Abstract
Human life expectancy increases, but the disease-free part of lifespan (healthspan) and the quality of life in old people may not show the same development. The situation poses considerable challenges to healthcare systems and economies, and calls for new strategies to increase healthspan and for sustainable future approaches to elder care. This call has motivated innovative research on the role of social relationships during ageing. Correlative data from clinical surveys indicate that social contact promotes healthy ageing, and it is time to reveal the causal mechanisms through experimental research. The fruit fly Drosophila melanogaster is a prolific model animal, but insects with more developed social behaviour can be equally instrumental for this research. Here, we discuss the role of social contact in ageing, and identify lines of study where diverse insect models can help uncover the mechanisms that are involved.
This article is part of the theme issue ‘Ageing and sociality: why, when and how does sociality change ageing patterns?’
Keywords: social contact, health, cognitive function, physical disability, inflammation, nutrition
1. Background
Our social environment can have a significant impact on our cognitive and physical ageing [1,2]. Clinical studies find that peoples' health and longevity correlate with a variety of social factors such as being married, living with extended family, social network size, social activities, social satisfaction and different social stressors [1,3–5]. These studies cover many health-related questions, but cognitive decline (i.e. dementia), physical disability, inflammation and nutrition are topics of particular interest [6–11]. Resolving the role of social environment in these four areas of health could lead to significant improvements in future strategies of elder care [12].
Cognitive decline is a symptom of many age-related neurodegenerative disorders such as dementia, Alzheimer's disease and Parkinson's disease. Dementia is not one disease but a syndrome of reduced cognitive functioning that goes beyond what is expected in successful ageing. Elderly that report being married, having many satisfying social relationships, and large social networks are less likely to be affected [1,3,13,14]. Social connectivity, however, does not protect against physical brain pathologies during ageing [13]. Social contact may thus enable cognitive functions to persist although the brain suffers from neurodegeneration. However, it is also possible that sociable people innately have brains that can endure more neurodegenerative damage independent of how much social contact they experience [15]. These competing explanations could be tested if peoples' private lives were manipulated so that social experiences no longer correlated with personal social inclinations. Such studies, however, would be difficult to perform—both technically and ethically.
Physical disability correlates with being alone or feeling alone during ageing [16,17]. Likewise, participation in a robust social network can have a protective effect on the prevalence and incidence of disability [18]. Perhaps psychosocial factors directly interfere with the physical health of old people [19,20], or perhaps lonely human beings are less active and as a result become frail [21–23]. These alternatives can be solved by surveying the activities of people that report different levels of loneliness. Physical activity, however, is usually self-reported and reporting can be biased by emotions like loneliness. Trained personnel can observe or standardize peoples’ physical activities, but imposed situations may have emotional side effects that also interfere with data interpretation [19]. Manipulating peoples' loneliness to study effects on health and activity is also generally unfeasible and unethical.
Pro-inflammatory mediators such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, cyclooxygenase-2 and inducible nitric oxide synthase are elevated in many diseases, and levels tend to increase during ageing [24,25]. Social stress is also a predictor of heightened inflammatory activity in middle-aged and old people [6,26], while positive social ties might attenuate inflammation-driven health problems [27,28]. Still, it is unclear how these correlations arise. Perhaps social relationships modulate peoples’ inflammatory status, or perhaps individuals with low pro-inflammatory activity are more sociable. One can resolve these alternatives by manipulative research that decouples peoples' social desires from their actual social experiences, but again, such experiments remain technically and ethically unfeasible.
Central obesity can increase the risk of cardiovascular disease, diabetes 2 and physical disability over the majority of the human lifespan [29,30]. Social relationships influence food intake, body image and weight: people adjust their food intake to mimic or please a social companion [31,32], eat more in larger groups [33], and gain weight when friends become heavier [34]. The regulation of these behaviours is incompletely understood [31], and there is limited attention on whether such phenomena of ‘behavioural modelling’ or ‘copying’ can be tapped into to improve nutrition in the elderly.
While connections are proposed, it is largely unresolved how social influences impact the biology of ageing. Experiments that go beyond correlation are few or missing, and it is practically and ethically difficult to design manipulative experiments with human participants. Nevertheless, the challenges imposed by our increasingly aged global human population require we address these knowledge gaps. It is thus timely to ask if animal models can help reveal mechanisms that connect social relationships to ageing outcomes. Here, we present a selection of insect systems that can be employed in ageing research, we propose lines of investigation that can take advantage of these models, and we discuss the relevance of expanding these comparisons to broader questions about the ecology and evolution of ageing.
2. Insect models of social behaviour
(a). A solitary insect, Drosophila melanogaster
Though they lack advanced social behaviour, fruit flies respond adaptively to other individuals (including dead conspecifics) and can therefore model (some) social processes [35–37]. One such paradigm is social niche construction, where flies shape their own environment through behaviour [38]. Males optimize mating success by constructing social milieus that match their ability to win aggressive encounters: winners benefit from aggressive niches, while losers benefit from foregoing aggression. Flies may also use social information to make reproductive choices: sexually inexperienced females copy the male choices of experienced females [39], and also prefer the food substrates they associate with mated females and their eggs for own egg-laying [40]. For both sexes, social interactions are associated with reduced lifespan the underlying physiological mechanisms are nuanced and vary between sexes [41,42].
Complementary to these behavioural paradigms, the fruit fly system has an impressive toolkit for neurogenetic analysis of behaviour [35]. Transgene fly stocks, RNA interference (RNAi) lines and other mutant Drosophila strains provide genetic models for age-related disorders such as Alzheimer's and Parkinson's disease [43–46]. Transgenesis is required to model human Alzheimer's pathology in the flies because insects do not form neurodegenerative tangles [47]. The Drosophila lines that model Parkinson's pathology, moreover, lose dopaminergic neurons and develop motor behavioural defects that do not occur during normal ageing in flies [48]. Such models can be combined with behavioural paradigms to study connections between social behaviour and age-related disorders [49,50]. In any case, these experiments should factor in that normal fly behaviours may not interface correctly with pathologies from introduced or changed genetic elements. Such mismatches could obscure the cause-effect relationships we want to study.
(b). A gregarious insect, Schistocerca gregaria
The desert locust (Schistocerca gregaria) can reversibly transform between a solitary phenotype that avoids conspecifics and a gregarious phenotype that forms large groups. Gregarious behaviour (tendency to stay in groups) is triggered by crowding: touch, visual and olfactory contact with others [51]. The behavioural phenotypes of gregarization occur within a few hours, while changes in body and brain morphology, sensory and endocrine physiology, colour and life-history strategy occur over multiple generations. These transgenerational changes occur because (female) crowding induces the epigenetic programming of eggs so new individuals hatch with more gregarious phenotypes [52]. With this mechanism, S. gregaria might provide a suitable system for exploring transgenerational physiological effects of social interactions, a phenomenon also observed in humans [53]. Today, expressed sequence tag databases provide a resource for locust genomic studies while the full genome sequence is pending. RNAi-mediated gene knockdowns are established [54,55], and additional functional genomic tools are underway.
(c). Social insects
Social insects share resources and reproduce cooperatively, forming communities or colonies with individuals of the same species [56]. The most advanced form of insect social living, called eusociality, is defined by division of labour between reproductives and helpers (workers), cooperative care for offspring, and overlapping generations in the colony. Highly eusocial behaviour is found in all living termites, the majority of ants, in honeybees, bumblebees and stingless bees, and the ambrosia beetle Austroplatypus incompertus. Several different forms of social organization are found in other social insects, and this diversity is widely used to probe proximate and ultimate mechanisms of social behaviour [36].
Insect division of labour has led to amazing organizational achievements in colonies (figure 1), and can be based on flexible behavioural biases between similar individuals as well as permanent task allocations among morphological specialized individuals [56]. Behaviour biases include care behaviour such as cleaning, tending and feeding others (nursing), and defensive activities like patrolling and attacking intruders (guarding). Morphologically similar workers of honeybees, stingless bees, several ants and wasps show a flexible division of labour for these and other social tasks like nest hygiene, construction, foraging, food handling and thermoregulation. Workers may initially nurse and later forage or defend the colony, so they perform several tasks during their lifetimes [60]. Permanent morphological specializations are seen in highly eusocial insects where reproductives are distinct from workers that are either functionally or completely sterile. Further morphological differences between workers are found in termites, some ants and a bee, and include enlarged mandibles and head armour in termite soldiers, and enlarged jaws and head in ants responsible for leaf-cutting [57]. Among the social insects, the honeybee worker is a well-established model in behavioural, neurobiological and gerontological research [61]. The extensive list of experimental paradigms in honeybee workers includes social learning, decision-making, learning and memory, and questions asked within the context of ageing take advantage of the flexible ageing trajectory of honeybee workers. This trajectory is partially determined by colony age demographics which can be manipulated under semi-wild conditions [62,63].
Figure 1.
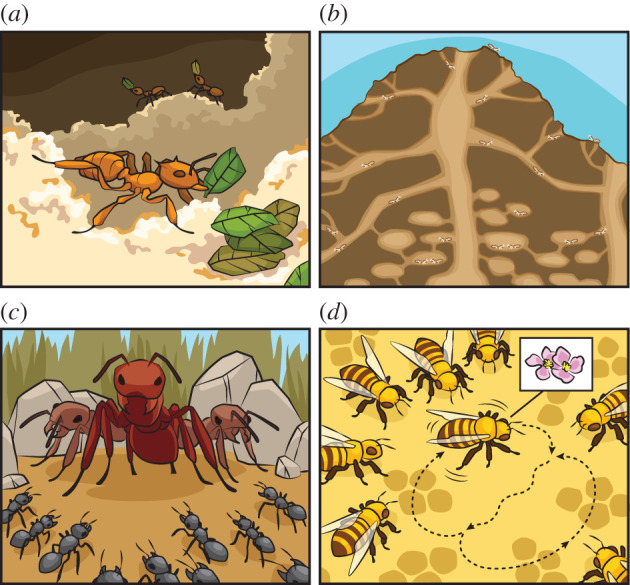
Division of labour in insects is associated with ‘cultural’ achievements such as (a) ‘agriculture’ in ants, termites and the ambrosia beetle that grow fungi inside their colonies, (b) ‘architecture’ in termites and ants that build cities with waste disposal systems, ventilation shafts, gardens, nurseries and other amenities [57], and (c) ‘warfare’ of ritualized tournaments, raiding and enslavement between ants [58]. The dance ‘language’ of honeybees (d), moreover, is a unique form of abstract communication between active foragers and new recruits, in which foragers use movement to communicate the value, direction and flight distance to food sources [59].
Although lacking the extensive genetic toolkit of Drosophila, several social insect genomes are either sequenced or in the process of being completed, including the honeybee Apis mellifera, the fire ant Solenopsis invicta, the red harvester ant Pogonomyrmex barbatus, the invasive Argentine ant Linepithema humile and the bumblebees Bombus terrestris and Bombus impatiens (see [60] for a commentary) [64]. The sequencing of social insect genomes has paved the way for researchers to use the CRISPR/Cas9 system to understand social insect physiology and behaviour. This genetic tool has been employed to understand the role of antennal lobe glomeruli in the social behaviour of the clonal raider ant, Ooceraea biroi, and the nutritional regulation of dimorphic size development of reproductive organs in honeybees [65,66]. In addition, RNAi-mediated gene knockdown is successful in eggs, larvae and adult workers honeybees [67–69], in female reproductives of S. invicta and Camponotus floridanus [70,71], and in larvae of the primitively eusocial wasp Polistes metricus [72]. Additionally, the genome biology of A. mellifera includes dynamic CpG DNA methylation, which is a crucial epigenetic system for gene regulation in vertebrates.
