was loaded with 2.5 µL of plasma (pooled from five individual larvae). M: Standard marker reference proteins (kDa). Infected plasma clearly shows different protein over-expression compared with non-injected controls.
3.2.5.3. Characterization and identification of immune peptides/proteins
Protein expression change in crude plasma of third instars was determined 24 h post-injection with P. larvae (ksuPL5) using MS to describe any induced antimicrobial peptide/proteins. Induced overexpressed protein of (Mr ≈ 80 kDa) compared with non-injected control was revealed by 1D-SDS-PAGE (Fig. 7-II). Matching the peptide/proteins sequences in the NCBI database against those of Apis mellifera and P. larvae standards as references revealed up to 243 peptides/proteins (listed in an additional file), of which the targeted PO subunit A3 (Apis mellifera; reference accession number NP_001011627.1) was quantified with a predicted Mr 80 kDa. The observed overexpression of PO in the plasma of P. larvae-injected broods compared with that of non-injected controls (Fig. 7-II) suggests proteolytic activation of PO in the hemolymph plasma can be only partially attributable to up-regulated expression. This is also clear in the humoral immunity results shown in Fig. (3-II) and the complete humoral melanization and nodulation responses shown in Fig. 5. The second detected protein was beta-1,3-glucan-binding protein 1 (Apis mellifera; reference accession number XP_001121634.2) of Mr 52 kDa, which increased upon injection compared with non-injected controls. The third characterized protein was the predicted serine protease 44 isoform X1 (Apis mellifera; reference accession number XP_016769182.1) of Mr 46 kDa that increased upon injection compared with non-injected controls. These results provide a clue to the vital processing required to carry out an in vivo bioassay in AFB-infected broods.
4. Discussion
To the best of our knowledge, this is the first study to reveal an inducible immune response in broods of the indigenous Saudi honeybee A. mellifera jemenitica. We investigated the possibility of producing safe and natural honeybee-derived immune peptides (antibiotics) as they would represent a powerful tool to probe biological systems and increase the selectivity of natural therapeutic antibiotics that can address AFB infection in apiaries. To achieve this aim, two objectives were set. The first was exploring the main cellular and humoral immune responses of the third larval instar of A. mellifera jemenitica against the AFB bacterium P. larvae (ksuPL5) and other standard bacterial strains. The second was purifying and characterizing potentially active anti-P. larvae immune peptides and verifying their therapeutic effect on P. larvae-infected first instars (those most susceptible to AFB infection) (Genersch et al., 2005). Given the significance of insect-derived, natural, safe, and wide-spectral antimicrobials, these findings have important implications for avoiding AFB-resistance and adverse impacts on the health and development of broods when using conventional antibiotics for AFB treatment.
Honey production and cross-pollination of agricultural and horticultural crops rely on healthy honeybees, which therefore have a major economic worldwide impact (Ellis and Munn, 2005). Recently, significant losses of honeybees as well as decline in global apiculture have been reported, both of which are linked directly to a large diversity of lethal microorganisms, including bacterial, viral, protozoal, fungal, and parasitic organisms (Genersch, 2010). AFB is the most lethal and devastating bacterial disease in honeybee broods globally (Evans, 2003, Genersch, 2010, Miyagi et al., 2000) and locally (Ansari et al., 2017a, 2017b). While burning infected bee colonies and hive materials remains the simplest and most practical way to control AFB, conventional antibiotics such as oxytetracycline are becoming manageable treatment options. However, serious concerns are linked to such antibiotics because of rapidly emerging AFB-resistance (Evans, 2003, Jarosz, 1995, Miyagi et al., 2000) and adverse effects on the health and development of honeybee broods (Thompson et al., 2005) and their beneficial gut bacterial fauna (Al-Ghamdi et al., 2020, Vásquez et al., 2012).
