Abstract
In this study, the phytochemical, phenolic, flavonoid and bioactive compounds were successfully screened from crude extract of Sargassum wightii by LC-MS analysis after NIST interpretation. Bacterial growth inhibition study result was shown with 24 mm zone inhibition at 200 µg/mL concentration against P. aeruginosa. The increased phenolic content was much closed to gallic acid and the range was observed at 250 μg/mL concentration. In addition, flavonoid contents of the algae extract was indicated more significant with rutin at 200 μg/mL. In result, both the phenolic and flavonoid contents of the extract were more correlated with gallic acid and rutin. Further, the total anti-oxidant and DPPH radical scavenging activities were shown increased activity at 200 μg/mL concentrations. Furthermore, the excellent anti-bacterial alteration result was observed at 200 μg/mL concentration by minimum inhibition concentration. Therefore, the result was revealed that the marine algae Sargassum wightii has excellent phytochemical and anti-oxidant activities, and it has improved anti-bacterial activity against P. aeruginosa.
Keywords: Marine algae, Phytochemical compounds, Anti-oxidant properties, Bacterial inactivation and minimum inhibition concentration
1. Introduction
The unpredictable marine environment is complex process and researcher’s great concern for marine biodiversity and identification of pharmaceutical products (Cai et al., 2020). It is most advantages environment due to the extraordinary nutrients and organic materials. It is produced excellent biodiversity, significant physical biography and increased seafood activations to human (El-Din and El-Ahwany, 2016). It mainly contained several factors including pH, temperature, salinity, additional nutrients, high organic content, velocity and more carbon and nitrogen sources (Ramachandran et al., 2019). The prediction of marine environment research was very low and only few researchers are working the prediction of marine environmental conditions. Sometimes, the intertidal changes were influenced the microbes, seaweed, sponges with unpredictable environmental factors. They all are possible to influence the production of unknown biological activities (Putri et al., 2019). Compared with terrestrial environment, the marine environment is more complex and increased concentration in all nutrients. Sometimes, the organic level is also increasing and decreasing in unpredictable range such as decrease or increase (Zheng et al., 2020). Further, the unpredictable environments increase the booster dose in pharmaceutical drugs and also marine organisms belonging to various levels of the ecological hierarchy from algae, bivalves, crustaceans and fishes (Mehdinezhad et al., 2016). Among the various sources, seaweeds are important algae providing enormous biological properties due to the rich polysaccharides, phytochemical derivatives and bioactive compounds (Angela et al., 2020). Sometimes, the alkaloid and flavonoid content also available very rich in marine algae. All these rich content are exhibited with more anti-oxidant activity. All these properties are detected based on the algae nature (Cyril et al., 2017).
Among the algal family, Sargassum wightii is a brown macroalgae comes under the order of family of Sargassaceae and order of Fucales with more polysaccharides contents (Raman et al., 2014). It has variety of species, presenting throughout the world in the nearest of temporal and tropical region. The habitat of Sargassum wightii is livening in shallow water and coral reefs. S. wightii is presenting in southeast Coast of Tamil Nadu, India and various other parts of Asia, it is highly reported for animal food, food ingredients and fertilizer usage (Dileepkumar et al., 2018). It has more flavonoid content leads to greater anti-oxidant activity suggesting ideal target for investigating presence of bio-molecules for various medical and industrial applications. In addition, the anti-oxidative properties of the O-hetrocyclic analogues from S. wightii have been reported previously by Sastry and Rao (1995). It has the effective enzyme-ligand interactions to synthesis novel kind of pharmacophores and nutraceuticals to combat multi drug resistant bacterial infections (Marudhupandi and Thangappan (2013)). Last 100 years, seaweed is a potential use in fertilizer, fodder and medicinal sources. In addition, these raw materials are used in industry by agar, align and carrageenan and also consumed largely as food in Asian countries. S. wightii is a large, most economically important and also ecologically dominant than other algae (Ramani et al., 2014).
Previously, the more polysaccharides compounds are frequently reported from Sargassum wightii with increased biological and chemical properties (v et al., 2016). Marine algae are three groups based on the pigment production, named as green, red and brown algae. These three algal groups are comes under the family of Chlorophyceae, Rhodophyceae and Phaeophyceae respectively (El-Din and El-Ahwany (2016)). All these algae are structurally diverse characters including rich phytochemical, pharmaceutical, and biomedical properties. Previously, researchers are reported that the algae and its derivatives have excellent biological activities including anti-oxidant, anti-bacterial, anti-fungal and anti-cancer activities (Dileepkumar et al., 2018). Importantly, the notable compounds form seaweeds are very effective against some of the biological activities including Sesquiterpenes, acyclic diterpenes, Atlantic, two diterpenoids with a novel skeleton, dictyterpenoids A and B, tetraterpenes, Oleanane-type triterpene (Raman et al., 2014, Angela et al., 2020, Cyril et al., 2017, Putri et al., 2019). Based on this above facts, we have focused on phytochemical properties of Sargassum wightii extract and its anti-oxidant properties against P. aeruginosa.