3. Lines of investigation
(a). Cognitive function
Cognitive function includes all mental processes that involve symbolic operations such as perception, complex learning and memory, and it encompasses awareness, thinking and capacity for judgement. It is discussed whether insects have cognitive capacities, but at least some species solve complex computational problems that including rule learning, non-elemental learning and delayed-matching-to-sample tasks [73–75]. These abilities are best documented in honeybees [76], which also solve intricate categorization tasks and show numerical processing abilities for numbers up to and including four [77,78]. Some complex brain functions known from honeybees are absent from Drosophila melanogaster and remain poorly explored in other insects [74]. It is not well resolved how these functions compare between insects and mammals, but central computational brain regions (figure 2) appear to be homologous between the animals [79–81].
Figure 2.

Central computational brain regions (orange) can be homologous between the insects and the vertebrates [79–81]: Stylized mushroom body neuropiles of a honeybee brain (a), and the human cortex (b).
(i). Physiological signatures of cognitive decline
Learning, memory and behavioural deficits are hallmarks of cognitive decline and associated with numerous age-related cognitive disorders in humans. Loneliness has been identified as a risk factor for Alzheimer's disease and dementia [82], while perceived social support and both physical and virtual (i.e. social media) social engagement associate with improvements in multiple aspects of cognitive function [83–85]. Similar relationships between ageing, cognitive decline, and the protective effects of social interaction can be found in the worker caste of A. mellifera. Honeybee workers begin to senescence approximately 14 days after their initial foraging flight. The age at which a worker takes its initial foraging flight is independent of chronological age, rather, determined by a combination of genetic, environmental, and social influences [61]. Senescent foragers tend to have decreased learning and memory ability compared to younger foragers and in-hive nurses, however, the progression of senescence itself can be modulated by social factors such as brood presence [86]. Fascinatingly, cognitive decline in honeybee workers can also be reversed by experimentally removing young in-hive bees from the colony (i.e. altering colony age demographics) (figure 3). This manipulation induces senescent honeybees to revert back to performing in-hive tasks such as brood care. This switch to tasks associated with young worker bees is accompanied by a relative increase in learning and memory ability over workers that continue foraging [87]. These unique ageing trajectories of honeybee workers make them a tractable insect model for elucidating mechanistic relationships between social contact and cognitive function, a pressing yet difficult-to-approach question in human studies.
Figure 3.
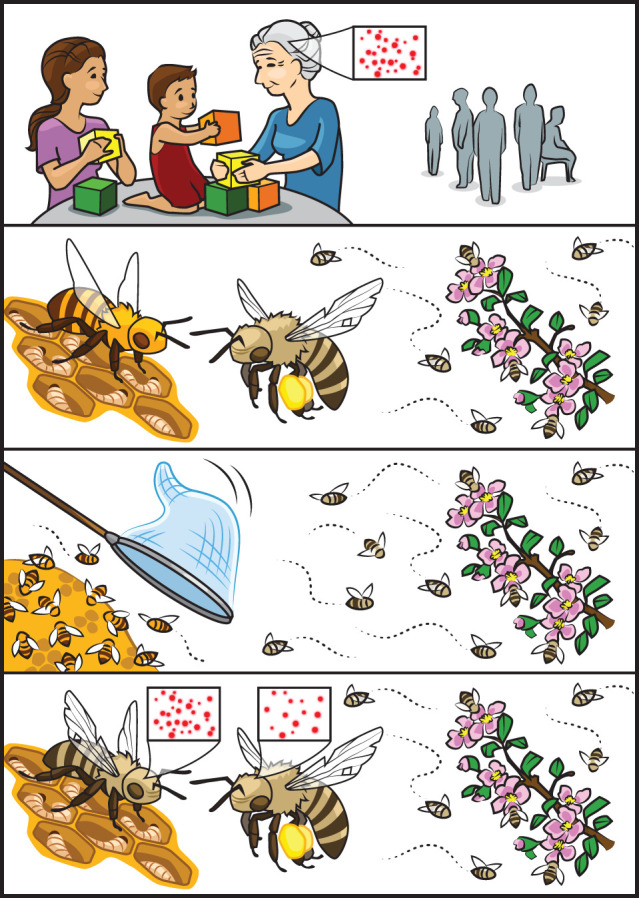
Peroxiredoxins (Prxs, red dots) can have protective effects in the ageing brain. It is not clear if Prxs respond to social contact between people (top panel), but one enzyme, Prx-6, is elevated in old bee brains that experience improved function after a social change. This social change involves the removal of young bees that do in-nest duties in the colony, such that old bees must switch from foraging to nursing larvae (mid panels). This task-switch is a behavioural reversion for the bees, because about all of them were nurses before they became foragers. Old reverted nurses that improve in brain function have greater than twofold higher Prx-6 levels than those bees that continue to forage (bottom panel).
Other social insects may serve as excellent models for understanding how social isolation impacts higher order cognitive processes necessary for the maintenance of social structures. Individuals of the paper wasp species, Polistes fuscatus, use their unique yellow and black facial markings as visual signals of individual identity, which they use to establish social dominance hierarchies within a colony [88]. Workers that are isolated from conspecifics during the first 6 days after eclosion do not develop individual recognition, which suggests that social interaction is a crucial component of brain development in young wasps [89]. A similar phenomenon is observed in ant species, where social isolation impairs the ability of ants to recognize individuals via hydrocarbon profile, as well as the hydrocarbon profiles themselves [90–92]. Individuals of the ant species, C. floridanus, reared in isolation have attenuated mushroom bodies, as well as reduced aggression when presented with a dead ant (a stimulus which induces an aggressive response to normally reared individuals) [93]. This suggests that social interaction is necessary for the proper development of the mushroom bodies, the seat of learning and memory in insects. Further studies on the interactions between social environment and brain development in insects may unravel fundamental concepts on how early social experiences can shape an individual's lifespan.
Brain senescence can manifest through a variety of means, including cellular senescence, inflammation, protein aggregation and neuronal damage [94,95]. These senescent processes can be triggered through a variety of genetic, disease or trauma-related events, and often underlie the cognitive impairments observed in both healthy ageing and age-related brain pathology [94]. An outstanding question in the social modulation of ageing is whether social interaction directly alleviates senescent processes (i.e. telomere shortening, tau aggregations), promotes resilience in cognitive function amidst senescent processes, or simply whether social individuals are intrinsically resilient to cognitive impairment. Experiments with the honeybee worker model seem to support the former two possibilities, while the minor workers of another eusocial insect, Pheidole dentata, support the latter. Similar to honeybee workers, P. dentata minors increase their task repertoire with age, eventually performing outside-nest tasks such as foraging [96]. However, aged P. dentata minors experience negligible senescence in brain structures that regulate task performance, and in fact display an increased ability to switch tasks given colony needs. Old P. dentata workers retain a full range of sensory abilities, as well as increased titers of serotonin and dopamine in the brain. These counterintuitive characteristics of older individuals may ultimately be related to the lower neurometabolic costs of individuals in highly integrated societies which can practice ‘collective cognition’ [97]. Although this ultimate hypothesis may not translate to humans, investigating the intrinsic resilience of P. dentata minors to senescence may illuminate fundamental proximate mechanisms that govern variation in our own intrinsic resilience to senescence.
(ii). Molecular signatures of cognitive decline
Protein kinase A (PKA) is a multi-functional family of cyclic AMP-dependent enzymes that can play a role in maturation of amyloid precursor proteins in Alzheimer's disease [98]. Brain ageing in Drosophila is also exacerbated by PKA activity in brain structures called mushroom bodies [99] that are homologous to vertebrate cortex (figure 2) [79]. It is unclear whether PKA can be modulated socially in the human brain, but levels are changed in brains from rats that experience chronic social defeat [100], as well as altered in the frontal cortex of people with regressive autism [101]. Changes in brain PKA gene expression can also regulate gregarization in desert locusts [102] and correlate with variation in social behaviour and division of labour between honeybees [103]. Thus, it can be worthwhile to investigate the social modulability of PKA and use insect models to explore its connections to cognitive ageing.
Peroxiredoxins (Prxs) are an enzyme family of sulfhydryl-dependent peroxidases. Six isozymes (Prx1–6) have been found in mammals. Prxs appear to have protective roles in neurodegenerative disorders involving oxidative and inflammatory stress, including Alzheimer's (Prx1, Prx2, Prx6), HIV-associated dementia (Prx3) and Huntington's disease (Prx1, Prx3, Prx6, 102, 125), and the enzymes are targets of drug discovery for treatments of dementia [104–107]. Perhaps similarly, elevated Prx6 levels correlate with improved brain function in old A. mellifera. Cognitive deficits develop in aged worker bees that forage for food, but foragers which revert to in-hive duties once (young) in-hive bees are removed from the colony show brain Prx6 levels that are more than twofold elevated (figure 3) [108]. It is not clear if Prx1–6 can respond to changes in peoples' social lives, but the human Prx enzymes show circadian changes [109] and circadian biology can often be modulated by social factors—in insects and people alike [110,111].
Excitatory amino acid transporter-2 (EAAT2) is another protein that correlates with recovery of brain performance after senesced foragers revert to in-nest tasks [108]. In contrast to Prx6 that is elevated, EAAT2 is reduced greater than twofold in reverted individuals that recover from ageing. EAAT2 is an important transporter of the excitatory neurotransmitter glutamate, and EAAT2 dysregulation has been implicated in Alzheimer's and Huntington's disease [112]. The regulation of EAAT2 has not been firmly connected to social factors in mammals, but old rats that experienced maternal separation can show elevated EAAT2 expression in the hippocampus [113]. Maternal separation is a juvenile stress paradigm that increases the risk of Alzheimer's pathology during ageing [114,115]. Perhaps research on insects can give more insight into how social relationships can affect EAAT2.
(b). Physical disability
This blanket term covers dysfunctions of limbs and fine or gross motor ability that leads to physical impairments, activity limitations and participation restrictions, which are common during ageing. Old people are also at higher risk of biomechanical wear, falling and fracturing [116,117]. Insects can experience similar losses of physical abilities, and old individuals often fly or walk less and spend more time stationary or in a supine position [118]. In Mediterranean fruit flies (Ceratitis capitata), the first occurrence of supine behaviour predicts remaining length of life, like the onset of physical disability correlates with time of death in elderly people [119]. Thus, it has been argued that insects can help identify common principles in the disablement process of ageing as well as assist in the discovery of preclinical markers of disablement [118,120].
Socially induced physical activity (SIPA) is motor activity that results from social motivation or contact. Physical activity protects against disability, reduces the risks of dementia and morbidity, and promotes health even in the oldest old humans (greater than or equal to 85 years, see (128) for an example) [121–123]. Emotional support from social networks correlates with better physical performance in elderly [124], and social motivation can impact the quantity and frequency of physical activity at any age [125]. Even so, research on D. melanogaster indicates that SIPA effects may be conditional on several factors (figure 4). Scientists used a mutant fly stock with genetic changes in antioxidant enzyme Cu/Zn superoxide. These flies are less stress resistant and shorter lived than normal flies [126]. Mutant lifespan and climbing ability, which served as a measure of physical performance, was improved by social contact with young, healthy, and normal (wild-type) individuals. Social contact with old wild-type flies, by contrast, did not prolong the mutant's lifespan. Disabling the young companion flies also had negative implications: mutant lifespan was less extended or even shortened, depending on how severely disabled the companions had become [126]. These results (figure 4) might imply that SIPA is less effective, less beneficial, or even harmful in social networks that are dominated by old or disabled individuals.
Figure 4.
