Abstract
Social insects seem to have overcome the almost universal trade-off between fecundity and longevity as queens can be highly fecund and at the same time reach lifespans of decades. By contrast, their non-reproducing workers are often short-lived. One hypothesis to explain the long lifespan of queens is that they are better protected against stress than their workers. However, evidence is controversial and experimental studies are scarce. We aimed at manipulating environmental stress and ageing by exposing colonies of the less-socially complex termite Cryptotermes secundus to temperature regimes that differed in variance. In contrast with expectation, constant temperatures imposed more stress than variable temperatures. Survival of queens and workers as well as queens' fecundity were partly reduced under constant conditions and both castes showed signs of ageing in the transcriptome signature under constant conditions. There was a clear oxidative stress defence signal under constant conditions that was, surprisingly, stronger for workers than queens. We discuss how our results relate to social complexity. We argue that workers that are totipotent to become reproductives, like in C. secundus, should invest more in ‘anti-ageing' mechanisms than sterile workers because the former can still reproduce and have not reached maturity yet.
This article is part of the theme issue ‘Ageing and sociality: why, when and how does sociality change ageing patterns?’
Keywords: ageing, life history, termites, sociality, temperature, oxidative stress
1. Introduction
Most animals show a trade-off between fecundity and longevity: high reproduction comes at the cost of a shorter lifespan and vice versa [1]. However, the reproductives of social insects seem to be a major exception: queens of ants, bees, wasps and termites often have extraordinary lifespans compared to their workers and to solitary-living reproducing insect females [2–5]. The difference in the queen to worker lifespan ratio varies between species but reproductives always seem to have evolved mechanisms to prevent ageing and maintain fertility. The exact proximate mechanisms and ultimate causes of this reversal are, however, only partly understood.
On the mechanistic level, there are different potential causes for ageing in animals. They include mechanisms related to the nutrient-sensing pathways TOR (target of rapamycin) and IIS (insulin/insulin-like growth factor 1 signalling), which link to fertility and longevity [2,6,7], hormones (especially juvenile hormone, JH, in insects) [8,9] and transposable elements (TEs) (e.g. [10–12]; for social insects see [13]). According to the free radical theory of ageing, ageing is mainly caused by the accumulation of oxidative stress [14,15]. Reactive oxygen species (ROS), which are produced by mitochondrial respiration or environmental stress, cause damage to macromolecules such as proteins, lipids or DNA [16,17]. The production of antioxidants can reduce such damage. It is currently proposed that an intricate balance between ROS production and antioxidant defence is essential for a long lifespan as ROS can also be beneficial at low levels [7,18,19].
Most ageing studies in social insects are correlative, comparing, for instance, gene expression in queens and workers. Yet, such correlative investigations are insufficient to test for causality and experimental studies are needed [20]. Experimental studies are generally rare, especially outside model organisms such as the fruit fly Drosophila melanogaster or the nematode Caenorhabditis elegans [21]. Only few social insect studies exist that aimed at increasing stress via the ambient environment. An example is Li et al. [22] who used temperature and humidity to cause oxidative stress in the bees Apis mellifera and Apis cerana. Also for solitary insects, the effect of environmental/temperature stress on ageing and/or fecundity has rarely been tested (e.g. [23,24]). Results for D. melanogaster show that both cold and hot temperatures, lead to reduced reproduction in favour of survival. As the ambient temperature is known to have a crucial impact on (social) insects [22–25], varying temperatures seems to be a promising means to manipulate environmental stress.
In the current study, we experimentally tested the hypothesis that increased environmental stress negatively affects the lifespan and fertility of termite queens and/or workers. As stressor, we used ambient temperature variation and as model species the tropical wood-dwelling termite Cryptotermes secundus (Kalotermitidae). C. secundus is socially less complex. It has small colonies of a few hundred individuals, comprising a few sterile soldiers (2–10 individuals), non-sterile workers, a queen and a king [26]. Being a worker is a transient stage in C. secundus. Workers are totipotent immatures that develop into winged dispersing reproductives (alates) that found new colonies, or into replacement reproductives if the same-sex reproductive of their natal colony dies [27,28].
During our long-term experiment that lasted 20 months, we investigated the effect of temperature variation as a stressor on ultimate fitness consequences as well as on proximate mechanisms. As fitness measures, we recorded fecundity and survival, the latter for queens and workers. To obtain insights into potentially underlying mechanisms, we did transcriptome analyses for queens and workers that had been kept under constant or variable conditions.
2. Material and methods
(a). Colony collection, maintenance and experimental design
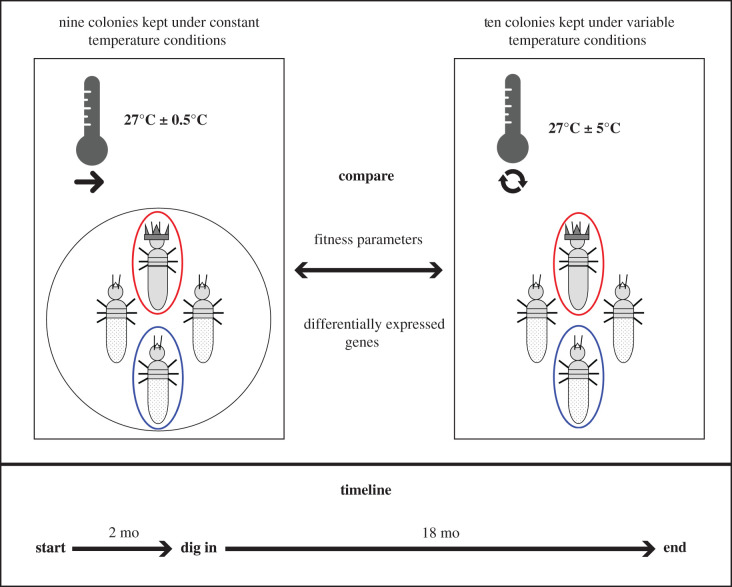
The C. secundus colonies used in this study were all collected in September 2011 in a mangrove forest near Palmerston-Channel Island in Darwin Harbour, Northern Territory, Australia (12°50′ S 131°00′ E), as described in [26]. Colonies were set up in Pinus radiata wood blocks providing abundant food conditions [26]. They were transported to Germany where they were kept in climate chambers as described in [27]. Before the experiment in 2015, over 20 colonies were set up from five stock colonies by placing 25 workers in wooden blocks of P. radiata providing abundant food conditions, as described in [29]. From these, not all developed into functional colonies with reproducing reproductives; finally, we had 19 experimental colonies. All these colonies had young reproductives of similar size and colony sizes that reflected 1-year-old colonies when the experiment started. Colonies were randomly allocated to the two temperature treatments only ensuring that, whenever possible, both treatments received colonies from each stock colony. The rationale for using experimental colonies that also came from the same stock colony was to test for genetic background effects and to obtain hints on genotype × treatment interactions. By generating and using experimental colonies that had the same genetic background (i.e. derived from the same stock colony: family members) as well as colonies with different genetic backgrounds (i.e. derived from different stock colonies: no-family members), we could test whether different genetic backgrounds respond differently to the treatment (i.e. temperature variation). All stock colonies had experienced the same environmental conditions before the experiment. They had been collected as monogamous, mature colonies (i.e. producing winged sexuals) in September 2011 from the same area in the field and kept in the laboratory under the same environmental conditions for 3.5 years before the experiment started. Additionally, all experimental colonies were very similar in their life history reflecting 1-year-old colonies. Thus, different responses between stock colonies to treatment are unlikely to be owing to environmental experience and colony life history. They rather hint at genetic variation being responsible for how well colonies can cope with temperature variation. This implies that there can be selection on this trait. As all stock colonies had been collected at the same geographic location, we studied part of the standing genetic variation present in this species' natural habitat. Nine colonies were kept at 27°C with