For safe and efficient control of AFB, several recent studies have been conducted to deploy two alternatives scenarios to conventional antibiotics (Mutinelli, 2003). The first is utilization of natural compounds and essential oils (Erler and Moritz, 2016, Kuzyšinová et al., 2016). However, these compounds have exhibited cytotoxic effects on honeybees. The second is development of insect-derived natural antibiotics (antimicrobial immune peptides). These antimicrobial peptides have proven to be basic and small molecules (Boman and Hultmark, 1987, Chen et al., 2009, Evans et al., 2006, Hoffmann, 1995) that work synergistically against pathogens to provide broad-spectrum coverage and rarely encounter bacterial resistance (Rajanbabu and Chen, 2011). In support of the second scenario, and following on our recent study of AFB in local apiaries for the first time in Saudi Arabia (Ansari et al., 2017a, 2017b), we conducted the present study to advance efforts to produce safe and natural honeybee-derived antibiotics against AFB. In this context, four points warrant clarification. First, as the only race of honeybees in Saudi Arabia (Al-Ghamdi et al., 2013), A. mellifera jemenitica is well-adapted to a semi-desert climate and has had a major impact on the national economy and biodiversity (Al-Ghamdi et al., 2013, Alqarni et al., 2011). Second, a locally isolated AFB strain, P. larvae (ksuPL5) (Ansari et al., 2017a, 2017b), was used to induce the immune responses. Third, the third instar was used for exploring its immune responses, as it is more tolerant to AFB (Chan et al., 2009), and hence purifying the induced immune peptides. Fourth, the first instar was used for in vivo immune peptide verification experiments as it is the most susceptible stage to AFB infection (Bastos et al., 2008, Chan et al., 2009).
Phenoloxidase (with a molecular mass ≈ 80 kDa) overexpression on P. larvae-injection is in accordance with the results of previous studies by Osawa et al. (2001), who concluded that the activity of this enzyme appears to correlate closely with larval development and susceptibility to infection. In insects, PO is present as pro-PO, the inactive precursor, which is usually activated by a steps of serine proteases into the essential form for a several processes, such as hardening of cuticle, healing of wounds, pigmentation and nodule formation (Gätschenberger et al., 2013, Lu et al., 2014, Zufelato et al., 2004). PO activity is considered an indicator of insects’ immunocompetence, and a significant increase in the activity of PO plasma in P. larvae-infected broods has been reported over time. This may be due to activation of PO from its zymogen (pro-PO) by immune receptor proteins on the surface of hemocytes and hemolymph (Osawa et al., 2001) and may explain the upregulation of PO against E. coli observed in the present investigation, although E. coli is not a natural bee pathogen. Moreover, activation of PO is a central step in the melanization response, which is the most important defensive mechanism in insect innate immunity, leading to the encapsulation of infectious agents (Lu et al., 2014). This, in turn, may explain the strong melanization response against P. larvae observed in the present study. Nodulation is the deposition of melanin in the formed nodules on the surface of encapsulated antigens and in hemolymph clots at the wound vicinity in insects (Koella and Sørensen, 2002, Li et al., 2019). The current study showed strong nodulation responses as early as 4 h and peaked 48 h post-P. larvae injection. Evidence supporting these results has been provided by several similar studies of different types of insects (Kayis et al., 2012, Lord et al., 2002, Mirhaghparast et al., 2015, Zhao et al., 2009).
The present study also reported lysozyme-like activity in the plasma of P. larvae-infected A. mellifera jemenitica third instars. Lysozymes are immune peptides that degrade the peptidoglycan shell bacteria (Freitak et al., 2007, Li et al., 2014, Masschalck and Michiels, 2003) during the initial cellular immune response (Randolt et al., 2008), resulting in the death of Gram-negative and -positive bacteria alike (Casteels et al., 1993, Randolt et al., 2008). This may explain the significant growth-inhibitory effect reported in the experimental Gram-positive AFB bacteria P. larvae and M. luteus, and the Gram-negative bacterium E. coli. Moreover, the constitutive lysozyme concentration we reported in plasma may indicate that a background level of activity plays a crucial role in defending against P. larvae, a conclusion supported by other studies of (Evans et al., 2006, Randolt et al., 2008). The upregulation of both PO and lysozymes could have contributed to the observed increase in total plasma protein content in P. larvae-infected larvae. These results suggest that lysozymes are an important weapons in honeybee defenses.