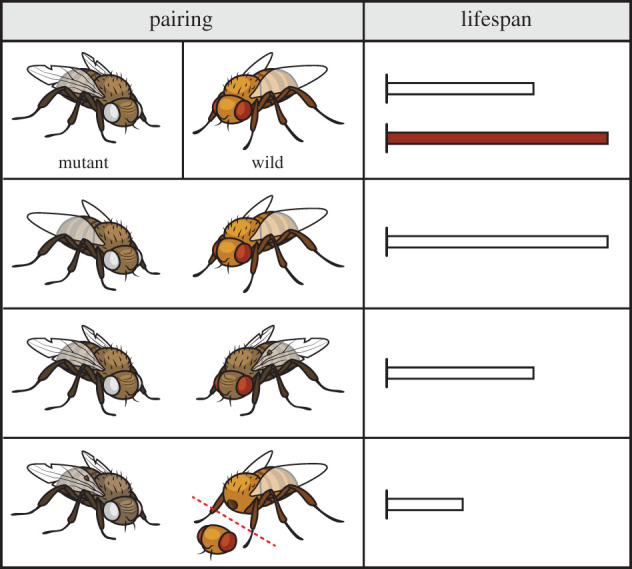
Mutant fruit flies (mutant) with genetic changes to antioxidant enzyme Cu/Zn superoxide are less stress resistant and live shorter lives than normal wild-type (wild) flies (top panel). Mutant lifespan is improved by social contact with young wild-type individuals, but not by contact with old wild-type flies (mid panel). Contact with disabled wild-type flies can shorten the lifespan of mutants even more (bottom panel).
Social role valorization (SRV) is a theory about the enablement of valued social roles for people. Social devaluation can lead elderly to devalue their residual capacities even further [127], and poor self-perceived abilities is a risk-factor of depression, immobility and health decline during the ageing process [128,129]. Improvements in physical fitness can directly counteract these symptoms by promoting a sense of self-perceived ability [130]. Interestingly, some insect societies have task systems that enable contributions from aged and disabled animals. In the leaf-cutter ant Atta cephalotes, workers divide labour based on size [57]. Tiny workers tend the fungus garden (figure 1), eggs and larvae, and they patrol the terrain around the colony in search of intruders. Larger workers are foragers that perform tasks of leaf-cutting and transport. Cutting is achieved by chewing, causing progressive teeth-wear that compromises old ants' ability to harvest plant material. Instead, the old ants transport leaves cut by other foragers [131].
The same Atta is known for its system of hazardous waste management. Waste materials from the colony are potentially infectious, and specific workers handle the waste following a convey belt model, with increasingly dangerous tasks being performed by older individuals [132]. This role allocation is functional: compared to young ants, the total lifespan of old individuals is minimally affected by the danger, because the remaining life-expectancy of old ants is short regardless of what they do. A similar rationale was put forward by the Skilled Veterans Corps after the 2011 nuclear crisis at the Fukushima power station in Japan. The Corps of retired engineers and professionals volunteered to stabilize the plant, arguing that they would be less impacted by long-term health risks than younger people [133]. Here, it was age that modulated task allocation, providing an important social role to a group traditionally at risk of social devaluation. In this context, SRV theory does not propose solutions, but provides a framework for satisfying the needs and motivations of old individuals in the society they belong to. Perhaps studies of groups at risk of social devaluation and their functional roles in insect societies can stimulate ideas of role-valorization in our own communities.
(c). Inflammatory responses
Inflammation attempts to eliminate harmful agents, irritants or damaged tissue, and initiate healing after cell injury. The process is part of the innate immune response that has conserved molecular mediators in humans and insects [134,135]. Many age-related disorders in people occur together with abnormally persistent inflammation, but elevated inflammatory activity (inflammaging) is also present in normal ageing, perhaps promoting neurodegeneration, depression and loss of muscle mass [25,136]. Pro-inflammatory markers that increase in elderly (TNF-α, IL-1β, reactive oxygen species, and others) can activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor that controls a battery of genes in innate immunity, inflammation and ageing. An NF-κB inhibitor was recently tested in D. melanogaster where it extended lifespan [137]. Overall, insects provide many tools for studying links between ageing and the immune system [138].
Sleep loss elevates NF-κB in both humans and D. melanogaster [139–141]. This increase could translate sleep disturbances into systemic inflammation and increase the risk of diseases. Ageing often comes with disruption to the daily sleep-wake cycle, with as many as 50% of elderly reporting difficulties in sleeping [142,143]. At the same time, stable relationship histories such as marriage or having a partner might promote human health span by positive effects on sleep [144,145]. Nuances exist in the relationship between marriage and health, as the act of staying married correlates with lower levels of inflammatory marker activity in older men, while women tend to have higher levels of inflammation if the quality of their marriage is low [146,147]. Social network support among elderly correlates with better sleep [148], while social activities combined with exercise can improve sleep patterns in nursing home and assisted living residences [149]. In Drosophila, cockroach and many social insects, circadian behaviours like sleep can be manipulated in social experiments [150–154]. Such methods could probe for causal connections, for instance between NF-κB regulation and clock-related proteins with roles in immunity and inflammation—such as the Prxs enzymes [155,156].
Social stress correlates with higher inflammatory marker activity in young, middle-aged and old adults [26,100]. This pattern is seen after stressful episodes and during chronic social stress [157]. Social relationships with conflicts, rejection, mistrust or instability could, thereby, contribute to low-grade systemic inflammation and poor health. It is, however, challenging to test this connection because many additional factors correlate with relationship qualities, including socio-economic status, environmental risks of aggression or depression, and several health behaviours including sleep [158]. Complex connections between social factors and innate immunity are also found in insects such as the ant Harpegnathos saltator, where individuals that achieve upward social mobility become highly resistant to infection and live longer lives while those that experience social stress have increased susceptibility to infection [159,160]. Possibly, links between social status and mobility, relationship qualities, stress, innate immunity and inflammation, can lead to a ‘spiraling’ (a positive or negative reinforcement) of individual health outcomes. In the former example of H. saltator, the outcome is positive, in the latter, negative. In another example from a different social insect, the outcome is also negative: honeybee workers with lipopolysaccharide (LPS)-induced systemic inflammation experience more frequent rejection by healthy social partners [161]. Social rejection causes physical injury, innate immune activation, and mortality in honeybees. LPS is also an inflammatory inducer in mammals [162], and the mammalian LPS-response can probably be modulated by social partners [163]. Stress associated with opposite-sex contact also exists in Drosophila, where both sexes suffer reduced lifespan upon interacting with the opposite sex [41,42]. The negative impact of social interaction on lifespan is greater in males, and interestingly, this effect is also seen in genetically masculinized female flies. [164]. Perhaps these connections can be better understood by further research on insect immunity and social interactions.
(d). Nutrition
Many elderly loose appetite and develop an anorectic syndrome [165,166], but an increasing number of obese individuals become elderly and these individuals may stay overweight for the rest of their lives [167]. At the same time, the body weight that confers maximal survival is not constant over the life-course: it is higher in old people than in middle-aged adults, and this ‘obesity-mortality paradox’ causes debate about criteria for recommending weight-management strategies to elderly [168]. Also, several social insects show significant and stable weight loss among old individuals and offer many opportunities for studying social aspects of diet including food consumption and food choice [169–171]. Drosophila moreover, can provide genetic models for a number of weight-related pathologies because the pathways involved in metabolic biology are highly conserved between the species [172].
Food consumption is often reduced after age 70–75 years and leads to loss of body fat and lean muscle. People with ‘anorexia of ageing’ are at risk of pathological weight loss, malnutrition, morbidity and mortality. This syndrome is not completely understood, but dysregulation of orexigenic (appetite increasing) and anorexigenic (appetite reducing) signal peptides from brain, pancreas, adipose tissue and gastrointestinal tract is likely to play a role [165,166]. Social relationships also affect elder nutrition and eating behaviour (figure 5). These patterns are not well explained mechanistically, but appetite-controlling molecules like the anorexigenic peptide hormone leptin can be influenced by social situations [175–177]. In honeybees, abdominal adiposity, carbohydrate metabolism and gustation is linked to signalling by juvenile hormone [69,169], which is similar in structure, function and mode of action to vertebrate thyroid hormone [178]. Juvenile hormone is sensitive to social interactions, and young workers kept in social isolation develop levels identical to those of old bees. Social contact with only 2–3 other workers is sufficient to normalize the hormone level [179]. Drosophila larvae also benefit from group feeding in natural conditions, as feeding aggregates are able to dig ‘wells’ into deeper, nutrient-rich layers of decaying plant matter. Groups tend to be larger when more closely related larvae are in the local environments, and more local kin is associated with higher survival rates and greater female weights (a robust marker of lifetime fecundity) [180]. Thus, the ability for a fly to recognize and cooperate with kin within a social environment may be beneficial for overall fitness (at least in females) and may serve as a useful model for the effects of group feeding on individual physiology.
Figure 5.
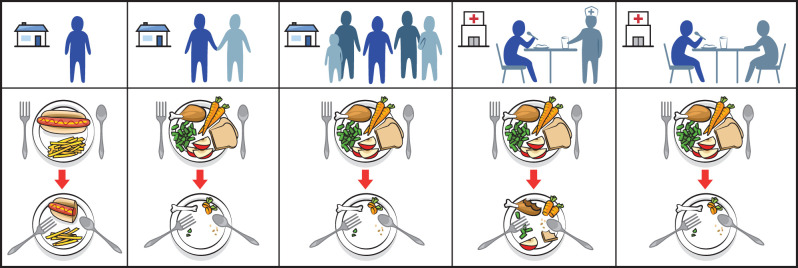
From left to right: old people who live alone or report feeling lonely consume fewer daily meals and total calories, tend to eat less protein, fruits and vegetables, and are at increased risk of being underweight and malnourished compared to those who have a partner or live in traditional family surroundings [173]. Hospitalized elderly, similarly, increase their intake of protein and total calories when a personal volunteer, rather than a professional nurse, socialize with them and assist with feeding at mealtimes [174].
Food choice is motivated by many factors including sensory appeal. Sensory capacities like taste and smell are often diminished during ageing [166,181]. Sensory changes can make familiar meals seem undesirable [182], and push elderly individuals to prefer meals with more intense flavours (i.e. salty, sweet), but which lack proper nutritional value [183]. Interestingly, research on humans and many animals show that social modelling (figure 6) can increase food acceptance for meals that are novel, different or varied [31,184]. Drosophila can use social modelling to make decisions about food [40,185], but there is surprisingly limited evidence for whether or not (having) choices is favoured by animals like insects [186].
Figure 6.
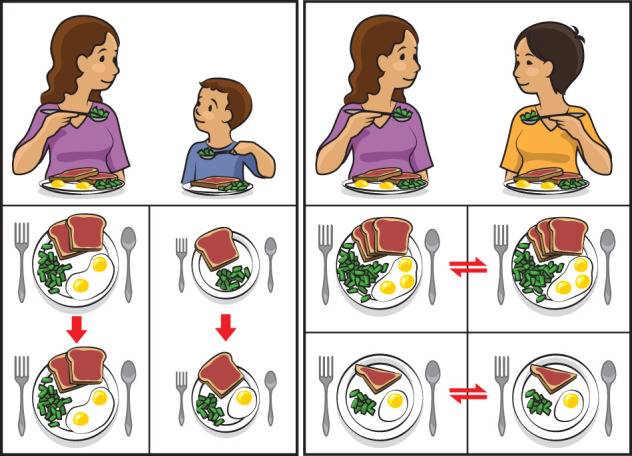
Young children accept novel foods more readily after observing their mother eating them (left panel), while adults modulate their food intake to match that of social companions (right panel). Perhaps care strategies that incorporate aspects of this social modelling can help maintain a varied and calorically sufficient diet in the elderly.
Grasshoppers can optimize development and growth if they mix their diet, but increased feeding time in mixtures suggest inefficient decision making when many options are available to animals [186,187]. Perhaps in contrast, social insects overcome the difficulty of multiple choices through collective decision making. These decisions are based on communication between animals with limited individual information. Each animal knows about one or perhaps some options, but together, the group has assessed many options and arrives at decisions together. In the ant Temnothorax rugatulus, for instance, groups handle dual versus multiple choices (eight options) with equal efficiency, while single ants are less able to make good decisions about eight options [188]. Honeybees, moreover, use collective decision making during foraging [189] but food-choice behaviour also has genetic components in the bee [190]. Genes involved in ovarian and insulin-associated pathways affect the bees' (sweet) taste and food foraging bias [191,192]. Similar relationships have been reported in humans [193,194], but it is unclear how these mechanisms respond to social contact.