a daily temperature range of ± 0.5°C (hereafter, constant conditions) and the other ten at 27°C with a daily temperature range of ± 5°C (hereafter, variable conditions). To accomplish these temperature conditions, we kept the colonies in high-end climate rooms, which can control temperature conditions with a precision of 0.1°C within the whole room, while keeping humidity constant. One room was programmed to have a mean of 27°C with a daily minimum of 26.5°C and a daily maximum of 27.5°C (constant conditions). The second room was programmed to also have a mean of 27°C but with a daily minimum of 22°C and a daily maximum of 32°C (variable conditions). These daily temperature cycles were maintained throughout the experiment with minimum temperatures at midnight and maximum temperatures at noon. The variable conditions are the standard conditions under which colonies are kept normally. Humidity was set to 70%, which is optimal for C. secundus [30]. Therefore, humidity and mean temperature were the same under both conditions, but the temperature variance differed. Colony sizes did not differ significantly between colonies allocated to constant versus variable conditions (Mann–Whitney-U Test: N = 19, W = 45, p = 1). The whole experiment lasted for 20 months (figure 1). During the first two months, the colonies were kept in a hole within the wood blocks to allow observation of the whole colony. During this period, the numbers of larvae and eggs were recorded three times a week to measure queen fecundity. After two months, colony composition was again recorded and the colonies were allowed to dig into the wood blocks. After another 18 months, the experiment ended. The blocks were split and the colony composition was recorded a third time. The rationale for choosing 18 months here was, first, that for this period it is possible to distinguish ‘old' reproductives (i.e. those that were present during the first part of the experiment) from ‘young' reproductives (i.e. those that developed during the second part of the experiment because an ‘old’ reproductive had died) by degree of cuticle sclerotization. This is not possible after longer periods. Therefore, it was possible to determine the survival of reproductives. Second, after 18 months, it is also still possible to distinguish ‘old’ workers (i.e. worker present during the first part of the experiment) from ‘young' workers (i.e. those that developed during the experiment and can be accounted to ‘queen fecundity') by their instar. During the nuptial flight periods (July–September), all colonies had been placed in air-permeable plastic bags to collect alates. The colony composition data included number and survival of the reproductives, number and instars of workers, alates, eggs and larvae (see electronic supplementary material, table S1).
Figure 1.
Experimental design of temperature manipulation. 19 colonies were used for the experiment that were either kept under constant (nine colonies; 27°C ± 0.5°C; thermometer with simple arrow) or variable (ten colonies; 27°C ± 5°C; thermometer with circular arrows) temperature conditions. For the first two months, colonies lived in a hole of their wood block, so that they were more exposed to temperature conditions. Then colony composition was recorded, and they were allowed to dig into their wood block. After another 18 months, the blocks were split, and colony composition was recorded again. At the end, queens that had survived the whole 20 months and one old worker per colony were collected for transcriptome analyses.
(b). Fitness estimates
The survival of the reproductives was assessed by their degree of cuticle sclerotization as it gets darker with age. When a reproductive had a light colour, the original reproductive (i.e. that present at the start of the experiment) had died during the experiment and was replaced by a new replacement reproductive (survival: no). A dark colouration showed the original reproductive had survived (survival: yes). As no reproductives died during the first part of the experiment (two months), we only compared reproductive survival at the end of the experiment.
For worker survival, we measured the survival after two months (until dig in) and for the whole experimental period (20 months). To do this, we determined the difference in the number of old workers (incl. alates) between two months (dig in), and respectively 20 months (end of the experiment), and the start of the experiment.
To test for an effect of temperature variance on queen fecundity, we had two output variables: (i) the total number of eggs produced during the first two months of the experiment (until dig in) and (ii) the sum of the number of eggs, larvae and young workers (i.e. all individuals born during the experiment) present at the end of the experiment (after 20 months). The first output variable directly measures queen fecundity, while the second output variable includes the survival of offspring born during the whole experiment.
(c). Statistical analysis of fitness data
All tests were two-tailed. Significance was defined at p < 0.05 and, when appropriate, the false discovery rate (FDR) approach [31] was applied to correct for multiple comparisons. Sample sizes and statistical tests are given in §3.
We used generalized linear models (GLM) followed by an ANOVA to analyse the effects of temperature variance (factor ‘treatment' with two levels ‘constant' and ‘variable'), genetic background (‘colony ID' of the five stock colonies) and their interaction. The GLMs for the survival of queens and both reproductives used the Binomial error family, the models for number of eggs or new offspring produced used Poisson error family, and the number of old workers surviving had Gaussian errors. Plots were generated with the package ggplot2 [32] implemented in R.
(d). Transcriptome preparation
To obtain more insights into potential stress and ageing effects as well as general molecular changes associated with changes in temperature variability, we generated transcriptomes of heads (plus prothorax) for queens and workers. Only those colonies in which both reproductives had survived the complete experimental period of 20 months were used for transcriptome analysis (see electronic supplementary material, table S1). Hence, samples were available only for two constant condition and six variable condition colonies. However, we could increase the sample size for constant conditions to six by using transcriptomes we had generated in another study (see below and electronic supplementary material, table S2). RNA was extracted from the head (plus prothorax) of the queen and of one arbitrarily chosen old worker of each colony. The rationale for choosing heads (plus prothorax) was that we thus generated transcriptomes of the brain, including the corpora allata. These parts provide important general information on ageing, such as neuronal senescence. In addition, ageing-related IIS and TOR as well as JH signalling occurs in these tissues [6,33]. By contrast, fat bodies would have revealed strong signals related to fecundity/vitellogenesis that differ between castes. Yet we were not interested in caste differences in this study but in ageing signals across castes. Thus, the head plus prothorax samples seemed most suited to compare ageing signals across castes. In line with this, the old-age marker genes that we used to test for effects of ageing (see below) had also been generated from the same tissues.
RNA extraction was realized following a standard in-house protocol (see electronic supplementary material, Methods). Briefly, individuals were killed on ice and the head plus prothorax was separated from thorax and abdomen. The head–prothorax samples were transferred into peqGold Tri FastTM (Peqlab) and homogenized with three zirconia beads in a Tissue Lyser II (Qiagen). Chloroform was added for protein precipitation. The aqueous phase containing the RNA was mixed with isopropanol and the received pellet was washed three times with 75% ethanol. The RNA pellet was dried and dissolved in nuclease-free water. Last, DNA was digested using the DNase I Amplification Grade (Sigma-Aldrich, Cat. No. AMPD1).
