Abstract
Standard evolutionary theory, supported by mathematical modelling of outbred, dispersed populations predicts that ageing is not an adaptation. We recently argued that in clonal, viscous populations, programmed organismal death could promote fitness through social benefits and has, in some organisms (e.g. Caenorhabditis elegans), evolved to shorten lifespan. Here, we review previous adaptive death theory, including consumer sacrifice, biomass sacrifice and defensive sacrifice types of altruistic adaptive death. In addition, we discuss possible adaptive death in certain semelparous fish, coevolution of reproductive and adaptive death, and adaptive reproductive senescence in C. elegans. We also describe findings from recent tests for the existence of adaptive death in C. elegans using computer modelling. Such models have provided new insights into how trade-offs between fitness at the individual and colony levels mean that senescent changes can be selected traits. Exploring further the relationship between adaptive death and social interactions, we consider examples where adaptive death results more from action of kin than from self-destructive mechanisms and, to describe this, introduce the term adaptive killing of kin.
This article is part of the theme issue ‘Ageing and sociality: why, when and how does sociality change ageing patterns?’
Keywords: adaptive death, ageing, altruism, Caenorhabditis elegans, kin selection, salmon
1. Introduction
Is ageing an adaptation? An early idea was that ageing benefits the species by removing worn out, old individuals, thereby increasing resource availability for those still able to reproduce. However, today, the consensus among evolutionary biologists is that senescence is not adaptive [1], but rather has evolved as the result of the decline in purifying selection with increasing age. Two plausible theories describe how this happens. First, the mutation accumulation theory reasons that the age decline in selection leads to accumulation of mutations with little effect on fitness earlier in life, but with detrimental effects in old age [2]. Second, the antagonistic pleiotropy theory proposes that ageing evolves owing to positive selection for gene variants with pleiotropic effects at different ages: promoting early-life fitness and but also with late-life detrimental effects [3]. Importantly, neither theory predicts that ageing per se evolves because it provides a selective advantage.
From a population genetic perspective, the impact of the decline in the force of natural selection on the evolution of ageing has been described mathematically [4,5]. Notably, these analyses were based on idealized Wright–Fisher populations (i.e. dispersed and out-crossing), and did not include social and ecological factors, including spatial structure, access to resources or dispersal. Also, the theory considers only the effect of natural selection, and does not account for other evolutionary mechanisms such as genetic drift, gene flow and mutation bias.
The mutation accumulation and antagonistic pleiotropy theories have been interpreted as implying that ageing is determined by many genes with small effects [3]. However, the existence of mutations that dramatically extend lifespan in the nematode Caenorhabditis elegans presented a challenge to the classic evolutionary theory. For example, mutation of the daf-2 insulin/IGF-1 receptor gene can more than double C. elegans lifespan [6]. This led to renewed speculation about possible adaptive benefits of ageing [7]. For example, it was suggested that the wild-type daf-2 allele ‘may have been selected because of its effects on aging if, for example, species whose members have short life spans prospered from increased genetic diversity or decreased competition between parents and offspring’ [8, p. 451].
2. Can organismal death ever promote fitness?
Could the existence of mutations that dramatically increase lifespan really reflect the presence of programmed ageing? To avoid the ambiguity in meaning of both the terms ‘ageing’ and ‘programmed’ [9], we will use instead the term ‘adaptive death’ to emphasize the proposal that death itself increases inclusive fitness for the focal individual [7].
In classical evolutionary theory, kin selection was incorporated into the model of age-structured populations [10], though it was not actually explored as a possible mechanism that could affect ageing. However, it was concluded that altruistic behaviour is more likely to evolve where social interactions involve a donor of low reproductive value and a recipient with a high one. Thus, where resources are limited (as they often are in nature), altruistic behaviour can evolve by kin selection to transfer resources from parents to offspring. Where social interactions occur, the fitness of an individual includes not only the number of its progeny, but also any increases it causes in the reproductive success of its closer relatives or kin (inclusive fitness). In principle, adaptive death could occur as an extreme form of altruism where parents sacrifice themselves to increase their inclusive fitness.
But is adaptive death a real thing? The behaviour of several computer models provided evidence that ageing can evolve through kin selection when dispersal is limited and reproductive capacity declines with age [11–13]. Thus, while classical theory predicts that ageing per se is a non-adaptive by-product of evolution, it does not rule out the possibility that ageing can be adaptive in some ecological conditions.
We recently elaborated a theory of adaptive death based on evolutionary theory, earlier discussion of the topic [14] and a survey of organisms in which adaptive death may occur. This has been described elsewhere [7,9], but, briefly, it argues that conditions exist for the emergence of adaptive death where closely related individuals dwell in discrete, viscous (non-dispersed) populations or colonies, such that colony-level (or inter-demic) selection can occur. Importantly, in viscous populations, greater levels of altruism can evolve owing to reduced risk of exploitation by non-altruist, non-kin cheaters. Adaptive death is expected to occur particularly in microorganisms that exist as clonal colonies, such as bacteria, yeast and some small metazoans such as free-living nematodes. We previously discussed in detail evidence for adaptive death in Saccharomyces cerevisiae and C. elegans [7,9]. In this essay, we explore further the biology of adaptive death in a wider range of highly social organisms, and present a number of new ideas about adaptive death.