Metabolic syndrome describes a group of disorders and conditions that increases the risk of cardiovascular disease, diabetes and mortality. The syndrome becomes progressively more common and poses many challenges to human quality of life, health and economy [195]. Metabolic syndrome is more prevalent in obese people [196], but even though obesity predicts higher mortality in 75–84 year-olds, it has protective effects from age 85 and onwards [168,197,198]. These results suggest that the oldest old should not be encouraged to lose weight or avoid obesity, although the obesity-mortality paradox of ageing remains an active area of study [197,199].
As core metabolic pathways regulating energy homeostasis are highly conserved, insect models have proven useful for unravelling aspects of this paradox. Drosophila can be made obese by feeding a high-sugar diet, high-fat diet, calorie restricted diet, or by genetic manipulation. Each of these manipulations acts on a different set of tissues and pathways linked to lifespan and can be used interchangeably to tease apart the effects of diet on lifespan [200]. Much of this research centres on the effects of diet on the highly conserved insulin/insulin-like growth factor (IIS). IIS reduction is associated with high-sugar and high-fat diets, similar to the diabetic phenotype in humans. However, whereas reduced insulin signalling in humans has negative health implications, its reduction in flies is associated with increased lifespan [172,200]. Here, the honeybee model may be better suited to unravel the connections between insulin signalling and lifespan regulation. Ihle et al. recently discovered that reduction in insulin signalling is associated with an accelerated behavioural transition to foraging, resulting in a reduction in total lifespan [201]. In a natural context, the worker transition to foraging is partially regulated by the needs of the colony, suggesting an interplay between social environment, metabolic pathways, and lifespan in honeybees.
4. Ecological processes
Ecology is the study of environmental systems that focus on relationships between living organisms and the natural world. Humans and many other advanced social species have unique strategies for how they interface with nature, perhaps particularly with regard to the scale by which they explore, shape, manage, culture and mine the environment for the extraction of resources (figure 1) [57,202]. From this extraction emerges economies based on the production, distribution and consumption of the resources, as well as derived goods and associated services. Economies create influential environmental systems that affect individual activities, resource availability, social status and health [203]. Ecology is often called the economy of nature, but it does not typically extend its scope to environments (like economies) that emerge from social organization. Nonetheless, we believe a focus on social systems is essential in a discussion of social impacts of/on the ecology of ageing. This is an established concept in elder care where ‘ecology of ageing’ often relates to environments that are highly organized, including neighbourhoods and other living-arrangements for ageing independently (see [188] for an overview) [204].
Never before in the history of the planet has the world contained so many elderly, or such a large fraction of them compared to young people [205]. This situation raises many questions in societies, including how economies are impacted by the elderly and how economic forces impact their lives, or ecology. It is commonly claimed that current elder care systems are unsustainable, and this concern has inspired research on potential solutions. One is ‘smart house’ (robotic) technologies for independent living that cut the costs of having actual (human) carers [206]. A different realization is that elderly represent a substantial and predictably growing consumer group [207]. National and international innovation, development and trade in healthcare services may thus become a major economic indicator in the future. If any of these solutions and predictions come through, it may profoundly change the environment of elderly.
Research on ageing in model animals is largely focused on the immediate biological and environmental causes of senescence. Such data do not provide a good basis for discussing how the economics of societies can impact the ecology of ageing. Economies of resource gathering and allocation, however, are studied in several social insect species [57,202]. Social insects use a variety of strategies for harvesting and processing natural resources that fuel colony growth, reproduction and survival. Materials and foods that are brought to the nest are often processed in one or several steps involving different worker groups, before the resulting goods are distributed to members or areas of the colony [60]. Old individuals typically have specific roles in these processing and distribution chains, defining their ecology of ageing.
Old individuals usually do risky tasks in insect societies (figure 7) [132,208–210]. This work includes foraging for food, construction materials, resins and water, as well as defensive strategies, waste handling and removal. These tasks expose the animals to wear, predators, and pathogens that are not encountered by other workers [211]. Old individuals may also be treated differently—and at least partly because of their exposures: old worker honeybees receive less social feeding than younger adults (reviewed in [194]) [210], whereas old worker ants contaminated with waste and similarly immune-compromised worker bees can be rejected by other colony members [212,213]. Such environments of risk and rejection are detrimental to lifespan [211]. However, the allocation of old, undernourished and infected individuals to this ecology is good colony economy because the animals' remaining life-expectancy is short—regardless. Social insect ‘economic policy’ is then, in short, to minimize physical costs in the young population and to balance resource use with remaining life-expectancy in the elderly.
Figure 7.

In honeybee societies, younger individuals (left panel) work in the protected nest while older individuals (right panel) take care of risky tasks like foraging. This age-associated division of labour is good colony economy. The total lifespan of old bees is less affected by the dangers of foraging because the remaining life-expectancies of the individuals are short (red arrow) regardless of the tasks they perform for the colony.
Statements like ‘protecting the young’ and ‘balance resource use’ can resonate with people. However, the ecology of ageing in social insect colonies is clearly not a useful model for the future of elder care in human societies. Most animal models come with advantages and disadvantages, and each system can only address specific questions [214]. Insect models can contribute to deciphering specific genetic, molecular and physiological mechanisms that connect social contact to ageing, while being more broadly useful in questions of how resource acquisition and allocation (economy) can impact the ecology of ageing.
5. Evolutionary processes
Species age at different time-scales [215]. Some animals and plants age rapidly, like the nematode worm Caenorhabditis elegans and the thale cress Arabidopsis thaliana that have lifespans of about 30 days. Others age slowly or even negligibly, like the rockfish Sebastes aleutianus and the bristlecone pine Pinus longaeva with lifespans of about 200 and 4500 years, respectively [215,216]. Evolutionary theories of ageing seek to explain such differences and why mortality increases with age. They predict that there is no selection for longevity beyond the period of reproductive capability (reviewed by [194]) [210]. Social species, however, can provide an exception. This is because post-reproductive individuals in social groups can gain (inclusive) fitness by helping their offspring reproduce. Lee developed a theory of ageing that accounts for helping in the form of ‘intergenerational resource transfers’ [217]. The theory linked an economic model for resource use in caring (such as feeding, warming, fanning and guarding) to predictions of age-specific mortality. Lee's formalism presents the force of selection on longevity as a weighted average of remaining fertility and remaining resource transfers (help) to be given to others, and, it shows that the remaining capacity to help at any age is what shapes senescence in social species with continuing care for offspring. This conclusion is in line with the ‘grandmother hypothesis’, which posits that grandmothers have enhanced their children's’ fitness and the survival of their grandchildren through helping [218].
Evolutionary implications of Lee's theory and the grandmother hypothesis are supported by results from pre-modern human populations, where women with prolonged post-reproductive lifespans had more grandchildren, and hence greater inclusive fitness [218]. It is, on the other hand, not demonstrated that the inclusive fitness benefits of grandmothering are large, and recently, it was suggested that the benefits of helping are not large enough to explain why women outlive their reproductive period by several decades [219]. Inclusive fitness theory has also been used to understand helping in social insects, and it is questioned whether inclusive fitness benefits are sufficient to explain that workers (sterile helpers) evolved [202]. These debates are still ongoing, and perhaps defines an area where scientists can come together to ask broader questions about the role of helping in the evolution of social life histories.
6. Concluding remarks
Geriatrics is becoming an increasingly important specialty, and research on ageing is an engaging, proactive science [220]. Although many ‘smart’ technologies are accessible to elder care, there is resistance against substituting ‘warm’ human hands with the ‘cold’ technology [221]. Our review indicates that some of this resistance can be appropriate. A number of studies reveal significant correlations between social factors, health and ageing in human populations, but it is difficult to extract specific and causal relationships from the clinical surveys. Senescence results from a cumulative imbalance between damage and repair, and insects may help reveal whether and how social processes can reduce damage and increase repair. This understanding may lead to new elder care strategies that can improve the health and quality of life for many people.
Acknowledgements
Thanks to Sabine Deviche for contributions to figures and graphic design, and to Christofer Bang for comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
T.P.Q. and G.V.A. contributed equally to this manuscript.
Competing Interests
We declare we have no competing interests.
Funding
The work was supported by the Norwegian Research Council (180504, 191699), The PEW Charitable Trust and the National Institute on Aging (PO1AG22500).