The prepared RNA samples were sent on dry ice to BGI Tech Solutions Co., Hong Kong. The sequencing was done at BGI Shenzhen (PR China). After quality control, BGI prepared cDNA libraries using the TruSeq RNA Library Prep Kit v. 2 (Illumina) following their internal and proprietary standard operating procedure. They performed paired-end 100 bp sequencing on an Illumina HiSeq 4000 platform, generating around 4GBases data per sample.
(e). RNA-seq data analysis
The quality of the transcriptome data was first checked with FastQC v. 0.11.7 [34]. Then the BGI in-house adapters were trimmed with Fastp 0.20.0 [35] and all reads shorter than 20 bp as well as all unpaired reads were removed. The reads were mapped against the C. secundus genome (accession number: PRJNA381866, [36]) with Hisat2 2.1.0 [37]. The resulting SAM-file was converted into a BAM-file for easier processing using SAMtools view 1.9 [38]. The obtained BAM-file was sorted according to name using SAMtools sort 1.9 [38]. The mapped reads were counted with the program HTSeq 0.11.2 (settings: inputformat: bam, type: gene, idattr: ID, mode: union, order: name, stranded: no) [39] and the unnormalized read count table was then analysed using the package DESeq2 [40] v. 1.18.1 in R (v. 3.4.4).
To increase the number of colonies for constant conditions from two to six, we used RNA-seq data from four additional colonies, which were kept under the same conditions in our laboratory and generated using the same protocol (provided by Karen Meusemann, see electronic supplementary material, table S2). These four colonies were generated and kept like the constant condition colonies from the current experiment, but they came from different stock colonies. The only differences were that the additional colonies had six months (instead of two months) until dig in and a total experimental time of 18 months (instead of 20 months). We consider these time differences justifiable, given a lifespan of at least 4 years for workers and of around 11 years for queens of C. secundus [41]. We also did not detect obvious differences between these four additional transcriptomes and the other two that were generated in the current study (see electronic supplementary material, figure S1).
We performed a gene expression analysis with caste and temperature as two independent variables using the generalized negative binominal model implemented in DESeq2, which internally corrects for different library sizes. Differentially expressed genes (DEGs) between temperature treatments were determined for each caste. p-values were calculated using Wald statistics and corrected for multiple testing using the FDR approach [31]. Normalized count data were used to perform principal component analysis (PCA) to check for caste-specific temperature effects on gene expression profiles (electronic supplementary material, Results, electronic supplementary material figure S1). Additionally, we did a GO enrichment analysis of DEGs using the topGO package [42] (see electronic supplementary material, Results).
To test whether changes in temperature variance affected ageing, we compared the treatment-related DEGs, separately for each caste, with an in-house gene list containing genes characterizing old C. secundus queens that can be considered as ageing markers (old-age genes, hereafter) ([41]; see electronic supplementary material, table S3). This list comprised genes that are upregulated in the head plus prothorax tissues of old queens (age: 10–11 years) that are likely to die soon (medium maximum longevity: 11 years; max longevity 13 years for C. secundus; [41]). These old-age genes were unrelated to fecundity. They reflect strong signs of stress, decline, defence and repair, at the transcriptional level of epigenetic control as well as at the posttranscriptional level with changes in TE-activity and the proteostasis network [41]. The latter depicts an upregulation of protein degradation, together with protein synthesis and protein folding, including many ribosomal genes [41]. We tested if the number of overlapping genes between the DEGs of queens, and workers respectively, and the old-age genes differed significantly between the treatments using contingency analyses. We used permutation tests and one-sample t-tests to check if the overlaps were significantly larger than expected by chance. Additionally, we also compared whether queens and workers differed in their relative proportion of old-age genes using contingency tests.
(f). Annotation
DEGs were identified by using the existing annotation of the C. secundus genome [36]. Additionally, they were annotated by performing a search against the Interpro Database (InterProScan-v5.35-74.0 [43]) and by performing a BLAST search against the fruit fly D. melanogaster and the fungus-growing termite Macrotermes natalensis using local blast [44] with a threshold E-value of 1 × 10−5. For D. melanogaster, gene annotation was retrieved from flybase [45]; for M. natalensis it was retrieved from Elsner et al. [13].
(g). Weighted gene co-expression network analysis
To obtain information on networks of co-expressed genes, three weighted gene co-expression network analyses (WGCNAs) were performed using the WGCNA package [46]. The counts of all genes obtained from HTSeq were transformed with the variance stabilizing transformation (vst) included in DESeq2 and used as input data. One analysis was performed with gene counts from queens, one with worker gene counts, and one with gene counts from both castes. For the latter, the influence of both temperature and caste was analysed.
A similarity matrix was constructed by calculating Pearson correlations between the expression values of all genes. Then, based on the similarity matrix, a signed weighted adjacency matrix was built. To get the necessary value of β, a soft-thresholding approach was used [46] (queens: β = 15, scale-free topology R2 = 0.89; workers: β = 20, scale-free topology R2 = 0.82; both castes combined: β = 14, scale-free topology R2 = 0.86).
A topological overlap matrix (TOM) was constructed using the adjacency matrix [47]. A hierarchical clustering tree was constructed using the dissimilarity measure (1 – TOM). Branches of the tree were cut using the Dynamic Hybrid Tree Cut algorithm [48] to define gene modules. The minimum module size was set to 30 and the modules were named after colour and analysis (e.g. ‘both-darkgreen' for the module ‘darkgreen' revealed in the analysis of both castes combined). Gene modules were merged based on the correlation of the module eigengenes (module cor ≥ 0.80), calculated with the function moduleEigengenes (default settings, [46]). Using the eigengenes, we tested for a correlation between modules and experimental temperature condition (in the analyses of queens, workers, and both castes combined) and for a correlation with caste (in the analysis of both castes combined). The modules that were significantly correlated with one or both factors were identified. All genes within the modules were annotated as described above, and their correlation coefficient and p-value were calculated.
3. Results
(a). Effect of temperature manipulation on fitness parameters
There was no significant difference in the survival of queens between constant and variable temperature conditions (binomial GLM, treatment: N = 19, p = 0.242). Also, the stock colony (genetic background) had no significant effect but its interaction with treatment was significant (stock colony: p = 0.300, interaction: p = 0.008), implying that queens coming from some genetic backgrounds survived temperature changes better than others (electronic supplementary material, figure S2). In colonies kept under constant conditions, six (66%) out of nine queens died while four (40%) out of 10 queens died under variable conditions. Similarly, the survival of both reproductives did not differ between temperature conditions but its interaction with stock colony had an effect (for details see electronic supplementary material, Results).