2. Materials and methods
2.1. Collection and processing of seaweed
The fresh, undamaged brown algae Sargassum wightii was collected on the Sea shores of Mandapam Region, Ramanathapuram District, Southeast coast of Tamil Nadu, India. The collected samples were washed by tap water and stored in ice box for transferred to lab. The collected Seaweeds were confirmed as Sargassum wightii after authenticated with Dr.N.Manoharan, Department of Marine Sciences, Bharathidasan University, Tiruchirapplli, Tamil Nadu, India. The collected seaweed were soaked on double distilled water containing beaker and washed three times for removal of surface contaminations. Following alcohol treatment was done for removal of free floating microorganisms on the seaweed surface. Finally, the washed sample was kept in dry for 15 days in room temperature at dark condition. After complete dry, the seaweed samples were ground to get powder form. After receive the thin powder using sieving, the sample was tightly packed and covered by black cloth for protected from sunlight and stored in 4 ℃ for further use.
2.2. Crude extract preparation
The powdered sample of Sargassum wightii was used to prepare the crude extract for further identifications using ethanol as a solvent used for get a better yield (Sastry and Rao (1995)). The 20 g of powder sample was mixed in 1 L ethanol solution for extraction of crude extract using Soxhlet apparatus at the required temperature until the color of the solvent become clear or 12 h. The crude extract of the sample was received after complete solvent removal using the rotary vacuum evaporator. Then, the extracted sample was filled in 50 g volume of container. Finally, the yield of 25 g powder material was calculated by the bellowed formula,
| (1) |
2.3. Phytochemical screening of crude extract
The complete profile of the crude extract including essential oils, phenol, alkaloids, flavonoids, tannins, terpenoids, alcohols, acids, esters, steroids, amino and organic compounds were qualitatively and quantitatively analyzed by LC-MS based on the previous report of Bibi and Mohamad Fawzi (2020). In LC-MS, 30 m × 200 μm × 0.30 μm of capillary column was fixed automatically, it made up of CP-Silicon 5 of chrome pack. In this process, 60℃ for 5 min was set as initially and extended up to 150 °C for 15 min. The injector coupled detector and constant temperature was maintained at up to 200–300 °C. Additionally, 1 mL/min flow rate with carrier gas of 30 cm/s with linier velocity were arranged. The flowed sample was used for detect the available chemical constituents of the tested seaweed of Sargassum wightii based on the peaks and their respective retention time and occupied percentages. The obtained peaks and their respective chemical derivatives were interpreted by NIST Wiley library of Bharathidasan University, Thiruchirappalli, Tamilnadu, India.
2.4. Antimicrobial activity of Sargassum wightii crude extract
The antimicrobial activity performance to identification of Sargassum wightii crude extract against bacteria was done by agar well diffusion experiment. This method was followed by earlier accepted procedure of Lin et al. (2019). Yesterday inoculated well grown P. aeruginosa culture was streaked on muller hinton agar plate with the help of sterile buds. Then 6 mm of 3 distance wells for first well of minimum concentration, second well of maximum of crude extract, third well of distilled water. Then, add 100 µL of crude extract properly in respective wells and kept in inside of the incubator one day. After, the zones of the wells were measured using measuring scale and compared with distilled water well for detect the anti-bacterial effect of Sargassum wightii crude extract.
2.5. Measurement of phenol and flavonoid properties
Based on the previous evidences, the phenol and flavonoid content of the purified crude compound of Sargassum wightii was calculated by folin-ciocalteu’s method, and the result was taken in UV–vis spectroscopy (Rajivgandhi et al., 2020a, Rajivgandhi et al., 2020b). The process of phenolic and flavonoid properties of the purified crude compound was done in a respective 50 mL test tubes including 1 mL of purified extract and 5 mL 10 fold dilution of folin–Ciocalteu’s reagent was reacted in one tube, and Na2 CO3 was mixed in the same tube after reaction. Next, all the sample in the same tube was shaken continuously with 10 min and held at half hour incubation. The reaction mixture content of the tube was measured at 540 nm by using UV-spectrometer. Additionally, the gallic acid with 50–250 µg/mL separately was used for negative control and same concentrations without extract of gallic acid was used for positive control. The result of 1 µg gallic acid was converted to 1 mg equal content of tested sample after comparison of triplicate result of test and control O.D values. Further, the colorimetric analysis result was confirmed the availability of flavonoid content after following the reported procedure of Dussossoy et al. (2011). As same as, in same tube 200 µL of purified extract plus 75 µL of sodium nitrate was taken clearly. Aliquot 1:1 concentration of 1 mg/mL mixed AlCL3 solution plus 1% of potassium iodide solution was added gradually into the wall of the test tube. Then, the mixture solution was shaking vigorously and kept in room temperature 30 min. After incubation, 540 nm of colorimeter was taken for control rutin for comparison and test sample with blank. The test and control samples were used in triplicate and resulted flavonoid content was calculated by 1 µg gallic acid that equal to 1 mg of tested sample.