HPLC-purified fractions of P. larvae-infected A. mellifera jemenitica third instars showed promising anti-P. larvae effects both in vitro and in vivo. Data from the in vivo assay revealed significantly higher survival rates in P. larvae-infected broods fed on a diet supplemented with peptides/proteins of fraction B compared with those fed on a fraction B–free diet. This may indicate that the proteins of fraction B may be effective honeybee-derived natural therapeutic antibiotics against AFB. In addition, MS and Orbitrap analysis data revealed PO of Mr 80 kDa, the beta-1,3-glucan-binding protein 1 of Mr 52 kDa, and the serine protease 44 isoform X1 of 46 kDa were the three primary antimicrobial immune peptides/proteins induced against the injected AFB causative agent, P. Larvae (ksuPL5).
In conclusion, insect immune peptides are promising candidates for the development of a new class of antibiotics (Hancock, 1997, Hetru et al., 1998, Lu and Chen, 2010, Silva, 2004). Analysis of their potential pharmacological properties and drug discovery efforts have recently been undertaken (Rajanbabu and Chen, 2011, Ratcliffe et al., 2011). Examples of insect-derived antibiotics have already been provided for clinical purposes (Chen et al., 2009, Nizet et al., 2001). Expression of these peptides in bacteria is by far the simplest and least expensive method of producing large amounts of such products (Li et al., 2014, Niu et al., 2008, Rao et al., 2004, Valore and Ganz, 1997). Observations of anti-P. larvae lysozyme-like, cecropin-like, PO activities, and nodulation responses, as well as in vivo anti-P. larvae activity detailed here provide direction for the production of bee-derived antibiotics that could help counter AFB infection while minimizing the chances of the emergence of AFB-resistance. The present study provides a framework for planning future studies of molecular characteristics and functional roles predicted and related AMPs in honeybees immune responses. Characterization of AMPs from both broods and adult honeybees may expand the arsenal of insect-derived antibiotics of therapeutic purposes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number (11-AGR2082-02). The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Ghamdi A., Al-Abbadi A.A., Khan K.A., Ghramh H.A., Ahmed A.M., Ansari M.J. In vitro antagonistic potential of gut bacteria isolated from indigenous honey bee race of Saudi Arabia against Paenibacillus larvae. J. Apic. Res. 2020:1–9. [Google Scholar]
- Al-Ghamdi A., Khan K.A., Ansari M.J., Almasaudi S.B., Al-Kahtani S. Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi J. Biolog. Sci. 2018;25:383–387. doi: 10.1016/j.sjbs.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi A.A., Nuru A., Khanbash M.S., Smith D.R. Geographical distribution and population variation of Apis mellifera jemenitica Ruttner. J. Apic. Res. 2013;52:124–133. [Google Scholar]
- Alqarni, A.S., Hannan, M.A., Owayss, A.A., Engel, M.S., 2011. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. ZooKeys, 83. [DOI] [PMC free article] [PubMed]
- Amaral I.M.R., Neto J.F.M., Pereira G.B., Franco M.B., Beletti M.E., Kerr W.E., Bonetti A.M., Ueira-Vieira C. Circulating hemocytes from larvae of Melipona scutellaris (Hymenoptera, Apidae, Meliponini): Cell types and their role in phagocytosis. Micron. 2010;41:123–129. doi: 10.1016/j.micron.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Amsterdam, D., 1996. Antibiotics in laboratory medicine, In: Wilkins, W.a. (Ed.), Susceptibility testing of antimicrobials in liquid media, 4th ed, Baltimore, M.