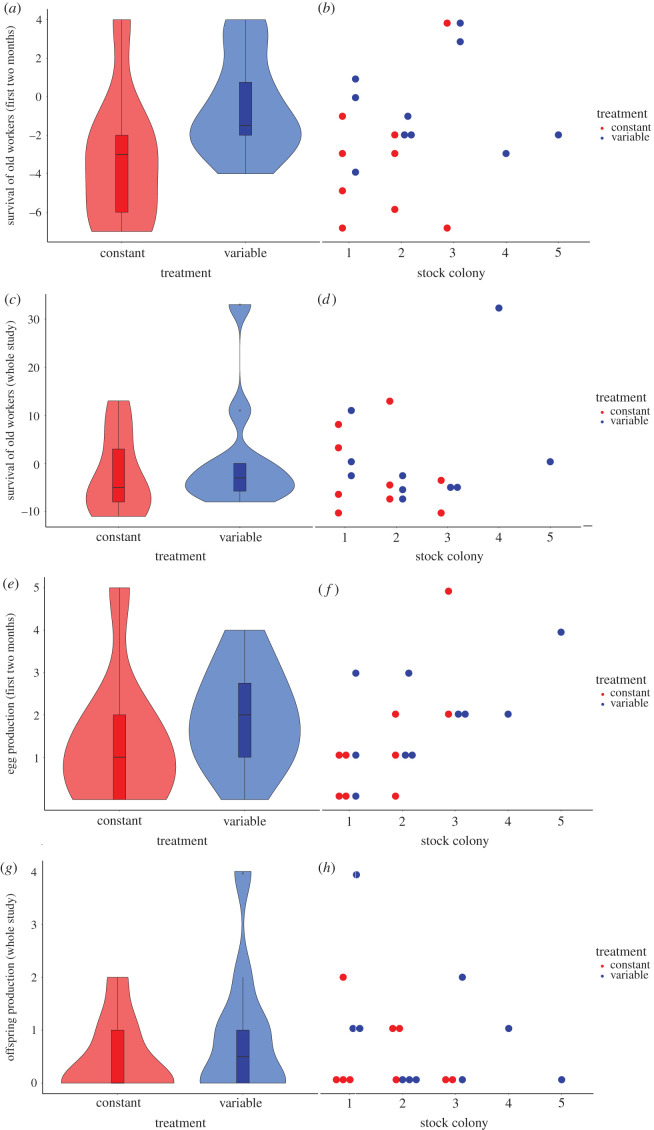
The survival of old workers did not differ significantly between temperature conditions. However, there was a trend that during the first two months period, fewer old workers survived under constant than variable conditions, which disappeared over the whole experimental time (Gaussian GLM, treatment: N = 19, first two months: F = 3.73, p = 0.080; total period: F = 1.15, p = 0.306; figure 2a,c). There was no effect of the stock colony or its interaction with treatment during the first two months (stock colony: F = 1.28, p = 0.335, interaction: F = 0.28, p = 0.756). Over the whole experimental period, there was a significant effect of stock colony but not of its interaction with treatment (stock colony: F = 5.35, p = 0.012, interaction: F = 0.74, p = 0.500), implying that old workers from some stock colonies survived better than those from other stock colonies, regardless of temperature conditions (figure 2b,d).
Figure 2.
Fitness results. All data are shown pooled per treatment (a,c,e,g) with boxplots indicating mean (black bar), interquartile range (box), and total data range (whiskers) and with the violins indicating the data distribution. For each variable, a second plot (b,d,f,h) shows the measurements of individual experimental colonies sorted by their genetic background (stock colony). Colonies under constant temperature conditions are given in red, those under variable conditions in blue. (a) The survival of old workers (i.e. those workers being present at the start of the experiment) seemed to be reduced under constant compared to variable temperature conditions during the first two months of the study period. (b) There was no significant stock colony effect (genetic background) nor an interaction between stock colony and treatment. (c) Over the whole study period of 20 months, the survival of old workers did not differ between temperature conditions. (d) Yet, workers from some genetic backgrounds survived less well than those from other backgrounds, while there was no genetic background × treatment interaction. (e) The number of eggs laid during the first two months did not differ between temperature conditions and (f) there was also no stock colony effect. (g) Over the whole study period, the total number of surviving offspring produced did not differ significantly between temperature conditions. (h) However, there was also significant stock colony × treatment interaction; the fecundity of queens from some stock colonies was more affected by changes in temperature conditions than those from other stock colonies.
Concerning fecundity, there was a no significant effect of temperature conditions on egg production during the first part of the experiment (Poisson GLM, treatment: N = 19, p = 0.332; figure 2e). Also, stock colony or its interaction with treatment had no effect (stock colony: p = 0.103, interaction: p = 0.276; figure 2f). Over the whole experimental period, the total fecundity did not differ (figure 2g) nor had stock colony an effect but its interaction with treatment was significant (Poisson GLM, treatment: N = 19, p = 0.223, stock colony: p = 0.231, interaction: p = 0.035; figure 2h). Queens of some genetic backgrounds had lower fecundity under constant than variable temperature conditions while queens from other genetic backgrounds had not.
(b). Effect of temperature manipulation on gene expression
The differential expression analysis revealed 574 temperature-associated DEGs for queens. About 20% (124 genes) of these DEGs were significantly upregulated in queens under variable conditions, while 80% (450 genes) of the DEGs were significantly upregulated under constant conditions. For workers, we found 1810 temperature-associated DEGs. Like in queens, fewer DEGs were upregulated under variable (552 DEGs, around 30%) than under constant conditions (1258 DEGs, around 70%).
(i). Overlap of differentially expressed genes with old-age genes
In contrast with our expectation, but in line with the fitness data, the gene expression data indicated that constant conditions were more stressful than variable conditions and resulted in faster ageing.
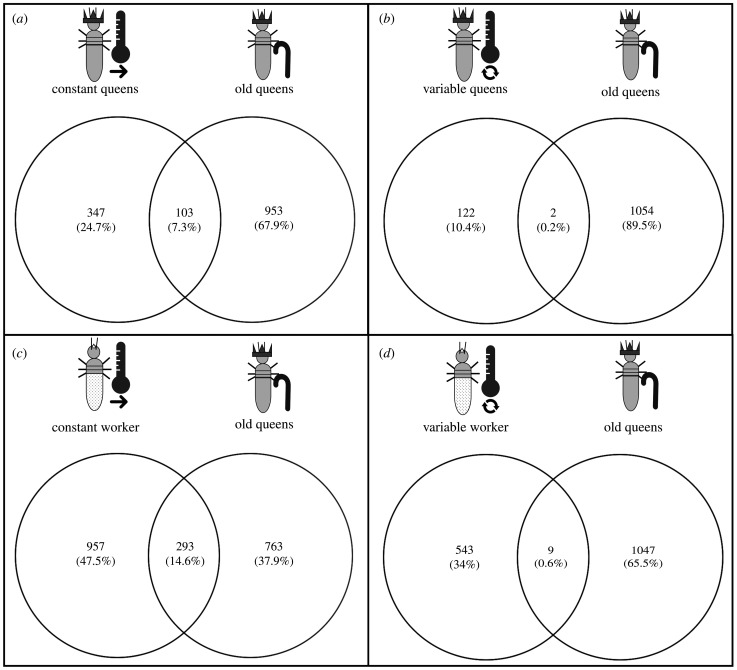
Comparing the overlap of temperature-associated DEGs with genes characterizing old C. secundus queens (old-age genes) revealed that termites living under constant conditions had a significantly higher proportion of old-age genes than those individuals living under variable conditions; this applied to queens (contingency analysis: p = 1.19 × 10−7; figure 3a,b) as well as workers ( p = 1.20 × 10−29; figure 3c,d). Comparing both castes, the overlap with old-age genes was significantly higher for workers than queens ( p = 2.54 × 10−4; figure 3). For both castes, more genes were shared with the old-age gene set than expected by chance under constant conditions (t-test: queens: t1,99 = −70.42, p = 2.22 × 10−86; workers: t1,99 = −172.90, p = 5.35 × 10−124) and fewer genes were shared than expected by chance under variable conditions (queens: t1,99 = 43.20, p = 4.72 × 10−66; workers: t1,99 = 85.06, p = 2.25 × 10−94).