2.6. Measurement of Anti-oxidant activity of purified extract
Phosphor-molybdenum experiment based procedure was used in this study to detect the total anti-oxidant activity in crude extract of Sargassum wightii (Maneesh and Chakraborty (2018)). Each 5 mL solution of sodium phosphate and ammonium molybdate was taken in a separate test tube. Additionally, 5 mL of H2SO4 was also taken in a test tube. Each 2 mL of all the three solution taken together in a same test tube and maintained at water bath at 97 ℃ for 30 min with shaking facility. After cool, 1 mL of well mixed solution was taken in a test tube, followed by 10 µL of Sargassum wightii crude extract was added into the same tube at 37 ℃ for 10 min. Finally, the anti-oxidant production of prepared solution was taken 600 nm O.D using UV-spectrophotometer. The result was compared with control ascorbic acid O.D, and noted the interpreted anti-oxidant result. In the result, 1 µg of ascorbic acid is equal to tested Sargassum wightii crude extract.
2.7. DPPH free radical scavenging assay
The ability of available radical scavenging activity in the crude extract of Sargassum wightii was calculated by DPPH assay (Putri et al., 2019). 150 µL DPPH and different concentration of crude extract of Sargassum wightii was taken together in the sterile test tube. Instead, the sterile test tube was filled with 5 µL of butylated hydroxyl toluene with DPPH was acted as an experiment control. Additionally, ethanol was used for control for this experiments. All the tubes were allowed to maintain at room temperature with 45 min. Finally, the formed anti-oxidant activity tubes were monitored under UV–vis spectrophotometer at O.D of 600 nm. The anti-oxidant result was noted after interpretation with control tube result using bellowed equation,
| (2) |
2.8. Minimum inhibition concentration
Overnight P. aeruginosa culture was used in this study with different purified extract concentration into the 96-well polystyrene plate wells by microtitre plate reader (Rajivgandhi et al., 2020a, Rajivgandhi et al., 2020b). Firstly, the confluent culture of P. aeruginosa volume was 10 µL in all the wells of 96-well polystyrene plate and 25–200 µg/mL of purified extract of Sargassum wightii was added into the same plate and slightly shaken. Then, the plate was put in inside of the incubator and set 37 ℃ with one day. After one day, the high and low turbidity of the wells were observed on the naked eye and followed by taken O.D values using spectrophotometer with triplicate. The result was MIC was considered as which well was shown with very low turbidity in very low concentration. That concentration was fixed as a MIC. The following formula was used to analyze the percentage conversion of identified test and control results. MIC is referred as the lowest concentration of extract was inhibited the highest bacterial growth.
Inhibition (%) = 100 (O.D purified extract of Sargassum wightii – O.D only pathogen) × 100 (2).