References
- 1.Lara E, Martín-María N, De la Torre-Luque A, Koyanagi A, Vancampfort D, Izquierdo A, Miret M. 2019. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res. Rev. 52, 7-16. ( 10.1016/j.arr.2019.03.002) [DOI] [PubMed] [Google Scholar]
- 2.Kotwal AA, Kim J, Waite L, Dale W. 2016. Social function and cognitive status: results from a US nationally representative survey of older adults. J. Gen. Intern. Med. 31, 854-862. ( 10.1007/s11606-016-3696-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Hu Z, Wei L, Ma Y, Liu Z, Copeland JR. 2011. Incident dementia in a defined older Chinese population. PLoS ONE 6, 1-7. ( 10.1371/journal.pone.0024817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlberg L, Mckee KJ. 2014. Correlates of social and emotional loneliness in older people: evidence from an English community study. Aging Ment. Health 18, 504-514. ( 10.1080/13607863.2013.856863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar A, Hamer M, McMunn A, Steptoe A. 2013. Social isolation and loneliness. Psychosom. Med. 75, 161-170. ( 10.1097/PSY.0b013e31827f09cd) [DOI] [PubMed] [Google Scholar]
- 6.Yang S-Y, et al. 2015. Leisure activities, apolipoprotein E e4 status, and the risk of dementia. J. Formos. Med. Assoc. 114, 1216-1224. ( 10.1016/j.jfma.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 7.Kulmala J, Nykänen I, Mänty M, Hartikainen S. 2014. Association between frailty and dementia: a population-based study. Gerontology 60, 16-21. ( 10.1159/000353859) [DOI] [PubMed] [Google Scholar]
- 8.Morris MC. 2016. Nutrition and risk of dementia: overview and methodological issues. Ann. N.Y. Acad. Sci. 1367, 31-37. ( 10.1111/nyas.13047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosso AL, Taylor JA, Tabb LP, Michael YL. 2013. Mobility, disability, and social engagement in older adults. J. Aging Health 25, 617-637. ( 10.1177/0898264313482489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin MR. 2014. Sleep and inflammation in resilient aging. Interface Focus 4, 20140009. ( 10.1098/rsfs.2014.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wysokiński A, Sobów T, Kłoszewska I, Kostka T. 2015. Mechanisms of the anorexia of aging—a review. Age (Omaha) 37, 81. ( 10.1007/s11357-015-9821-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liggins C, Pryor L, Bernard MA. 2010. Challenges and opportunities in advancing models of care for older adults: an assessment of the national institute on aging research portfolio. J. Am. Geriatr. Soc. 58, 2345-2349. ( 10.1111/j.1532-5415.2010.03157.x) [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. 2006. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 5, 406-412. ( 10.1016/S1474-4422(06)70417-3) [DOI] [PubMed] [Google Scholar]
- 14.Youm Y, Laumann EO, Ferraro KF, Waite LJ, Kim HC, Park Y-R, Chu SH, Joo W-, Lee JA. 2014. Social network properties and self-rated health in later life: comparisons from the Korean social life, health, and aging project and the national social life, health and aging project. BMC Geriatr. 14, 102. ( 10.1186/1471-2318-14-102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillai JA, Verghese J. 2009. Social networks and their role in preventing dementia. Indian J. Psychiatry 51, S22. [PMC free article] [PubMed] [Google Scholar]
- 16.Lund R, Nilsson CJ, Avlund K. 2010. Can the higher risk of disability onset among older people who live alone be alleviated by strong social relations? A longitudinal study of non-disabled men and women. Age Ageing 39, 319-326. ( 10.1093/ageing/afq020) [DOI] [PubMed] [Google Scholar]
- 17.Mushtaq R, Shoib S, Shah T, Mushtaq S. 2014. Relationship between loneliness, psychiatric disorders and physical health? A review on the psychological aspects of loneliness. J. Clin. Diagn. Res. 8, WE01-WE04. ( 10.1111/crj.12028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escobar-Bravo MÁ, Puga-González D, Martín-Baranera M. 2012. Protective effects of social networks on disability among older adults in Spain. Arch. Gerontol. Geriatr. 54, 109-116. ( 10.1016/j.archger.2011.01.008) [DOI] [PubMed] [Google Scholar]
- 19.Buchman AS, Boyle PA, Wilson RS, James BD, Leurgans SE, Arnold SE, Bennett DA. et al. 2010. Loneliness and the rate of motor decline in old age: the rush memory and aging project, a community-based cohort study. BMC Geriatr. 10, 77. ( 10.1186/1471-2318-10-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fancourt D, Steptoe A. 2018. Physical and psychosocial factors in the prevention of chronic pain in older age. J. Pain 19, 1385-1391. ( 10.1016/j.jpain.2018.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petitte T, Mallow J, Barnes E, Petrone A, Barr T, Theeke L. 2015. Physical conditions in adults. Open Psychol. J. 8, 113-132. ( 10.2174/1874350101508010113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris ZA. 2019. Loneliness as a predictor of work disability onset among nondisabled, working older adults in 14 countries. J. Aging Health 32, 554-563. ( 10.1177/0898264319836549) [DOI] [PubMed] [Google Scholar]
- 23.Schrempft S, Jackowska M, Hamer M, Steptoe A. 2019. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health 19, 1-10. ( 10.1186/s12889-019-6424-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rea IM, Marshall C, Darby J, Wei W, Lyons AB, Körner H. 2018. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 9, 1-28. ( 10.3389/fimmu.2018.00001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt KJ, Walsh BM, Voegeli D, Roberts HC. 2010. Inflammation in aging part 1: physiology and immunological mechanisms. Biol. Res. Nurs. 11, 245-252. ( 10.1177/1099800409352237) [DOI] [PubMed] [Google Scholar]
- 26.McDade TW, Hawkley LC, Cacioppo JT. 2006. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom. Med. 68, 376-381. ( 10.1097/01.psy.0000221371.43607.64) [DOI] [PubMed] [Google Scholar]
- 27.Tomfohr LM, Edwards KM, Madsen JW, Mills PJ. 2015. Social support moderates the relationship between sleep and inflammation in a population at high risk for developing cardiovascular disease. Psychophysiology 52, 1689-1697. ( 10.1111/psyp.12549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman EM. 2011. Sleep quality, social well-being, gender, and inflammation: an integrative analysis in a national sample. Ann. N.Y. Acad. Sci. 1231. 23. ( 10.1111/j.1749-6632.2011.06040.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. 2014. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J. Clin. Endocrinol. Metab. 99, 462-468. ( 10.1210/jc.2013-2832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang EJ, Lim S, Lim J-Y, Kim KW, Jang HC, Paik N-J. 2012. Association between muscle strength and metabolic syndrome in older Korean men and women: the Korean longitudinal study on health and aging. Metabolism. 61, 317-324. ( 10.1016/j.metabol.2011.07.005) [DOI] [PubMed] [Google Scholar]
- 31.Cruwys T, Bevelander KE, Hermans RCJ. 2015. Social modeling of eating: a review of when and why social influence affects food intake and choice. Appetite 86, 3-18. ( 10.1016/j.appet.2014.08.035) [DOI] [PubMed] [Google Scholar]
- 32.Hermans RCJ, Lichtwarck-Aschoff A, Bevelander KE, Herman CP, Larsen JK, Engels RCME. 2012. Mimicry of food intake: the dynamic interplay between eating companions. PLoS ONE 7, e31027. ( 10.1371/journal.pone.0031027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Castro JM, Brewer EM. 1992. The amount eaten in meals by humans is a power function of the number of people present. Physiol. Behav. 51, 121-125. ( 10.1016/0031-9384(92)90212-K) [DOI] [PubMed] [Google Scholar]
- 34.Salvy S, De K, Bowker JC, Hermans RCJ. 2012. Physiology & behavior influence of peers and friends on children's and adolescents’ eating and activity behaviors. Physiol. Behav. 106, 369-378. ( 10.1016/j.physbeh.2012.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camiletti AL, Thompson GJ. 2016. Drosophila as a genetically tractable model for social insect behavior. Front. Ecol. Evol. 4, 40. ( 10.3389/fevo.2016.00040) [DOI] [Google Scholar]
- 36.Sokolowski MB. 2010. Social interactions in ‘simple’ model systems. Neuron 65, 780-794. ( 10.1016/j.neuron.2010.03.007) [DOI] [PubMed] [Google Scholar]
- 37.Chakraborty TS, Gendron CM, Lyu Y, Munneke AS, Demarco MN, Hoisington ZW, Pletcher SD. 2019. Sensory perception of dead conspecifics induces aversive cues and modulates lifespan through serotonin in Drosophila. Nat. Commun. 10, 2365. ( 10.1038/s41467-019-10285-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saltz JB. 2016. Genetic variation in social environment construction influences the development of aggressive behavior in Drosophila melanogaster. Nat. Publ. Gr. 118, 340-347. ( 10.1038/hdy.2016.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mery F, Varela SAM, Danchin É, Blanchet S, Parejo D, Coolen I, Wagner RH. et al. 2009. Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730-734. ( 10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- 40.Sarin S, Dukas R. 2009. Social learning about egg-laying substrates in fruitflies. Proc. R. Soc. B 276, 4323-4328. ( 10.1098/rspb.2009.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leech T, Sait SM, Bretman A. 2017. Sex-specific effects of social isolation on ageing in Drosophila melanogaster. J. Insect Physiol. 102, 12-17. ( 10.1016/j.jinsphys.2017.08.008) [DOI] [PubMed] [Google Scholar]
- 42.Leech T, Evison SEF, Armitage SAO, Sait SM, Bretman A. 2019. Interactive effects of social environment, age and sex on immune responses in Drosophila melanogaster. J. Evol. Biol. 32, 1082-1092. ( 10.1111/jeb.13509) [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, et al. 2014. A Drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell 159, 200-214. ( 10.1016/j.cell.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGurk L, Berson A, Bonini NM. 2015. Drosophila as an in vivo model for human neurodegenerative disease. Genetics 201, 377-402. ( 10.1534/genetics.115.179457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prüßing K, Voigt A, Schulz JB. 2013. Drosophila melanogaster as a model organism for Alzheimer's disease. Molecular Neurodegeneration 8, 35. ( 10.1186/1750-1326-8-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu B, Vogel H. 2009. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. Mech. Dis. 4, 315-342. ( 10.1146/annurev.pathol.3.121806.151529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun M, Chen L. 2015. Studying tauopathies in Drosophila: a fruitful model. Exp. Neurol. 274, 52-57. ( 10.1016/j.expneurol.2015.03.029) [DOI] [PubMed] [Google Scholar]
- 48.Navarro JA, Heßner S, Yenisetti SC, Bayersdorfer F, Zhang L, Voigt A, Schneuwly S, Botella JA. 2014. Analysis of dopaminergic neuronal dysfunction in genetic and toxin-induced models of Parkinson's disease in Drosophila. J. Neurochem. 131, 369-382. ( 10.1111/jnc.12818) [DOI] [PubMed] [Google Scholar]
- 49.Ruland C, Berlandi J, Eikmeier K, Weinert T, Lin FJ, Ambree O, Seggewiss J, Paulus W, Jeibmann A. 2018. Decreased cerebral Irp-1B limits impact of social isolation in wild type and Alzheimer's disease modeled in Drosophila melanogaster. Genes, Brain Behav. 17, 1-9. ( 10.1111/gbb.12451) [DOI] [PubMed] [Google Scholar]
- 50.Schoenfeld BP, et al. 2013. The Drosophila DmGluRA is required for social interaction and memory. Front. Pharmacol. 4, 1-10. ( 10.3389/fphar.2013.00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Z, Guo X, Lei H, Li T, Hao S, Kang L. 2015. Octopamine and tyramine respectively regulate attractive and repulsive behavior in locust phase changes. Sci. Rep. 5, 1-11. ( 10.1038/srep08036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ernst UR, Van Hiel MB, Depuydt G, Boerjan B, De Loof A, Schoofs L. 2015. Epigenetics and locust life phase transitions. J. Exp. Biol. 218, 88-99. ( 10.1242/jeb.107078) [DOI] [PubMed] [Google Scholar]
- 53.Ambeskovic M, Roseboom TJ, Metz GAS. 2017. Transgenerational effects of early environmental insults on aging and disease incidence. Neurosci. Biobehav. Rev. 117, 297-316. ( 10.1016/j.neubiorev.2017.08.002) [DOI] [PubMed] [Google Scholar]
- 54.Sugahara R, Saeki S, Jouraku A, Shiotsuki T, Tanaka S. 2015. Knockdown of the corazonin gene reveals its critical role in the control of gregarious characteristics in the desert locust. J. Insect Physiol. 79, 80-87. ( 10.1016/j.jinsphys.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 55.Badisco L, et al. 2011. Microarray-based transcriptomic analysis of differences between long-term gregarious and solitarious desert locusts. PLoS ONE 6, e28110. ( 10.1371/journal.pone.0028110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler W. 1928. The social insects. London, UK: Harcourt, Brace & Co. [Google Scholar]
- 57.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 58.Hölldobler B. 1976. Tournaments and slavery in a desert ant. Science 192, 912-914. ( 10.1126/science.192.4242.912) [DOI] [PubMed] [Google Scholar]
- 59.Simpson J, von Frisch K. 1969. The dance language and orientation of bees. J. Anim. Ecol. 38, 460. ( 10.2307/2785) [DOI] [Google Scholar]
- 60.Seeley TD. 1982. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 11, 287-293. ( 10.1007/BF00299306) [DOI] [Google Scholar]
- 61.Quigley TP, Amdam GV, Rueppell O. 2018. Honeybee workers as models of aging. In Conn's handbook of models for human aging (ed. PM Conn), pp. 533-547. Burlington, MA: Elsevier Inc. [Google Scholar]
- 62.Munch D, Baker N, Rasmussen EMK, Shah AK, Kreibich CD, Heidem LE. 2013. Obtaining specimens with slowed, accelerated and reversed aging in the honey bee model. J. Vis. Exp. 78, e50550. ( 10.3791/50550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheiner R, et al. 2013. Standard methods for behavioural studies of Apis mellifera. J. Apic. Res. 52, 1-58. ( 10.3896/IBRA.1.52.4.04) [DOI] [Google Scholar]