Figure 3.
Overlap of temperature-associated differentially expressed genes (DEGs) with genes characteristic for old queens (i.e. old-age genes). The Venn diagrams show the number of DEGs that were upregulated in queens under (a) constant and (b) variable conditions and their overlap with old-age genes (queen symbol with walking stick). The corresponding diagrams for workers are shown in (c) for constant and (d) for variable conditions. Constant conditions are symbolized by a thermometer with a simple arrow, variable conditions by a thermometer with a circular arrow.
(ii). Differentially expressed genes in queens and their co-expression pattern
For queens, some expression signals were associated with IIS and TOR signalling, especially under constant temperature conditions (see electronic supplementary material, figure S3a). Under constant conditions, there was also an upregulation of some genes linked to oxidative stress. The IIS-related genes bigmax (Csec_G01223) and no child left behind (Csec_G08181), which are involved in the fruit fly in sugar-dependent gene regulation and in insulin-related peptide response, respectively, were upregulated under constant conditions, in addition to 55 ribosomal-related genes (approx. 12% of DEGs; electronic supplementary material, table S4).
These genes were co-expressed in a single WGCNA module, queen-darkgrey, which was positively associated with constant conditions (p = 0.010, r = 0.700; see also electronic supplementary material, Results). Besides bigmax and no child left behind, it included the IIS-related genes protein phosphatase 2A B' (PP2A B', Csec_G07375) and twins (Csec_G00373), which were all significantly upregulated under constant conditions (for all: p ≤ 0.027, r ≥ 0.633; electronic supplementary material, figure S3a, electronic supplementary material, table S5). In addition, some genes related to oxidative stress, such as a peroxiredoxin prdx4 (Csec_G09390, p = 0.025, r = 0.641) and a calmodulin (Csec_G04697, p = 0.034, r = 0.613), were co-expressed in this module (electronic supplementary material, table S5).
For DEGs upregulated under variable conditions, we did not find such a clear pattern (electronic supplementary material, table S4). The module queen-brown was positively associated with variable conditions (p = 0.010, r = 0.700; electronic supplementary material, table S5) (see also electronic supplementary material, Results). It contained more than 5000 genes, including among them also several genes linked to IIS and TOR signalling, including Tor (Csec_G19105) itself (p = 0.039, r = 0.601; electronic supplementary material, figure S3a, electronic supplementary material, table S5).
(iii). Differentially expressed genes in workers and their co-expression pattern
For workers, we found a strong upregulation of genes related to oxidative stress under constant conditions. Like in the queens, we also detected some TOR- and IIS-related genes, especially under constant conditions (see electronic supplementary material, figure S3b).
Several DEGs that can be linked to oxidative stress were upregulated under constant conditions in workers. They included one catalase (Csec_G02642), the peroxiredoxin prdx4 (Csec_G09390), several heat-shock genes (hsp83, Csec_G12291; hsp70, Csec_G03871; hsp60, Csec_G12606), a stress-induced phosphoprotein (Csec_G00459), a gene classified as eiger (Csec_G14519) as well as Neofem3, a vitellogenin (Csec_G11247; electronic supplementary material, table S4). Additionally, 86 ribosomal-related genes (approx. 7% of all constant worker DEGs) were upregulated under constant conditions (electronic supplementary material, table S4).
Most of these genes were co-expressed in the module worker-greenyellow, which was significantly associated with constant temperature conditions (p = 6 × 10−4, r = 0.870; electronic supplementary material, table S5). Prdx4, hsp83, hsp60, the stress-induced phosphoprotein Csec_G00459 as well as eiger were significantly positively associated with constant temperature conditions (for all: p ≤ 0.007, r ≥ 0.730; electronic supplementary material, table S5). Many ribosomal-related genes were also located in this module as well as some TOR/IIS genes (twins: p = 0.009, r = 0.716; pras40: p = 0.008, r = 0.725; PP2A_subA: p = 0.008, r = 0.721; electronic supplementary material, table S5; electronic supplementary material figure S3b).
Under variable conditions, we found—as in queens—no clear patterns in the DEGs (electronic supplementary material, table S4) but a slight IIS/TOR signal associated with the module worker-grey60, which was significantly associated with variable temperature conditions (p = 4 × 10−3, r = 0.790; electronic supplementary material, table S5). This module included Tor, rictor (Csec_G09447) and inaE (Csec_G05993), which were significantly positively associated with variable temperatures (for all: p ≤ 0.045, r ≥ 0.587; electronic supplementary material, table S5, electronic supplementary material figure S3b).
(iv). Effects that were consistent across castes
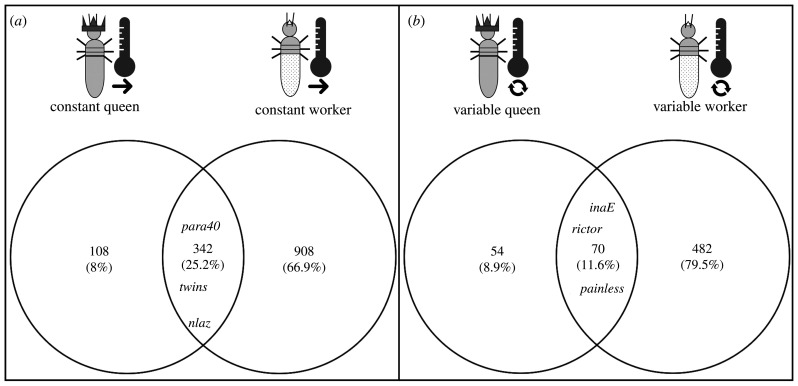
Across both castes, a comparison of the DEGs in queens and workers showed that in total 412 genes changed their expression consistently with treatment (figure 4). Three hundred and forty-two genes were more highly expressed under constant temperatures (electronic supplementary material, table S4) and 70 under variable temperature conditions (electronic supplementary material, table S4). In both cases, the log2 values showed similar patterns (electronic supplementary material, table S4), meaning that genes which differed strongly between treatments in workers also strongly differed in queens. No gene had opposing expression patterns between the two castes.
Figure 4.
Temperature-associated differentially expressed genes (DEGs) shared between queens and workers. The Venn diagrams show the number of DEGs that were upregulated in both castes (a) under constant (thermometer with a simple arrow) and (b) under variable conditions (thermometer with a circular arrow), highlighting some striking genes. Consistently across castes, 342 DEGs were upregulated under constant conditions and 70 DEGs under variable conditions.