- Anjum S.I., Shah A.H., Aurongzeb M., Kori J., Azim M.K., Ansari M.J., Bin L. Characterization of gut bacterial flora of Apis mellifera from north-west Pakistan. Saudi J. Biolog. Sci. 2018;25(2):388–392. doi: 10.1016/j.sjbs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Nuru A., Ahmed A.M., Ayaad T.H., Khan K.A., Al-Waili N. Diagnosis and molecular detection of Paenibacillus larvae, the causative agent of American foulbrood in honey bees in Saudi Arabia. Int. J. Trop. Insect Sci. 2017;37:137–148. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Khan K.A., Alqarni A.S., Kaur M., Al-Waili N. In vitro evaluation of the effects of some plant essential oils on Ascosphaera apis, the causative agent of Chalkbrood disease. Saudi J. Biolog. Sci.: SI: Curr. Res. Apiculture. 2017;24(5):1004–1011. doi: 10.1016/j.sjbs.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupinel P., Fortini D., Dufour H., Tasei J., Michaud B., Odoux J., Pham-Delegue M. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 2005;58:107. [Google Scholar]
- Ayaad T.H., Shaker G.H., Almuhnaa A.M. Isolation of antimicrobial peptides from Apis florae and Apis carnica in Saudi Arabia and investigation of the antimicrobial properties of natural honey samples. J. King Saud Univ.-Sci. 2012;24:193–200. [Google Scholar]
- Ayaad T.H., Ahmed A.M., Al-Ghamdi M.S., Siddiqi N.J., Al-Ghamdi A.A., Ansari M.J., Mohamed A.A. Histopathological impact in the larval gut of the honeybee, Apis mellifera jemenitica, upon infection with the American foulbrood bacterium, Paenibacillus larvae. Indian J. Pharmaceut. Educ. Res. 2018;52:268–276. [Google Scholar]
- Bastos E.M.A., Simone M., Jorge D.M., Soares A.E.E., Spivak M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008;97:273–281. doi: 10.1016/j.jip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Boman H.G., Hultmark D. Cell-free immunity in insects. Ann. Rev. Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brogden K.A., Ackermann M., McCray P.B., Jr, Tack B.F. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents. 2003;22:465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Tempst P. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera) J. Biol. Chem. 1993;268:7044–7054. [PubMed] [Google Scholar]
- Chan Q.W., Melathopoulos A.P., Pernal S.F., Foster L.J. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC genomics. 2009;10:387. doi: 10.1186/1471-2164-10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhu F., Cao Y., Qiao S. Novel expression vector for secretion of cecropin AD in Bacillus subtilis with enhanced antimicrobial activity. Antimicrob. Agents Chemother. 2009;53:3683–3689. doi: 10.1128/AAC.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.P., Pettis J.S., Zhao Y., Liu X., Tallon L.J., Sadzewicz L.D., Li R., Zheng H., Huang S., Zhang X. Genome sequencing and comparative genomics of honey bee microsporidia, Nosema apis reveal novel insights into host-parasite interactions. BMC Genomics. 2013;14:451. doi: 10.1186/1471-2164-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G., Müller H.-M., Levashina E.A., Kafatos F.C. Innate immune defense against malaria infection in the mosquito. Curr. Opin. Immunol. 2001;13:79–88. doi: 10.1016/s0952-7915(00)00186-2. [DOI] [PubMed] [Google Scholar]