- 64.Gadagkar R. 2011. The birth of ant genomics. Proc. Natl Acad. Sci. USA 108, 5477-5478. ( 10.1073/pnas.1100765108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trible W, Olivos-Cisneros L, Mckenzie SK, Saragosti J, Chang N-C, Matthews BJ, Oxley PR, Kronauer DJC. 2017. orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727-735.e10. ( 10.1016/j.cell.2017.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roth A, Vleurinck C, Netschitailo O, Bauer V, Otte M, Kaftanoglu O, Page RE, Beye M. 2019. A genetic switch for worker nutrition-mediated traits in honeybees. PLoS Biol. 17, e3000171. ( 10.1371/journal.pbio.3000171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amdam GV, Simões ZL, Guidugli KR, Norberg K, Omholt SW. 2003. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnol. Jan 203, 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel A, Fondrk MK, Kaftanoglu O, Emore C, Hunt G, Frederick K, Amdam GV. 2007. The making of a queen: tor pathway is a key player in diphenic caste development. PLoS ONE 2, 1-7. ( 10.1371/journal.pone.0000509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Brent CS, Fennern E, Amdam GV. 2012. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 8, e1002779. ( 10.1371/journal.pgen.1002779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu HL, Vinson SB, Pietrantonio PV. 2009. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 276, 3110-3123. ( 10.1111/j.1742-4658.2009.07029.x) [DOI] [PubMed] [Google Scholar]
- 71.Ratzka C, Gross R, Feldhaar H. 2013. Systemic gene knockdown in Camponotus floridanus workers by feeding of dsRNA. Insectes Soc. 60, 475-484. ( 10.1007/s00040-013-0314-6) [DOI] [Google Scholar]
- 72.Hunt JH, Mutti NS, Havukainen H, Henshaw MT, Amdam GV. 2011. Development of an RNA interference tool, characterization of its target, and an ecological test of caste differentiation in the eusocial wasp Polistes. PLoS ONE 6, e26641. ( 10.1371/journal.pone.0026641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dyer AG. 2012. The mysterious cognitive abilities of bees: why models of visual processing need to consider experience and individual differences in animal performance. J. Exp. Biol. 215, 387-395. ( 10.1242/jeb.038190) [DOI] [PubMed] [Google Scholar]
- 74.Young JM, Wessnitzer J, Armstrong JD, Webb B. 2011. Elemental and non-elemental olfactory learning in Drosophila. Neurobiol. Learn. Mem. 96, 339-352. ( 10.1016/j.nlm.2011.06.009) [DOI] [PubMed] [Google Scholar]
- 75.Giurfa M. 2015. Learning and cognition in insects. Wiley Interdiscip. Rev. Cogn. Sci. 6, 383-395. ( 10.1002/wcs.1348) [DOI] [PubMed] [Google Scholar]
- 76.Srinivasan MV. 2010. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 203, 1963-1986. ( 10.1146/annurev.ento.010908.164537) [DOI] [PubMed] [Google Scholar]
- 77.Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG. 2018. Numerical ordering of zero in honey bees. Science 360, 1124-1126. ( 10.1126/science.aar4975) [DOI] [PubMed] [Google Scholar]
- 78.Avarguès-Weber A, Dyer AG, Combe M, Giurfa M. 2012. Simultaneous mastering of two abstract concepts by the miniature brain of bees. Proc. Natl Acad. Sci. USA 109, 7481-7486. ( 10.1073/pnas.1202576109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strausfeld NJ, Hirth F. 2013. Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157-161. ( 10.1126/science.1231828) [DOI] [PubMed] [Google Scholar]
- 80.Sweeney LB, Luo L. 2010. Fore brain: a hint of the ancestral cortex. Cell 142, 679-681. ( 10.1016/j.cell.2010.08.024) [DOI] [PubMed] [Google Scholar]
- 81.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800-809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 82.Sundström A, Adolfsson AN, Nordin M, Adolfsson R. 2020. Loneliness increases the risk of all-cause dementia and Alzheimer's disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 75, 919-926. ( 10.1093/geronb/gbz139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, Livingston G. 2019. Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Med. 16, 1-18. ( 10.1371/journal.pmed.1002862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myhre JW, Mehl MR, Glisky EL. 2017. Cognitive benefits of online social networking for healthy older adults. J. Gerontol. - Ser. B Psychol. Sci. Soc. Sci. 72, 752-760. ( 10.1093/geronb/gbw025) [DOI] [PubMed] [Google Scholar]
- 85.Evans IEM, Martyr A, Collins R, Brayne C, Clare L. 2019. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J. Alzheimer's Dis. 70, S119-S144. ( 10.3233/JAD-180501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munch D, Kreibich CD, Amdam GV. 2013. Aging and its modulation in a long-lived worker caste of the honey bee. J. Exp. Biol. 216, 1638-1649. ( 10.1242/jeb.078915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker N, Wolschin F, Amdam GV. 2012. Age-related learning deficits can be reversible in honeybees Apis mellifera. Exp. Gerontol. 47, 764-772. ( 10.1016/j.exger.2012.05.011) [DOI] [PubMed] [Google Scholar]
- 88.Tibbetts EA. 2002. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B 269, 1423-1428. ( 10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tibbetts EA, Desjardins E, Kou N, Wellman L. 2019. Social isolation prevents the development of individual face recognition in paper wasps. Anim. Behav. 152, 71-77. ( 10.1016/j.anbehav.2019.04.009) [DOI] [Google Scholar]
- 90.Boulay R, Lenoir A. 2001. Social isolation of mature workers affects nestmate recognition in the ant Camponotus fellah. Behav. Processes 55, 67-73. ( 10.1016/S0376-6357(01)00163-2) [DOI] [PubMed] [Google Scholar]
- 91.Lenoir A, Cuisset D, Hefetz A. 2001. Effects of social isolation on hydrocarbon pattern and nestmate recognition in the ant Aphaenogaster senilis (Hymenoptera, Formicidae). Insectes Soc. 48, 101-109. ( 10.1007/PL00001751) [DOI] [Google Scholar]
- 92.Boulay R. 1999. Social isolation in ants: evidence of its impact on survivorship and behavior in Camponotus fellah (Hymenoptera, Formicidae). Sociobiology 33, 111-124. [Google Scholar]
- 93.Seid MA, Junge E. 2016. Social isolation and brain development in the ant Camponotus floridanus. Sci. Nat. 103, 10-15. ( 10.1007/s00114-016-1335-6) [DOI] [PubMed] [Google Scholar]
- 94.Stephenson J, Nutma E, van der Valk P, Amor S. 2018. Inflammation in CNS neurodegenerative diseases. Immunology 154, 204-219. ( 10.1111/imm.12922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boccardi V, Pelini L, Ercolani S, Ruggiero C, Mecocci P. 2015. From cellular senescence to Alzheimer's disease: the role of telomere shortening. Ageing Res. Rev. 22, 1-8. ( 10.1016/j.arr.2015.04.003) [DOI] [PubMed] [Google Scholar]
- 96.Seid MA, Traniello JFA. 2006. Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav. Ecol. Sociobiol. 60, 631-644. ( 10.1007/s00265-006-0207-z) [DOI] [Google Scholar]
- 97.Giraldo YM, et al. 2016. Lifespan behavioural and neural resilience in a social insect. Proc. R. Soc. B 283, 20152603. ( 10.1098/rspb.2015.2603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang D, et al. 2013. B2 adrenergic receptor, protein kinase A (PKA) and c-Jun N-terminal Kinase (JNK) signaling pathways mediate tau pathology in Alzheimer disease models. J. Biol. Chem. 288, 10 298-10 307. ( 10.1074/jbc.M112.415141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamazaki D, Horiuchi J, Miyashita T, Saitoe M. 2010. Acute inhibition of PKA activity at old ages ameliorates age-related memory impairment in Drosophila. J. Neurosci. 30, 15573-15577. ( 10.1523/JNEUROSCI.3229-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L, Shi L, Yu J, Zhang Y. 2016. Activation of protein kinase A in the amygdala modulates anxiety-like behaviors in social defeat exposed mice. Mol. Brain 9, 1-13. ( 10.1186/s13041-015-0181-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji L, Chauhan V, Flory MJ, Chauhan A. 2011. Brain region-specific decrease in the activity and expression of protein kinase a in the frontal cortex of regressive autism. PLoS ONE 6, e23751. ( 10.1371/journal.pone.0023751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ott SR, Verlinden H, Rogers SM, Brighton CH, Quah PS, Vleugels RK, Verdonck R, Vanden Broeck J. 2012. Critical role for protein kinase A in the acquisition of gregarious behavior in the desert locust. Proc. Natl Acad. Sci. USA 109, E381-E387. ( 10.1073/pnas.1114990109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Humphries MA, Müller U, Fondrk MK, Page RE. 2003. PKA and PKC content in the honey bee central brain differs in genotypic strains with distinct foraging behavior. J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol. 189, 555-562. ( 10.1007/s00359-003-0433-z) [DOI] [PubMed] [Google Scholar]
- 104.Kim IK, Lee KJ, Rhee S, Seo SB, Pak JH. 2013. Protective effects of peroxiredoxin 6 overexpression on amyloid β-induced apoptosis in PC12 cells. Free Radic. Res. 47, 836-846. ( 10.3109/10715762.2013.833330) [DOI] [PubMed] [Google Scholar]
- 105.Kim B, Park J, Chang K, Lee D. 2016. Free radical biology and medicine peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK – Drp1-mediated mitochondrial fragmentation. Free Radic. Biol. Med. 90, 184-194. ( 10.1016/j.freeradbiomed.2015.11.015) [DOI] [PubMed] [Google Scholar]
- 106.Ruszkiewicz J, Albrecht J. 2015. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem. Int. 88, 66-72. ( 10.1016/j.neuint.2014.12.012) [DOI] [PubMed] [Google Scholar]
- 107.Pitts A, Dailey K, Newington JT, Chien A, Arseneault R, Cann T, Thompson LM, Cumming RC. 2012. Dithiol-based compounds maintain expression of antioxidant protein peroxiredoxin 1 that counteracts toxicity of mutant huntingtin. J. Biol. Chem. 287, 22 717-22 729. ( 10.1074/jbc.M111.334565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amdam GV. 2011. Social context, stress, and plasticity of aging. Aging Cell 10, 18-27. ( 10.1111/j.1474-9726.2010.00647.x) [DOI] [PubMed] [Google Scholar]
- 109.Edgar RS, et al. 2012. Peroxiredoxins are conserved markers of circadian rhythms. Nature 485, 459-464. ( 10.1038/nature11088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roenneberg T, Merrow M. 2016. The circadian clock and human health. Curr. Biol. 26, R432-R443. ( 10.1016/j.cub.2016.04.011) [DOI] [PubMed] [Google Scholar]
- 111.Bloch G, Herzog ED, Levine JD, Schwartz WJ. 2013. Socially synchronized circadian oscillators. Proc. R. Soc. B 280, 20130035. ( 10.1098/rspb.2013.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takahashi K, Foster JB, Lin C-LG. 2015. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell. Mol. Life Sci. 72, 3489-3506. ( 10.1007/s00018-015-1937-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, Ramírez MJ. 2012. Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology 62, 1944-1953. ( 10.1016/j.neuropharm.2011.12.019) [DOI] [PubMed] [Google Scholar]
- 114.Hui J, Feng G, Zheng C, Jin H, Jia N. 2017. Maternal separation exacerbates Alzheimer's disease-like behavioral and pathological changes in adult APPswe/PS1dE9 mice. Behav. Brain Res. 318, 18-23. ( 10.1016/j.bbr.2016.10.030) [DOI] [PubMed] [Google Scholar]
- 115.Martisova E, Aisa B, Guerenu G, Javier Ramirez M. 2013. Effects of early maternal separation on biobehavioral and neuropathological markers of Alzheimer's disease in adult male rats. Curr. Alzheimer Res. 10, 420-432. ( 10.2174/1567205011310040007) [DOI] [PubMed] [Google Scholar]
- 116.Wihlborg A, Englund M, Åkesson K, Gerdhem P. 2015. Fracture predictive ability of physical performance tests and history of falls in elderly women: a 10-year prospective study. Osteoporos. Int. 26, 2101-2109. ( 10.1007/s00198-015-3106-1) [DOI] [PubMed] [Google Scholar]
- 117.da Cruz DMC, Lima TDC, Nock LJ, Figueiredo MDO, Paulisso DC. 2017. Relationships between falls, age, independence, balance, physical activity, and upper limb function in elderly Brazilians. Cogent. Med. 4, 1-11. ( 10.1080/2331205X.2017.1328030) [DOI] [Google Scholar]
- 118.Gaitanidis A, Dimitriadou A, Dowse H, Sanyal S, Duch C, Consoulas C. 2019. Longitudinal assessment of health-span and pre-death morbidity in wild type Drosophila. Aging (Albany NY) 11, 1850-1873. ( 10.18632/aging.101880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shubert TE. 2011. Evidence-based exercise prescription for balance and falls prevention: a current review of the literature. J. Geriatr. Phys. Ther. 34, 100-108. ( 10.1519/JPT.0b013e31822938ac) [DOI] [PubMed] [Google Scholar]
- 120.Carey JR, Papadopoulos N, Kouloussis N, Katsoyannos B, Muller H, Wang J, Tseng Y. 2006. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol. 41, 93-97. ( 10.1016/j.exger.2005.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Groot C, Hooghiemstra AM, Raijmakers PGHM, Van Berckel BNM, Scheltens P, Scherder EJA, Van Der Flier WM, Ossenkoppele R. 2016. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res. Rev. 25, 13-23. ( 10.1016/j.arr.2015.11.005) [DOI] [PubMed] [Google Scholar]
- 122.Sumic A, Michael YL, Carlson NE, Howieson DB, Kaye JA. 2007. Physical activity and the risk of dementia in oldest old. J. Aging Health 19, 242-259. ( 10.1177/0898264307299299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gajewski PD, Falkenstein M. 2016. Physical activity and neurocognitive functioning in aging - a condensed updated review. Eur. Rev. Aging Phys. Act. 13, 1-7. ( 10.1186/s11556-016-0161-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seeman TE, Berkman LF, Charpentier PA, Blazer DG, Albert MS, Tinetti ME. 1995. Behavioral and psychosocial predictors of physical performance: MacArthur studies of successful aging. J. Gerontol. - Ser. A Biol. Sci. Med. Sci. 50, M177-M183. ( 10.1093/gerona/50A.4.M177) [DOI] [PubMed] [Google Scholar]
- 125.Gabriele JM, Walker MS, Gill DL, Harber KD, Fisher EB. 2005. Differentiated roles of social encouragement and social constraint on physical activity behavior. Ann. Behav. Med. 29, 210-215. ( 10.1207/s15324796abm2903_7) [DOI] [PubMed] [Google Scholar]
- 126.Ruan H, Wu CF. 2008. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proc. Natl Acad. Sci. USA 105, 7506-7510. ( 10.1073/pnas.0711127105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greenblum J. 1984. Age and capacity devaluation: a replication. Soc. Sci. Med. 19, 1181-1187. ( 10.1016/0277-9536(84)90368-X) [DOI] [PubMed] [Google Scholar]
- 128.Jääskeläinen A, Kausto J, Seitsamo J, Ojajarvi A, Nygård CH, Arjas E, Leino-Arjas P. 2016. Work ability index and perceived work ability as predictors of disability pension: a prospective study among Finnish municipal employees. Scand. J. Work. Environ. Heal. 42, 490-499. ( 10.5271/sjweh.3598) [DOI] [PubMed] [Google Scholar]
- 129.Tsai YF. 2006. Self-care management and risk factors for depressive symptoms among elderly nursing home residents in Taiwan. J. Pain Symptom Manage. 32, 140-147. ( 10.1016/j.jpainsymman.2006.02.008) [DOI] [PubMed] [Google Scholar]
- 130.Kaarna J. 2016. Association of physical fitness on self-perceived work ability on working aged people. Masters thesis. See https://jyx.jyu.fi/handle/123456789/52142.