Unsurprisingly, the differential expression pattern was very similar to that described above, with genes related to oxidative stress being upregulated under constant conditions and a co-expression of these genes with some TOR and IIS genes was visible (for more details see electronic supplementary material, Results; electronic supplementary material table S4; electronic supplementary material table S5).
4. Discussion
In contrast with our expectation, constant temperatures inflicted larger stress on C. secundus colonies than variable temperatures. The transcriptome data revealed signs of faster ageing and more stress under constant than variable temperatures (figures 3 and 4). Additionally, the fitness proxies showed that workers had a trend to have reduced survival during the first two month period under constant than variable temperatures (figure 2a,b). Furthermore, depending on from which stock colony a queen originated (i.e. genetic background), queens' survival and fecundity were negatively affected by constant temperature conditions (figure 2f,h; electronic supplementary material, figure S2). For instance, queens of most stock colonies survived less well and produced fewer offspring under constant than variable conditions. However, this was not the case for queens from stock colony 2. As the prior environmental conditions (with exception of our temperature treatment) and colony life histories were very similar for all colonies (see §2) and as we could not detect any obvious trait that distinguished stock colony 2 from the other stock colonies, this hints at genetic differences underlying the different response. Currently, we do not know the genetic differences between the stock colonies, but we think it is interesting to note that differential responses exist, and that there might be standing genetic variation in how to cope with temperature variability. As costs for DNA sequencing are constantly decreasing, future studies could aim to determine the underlying genetic differences.
Reasons for the unexpected finding of a negative impact of constant temperatures may be that termites do not face such constant conditions in nature and hence are adapted to variable conditions, which may be crucial for some physiological processes. In line with this, casual observations during the experiment showed that termites were more active under variable than constant temperatures (D. Schnaiter 2015, pers. comm.). Yet, temperature measurements in nests of tropical fungus-growing Macrotermes termites that experience similar ambient temperature conditions as C. secundus show that nest temperatures only differ by ±1°C during the daily and annual cycle [49–51]. Maybe C. secundus experiences higher fluctuations as they nest inside wood and do not build elaborate mounds as Macrotermes does. Additionally, colony sizes are four orders of magnitude smaller in wood-dwelling termites like C. secundus (a few hundred individuals) than in Macrotermes species (few million individuals). For the latter, it has been shown that colony size affects temperature variability in the nest [52]. However, nests of the Australian termite Coptotermes frenchi, which inhabits living trees, experience annual temperature variations from 27 to 36°C, though diurnal fluctuations are small [53].
We also had kept the stock colonies before the experiment under variable conditions so that colonies might have been acclimatized to these conditions, and therefore, we found constant conditions to result in reduced fitness proxies and gene expression profiles indicative of (i) faster ageing and (ii) more stress.
(a). Ageing signals
The comparison with genes characteristic for old C. secundus queens (old-age genes; electronic supplementary material, table S3) revealed for both castes more old-age genes under constant than variable conditions (figure 3). Additionally, the degree of overlap was significantly larger (or smaller) than expected by chance (figure 3). Workers showed a significantly stronger overlap with the old-age genes than queens (14.6% versus 7.3% of all DEGs upregulated under constant conditions; figure 3). Such a strong overlap for workers may be surprising given that the old-age gene list was derived for queens. These old-age genes are unrelated to fecundity but represent an old-age signature of the brain. It reflects processes of physiological upheaval, characterized by signs of stress, decline, defence and repair, at the transcriptional level of epigenetic control as well as at the posttranscriptional level with changes in TE activity and the proteostasis network ([41]; electronic supplementary material, table S3). The many ribosomal genes that we found to be upregulated under constant conditions are part of this ageing signature. As this ageing signature comprises rather general physiological processes, unlinked to caste-specific functions such as reproduction of the queen, we argue that it is a sign of ageing in the workers as well. The notion that constant temperatures had more negative consequences and probably induced faster ageing than variable temperatures is also supported by the expression of genes from the ageing-related TOR and IIS pathways (electronic supplementary material, figure S3a,b). In both castes, essential genes from the TOR pathway are downregulated under constant conditions, including rictor in both castes and Tor in queens. Such a downregulation of the TOR pathway can be a sign of stress and is associated with faster ageing, for instance in D. melanogaster (e.g. [6,54,55]). The concurrent treatment-dependent differential expression of pras40 in both castes links to the IIS pathway in which the expression of several genes was also affected. These genes included the Akt regulator PP2A in both castes and Foxo in workers.
(b). Oxidative stress
We also detected an upregulation of genes related to defence against oxidative stress under constant conditions, especially in workers. These included several heat-shock genes, a catalase, a peroxiredoxin and, strikingly, the vitellogenin Neofem3. The latter is supposed to be involved in vitellogenesis in C. secundus [29,56]. The upregulation of Neofem3 in workers under constant conditions may imply that convergently to the honeybee, A. mellifera [57], vitellogenin might also function as antioxidant in the termite C. secundus.
Oxidative stress has been proposed as a major cause of ageing [14,15]. How it contributes to explain the long life of social insect queens is less clear ([58,59]; see also [60]). Some studies found higher levels of antioxidants in queens compared to workers (e.g. [61,62]), others did not (e.g. [63]).
The strong upregulation of oxidative stress response genes in C. secundus workers (electronic supplementary material, table S4) may seem surprising given that queens, as a colony's ‘germ line', are supposed to have evolved better protection, facilitating a long lifespan. Yet workers in C. secundus, like in all wood-dwelling termites, are totipotent immatures that develop into reproductives [28] and life-history theory predicts strong selection to reach maturity [64]. Hence, we expect investment into antioxidants in these totipotent termite workers that have a high reproductive value (i.e. expected contribution of individuals of a given age to the population through both current and future reproduction; [65]). In line, a former study on C. secundus also found few ageing signals in workers when comparing caste-specific ageing pattern at the transcriptome level [66]. Accordingly, different selection regimes may explain the difference between our results and those for the subterranean termite Reticulitermes speratus [61] in which workers cannot develop into winged sexuals (though they can still reproduce as replacement reproductives). Tasaki et al. [61] found a higher expression of antioxidant genes in queens than workers using qRT-PCR, which they interpret to explain the long life of termite queens. By contrast, like in [66], we did not find antioxidants to be overexpressed in queens compared to workers or associated with caste-related modules (electronic supplementary material, table S4, electronic supplementary material table S5). Thus, differences in workers' ability to reproduce may explain different ageing and investment patterns between social insect species. This factor has largely been neglected so far. Differences in workers' reproductive potential seem to align with social complexity in termites [5] and social Hymenoptera [67]. More comparative research is needed to test whether caste differences in anti-ageing mechanisms and associated ageing patterns can be explained by workers' reproductive potential. Supporting evidence for the latter is indicated by a review on termites [5], which summarizes the longevity of workers and queens along with workers' reproductive potential.