3. Stress, damage and adaptive death
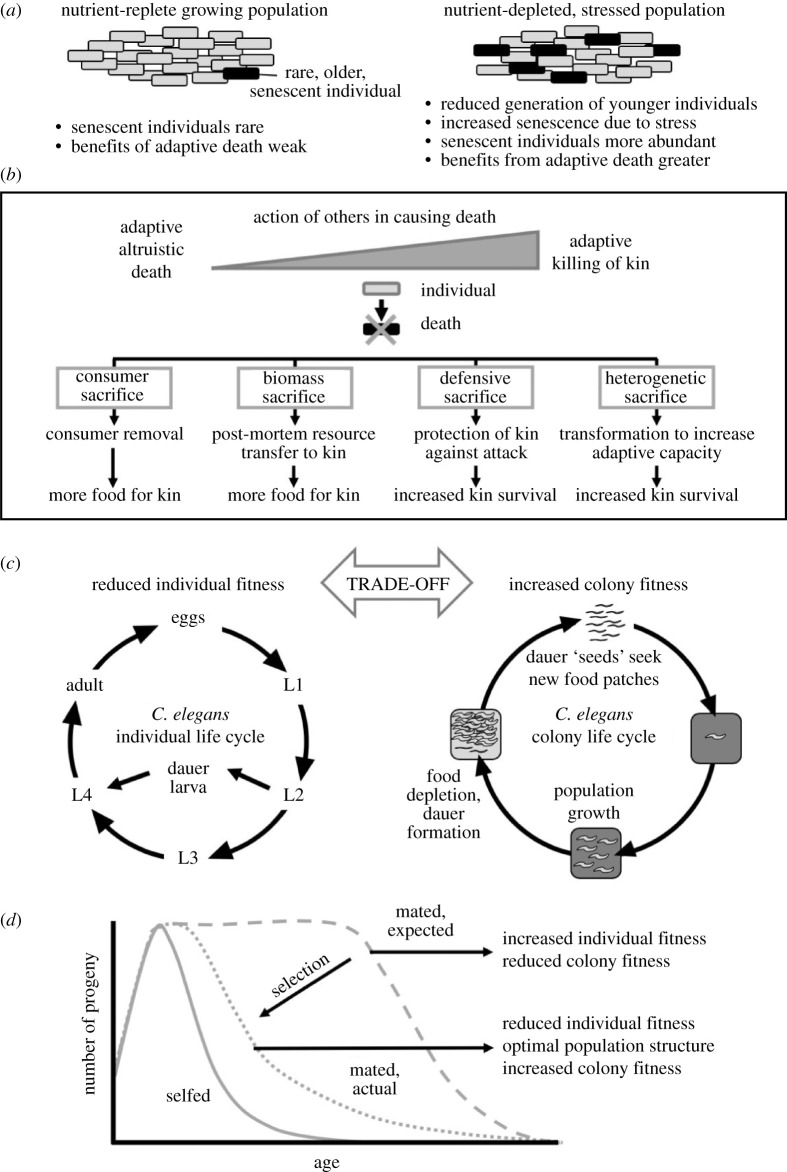
Fitness benefits from adaptive death are only expected to exist where a significant proportion of a population is older [15]. The proportion of old individuals is predicted to be low in exponentially growing populations, but to increase in populations whose growth rate has stalled, e.g. owing to nutrient depletion, pathogens or other insults. Thus, an expectation is that adaptive death is more beneficial in stressed populations. Molecular damage accumulation can contribute to ageing, including reproductive decline [16]. Thus, stressful conditions may both accelerate ageing and increase the benefits of adaptive death in a colonial milieu (figure 1a).
Figure 1.
How reduction of individual fitness can increase colony-level fitness. (a) The fitness benefits of adaptive death are expected to be greater in populations with less reproduction and more stress. (b) Adaptive altruistic death and adaptive killing of kin. Adaptive death can be triggered by social interactions with kin. There exists a graded spectrum between adaptive altruistic death resulting from self-destruction, and adaptive killing of kin caused entirely by others. This spectrum ranges from subtle social cues to trigger death, through cannibalism toxins, to murder and predation. (c) Trade-offs between fitness traits at the level of the individual and the colony: reduction of individual fitness traits (including adaptive death) can increase colony fitness, particularly by reducing futile food consumption (that which does not increase dauer yield) [17]. (d) Hypothesis: selection to minimize futile food consumption could explain how mechanistically programmed reproductive senescence in Caenorhabditis promotes colony fitness (see main text for explanation).
4. Adaptive death in colonial unicells
Many unicellular organisms grow as clonal colonies in which individual cells have limited motility. In some species, colonies exhibit properties of primitive multicellular organisms, where some cells differentiate and perform altruistic functions. For example, S. cerevisiae exhibit programmed cell death driven by molecular mechanisms similar to those in multicellular organisms [18]. As nutrient availability declines in ageing yeast colonies, some cells in the centre of the colony undergo programmed cell death triggered by an ammonia signalling gradient, apparently to supply nutrients to the younger kin growing at the colony's edge [19,20]. We have defined this form of adaptive death as biomass sacrifice since the altruists feed their kin with their own biomass [7]. However, it is also possible in principle that consumer sacrifice is operative, where older individuals die in order to cease consumption, thereby increasing nutrient availability for kin. Consumer sacrifice is similar to the adaptive value attributed to ageing by August Weismann in the nineteenth century [1]. (see Glossary for a summary of recent and new terminology relating to adaptive death)
Adaptive death also occurs in a number of bacterial species, for example, in the social predator Myxococcus xanthus [21]. Upon nutrient depletion, these bacteria aggregate to form a fruiting body, which can contain up to 100 000 cells. During fruiting body development, approximately 80% of cells die as a result of altruistic lysis, providing nutrients by biomass sacrifice to the remaining 20% of cells, which develop into spores [22].
In Escherichia coli, toxin–antitoxin systems can be activated in response to nutrient depletion or other stressors, and promote biomass sacrifice adaptive death that increases colony fitness [23]. Toxin–antitoxin systems are also employed by many bacterial species to prevent the spread of bacteriophage infection [24], an example of defensive sacrifice [7,9]; thus, adaptive death benefits do not only stem from nutritional improvements.