- 131.Schofield RMS, Emmett KD, Niedbala JC, Nesson MH. 2011. Leaf-cutter ants with worn mandibles cut half as fast, spend twice the energy, and tend to carry instead of cut. Behav. Ecol. Sociobiol. 65, 969-982. ( 10.1007/s00265-010-1098-6) [DOI] [Google Scholar]
- 132.Bot ANM, Currie CR, Hart AG, Boomsma JJ. 2001. Waste management in leaf-cutting ants. Ethol. Ecol. Evol. 13, 225-237. ( 10.1080/08927014.2001.9522772) [DOI] [Google Scholar]
- 133.Buerk R. 2011. Japan pensioners volunteer to tackle nuclear crisis. 31 May 2011, BBC News, Tokyo. See https://www.bbc.com/news/world-aisa-pacific-13598607.
- 134.Stokes BA, Yadav S, Shokal U, Smith LC, Eleftherianos I. 2015. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front. Microbiol. 6, 1-12. ( 10.3389/fmicb.2015.00019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lich JD, Ting JPY. 2007. CATERPILLER (NLR) family members as positive and negative regulators of inflammatory responses. Proc. Am. Thorac. Soc. 4, 263-266. ( 10.1513/pats.200701-022AW) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fulop T, Witkowski JM, Olivieri F, Larbi A. 2018. The integration of inflammaging in age-related diseases. Semin. Immunol. 40, 17-35. ( 10.1016/j.smim.2018.09.003) [DOI] [PubMed] [Google Scholar]
- 137.Moskalev A, Shaposhnikov M. 2011. Pharmacological inhibition of NF-κB prolongs lifespan of Drosophila melanogaster. Aging (Albany NY) 3, 391. ( 10.18632/aging.100314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramsden S, Cheung YY, Seroude L. 2008. Functional analysis of the Drosophila immune response during aging. Aging Cell 7, 225-236. ( 10.1111/j.1474-9726.2008.00370.x) [DOI] [PubMed] [Google Scholar]
- 139.Aho V, et al. 2013. Partial sleep restriction activates immune response-related gene expression pathways: experimental and epidemiological studies in humans. PLoS ONE 8, e77184. ( 10.1371/journal.pone.0077184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jones SV, Kounatidis I. 2017. Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 8, 201701805 ( 10.3389/fimmu.2017.01805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. 2007. Interaction between sleep and the immune response in Drosophila: a role for the NFκB relish. Sleep 30, 389-400. ( 10.1093/sleep/30.4.389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mattis J, Sehgal A. 2016. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metab. 27, 192-203. ( 10.1016/j.tem.2016.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Crowley K. 2011. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 21, 41-53. ( 10.1007/s11065-010-9154-6) [DOI] [PubMed] [Google Scholar]
- 144.Meadows R, Arber S. 2015. Marital status, relationship distress, and self-rated health. J. Health Soc. Behav. 56, 341-355. ( 10.1177/0022146515593948) [DOI] [PubMed] [Google Scholar]
- 145.Chen JH, Waite LJ, Lauderdale DS. 2015. Marriage, relationship quality, and sleep among U.S. older adults. J. Health Soc. Behav. 56, 356-377. ( 10.1177/0022146515594631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sbarra DA. 2009. Marriage protects men from clinically meaningful elevations in c-reactive protein: results from the national social life, health, and aging project (NSHAP). Psychosom. Med. 71, 828. ( 10.1097/PSY.0b013e3181b4c4f2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donoho CJ, Crimmins EM, Seeman TE. 2013. Marital quality, gender, and markers of inflammation in the MIDUS cohort. J. Marriage Fam. 75, 127-141. ( 10.1111/j.1741-3737.2012.01023.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kent RG, Uchino BN, Cribbet MR, Bowen K, Smith TW. 2015. Social relationships and sleep quality. Ann. Behav. Med. 49, 912-917. ( 10.1007/s12160-015-9711-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Richards KC, et al. 2011. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: randomized controlled trial. J. Am. Geriatr. Soc. 58, 214-223. ( 10.1111/j.1532-5415.2010.03246.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fuchikawa T, Eban-Rothschild A, Nagari M, Shemesh Y, Bloch G. 2016. Potent social synchronization can override photic entrainment of circadian rhythms. Nat. Commun. 7, 1-10. ( 10.1038/ncomms11662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eban-Rothschild A, Bloch G. 2012. Social influences on circadian rhythms and sleep in insects. Adv. Genetics 77, 1-32. [DOI] [PubMed] [Google Scholar]
- 152.Dubowy C, Sehgal A. 2017. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205, 1373-1397. ( 10.1534/genetics.115.185157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lone SR, Sharma VK. 2011. Timekeeping through social contacts: social synchronization of circadian locomotor activity rhythm in the carpenter ant Camponotus paria. Chronobiol. Int. 28, 862-872. ( 10.3109/07420528.2011.622676) [DOI] [PubMed] [Google Scholar]
- 154.Knadler JJ, Page TL. 2009. Social interactions and the circadian rhythm in locomotor activity in the cockroach Leucophaea maderae. Chronobiol. Int. 26, 415-429. ( 10.1080/07420520902876634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klichko VI, Orr WC, Radyuk SN. 2016. The role of peroxiredoxin 4 in inflammatory response and aging. Biochim. Biophys. Acta - Mol. Basis Dis. 1862, 265-273. ( 10.1016/j.bbadis.2015.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Knoops B, Argyropoulou V, Becker S, Ferté L, Kuznetsova O. 2016. Multiple roles of peroxiredoxins in inflammation. Mol. Cells 39, 60-64. ( 10.14348/molcells.2016.2341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain. Behav. Immun. 64, 208-219. ( 10.1016/j.bbi.2017.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller GE, Rohleder N, Cole SW. 2009. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom. Med. 72, 57. ( 10.1097/PSY.0b013e318190d7de) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schneider SA, Schrader C, Wagner AE, Boesch-Saadatmandi C, Liebig J, Rimbach G, Roeder T. 2011. Stress resistance and longevity are not directly linked to levels of enzymatic antioxidants in the ponerine ant Harpegnathos saltator. PLoS ONE 6, e14601. ( 10.1371/journal.pone.0014601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schneider SA, Marchetti L, Parlanti P, Landi S, Tonazzini I, Cecchini M, Piazza V, Gemmi M. 2016. Social stress increases the susceptibility to infection in the ant Harpegnathos saltator. Sci. Rep. 6, 1-7. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Richard FJ, Aubert A, Grozinger CM. 2008. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 6, 50. ( 10.1186/1741-7007-6-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Forn-Cuní G, Varela M, Pereiro P, Novoa B, Figueras A. 2017. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci. Rep. 7, 1-9. ( 10.1038/srep41905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR. 2017. In Sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology 42, 242-253. ( 10.1038/npp.2016.141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flintham EO, Yoshida T, Smith S, Pavlou HJ, Goodwin SF, Carazo P, Wigby S. 2018. Interactions between the sexual identity of the nervous system and the social environment mediate lifespan in Drosophila melanogaster. Proc. R. Soc. B 285, 20181450. ( 10.1098/rspb.2018.1450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moss C, Dhillo WS, Frost G, Hickson M. 2012. Gastrointestinal hormones: the regulation of appetite and the anorexia of ageing. J. Hum. Nutr. Diet 25, 3-15. ( 10.1111/j.1365-277X.2011.01211.x) [DOI] [PubMed] [Google Scholar]
- 166.Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, Sisto A, Marzetti E. 2016. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients 8, 69. ( 10.3390/nu8020069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cetin DC, Nasr G. 2014. Obesity in the elderly: more complicated than you think. Cleve. Clin. J. Med. 81, 51-61. ( 10.3949/ccjm.81a.12165) [DOI] [PubMed] [Google Scholar]
- 168.dos Santos RR, Bicalho MAC, Mota P, de Oliveira DR, de Moraes EN. 2013. Obesity in the elderly. Rev. Médica Minas Gerais 23, 64-73. ( 10.5935/2238-3182.20130011) [DOI] [Google Scholar]
- 169.Ament SA, Chan QW, Wheeler MM, Nixon SE, Johnson SP, Rodriguez-Zas SL, Foster LJ, Robinson GE. 2011. Mechanisms of stable lipid loss in a social insect. J. Exp. Biol. 214, 3808-3821. ( 10.1242/jeb.060244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Paoli PP, Donley D, Stabler D, Saseendranath A, Nicolson SW, Simpson SJ, Wright GA. 2014. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 46, 1449-1458. ( 10.1007/s00726-014-1706-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pankiw T, Nelson M, Page RE, Fondrk MK. 2004. The communal crop: modulation of sucrose response thresholds of pre-foraging honey bees with incoming nectar quality. Behav. Ecol. Sociobiol. 55, 286-292. ( 10.1007/s00265-003-0714-0) [DOI] [Google Scholar]
- 172.Gáliková M, Klepsatel P. 2018. Obesity and aging in the Drosophila model. Int. J. Mol. Sci. 19, 1896. ( 10.3390/ijms19071896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ramic E, Pranjic N, Batic-Mujanovic O, Karic E, Alibasic E, Alic A. 2011. The effect of loneliness on malnutrition in elderly population. Med. Arh. 65, 92. [PubMed] [Google Scholar]
- 174.Young AM, Mudge AM, Banks MD, Ross LJ, Daniels L. 2013. Encouraging, assisting and time to EAT: improved nutritional intake for older medical patients receiving protected mealtimes and/or additional nursing feeding assistance Q. Clin. Nutr. 32, 543-549. ( 10.1016/j.clnu.2012.11.009) [DOI] [PubMed] [Google Scholar]
- 175.Jaremka LM, Belury MA, Andridge RR, Malarkey WB, Glaser R, Christian L, Emery CF, Kiecolt-Glaser JK. 2014. Interpersonal stressors predict ghrelin and leptin levels in women. Psychoneuroendocrinology 48, 178-188. ( 10.1016/j.psyneuen.2014.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kohlboeck G, et al. 2014. Peer problems are associated with elevated serum leptin levels in children. Psychol. Med. 44, 255-265. ( 10.1017/S003329171300069X) [DOI] [PubMed] [Google Scholar]