- Djukic M., Brzuszkiewicz E., Fünfhaus A., Voss J., Gollnow K., Poppinga L., Liesegang H., Garcia-Gonzalez E., Genersch E., Daniel R. How to kill the honey bee larva: genomic potential and virulence mechanisms of Paenibacillus larvae. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I., Gökçen F., Felföldi G., Millichap P.J., Trenczek T.E., Ffrench-Constant R.H., Reynolds S.E. The immunoglobulin family protein Hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell. Microbiol. 2007;9:1137–1147. doi: 10.1111/j.1462-5822.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- Ellis J.D., Munn P.A. The worldwide health status of honey bees. Bee world. 2005;86:88–101. [Google Scholar]
- Erler S., Moritz R.F. Pharmacophagy and pharmacophory: mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera) Apidologie. 2016;47:389–411. [Google Scholar]
- Evans J.D. Diverse origins of tetracycline resistance in the honey bee bacterial pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2003;83:46–50. doi: 10.1016/s0022-2011(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Evans J., Aronstein K., Chen Y.P., Hetru C., Imler J.L., Jiang H., Kanost M., Thompson G., Zou Z., Hultmark D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitak D., Wheat C.W., Heckel D.G., Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez E., Genersch E. Honey bee larval peritrophic matrix degradation during infection with Paenibacillus larvae, the aetiological agent of A merican foulbrood of honey bees, is a key step in pathogenesis. Environ. Microbiol. 2013;15:2894–2901. doi: 10.1111/1462-2920.12167. [DOI] [PubMed] [Google Scholar]
- Gätschenberger H., Azzami K., Tautz J., Beier H. Antibacterial immune competence of honey bees (Apis mellifera) is adapted to different life stages and environmental risks. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genersch E. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010;87:87–97. doi: 10.1007/s00253-010-2573-8. [DOI] [PubMed] [Google Scholar]
- Genersch E., Ashiralieva A., Fries I. Strain-and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Appl. Environ. Microbiol. 2005;71:7551–7555. doi: 10.1128/AEM.71.11.7551-7555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghramh, H.A., Khan, K.A., Ahmed, Z., Ansari, M.J., 2020. Quality evaluation of Saudi honey harvested from the Asir province by using high-performance liquid chromatography (HPLC). Saudi journal of biological sciences. [DOI] [PMC free article] [PubMed]
- Hancock R.E. Peptide antibiotics. The Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Hetru C, Hoffmann D, Bulet P, 1998. Antimicrobial peptides from insects, In: Brey PT, Hultmark D (Eds.), Molecular mechanisms of immune responses in insects. Chapman & Hall, London, pp. 40–66.
- Hoffmann J.A. Innate immunity of insects. Curr. Opin. Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Hultmark, D., 1998. Quantification of antimicrobial activity, using the inhibition-zone assay, In: Wissner A, D.G., Marmaras VJ, Morishimma I, Sugumaran M, Yamakawa M, (Ed.), Techniques in insect immunology. USA press.
- Irving P., Troxler L., Hetru C. Is innate enough? The innate immune response in Drosophila. C.R. Biol. 2004;327:557–570. doi: 10.1016/j.crvi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jarosz J. Haemolymph immune proteins protect the insect body cavity from invading bacteria. Comp. Biochem. Physiol. C: Pharmacol. Toxicol. Endocrinol. 1995;111:213–220. [Google Scholar]
- Jin Y., Manabe T. High-efficiency protein extraction from polyacrylamide gels for molecular mass measurement by matrix-assisted laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis. 2005;26:1019–1028. doi: 10.1002/elps.200410187. [DOI] [PubMed] [Google Scholar]
- Kayis, T., Emre, M., Coskun, M., 2012. Effects of diazinon on antioxidant enzymes and adult emergence of the parasitoid Pimpla turionellae L.(Hymenoptera: Ichneumonidae).