Quorum sensing communication can also coordinate adaptive death in bacterial populations and biofilms. In this context, actions of various signalling molecules have been described, including extracellular death factors in E. coli [25], Spo0A-P factor during sporulation in Bacillus subtilis [26], and competence-stimulating peptide in Streptococcus pneumoniae [27].
(a). Adaptive altruistic death and adaptive killing of kin: two ends of a spectrum
The behaviour of the bacterium B. subtilis provides an interesting perspective on the adaptive death concept. Something akin to adaptive death occurs as a mechanism by which B. subtilis delays the resource-intensive process of sporulation in biofilms facing a lack of nutrients [28]. In response to starvation, B. subtilis adopts a bet-hedging strategy in which cells differentiate in one of two ways: either they sporulate or they continue growth by subsisting on alternative metabolites [29]. Interestingly, sporulating cells can produce the so-called cannibalism toxins that kill their non-sporulating kin, thereby providing sporulators with nutrients that enable them either to survive until conditions improve or commit to spore maturation if they worsen.
Here, the B. subtilis cells that die likely experience inclusive fitness benefits through adaptive death. However, this differs from adaptive death as previously conceived [7], where individuals undergo programmed death altruistically to benefit their kin. By contrast, in B. subtilis, the dying cell is not so much an altruist as a victim of fratricide (or cannibalism). In The Voyage of the Beagle, Darwin describes how, in times of food scarcity, Fuegian Indians would sometimes hunt down and eat their elderly relatives (usually women) [30], an example of senicide. This we would describe as predation (or murder) rather than adaptive altruistic death. Yet by being murdered and eaten the unfortunate old women could have experienced inclusive fitness benefits, as shown in spider matriphagy [31].
Here one can, in theoretical terms, broadly define a continuum of adaptive death between two extreme cases. At one extreme, an individual within a community self-destructs to benefit their kin (adaptive altruistic death); at the other, an individual is killed by their kin for their benefit. In both cases, death increases the inclusive fitness of the deceased. As one moves along the continuum from suicide to killing (figure 1b), the agency (i.e. active role) of the one that dies decreases, and the agency of the beneficiaries increases, initially through harmful social cues and signals, and eventually through weapons (e.g. toxins). According to this account, to be defined as such, adaptive altruistic death must entail a substantial element of agency by the one that dies, i.e. it must result predominantly from self-destruction (suicide). By contrast in adaptive killing of kin, death is largely caused by action of others (figure 1b). But both are forms of adaptive death.
Distinguishing adaptive altruistic death from adaptive killing of kin is easy in some cases but difficult in others. For example, in S. cerevisiae, the ammonia gradient that provides a social cue for the decision by individual cells to undergo adaptive death is a property of the overall colony [20]. This appears not to be a case of one group of cells killing another, and so may be defined as altruistic, adaptive death. By contrast, in B. subtilis, the toxins produced by sporulating cells (SKF peptide and SdpC protein) appear to be relatively non-specific in their action, inducing leakage in cellular membranes. SdpC also acts as a signalling protein increasing susceptibility of non-sporulating cells to toxins [28]. Resistance to this toxin in sporulating cells is, at least partly, determined by pumping toxins outside the cell [32,33]. Thus, this appears to be an example of adaptive killing of kin.
(b). Enhancing adaptability of kin: heterogenetic adaptive death
A different mode of adaptive death is seen among Streptococcus and Vibrio species [34]. For example, during exponential growth in S. pneumoniae, accumulation of competence-stimulating peptide causes a subpopulation of cells to acquire competence (ability to uptake exogenous DNA), and also causes them to produce bacteriocins that selectively kill non-competent cells [27]. The death of the latter provides competent kin with new genetic material (new as a result of earlier mutagenesis), which is thought to improve survival in a changing environment [35]. Given the type of benefit provided by such adaptive killing of kin, it may be described as heterogenetic adaptive death. Heterogenetic adaptive death also appears to occur in other bacterial species capable of natural competence [29,36].
5. Adaptive death in colonial metazoans
Adaptive death appears to be relatively common in colonial unicellular organisms, but does it occur in colonial metazoans? We recently argued that C. elegans can be considered to be a colonial organism in which adaptive death could evolve owing to benefits from consumer sacrifice [7]. In its boom-and-bust ecology (figure 1c), a food source (typically a rotten plant stem) is encountered by dauer larva propagules (typically 3–7) [37]. These then develop into adults, reproduce and generate new dauers for dispersal and further colony establishment [38]. Caenorhabditis elegans populations are largely clonal, consisting of self-fertilizing hermaphrodites. An important detail is that C. elegans hermaphrodites are protandrous (generating first sperm and then oocytes); the resulting limitation to sperm number leads to cessation of reproduction only 2–3 days after sexual maturity, potentially favouring adaptive death [7].
To probe further the plausibility of adaptive death in C. elegans, we recently created a computer model based on an approximation of C. elegans life history [17]. This involved colonization and consumption of a food patch, with colony fitness measured as dauer yield. The behaviour of simulations showed that, as predicted, colony fitness was increased by shorter life when reproductive span was short (cf. protandry). Moreover, shorter life was more beneficial when adult food consumption was higher, i.e. death is more adaptive when parents are greedier [17].
(a). Minimizing futile food consumption to maximize Caenorhabditis elegans colony fitness
Caenorhabditis elegans adults show a marked age decline in feeding rate that begins within days of sexual maturation [39]. In model simulations, a sharp age decline in feeding rate increased colony fitness [17]. This suggests that the age decline in feeding rate in C. elegans may represent adaptive ageing, i.e. a functional decline that may decrease individual fitness but enhances colony fitness by increasing food availability for kin. This illustrates how consumer sacrifice can be effected by adaptive behavioural ageing as well as adaptive death.