- 177.Häfner S, Zierer A, Emeny RT, Thorand B, Herder C, Koenig W, Rupprecht R, Ladwig KH. 2011. Social isolation and depressed mood are associated with elevated serum leptin levels in men but not in women. Psychoneuroendocrinology 36, 200-209. ( 10.1016/j.psyneuen.2010.07.009) [DOI] [PubMed] [Google Scholar]
- 178.Flatt T, Tu MP, Tatar M. 2005. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 27, 999-1010. ( 10.1002/bies.20290) [DOI] [PubMed] [Google Scholar]
- 179.Huang ZY, Robinson GE. 1992. Honeybee colony integration: worker-worker interactions mediate hormonally regulated plasticity in division of labor. Proc. Natl Acad. Sci. USA 89, 11 726-11 729. ( 10.1073/pnas.89.24.11726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khodaei L, Long TAF. 2019. Kin recognition and co-operative foraging in Drosophila melanogaster larvae. J. Evol. Biol. 32, 1352-1361. ( 10.1111/jeb.13531) [DOI] [PubMed] [Google Scholar]
- 181.Bhattacharyya N, Kepnes LJ. 2015. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope 125, 1102-1106. ( 10.1002/lary.24999) [DOI] [PubMed] [Google Scholar]
- 182.Sergi G, Bano G, Pizzato S, Veronese N, Manzato E. 2017. Taste loss in the elderly: possible implications for dietary habits. Crit. Rev. Food Sci. Nutr. 57, 3684-3689. ( 10.1080/10408398.2016.1160208) [DOI] [PubMed] [Google Scholar]
- 183.Baugreet S, Hamill RM, Kerry JP, McCarthy SN. 2017. Mitigating nutrition and health deficiencies in older adults: a role for food innovation? J. Food Sci. 82, 848-855. ( 10.1111/1750-3841.13674) [DOI] [PubMed] [Google Scholar]
- 184.Birch LL. 1989. Effects of experience on the modification of food acceptance patterns. Ann. N. Y. Acad. Sci. 561, 209-216. ( 10.1111/j.1749-6632.1989.tb20983.x) [DOI] [PubMed] [Google Scholar]
- 185.Lihoreau M, Clarke IM, Buhl J, Sumpter DJT, Simpson SJ. 2016. Collective selection of food patches in Drosophila. J. Exp. Biol. 219, 668-675. ( 10.1242/jeb.127431) [DOI] [PubMed] [Google Scholar]
- 186.Hutchinson JMC. 2005. Is more choice always desirable? Evidence and arguments from leks, food selection, and environmental enrichment. Biol. Rev. Camb. Phil. Soc. 80, 73-92. ( 10.1017/S1464793104006554) [DOI] [PubMed] [Google Scholar]
- 187.Bernays EA, Bright KL. 2001. Food choice causes interrupted feeding in the generalist grasshopper Schistocerca americana: further evidence for inefficient decision-making. J. Insect Physiol. 47, 63-71. ( 10.1016/S0022-1910(00)00090-1) [DOI] [PubMed] [Google Scholar]
- 188.Sasaki T, Granovskiy B, Mann RP, Sumpter DJT, Pratt SC. 2013. Ant colonies outperform individuals when a sensory discrimination task is difficult but not when it is easy. Proc. Natl Acad. Sci. USA 110, 13 769-13 773. ( 10.1073/pnas.1304917110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Seeley TD, Camazine S, Sneyd J. 1991. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 28, 277-290. ( 10.1007/BF00175101) [DOI] [Google Scholar]
- 190.Page RE, Fondrk MK. 1995. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behav. Ecol. Sociobiol. 36, 135-144, ( 10.1007/BF00170718) [DOI] [Google Scholar]
- 191.Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. 2007. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5, 0673-0677. ( 10.1371/journal.pbio.0050062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y, Mutti NS, Ihle KE, Siegel A, Dolezal AG, Kaftanoglu O, Amdam GV. 2010. Down-regulation of honey bee IRS gene biases behavior toward food rich in protein. PLoS Genet. 38, 63-70. ( 10.1371/journal.pgen.1000896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Loper HB, La Sala M, Dotson C, Steinle N. 2015. Taste perception, associated hormonal modulation, and nutrient intake. Nutr. Rev. 73, 83-91. ( 10.1093/nutrit/nuu009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Belzer LM, Smulian JC, Lu SE, Tepper BJ. 2009. Changes in sweet taste across pregnancy in mild gestational diabetes mellitus: relationship to endocrine factors. Chem. Senses 34, 595-605. ( 10.1093/chemse/bjp041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Finucane MM, et al. 2011. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 377, 557-567. ( 10.1016/S0140-6736(10)62037-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Emanuela F, Grazia M, Marco DR, Maria Paola L, Giorgio F, Marco B. 2012. Inflammation as a link between obesity and metabolic syndrome. J. Nutr. Metabolism 2012, 1-7. ( 10.1155/2012/476380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Canning KL, Brown RE, Jamnik VK, Kuk JL. 2014. Relationship between obesity and obesity-related morbidities weakens with aging. J. Gerontol. - Ser. A Biol. Sci. Med. Sci. 69, 87-92. ( 10.1093/gerona/glt026) [DOI] [PubMed] [Google Scholar]
- 198.Cohen-Mansfield J, Perach R. 2011. Is there a reversal in the effect of obesity on mortality in old age? J. Aging Res. 2011, 765071. ( 10.4061/2011/765071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xia JY, Lloyd-Jones DM, Khan SS. 2019. Association of body mass index with mortality in cardiovascular disease: new insights into the obesity paradox from multiple perspectives. Trends Cardiovasc. Med. 29, 220-225. ( 10.1016/j.tcm.2018.08.006) [DOI] [PubMed] [Google Scholar]
- 200.Evangelakou Z, Manola M, Gumeni S, Trougakos IP. 2019. Nutrigenomics as a tool to study the impact of diet on aging and age-related diseases: the Drosophila approach. Genes Nutr. 14, 1-18. ( 10.1186/s12263-019-0638-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ihle KE, Mutti NS, Kaftanoglu O, Amdam GV. 2019. Insulin receptor substrate gene knockdown accelerates behavioural maturation and shortens lifespan in honeybee workers. Insects 10, 390. ( 10.3390/insects10110390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hölldobler B, Wilson EO. 2008. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: W.W. Norton. [Google Scholar]
- 203.Lantz PM, Pritchard A. 2010. Socioeconomic indicators that matter for population health. Prev. Chronic Dis. 7, A74. [PMC free article] [PubMed] [Google Scholar]
- 204.Wahl HW, Iwarsson S, Oswald F. 2012. Aging well and the environment: toward an integrative model and research agenda for the future. Gerontologist 52, 306-316. ( 10.1093/geront/gnr154) [DOI] [PubMed] [Google Scholar]
- 205.Harper S. 2014. Economic and social implications of aging societies. Science 346, 587-591. ( 10.1126/science.1254405) [DOI] [PubMed] [Google Scholar]
- 206.Majumder S, Aghayi E, Noferesti M, Memarzadeh-Tehran H, Mondal T, Pang Z, Deen M. 2017. Smart homes for elderly healthcare—recent advances and research challenges. Sensors 17, 2496. ( 10.3390/s17112496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arensberg MB. 2018. Population aging: opportunity for business expansion, an invitational paper presented at the Asia-Pacific Economic Cooperation (APEC) International Workshop on Adaptation to Population Aging Issues, 17 July 2017, Ha Noi, Viet Nam. J. Health. Popul. Nutr. 37, 7. ( 10.1186/s41043-018-0138-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yanagihara S, Suehiro W, Mitaka Y, Matsuura K. 2018. Age-based soldier polyethism: old termite soldiers take more risks than young soldiers. Biol. Lett. 14, 20180025. ( 10.1098/rsbl.2018.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kramer BH, Schaible R. 2013. Life span evolution in eusocial workers: a theoretical approach to understanding the effects of extrinsic mortality in a hierarchical system. PLoS ONE 8, e61813. ( 10.1371/journal.pone.0061813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Amdam GV, Page RE. 2005. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Ageing Res. Rev. 4, 398-408. ( 10.1016/j.arr.2005.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shorter JR, Rueppell O. 2012. A review on self-destructive defense behaviors in social insects. Insectes Soc. 59, 1-10. ( 10.1007/s00040-011-0210-x) [DOI] [Google Scholar]
- 212.Hart AG, Ratnieks FLW. 2001. Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 49, 387-392. ( 10.1007/s002650000312) [DOI] [Google Scholar]
- 213.Baracchi D, Fadda A, Turillazzi S. 2012. Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J. Insect Physiol. 58, 1589-1596. ( 10.1016/j.jinsphys.2012.09.014) [DOI] [PubMed] [Google Scholar]
- 214.Amdam GV, Seehuus SC. 2006. Order, disorder, death: lessons from a superorganism. Adv. Cancer Res. 95, 31-60. ( 10.1016/S0065-230X(06)95002-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Finch CE. 1994. Longevity, senescence, and the genome. Chicago, IL: University of Chicago Press. [Google Scholar]
- 216.Finch CE. 2009. Update on slow aging and negligible senescence - a mini-review. Gerontology 55, 307-313. ( 10.1159/000215589) [DOI] [PubMed] [Google Scholar]
- 217.Lee RD. 2003. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc. Natl Acad. Sci. USA 100, 9637-9642. ( 10.1073/pnas.1530303100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. 2004. Fitness benefits of prolonged post-reproductive lifespan in women. Nature 428, 178-181. ( 10.1038/nature02367) [DOI] [PubMed] [Google Scholar]
- 219.Kachel AF, Premo LS, Hublin JJ. 2011. Grandmothering and natural selection. Proc. R. Soc. B 278, 384-391. ( 10.1098/rspb.2010.1247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vaupel JW. 2010. Biodemography of human ageing. Nature 464, 536-542. ( 10.1038/nature08984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aanesen M, Lotherington AT, Olsen F. 2011. Smarter elder care? A cost-effectiveness analysis of implementing technology in elder care. Health Informatics J. 17, 161-172. ( 10.1177/1460458211409716) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.