- Khan K.A., Al-Ghamdi A.A., Ghramh H.A., Ansari M.J., Ali H., Alamri S.A., Al-Kahtani S.N., Adgaba N., Qasim M., Hafeez M. Structural diversity and functional variability of gut microbial communities associated with honeybees. Microb. Pathog. 2020;138 doi: 10.1016/j.micpath.2019.103793. [DOI] [PubMed] [Google Scholar]
- Koella J., Sørensen F. Effect of adult nutrition on the melanization immune response of the malaria vector Anopheles stephensi. Med. Vet. Entomol. 2002;16:316–320. doi: 10.1046/j.1365-2915.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- Kotthoff, U., Wappler, T., Engel, M.S., 2011. Miocene honey bees from the Randeck Maar of southwestern Germany (Hymenoptera, Apidae). ZooKeys, 11. [DOI] [PMC free article] [PubMed]
- Kuzyšinová K., Mudroňová D., Toporčák J., Molnár L., Javorský P. The use of probiotics, essential oils and fatty acids in the control of American foulbrood and other bee diseases. J. Apic. Res. 2016;55:386–395. [Google Scholar]
- Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed]
- Laughton A.M., Boots M., Siva-Jothy M.T. The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J. Insect Physiol. 2011;57:1023–1032. doi: 10.1016/j.jinsphys.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim J., Hwang S., Lee W., Yoon H., Lee H., Hong S. High-level expression of antimicrobial peptide mediated by a fusion partner reinforcing formation of inclusion bodies. Biochem. Biophys. Res. Commun. 2000;277:575–580. doi: 10.1006/bbrc.2000.3712. [DOI] [PubMed] [Google Scholar]
- Li T., Yan D., Wang X., Zhang L., Chen P. Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli. Insects. 2019;10:301. doi: 10.3390/insects10090301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: progress and challenges. Vaccines. 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J.C., Anderson S., Stanley D.W. Eicosanoids mediate Manduca sexta cellular response to the fungal pathogen Beauveria bassiana: a role for the lipoxygenase pathway. Arch. Insect Biochem. Physiol.: Publ. Collab. Entomolog. Soc. Am. 2002;51:46–54. doi: 10.1002/arch.10049. [DOI] [PubMed] [Google Scholar]
- Lu A., Zhang Q., Zhang J., Yang B., Wu K., Xie W., Luan Y.-X., Ling E. Insect prophenoloxidase: the view beyond immunity. Front. Physiol. 2014;5:252. doi: 10.3389/fphys.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Chen Z.-W. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides. 2010;31:44–50. doi: 10.1016/j.peptides.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Masschalck B., Michiels C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- Meixner M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010;103:S80–S95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Michalski, A., Damoc, E., Hauschild, J.-P., Lange, O., Wieghaus, A., Makarov, A., Nagaraj, N., Cox, J., Mann, M., Horning, S., 2011. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cellur. Proteomics 10. [DOI] [PMC free article] [PubMed]
- Mirhaghparast S.K., Zibaee A., Hoda H., Sendi J.J. Changes in cellular immune responses of Chilo suppressalis Walker (Lepidoptera: Crambidae) due to pyriproxyfen treatment. Journal of Plant Protection. Research. 2015 [Google Scholar]
- Miyagi T., Peng C.Y., Chuang R.Y., Mussen E.C., Spivak M.S. Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States. J. Invertebr. Pathol. 2000;75:95–96. doi: 10.1006/jipa.1999.4888. [DOI] [PubMed] [Google Scholar]
- Morrison D.A. How to improve statistical analysis in parasitology research publications. Int. J. Parasitol. 2002;8:1065–1070. doi: 10.1016/s0020-7519(02)00064-4. [DOI] [PubMed] [Google Scholar]
- Mundo M.A., Padilla-Zakour O.I., Worobo R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004;97:1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Mutinelli F. European legislation governing the authorization of veterinary medicinal products with particular reference to the use of drugs for the control of honey bee diseases. Apiacta. 2003;38:156–168. [Google Scholar]
- Niu M., Li X., Wei J., Cao R., Zhou B., Chen P. The molecular design of a recombinant antimicrobial peptide CP and its in vitro activity. Protein Expr. Purif. 2008;57:95–100. doi: 10.1016/j.pep.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Nizet, V., Ohtake, T., Lauth, X., Trowbridge, J., Rudisill, J., Dorschner, R.A., Pestonjamasp, V., Piraino, J., Huttner, K., Gallo, R.L., 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457. [DOI] [PubMed]