This and other properties of the model draw attention to a previously little-considered feature of C. elegans ecology: how colony fitness is increased by maximizing efficiency of conversion of food into dauers. This requires minimization of non-productive food consumption, which can be achieved in a variety of ways: not only by killing off post-reproductive adults, but also by tuning down their food consumption, and by optimizing population structure to avoid an excess of larvae that will starve before reaching the dauer stage. Thus, optimization of fitness involves trade-offs between individual fitness and colony fitness: if the latter is increased by reducing the former, then reduced individual fitness may be favoured by natural selection (figure 1c). Our modelling supports the view that the consumer sacrifice type of adaptive death at least partly accounts for the very short lifespan of C. elegans.
(b). Programmed reproductive senescence in Caenorhabditis to increase colony fitness?
One unexpected behaviour of the model was that where fecundity was high, shorter reproductive span could increase colony fitness, apparently because it results in a population structure that reduces futile food consumption [17]. This could explain paradoxical findings reported more than a decade ago, as follows. In selfing hermaphrodites, reproduction ceases after only 2–3 days owing to sperm depletion, but mating with males can provide sufficient sperm to sustain reproduction for many more days. One would, therefore, expect that mating would result in sustained progeny production rate, but in fact it shows a rapid age decline [40] (figure 1d). This is also true for females from the gonochoristic species Caenorhabditis remanei [41]. This reproductive decline in C. elegans is accompanied by visible deterioration and atrophy of the gonad [40,42]. Thus natural selection appears to have favoured early reproductive senescence; as put by Hughes et al. [40, 0264]: ‘C. elegans is not engineered to generate the maximum possible number of progeny. We speculate that there is an optimal number of F1 progeny, and reproductive aging contributes to the ability of the animal to generate the optimal progeny number’. Consistent with this, the behaviour of our in silico model suggests that early reproductive senescence shapes colony population structure in order to minimize futile food consumption, thereby maximizing colony fitness.
6. Does adaptive death occur in semelparous fish?
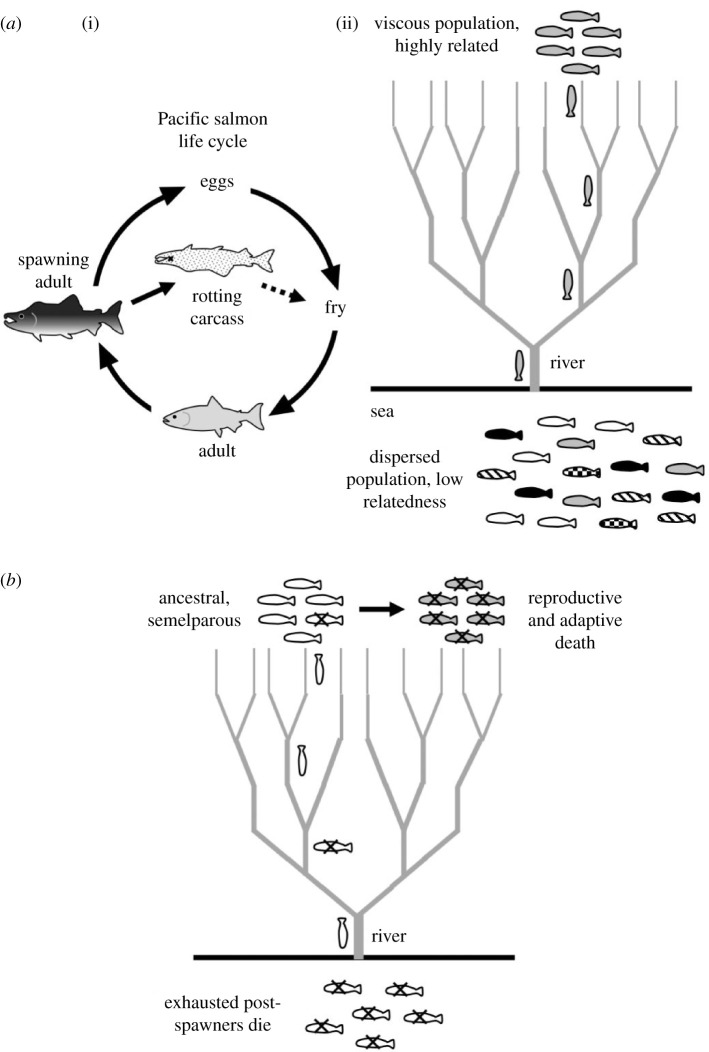
Is adaptive death restricted to colonial microorganisms or could it evolve in more complex taxa, such as vertebrates? It has been suggested that adaptive death occurs in some species of salmon [43,44]. Rapid death following reproduction is well documented as part of the semelparous life history of Pacific salmon such as Oncorhynchus nerka, and is usually interpreted as a non-adaptive by-product of massive reproductive effort (reproductive death) [45]. However, it has also been suggested that the presence of large numbers of decomposing salmon carcasses in the nutrient-poor upper reaches of rivers and streams provides nutrients, particularly nitrogen and phosphorus, that can support growth of phytoplankton and zooplankton upon which salmon fry feed [44] (figure 2a). Salmon fry (Oncorhynchus spp.) have also been observed to directly consume adult carcass biomass [46]. Notably, the death rate from starvation in salmon fry can reach 90%, underscoring the potential benefits from carcasses [47]. Thus, adult salmon could exhibit biomass sacrifice adaptive death.
Figure 2.