Besides the workers' reproductive potential, the strong upregulation of genes involved in defence against oxidative stress in C. secundus workers might be explained by a potentially stronger negative effect of constant temperatures on workers than queens (figure 3). Accordingly, workers would require better protection and hence a stronger upregulation of oxidative defence mechanisms was observed in workers than in queens. These two explanations are not mutually exclusive but complementary as only workers with a high reproductive value should invest in an upregulation of stress defence genes.
5. Conclusion
Our results demonstrated that constant temperatures induced stress to C. secundus colonies. Both queens and workers, were affected. As totipotent immatures, C. secundus workers have a high reproductive value, relative to species with sterile workers. Accordingly, they showed a stronger upregulation of genes involved in defence against oxidative stress than queens. Our study implies that workers' reproductive value, as reflected in workers' reproductive potential, should be considered in studies on ageing in social insects. It may not only explain inconsistencies in results about the role of oxidative stress, but it may also explain variation in caste-specific longevity across social insect species. As workers' reproductive potential generally declines with increasing degree of social complexity, we predict reduced investment into the anti-ageing mechanism in workers and associated with this an increase in the queen to worker longevity ratio.
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (DFG) by two grants to JK (DFG; KO1895/16-1; KO1895/19-2) within the Research Unit FOR2281. We thank Florentine Schaub for assistance in the field and wet laboratory, Karen Meusemann for help with bioinformatic analyses, Daniela Schnaiter for carefully looking after the termite colonies, and two anonymous referees for helpful comments. Charles Darwin University (Australia), and especially S. Garnett and the Horticulture and Aquaculture team, provided logistic support to collect C. secundus. The Parks and Wildlife Commission, Northern Territory, the Department of the Environment, Water, Heritage and the Arts gave permission to collect (Permit number 36401) and export (Permit WT2010-6997) the termites. The study was conducted in accordance with the Nagoya protocol.
Ethics
Research was performed according to guidelines for good scientific conduct. Animals were collected according to the Nagoya protocol.
The Parks and Wildlife Commission, Northern Territory, the Department of the Environment, Water, Heritage and the Arts gave permission to collect (Permit number 36401) and export (Permit WT2010-6997) the termites.
Data accessibility
Most of the data are within the manuscript and its Supporting Information files. Raw sequencing reads are deposited on NCBI (BioProject Accession PRJNA655108).
Authors' contributions
V.R. carried out data analyses and drafted the manuscript; J.K. obtained funding, designed the study, carried out data analyses and drafted the manuscript. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Deutsche Forschungsgemeinschaft (D.F.G.) by two grants to J.K. (DFG; KO1895/16-1; KO1895/19-2) within the Research Unit FOR2281.
References
- 1.Stearns SC. 2000. Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87, 476-486. ( 10.1007/s001140050763) [DOI] [PubMed] [Google Scholar]
- 2.Heinze J, Schrempf A. 2008. Aging and reproduction in social Insects – a mini-review. Gerontology 54, 160-167. ( 10.1159/000122472) [DOI] [PubMed] [Google Scholar]
- 3.Keller L, Genoud M. 1997. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature 389, 958-960. ( 10.1038/40130) [DOI] [Google Scholar]
- 4.Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insect Soc. 45, 235-246. ( 10.1007/s000400050084) [DOI] [Google Scholar]
- 5.Korb J, Thorne B. 2017. Sociality in Termites. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 124-153. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Partridge L, Alic N, Bjedov I, Piper MDW. 2011. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 46, 376-381. ( 10.1016/j.exger.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gems D, Partridge L. 2013. Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. 75, 621-644. ( 10.1146/annurev-physiol-030212-183712) [DOI] [PubMed] [Google Scholar]
- 8.Flatt T, Tu M-P, Tatar M. 2005. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 27, 999-1010. ( 10.1002/bies.20290) [DOI] [PubMed] [Google Scholar]
- 9.Toivonen JM, Partridge L. 2009. Endocrine regulation of aging and reproduction in Drosophila. Mol. Cell. Endocrinol. 299, 39-50. ( 10.1016/j.mce.2008.07.005) [DOI] [PubMed] [Google Scholar]
- 10.Maxwell PH, Burhans WC, Curcio MJ. 2011. Retrotransposition is associated with genome instability during chronological aging. Proc. Natl Acad. Sci. USA 108, 20 376-20 381. ( 10.1073/pnas.1100271108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Cecco M, Criscione SW, Peterson AL, Neretti N, Sedivy JM, Kreiling JA.. 2013. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging 5, 867-883. ( 10.18632/aging.100621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood JG, et al. 2016. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl Acad. Sci. USA 113, 11 277-11 282. ( 10.1073/pnas.1604621113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsner D, Meusemann K, Korb J. 2018. Longevity and transposon defense, the case of termite reproductives. Proc. Natl Acad. Sci. USA 115, 5504-5509. ( 10.1073/pnas.1804046115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harman D. 1956. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 11, 298-300. ( 10.1093/geronj/11.3.298) [DOI] [PubMed] [Google Scholar]
- 15.Goto S. 2015. The biological mechanisms of aging: a historical and critical overview. In Aging mechanisms (eds Mori N, Mook-Jung I), pp. 3-27. Tokyo, Japan: Springer. [Google Scholar]
- 16.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737-1745. ( 10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selman C, Blount JD, Nussey DH, Speakman JR. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570-577. ( 10.1016/j.tree.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 18.Gapper C, Dolan L. 2006. Control of plant development by reactive oxygen species. Plant Physiol. 141, 341-345. ( 10.1104/pp.106.079079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamanaka RB, Chandel NS. 2010. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35, 505-513. ( 10.1016/j.tibs.2010.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korb J. 2016. Why do social insect queens live so long? Approaches to unravel the sociality-aging puzzle. Curr. Opin. Insect Sci. 16, 104-107. ( 10.1016/j.cois.2016.06.004) [DOI] [PubMed] [Google Scholar]
- 21.Hansen M, Flatt T, Aguilaniu H. 2013. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 17, 10-19. ( 10.1016/j.cmet.2012.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, et al. 2019. Tolerance and response of two honeybee species Apis cerana and Apis mellifera to high temperature and relative humidity. PLoS ONE 14, e0217921. ( 10.1371/journal.pone.0217921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meiselman MR, Kingan TG, Adams ME. 2018. Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. BMC Biol. 16, 18. ( 10.1186/s12915-018-0484-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall KE, Sinclair BJ. 2009. Repeated stress exposure results in a survival–reproduction trade-off in Drosophila melanogaster. Proc. R. Soc. B 277, 963-969. ( 10.1098/rspb.2009.1807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klepsatel P, Wildridge D, Gáliková M. 2019. Temperature induces changes in Drosophila energy stores. Sci. Rep. 9, 5239. ( 10.1038/s41598-019-41754-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korb J, Lenz M. 2004. Reproductive decision-making in the termite, Cryptotermes secundus (Kalotermitidae), under variable food conditions. Behav. Ecol. 15, 390-395. ( 10.1093/beheco/arh033) [DOI] [Google Scholar]