- Osawa K., Miyazaki K., Shimura S., Okuda J., Matsumoto M., Ooshima T. Identification of cariostatic substances in the cacao bean husk: their anti-glucosyltransferase and antibacterial activities. J. Dent. Res. 2001;80:2000–2004. doi: 10.1177/00220345010800111001. [DOI] [PubMed] [Google Scholar]
- Rajanbabu V., Chen J.-Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides. 2011;32:415–420. doi: 10.1016/j.peptides.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Randolt K., Gimple O., Geissendörfer J., Reinders J., Prusko C., Mueller M.J., Albert S., Tautz J., Beier H. Immune-related proteins induced in the hemolymph after aseptic and septic injury differ in honey bee worker larvae and adults. Arch. Insect Biochem. Physiol.: Publ. Collab. Entomolog. Soc. Am. 2008;69:155–167. doi: 10.1002/arch.20269. [DOI] [PubMed] [Google Scholar]
- Rao X.C., Li S., Hu J.C., Jin X.L., Hu X.M., Huang J.J., Chen Z.J., Zhu J.M., Hu F.Q. A novel carrier molecule for high-level expression of peptide antibiotics in Escherichia coli. Protein Expr. Purif. 2004;36:11–18. doi: 10.1016/j.pep.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Ratcliffe N.A., Mello C.B., Garcia E.S., Butt T.M., Azambuja P. Insect natural products and processes: new treatments for human disease. Insect Biochem. Mol. Biol. 2011;41:747–769. doi: 10.1016/j.ibmb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Sheehan G., Farrell G., Kavanagh K. Immune priming: the secret weapon of the insect world. Virulence. 2020;11:238–246. doi: 10.1080/21505594.2020.1731137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L. Antimicrobial peptides from animals: focus on drug discovery. Lett. Drug Des. Discovery. 2004;1:230–236. [Google Scholar]
- Thompson H.M., Waite R.J., Wilkins S., Brown M.A., Bigwood T., Shaw M., Ridgway C., Sharman M. Effects of European foulbrood treatment regime on oxytetracycline levels in honey extracted from treated honeybee (Apis mellifera) colonies and toxicity to brood. Food Addit. Contam. 2005;22:573–578. doi: 10.1080/02652030500089986. [DOI] [PubMed] [Google Scholar]
- Tsakas S., Marmaras V. Insect immunity and its signalling: an overview. Invertebrate Survival Journal. 2010;7:228–238. [Google Scholar]
- Tzou P., De Gregorio E., Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host–pathogen interactions. Curr. Opin. Microbiol. 2002;5:102–110. doi: 10.1016/s1369-5274(02)00294-1. [DOI] [PubMed] [Google Scholar]
- Valore E.V., Ganz T. Laboratory production of antimicrobial peptides in native conformation. Antibacterial peptide protocols. Springer. 1997:115–131. doi: 10.1385/0-89603-408-9:115. [DOI] [PubMed] [Google Scholar]
- Vandenberg J., Shimanuki H. Technique for rearing worker honeybees in the laboratory. J. Apic. Res. 1987;26:90–97. [Google Scholar]
- Vásquez A., Forsgren E., Fries I., Paxton R.J., Flaberg E., Szekely L., Olofsson T.C. Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasami G., Sowa J.R., Jr A rapid, acetonitrile-free, HPLC method for determination of melamine in infant formula. Anal. Chim. Acta. 2010;665:227–230. doi: 10.1016/j.aca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Yue D., Nordhoff M., Wieler L.H., Genersch E. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera) Environ. Microbiol. 2008;10:1612–1620. doi: 10.1111/j.1462-2920.2008.01579.x. [DOI] [PubMed] [Google Scholar]
- Zhao F., Stanley D., Wang Y., Zhu F., Lei C.-L. Eicosanoids mediate nodulation reactions to a mollicute bacterium in larvae of the blowfly, Chrysomya megacephala. J. Insect Physiol. 2009;55:192–196. doi: 10.1016/j.jinsphys.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Zufelato M.S., Lourenço A.P., Simões Z.L., Jorge J.A., Bitondi M.M. Phenoloxidase activity in Apis mellifera honey bee pupae, and ecdysteroid-dependent expression of the prophenoloxidase mRNA. Insect Biochem. Mol. Biol. 2004;34:1257–1268. doi: 10.1016/j.ibmb.2004.08.005. [DOI] [PubMed] [Google Scholar]
Further Reading
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N., Nuru A., Sharma D., Khan K.A., Kaur M., Omer M. In vitro evaluation of the effects of essential plant oils on Paenibacillus larvae, the causative agent of American foulbrood. Biotechnol. Biotechnol. Equip. 2016;30(1):49–55. [Google Scholar]