Hypothesis: evolution of adaptive death in Pacific salmon. (a) (i) Pacific salmon life cycle, including hypothetical biomass sacrifice adaptive death [44]. Dotted arrow: post-mortem resource transfer from post-spawning adult to fry (juvenile fish). (ii) How Pacific salmon could meet the conditions for evolution of adaptive death: viscous populations of closely related individuals. Thanks to their homing capacity, closely related groups of salmon leave the dispersed, outbred population in the sea and concentrate within their natal streams. (b) The double death hypothesis: adaptive death co-evolved with semelparous reproductive death. The figure describes a hypothesis about how adaptive death evolved in Pacific salmon from a purely semelparous ancestor. In the ancestral species (as in modern Atlantic salmon, Salmo salar), exhausted post-spawning adults swim or are carried down-river with only a small chance of further reproduction. Evolution of mechanisms to cause rapid death after spawning increases fitness via biomass sacrifice adaptive death (nutrients from decomposing carcasses increase survival of fry). Given the low fitness of post-spawning adults, evolution of adaptive death involves only a small loss of individual fitness.
But is adaptive death in salmon really plausible from an evolutionary perspective? We have argued that adaptive death can evolve in clonal, viscous populations, but salmon are dioecious and do not exist as clonal populations. However, at the time of putative adaptive death, large numbers of closely related individuals do form relatively viscous populations.
Most salmon hatch and develop in streams, and then migrate to the sea where they hunt and grow for up to several years before returning to fresh water to spawn [48]. Salmon are famous for their ability to return to the streams of their birth, apparently using magnetoreceptive navigation to locate their home coastal area, and then olfactory cues to locate their natal stream [49]. The proportion of returning adults that correctly locate the stream of their birth varies between species, from 73 to 98% [50,51]. The rates of successful homing and selection on the one side (isolation) and straying (homogenization) determine genetic structure and evolution of salmon. The fact that the strays are less fit at their new locations is further evidence of reproductive isolation [52]. The presence in different rivers of distinct salmon subpopulations with unique local adaptations is taken into account in salmon conservation strategy [53]. For example, steelhead salmon from the Columbia River in Oregon are resistant to the cnidarian parasite Ceratonova shasta, whereas steelhead from the Siletz River experience up to 98% mortality from the parasite [54]. Relative gene diversity within populations between years is almost negligible (0.03–0.2%), consistent with enduring existence of distinct local populations [55]. Moreover, there is evidence that even local salmon populations are not panmictic (all can mate with all), but exist as metapopulations with hierarchical structure [56,57]. Taken together, these findings imply that adult Pacific salmon returning to spawn in their streams of origin are highly related to one another. Moreover, the decreasing volume of streams as salmon swim up-river cause populations to become increasingly viscous. We suggest that high relatedness and population viscosity in spawning salmon populations help ensure that benefits from parental biomass sacrifice are received by kin (figure 2a). This might also apply to lampreys, where, again, death occurs immediately after spawning and carcasses bring nutrients to the streams in which they have spawned [58]. The possible occurrence of adaptive death in salmon and lampreys, and indeed other species exhibiting natal phylopatry [14], warrants further investigation.
7. Coevolution of reproductive death and adaptive death: double death
Recent work from our research group suggests that, like salmon, C. elegans hermaphrodites exhibit semelparous reproductive death as well as adaptive death [7,17,59]. This could either be a remarkable coincidence, or reflect coevolution of the two traits. One possibility is that adaptive death more readily evolves in organisms that undergo reproductive death [7]. According to Hamilton's rule, an altruistic behaviour can be favoured by natural selection when rB > C, where r is the relatedness, B is the benefit to the recipient and C is the cost to the donor [60]. After reproduction in semelparous organisms, their individual fitness becomes very small or negligible, and, therefore, so does C. This means that adaptive death involves little or no individual fitness cost, but only potential inclusive fitness benefits; similar reasoning was used recently to account for the evolution of mass cell suicide in E. coli [61], an example of defensive sacrifice false adaptive death [7]. In the case of Pacific salmon, this suggests that their ancestors will have reproduced and then swum away to gradually die down-river, but natural selection favoured demes where death occurred rapidly at the site of spawning (figure 2b). These arguments predict that adaptive death is likely to occur in other semelparous organisms that are non-clonal and non-colonial, and that other examples of such double death (reproductive and adaptive death) exist in nature.
8. Adaptive death: theoretical approaches
The existence of adaptive death in clonal populations can be explained by kin selection since clonal colonies can be considered as ‘super-organisms’. Here, because relatedness r = 1, Hamilton's rule rB > C becomes B > C and is more easily satisfied, and the cost C of the focal individual's death is outweighed by the benefit B of many kin. As shown by Travis [13] using computer models, this can also be true in spatially and age-structured populations (individuals were stratified by age as their reproductive value decreased with age). The main condition for kin selection to operate in Travis's model was low dispersal as a way for a focal individual to pass resources (a cell in the grid) to its relatives.
An alternative and complementary approach to kin selection to address the mechanisms of natural selection is multilevel selection (MLS). Despite being rejected conventionally in the 1970–1980s, this approach is developing and has received more support recently [62]. The condition for MLS can be deduced from the Price equation as = cov(W, G) + cov(ΔW, ΔG) > 0, where is the mean fitness of the parent population (here the circumflex (or hat) denotes the mean), Δ is the change in the mean gene frequency between offspring and parental populations, cov(W, G) is the term in the equation responsible for between-group selection and cov(ΔW, ΔG) is the term in the equation responsible for within-group selection. The mathematical equivalency of MLS and kin selection was recently demonstrated [63].
The two approaches are complementary in the sense that kin selection is more convenient when trying to establish a phenotype optimum, whereas MLS shows more clearly the strength of selection [64]. The MLS approach can be difficult to interpret as the benefit at the group level can be diminished by within-group selection. This is why group adaptation is more difficult to prove and one of the requirements for its justification is the absence of selection within the group [65]. Where it is possible is in clonal populations, e.g. colonies of bacteria, yeast and even multicellular C. elegans, as described in this review.