- 27.Korb J, Katrantzis S. 2004. Influence of environmental conditions on the expression of the sexual dispersal phenotype in a lower termite: implications for the evolution of workers in termites. Evol. Dev. 6, 342-352. ( 10.1111/j.1525-142X.2004.04042.x) [DOI] [PubMed] [Google Scholar]
- 28.Korb J. 2007. Workers of a drywood termite do not work. Front. Zool. 4, 7. ( 10.1186/1742-9994-4-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weil T, Rehli M, Korb J. 2007. Molecular basis for the reproductive division of labour in a lower termite. BMC Genomics 8, 198-199. ( 10.1186/1471-2164-8-198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korb J, Schmidinger S. 2004. Help or disperse? Cooperation in termites influenced by food conditions. Behav. Ecol. Sociobiol. 56, 89-95. ( 10.1007/s00265-004-0757-x) [DOI] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 57, 289-300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 32.Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 33.Cao JQ, Tong WS, Yu HY, Tobe SS, Bendena WG, Hui JHL. 2017. The role of MicroRNAs in Drosophila regulation of insulin-like peptides and ecdysteroid signalling: where are we now? Adv. In Insect Phys. 53, 55-85. ( 10.1016/bs.aiip.2017.02.002) [DOI] [Google Scholar]
- 34.Andrews S. 2015. FastQC: A quality control tool for high throughput sequence data. Cambridge, UK: Babraham Institute. See https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Google Scholar]
- 35.Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884-i890. ( 10.1093/bioinformatics/bty560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison MC, et al. 2018. Hemimetabolous genomes reveal molecular basis of termite eusociality. Nat. Ecol. Evol. 2, 1-12. ( 10.1038/s41559-017-0459-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357-360. ( 10.1038/nmeth.3317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, et al. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078-2079. ( 10.1093/bioinformatics/btp352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anders S, Pyl PT, Huber W. 2015. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166-169. ( 10.1093/bioinformatics/btu638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 31-21. ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monroy Kuhn JM, Meusemann K, Korb J. 2020. Disentangling the aging network of a termite queen. bioRxiv 2020.12.19.423576. ( 10.1101/2020.12.19.423576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexa A, Rahnenführer J. 2016. topGO: Enrichment Analysis for Gene Ontology. R package version 2.42.0. ( 10.18129/B9.bioc.topGO) [DOI]
- 43.Quevillon E, et al. 2005. InterProScan: protein domains identifier. Nucleic Acids Res. 33(Web Server issue), W116-W120. ( 10.1093/nar/gki442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403-410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 45.Goodman JL, et al. 2019. FlyBase 2.0: the next generation. Nucleic Acids Res. 47, D759-D765. ( 10.1093/nar/gky1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langfelder P, Horvath S. 2008. WGCNA: a R package for weighted correlation network analysis. BMC Bioinf. 9, 559. ( 10.1186/1471-2105-9-559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yip AM, Horvath S. 2007. Gene network interconnectedness and the generalized topological overlap measure. BMC Bioinf. 8, 1551. ( 10.1186/1471-2105-8-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langfelder P, Zhang B, Horvath S. 2008. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719-720. ( 10.1093/bioinformatics/btm563) [DOI] [PubMed] [Google Scholar]
- 49.Lüscher M. 1961. Air-conditioned termite nests. Sci. Am. 205, 138-147. ( 10.2307/24937012) [DOI] [Google Scholar]
- 50.Korb J, Linsenmair KE. 1998. The effects of temperature on the architecture and distribution of Macrotermes bellicosus (Isoptera, Macrotermitinae) mounds in different habitats of a West African Guinea savanna. Insectes Soc. 45, 51-65. ( 10.1007/s000400050068) [DOI] [Google Scholar]
- 51.Korb J, Linsenmair KE. 2000. Ventilation of termite mounds: new results require a new model. Behav. Ecol. 11, 486-494. ( 10.1093/beheco/11.5.486) [DOI] [Google Scholar]
- 52.Korb J, Linsenmair KE. 2000. Thermoregulation of termite mounds: what role does ambient temperature and metabolism of the colony play? Insectes Soc.. 47, 357-363. ( 10.1007/PL00001731) [DOI] [Google Scholar]
- 53.Greaves T. 1964. Temperature studies of termite colonies in living trees. Aust. J. Zool. 12, 250-262. ( 10.1071/zo9640250) [DOI] [Google Scholar]
- 54.Katewa SD, Kapahi P. 2011. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp. Gerontol. 46, 382-390. ( 10.1016/j.exger.2010.11.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scialò F, et al. 2015. Target of rapamycin activation predicts lifespan in fruit flies. Cell Cycle 14, 2949-2958. ( 10.1080/15384101.2015.1071745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korb J. 2016. Genes underlying reproductive division of labor in termites, with comparisons to social Hymenoptera. Front. Ecol. Evol. 4, 76. ( 10.3389/fevo.2016.00045) [DOI] [Google Scholar]
- 57.Seehuus S-C, Norberg K, Gimsa U, Krekling T, Amdam GV. 2006. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl Acad. Sci. USA 103, 962-967. ( 10.1073/pnas.0502681103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Verges J, Nehring V.. 2016. A critical look at proximate causes of social insect senescence: damage accumulation or hyperfunction? Curr. Opin. Insect Sci. 16, 69-75. ( 10.1016/j.cois.2016.05.003) [DOI] [PubMed] [Google Scholar]
- 59.Lucas E, Keller L. 2017. Explaining extraordinary lifespans: the proximate and ultimate causes of differential lifespan in social insects. In The evolution of senescence in the tree of life (eds Shefferson RP, Jones OR, Salguero-Gómez R), pp. 1-23. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 60.Kramer BH, et al. 2021. Oxidative stress and senescence in social insects: a significant but inconsistent link? Phil. Trans. R. Soc. B 376, 20190732. ( 10.1098/rstb.2019.0732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tasaki E, Kobayashi K, Matsuura K, Iuchi Y. 2017. An efficient antioxidant system in a long-lived termite queen. PLoS ONE 12, e0167412. ( 10.1371/journal.pone.0167412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corona M, Hughes KA, Weaver DB, Robinson GE. 2005. Gene expression patterns associated with queen honey bee longevity. Mech. Ageing Dev. 126, 1230-1238. ( 10.1016/j.mad.2005.07.004) [DOI] [PubMed] [Google Scholar]
- 63.Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. 2004. Decreased expression of Cu–Zn superoxide dismutase 1 in ants with extreme lifespan. Proc. Natl Acad. Sci. USA 101, 3486-3489. ( 10.1073/pnas.0400222101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charlesworth B. 1980. Evolution in age-structured populations. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: The Claredon Press. [Google Scholar]
- 66.Monroy KJM, Meusemann K, Korb J. 2019. Long live the queen, the king and the commoner? Transcript expression differences between old and young in the termite Cryptotermes secundus. PLoS ONE 14, e0210371. ( 10.1371/journal.pone.0210371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korb J, Heinze J. 2016. Major hurdles for the evolution of sociality. Annu. Rev. Entomol. 61, 297-316. ( 10.1146/annurev-ento-010715-023711) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the data are within the manuscript and its Supporting Information files. Raw sequencing reads are deposited on NCBI (BioProject Accession PRJNA655108).