Most biological populations are structured by class (e.g. sex, age, caste), so the fitness of a social group can be changed owing to rearrangement of classes, which is not relevant for genes and natural selection. A recent approach makes it possible to account for the difference in social group members' reproductive quality and considers covariates between classes of different groups rather than groups themselves [66]. While considering adaptive death in clonal populations is relatively straightforward, application of MLS to populations with class structure and lower level of relatedness might be useful. Gardner [66] also makes a distinction between ‘aggregate’ (where group value is the mean of individuals' values in the group) and ‘emergent’ (where group values, such as sex ratio, or food source size in the case of C. elegans, are difficult to define at the individual level) features of groups, and argues that the genetic theory of MLS can account for ‘emergent’ traits [66].
Kin selection and MLS are complementary approaches. However, kin selection is currently the better developed and more widely used approach, and, importantly, allows the focal individuals can be defined. It is more general than MLS as it does not need group selection to operate; also, when groups exist they can overlap in the kin selection approach, which is not true for MLS. Both approaches could potentially be applied to establish whether adaptive death is able to evolve in non-clonal populations where there is a population structure, lack of resources and opportunities for transfer of resources from parents to their kin.
9. Conclusion
This essay illustrates how high levels of social interaction can lead to suicide and murder that promotes inclusive fitness. Adaptive death is a form of extreme altruism that currently appears to be largely restricted to organisms showing a level of relatedness, social organization and physical association so high that they possess features of higher order individuals: forming colonies of unicells with some metazoan features, or colonies of metazoans with some super-organismal features. It is part of a broader phenomenon, where colony-level fitness is enhanced by loss of fitness at the individual level. The apparent presence of adaptive death in semelparous fish suggests that there may be other interesting examples of such socially mediated, indirect fitness benefits of ageing or death in the animal and plant kingdoms.
Acknowledgements
We thank J. Bähler, N. Bayyoud, A. F. G. Bourke, H. Chapman, C. C. Kern, C. R .L. Thompson and Y. Zhao for useful discussion and critical comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
E.R.G. and D.G. wrote the manuscript, and D.G. created the figures.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Wellcome Trust Strategic Award (098565/Z/12/Z), and a Wellcome Trust Investigator Award (215574/Z/19/Z).
Glossary of key terms
- Adaptive altruistic death
A form of adaptive death where the suicide of a focal individual is a selected trait, and death leads to its increased inclusive fitness (or suicide of altruists to increase the fitness of the whole group if group selection theory is applied).
- Adaptive killing of kin (new term)
A form of adaptive death where the killing of a focal individual by its kin is a selected trait, which increases inclusive fitness for the victim (or killing of a less fit subgroup to increase the fitness of the whole group if group selection theory is applied).
- Adaptive reproductive senescence (new term)
Where programmed reproductive senescence in individuals promotes inclusive or group (colony) fitness, e.g. by the optimization of population structure to maximize efficiency of resource use for propagule production [17].
- Biomass sacrifice
A mechanism by which adaptive death promotes fitness, where death enhances transfer of an individual's own biomass to its kin, thereby increasing reproduction of kin. This increases the inclusive fitness of the focal individual, and (from another perspective) increases group (colony) fitness [7].
- Consumer sacrifice
A mechanism by which adaptive death promotes fitness, whereby an individual decreases its food consumption to increase food availability for its kin, thereby increasing their reproduction. This increases the inclusive fitness of the focal individual, and (from another perspective) increases group (colony) fitness [7].
- Defensive sacrifice
A mechanism by which adaptive death promotes fitness, where by dying an individual protects its kin from attack, e.g. by predators, host immunity or pathogens [7].
- Double death (new term)
Where both semelparous reproductive death and adaptive death occur in the same organism, proposed to occur in C. elegans [7] and Pacific salmon (this essay). In species that undergo reproductive death, adaptive death after reproduction involves zero fitness cost and hence is expected to evolve more easily.
- Heterogenetic adaptive death (new term)
A mechanism by which adaptive killing of an individual passes its genetic material to its kin killers, which increases their adaptability and therefore the inclusive fitness of the victim (or whole group fitness if group selection is considered).
References
- 1.Rose MR. 1991. Evolutionary biology of aging. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Medawar PB. 1952. An unsolved problem of biology. London, UK: H.K. Lewis. [Google Scholar]
- 3.Williams GC. 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution 11, 398-411. ( 10.1111/j.1558-5646.1957.tb02911.x) [DOI] [Google Scholar]
- 4.Hamilton WD. 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12-45. ( 10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 5.Charlesworth B. 1994. Evolution in age-structured populations, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Kenyon C, Chang J, Gensch E, Rudener A, Tabtiang R. 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461-464. ( 10.1038/366461a0) [DOI] [PubMed] [Google Scholar]
- 7.Lohr J, Galimov ER, Gems D. 2019. Does senescence promote fitness in Caenorhabditis elegans by causing death? Ageing Res. Rev. 50, 58-71. ( 10.1016/j.arr.2019.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon C. 2005. The plasticity of aging: insights from long-lived mutants. Cell 120, 449-460. ( 10.1016/j.cell.2005.02.002) [DOI] [PubMed] [Google Scholar]
- 9.Galimov ER, Lohr JN, Gems D. 2019. When and how can death be an adaptation? Biochemistry 84, 1433-1437. ( 10.1134/S0006297919120010) [DOI] [PubMed] [Google Scholar]
- 10.Charlesworth B, Charnov EL. 1981. Kin selection in age-structured populations. J. Theor. Biol. 88, 103-119. ( 10.1016/0022-5193(81)90330-1) [DOI] [PubMed] [Google Scholar]
- 11.Markov AV. 2012. Can kin selection facilitate the evolution of the genetic program of senescence? Biochemistry 77, 733-741. ( 10.1134/S0006297912070061) [DOI] [PubMed] [Google Scholar]
- 12.Dytham C, Travis JMJ. 2006. Evolving dispersal and age at death. Oikos 113, 530-538. ( 10.1111/j.2006.0030-1299.14395.x) [DOI] [Google Scholar]
- 13.Travis JMJ. 2004. The evolution of programmed death in a spatially structured population. J. Gerontol . 59A, 301-305. ( 10.1093/gerona/59.4.B301) [DOI] [PubMed] [Google Scholar]
- 14.Bourke AFG. 2007. Kin selection and the evolutionary theory of aging. Annu. Rev. Ecol. Evol. Syst. 38, 103-128. ( 10.1146/annurev.ecolsys.38.091206.095528) [DOI] [Google Scholar]
- 15.Zhao X, Promislow DEL. 2019. Senescence and ageing. In The Oxford handbook of evolutionary medicine (eds Brüne M, Schiefenhövel W), pp. 167-208. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Ogrodnik M, Salmonowicz H, Gladyshev VN. 2019. Integrating cellular senescence with the concept of damage accumulation in aging: relevance for clearance of senescent cells. Aging Cell 18, e12841. ( 10.1111/acel.12841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galimov ER, Gems D. 2020. Shorter life and reduced fecundity can increase colony fitness in virtual C. elegans. Aging Cell 19, e13141. ( 10.1111/acel.13141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fröhlich K.-U, Madeo F. 2000. Apoptosis in yeast—a monocellular organism exhibits altruistic behaviour. FEBS Lett. 473, 6-9. ( 10.1016/S0014-5793(00)01474-5) [DOI] [PubMed] [Google Scholar]
- 19.Váchová, L., Čáp M, Palková Z. 2012. Yeast colonies: a model for studies of aging, environmental adaptation, and longevity. Oxid. Med. Cell. Longev. 2012, 601836. ( 10.1155/2012/601836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Váchová, L, Palková, Z. 2005. Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia. J. Cell Biol. 169, 711-717. ( 10.1083/jcb.200410064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. 2016. Myxobacteria: moving, killing, feeding, and surviving together. Front. Microbiol. 7, 781. ( 10.3389/fmicb.2016.00781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nariya H, Inouye M. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 132, 55-66. ( 10.1016/j.cell.2007.11.044) [DOI] [PubMed] [Google Scholar]
- 23.Allocati N, Masulli M, Di Ilio C, De Laurenzi V. 2015. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis. 6, e1609. ( 10.1038/cddis.2014.570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GP. 2009. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc. Natl Acad. Sci. USA 106, 894-899. ( 10.1073/pnas.0808832106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popp PF, Mascher T. 2019. Coordinated cell death in isogenic bacterial populations: sacrificing some for the benefit of many? J. Mol. Biol. 431, 4656-4669. ( 10.1016/j.jmb.2019.04.024) [DOI] [PubMed] [Google Scholar]
- 26.Veening J-W, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 62, 193-210. ( 10.1146/annurev.micro.62.081307.163002) [DOI] [PubMed] [Google Scholar]
- 27.Guiral S, Mitchell TJ, Martin B, Claverys J-P. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl Acad. Sci. USA 102, 8710-8715. ( 10.1073/pnas.0500879102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González-Pastor JE, Hobbs EC, Losick R. 2003. Cannibalism by sporulating bacteria. Science 301, 510-513. ( 10.1126/science.1086462) [DOI] [PubMed] [Google Scholar]
- 29.López D, Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol. Rev. 34, 134-149. ( 10.1111/j.1574-6976.2009.00199.x) [DOI] [PubMed] [Google Scholar]
- 30.Darwin C. 1860. The voyage of the Beagle. Ware, UK: Wordsworth. [Google Scholar]
- 31.Evans TA, Wallis EJ, Elgar MA. 1995. Making a meal of mother. Nature 376, 299. ( 10.1038/376299a0)7630393 [DOI] [Google Scholar]
- 32.Ellermeier CD, Hobbs EC, Gonzalez-Pastor JE, Losick R. 2006. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell 124, 549-559. ( 10.1016/j.cell.2005.11.041) [DOI] [PubMed] [Google Scholar]
- 33.Meade E, Slattery MA, Garvey M. 2020. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: resistance is futile? Antibiotics 9, 32. ( 10.3390/antibiotics9010032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veening J.-W, Blokesch M. 2017. Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat. Rev. Microbiol. 15, 621. ( 10.1038/nrmicro.2017.66) [DOI] [PubMed] [Google Scholar]
- 35.Wei H, Håvarstein LS. 2012. Fratricide is essential for efficient gene transfer between pneumococci in biofilms. Appl. Environ. Microbiol. 78, 5897-5905. ( 10.1128/AEM.01343-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blokesch M. 2016. Natural competence for transformation. Curr. Biol. 26, R1126-R1130. ( 10.1016/j.cub.2016.08.058) [DOI] [PubMed] [Google Scholar]
- 37.Richaud A, Zhang G, Lee D, Lee J, Félix MA. 2018. The local coexistence pattern of selfing genotypes in Caenorhabditis elegans natural metapopulations. Genetics 208, 807-821. ( 10.1534/genetics.117.300564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulenburg H, Félix MA. 2017. The natural biotic environment of Caenorhabditis elegans. Genetics 206, 55-86. ( 10.1534/genetics.116.195511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Xiong C, Kornfeld K. 2004. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 101, 8084-8089. ( 10.1073/pnas.0400848101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes SE, Evason K, Xiong C, Kornfeld K. 2007. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 3, e25. ( 10.1371/journal.pgen.0030025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwoinska MK, Kolm N, Maklakov AA. 2013. Sex differences in cognitive ageing: testing predictions derived from life-history theory in a dioecious nematode. Exp. Gerontol. 48, 1469-1472. ( 10.1016/j.exger.2013.09.008) [DOI] [PubMed] [Google Scholar]
- 42.de la Guardia Y, Gilliat AF, Hellberg J, Rennert P, Cabreiro F, Gems D.. 2016. Run-on of germline apoptosis promotes gonad senescence in C. elegans. Oncotarget 7, 39082. ( 10.18632/oncotarget.9681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilby RE, Fransen BR, Bisson PA, Walter JK. 1998. Response of juvenile coho salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss) to the addition of salmon carcasses to two streams in southwestern Washington U.S.A. Can. J. Fish. Aquat. Sci. 55, 1909-1918. ( 10.1139/f98-094) [DOI] [Google Scholar]
- 44.Cederholm CJ, Kunze MD, Murota T, Sibatani A. 1999. Pacific salmon carcasses: essential contributions of nutrients and energy for aquatic and terrestrial ecosystems. Fisheries 24, 6-15. () [DOI] [Google Scholar]
- 45.Finch CE. 1990. Rapid senescence and sudden death. In Longevity, senescence and the genome, pp. 43-119. Chicago, IL: University of Chicago Press. [Google Scholar]
- 46.Piorkowski RJ. 1995. Ecological effects of spawning salmon on several southcentral Alaskan streams. Fairbanks, AL: University of Alaska. [Google Scholar]
- 47.Jonsson B, Jonsson N. 2011. Habitats as template for life histories. In Ecology of Atlantic salmon and brown trout (eds Jonsson B, Jonsson N), pp. 1-21. Berlin, Germany: Springer. [Google Scholar]
- 48.Willson MF. 1997. Variation in salmonid life histories: patterns and perspectives. Portland, OR: US Department of Agriculture, Forest Service. [Google Scholar]
- 49.Lohmann KJ, Lohmann CM. 2019. There and back again: natal homing by magnetic navigation in sea turtles and salmon. J. Exp. Biol. 222, jeb184077. ( 10.1242/jeb.184077) [DOI] [PubMed] [Google Scholar]
- 50.Quinn TP, Kinnison MT, Unwin MJ. 2001. Evolution of chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica 112–113, 493-513. ( 10.1023/A:1013348024063) [DOI] [PubMed] [Google Scholar]
- 51.Shapovalov L, Taft AC. 1954. The life histories of the steelhead rainbow trout (Salmo gairdneri gairdneri) and silver salmon (Oncorhynchus kisutch): with special reference to Waddell Creek, California, and recommendations regarding their management. Sacramento, CA: California Department of Fish and Game. [Google Scholar]
- 52.Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. 2000. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science 290, 516-518. ( 10.1126/science.290.5491.516) [DOI] [PubMed] [Google Scholar]
- 53.Ricker W. 1972. Hereditary and environmental factors affecting certain salmonid populations. In The stock concept in Pacific salmon (eds Simon C, Larkin P), pp. 19-160. Vancouver, Canada: University of British Columbia. [Google Scholar]
- 54.Buchanan DV, Sanders JE, Zinn JL, Fryer JL. 1983. Relative susceptibility of four strains of summer steelhead to infection by Ceratomyxa shasta. Trans. Am. Fish. Soc. 112, 541-543. () [DOI] [Google Scholar]
- 55.Ryman N. 1983. Patterns of distribution of biochemical genetic variation in salmonids: differences between species. Aquaculture 33, 1-21. ( 10.1016/0044-8486(83)90382-4) [DOI] [Google Scholar]
- 56.Altukhov YP, Salmenkova E. 1994. Straying intensity and genetic differentiation in salmon populations. Aquacult. Res. 25, 99-120. ( 10.1111/are.1994.25.s2.99) [DOI] [Google Scholar]
- 57.Dionne M, Caron F, Dodson JJ, Bernatchez L. 2009. Comparative survey of within-river genetic structure in Atlantic salmon; relevance for management and conservation. Conserv. Genet. 10, 869-879. ( 10.1007/s10592-008-9647-5) [DOI] [Google Scholar]
- 58.Weaver DM, Coghlan SM, Zydlewski J, Hogg RS, Canton M. 2015. Decomposition of sea lamprey Petromyzon marinus carcasses: temperature effects, nutrient dynamics, and implications for stream food webs. Hydrobiologia 760, 57-67. ( 10.1007/s10750-015-2302-5) [DOI] [Google Scholar]
- 59.Gems D, Kern CC, Nour J, Ezcurra M. 2020. Semelparity and reproductive death in Caenorhabditis elegans. Preprints 2020, 2020110019. ( 10.20944/preprints202011.0019.v1). [DOI] [Google Scholar]
- 60.Hamilton WD. 1964. The genetical evolution of social behaviour. J. Theor. Biol. 7, 1-52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 61.Granato ET, Foster KR. 2020. The evolution of mass cell suicide in bacterial warfare. Curr. Biol. 30, 2836-2843. ( 10.1016/j.cub.2020.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodnight CJ. 2015. Multilevel selection theory and evidence: a critique of Gardner, 2015. J. Evol. Biol. 28, 1734-1746. ( 10.1111/jeb.12685) [DOI] [PubMed] [Google Scholar]
- 63.Lehtonen J. 2016. Multilevel selection in kin selection language. Trends Ecol. Evol. 31, 752-762. ( 10.1016/j.tree.2016.07.006) [DOI] [PubMed] [Google Scholar]
- 64.Kramer J, Meunier J. 2016. Kin and multilevel selection in social evolution: a never-ending controversy? F1000Research 5, 776. ( 10.12688/f1000research.8018.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardner A, Grafen A. 2009. Capturing the superorganism: a formal theory of group adaptation. J. Evol. Biol. 22, 659-671. ( 10.1111/j.1420-9101.2008.01681.x) [DOI] [PubMed] [Google Scholar]
- 66.Gardner A. 2015. The genetical theory of multilevel selection. J. Evol. Biol. 28, 305-319. ( 10.1111/jeb.12566) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.