3. Result
3.1. Phytochemical screening of crude extract of Sargassum wightii
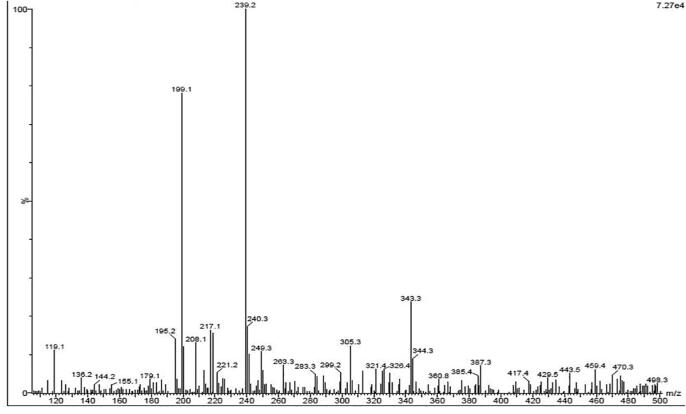
The LC-MS peaks of the Sargassum wightii was showed with more peaks that including 30% of phytochemical derivatives, 20% of phenol, flavonoid, alkaloid, 40% bioactive compounds and other organic compounds of aromatic hydrocarbons. Among the all peaks and their respective chemical composition, the retention time, occupation area, occupation percentages are effectively identified. The retention time, occupied area and occupied percentages of Sargassum wightii extract was correlated with respective 25 peaks of previous reports with anti-oxidant and biological activities, and also confirmed by NIST data base of the Bharathidasan University Wiley library (Fig. 1). In the result, the important peaks of 2,6-Di-tert-butyl, 1,4-benzoquinone, benzene, 1,1′-[oxybis(methylene)]bis, dodecanoic acid, phenol Oleanolic acid, 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3, hexadecane, (1-hydroxypenta-2,4-dien1-yl)oxy)anthracene-9,10-dione, trifluoroacetoxy hexadecane were observed based on the previously reported anti-microbial activities. This result was indicated that the chosen Sargassum wightii extract was suitable seaweed for inhibition of pathogens. Very recently, was reported that the seaweed synthesized chemical and aromatic compounds have extra ordinary anti-bacterial properties. This statement was agreed by Rajivgandhi et al., 2020a, Rajivgandhi et al., 2020b, the increased biological activities of marine sources depends on unpredicted environmental sources like extreme temperature, carbon, nitrogen content, pH, salinity and other organic nutrients. Seaweed mediated phytochemical derivatives have the ability to inhibit in inside of the pathogens and it altered the virulence genes like quorum sensing and biofilm formation. The anti-bacterial activity effect was increased in the Sargassum wightii extract against multi drug resistant bacterial due to the influence of different environmental conditions (Raman et al., 2014, Xinjun et al., 2020). The LC-MS was used to detect the complete phytochemical derivatives, aromatic hydrocarbons, and other polysaccharide mediated compounds. Recent reports of our previous study was supported to present result and it confirmed that the algae Turbinaria ornate and Caulerpa taxifolia have more phytochemical derivatives and it shown more anti-bacterial activity against multi drug resistant bacteria (Naiyf et al., 2020, Danjie et al., 2020).
Fig. 1.
Phytochemical screening of available chemical compounds of Sargassum wightii extract by LC-MS analysis.
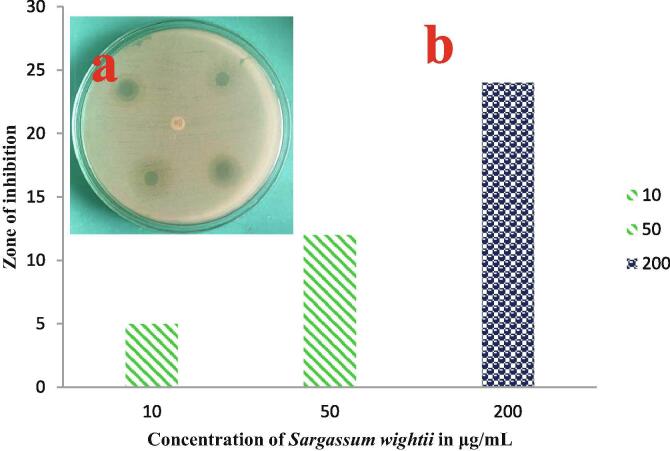
3.2. Antimicrobial activity of P. aeruginosa by Sargassum wightii crude extract
The zones of maximum and minimum inhibitions of the wells were shown with 12 and 24 mm against P. aeruginosa containing plate. In this assay, the 50 µg/mL for minimum and 200 µg/mL for maximum concentration were used respectively (Fig. 2). The result was suggested that the crude extract of Sargassum wightii has antimicrobial activity. These zones and their respective concentrations are surprisingly very excellent compared with previously reported crude extract of Sargassum wightii (Hanjabam et al., 2019). This may be increased due to the under estimated environment of marine habitat including pH, temperature, organic materials, surface light observation, NaCl, car bon and nitrogen sources (Kumar and Sahoo (2017)). This information as agreed by Antonisamy et al. (2012) and changed environmental factors were influenced the biological properties. Sometimes, the bioactive compounds, phytochemical derivatives were also very improved activities due to the influence of unpredictable environment (Anjana et al., 2014). Similar result was also reported by previous report of crude extract of Sargassum wightii against gram negative bacteria with higher biological activities. Sargassum wightii has more polysaccharides compounds compared with other algae, this polysaccharides have the ability to invade inside of the pathogens and damaged the nucleus effectively. After enter the nucleus, it affected entire bacteria and arrested the cell cycle growth that leads to cell death (Kumar et al., 2015). Finally, the result was suggested that the Sargassum wightii has increased antimicrobial activity against gram negative bacteria compared to other algal extract.
Fig. 2.
Detection of antimicrobial efficacy of Sargassum wightii crude extract against P. aeruginosa.
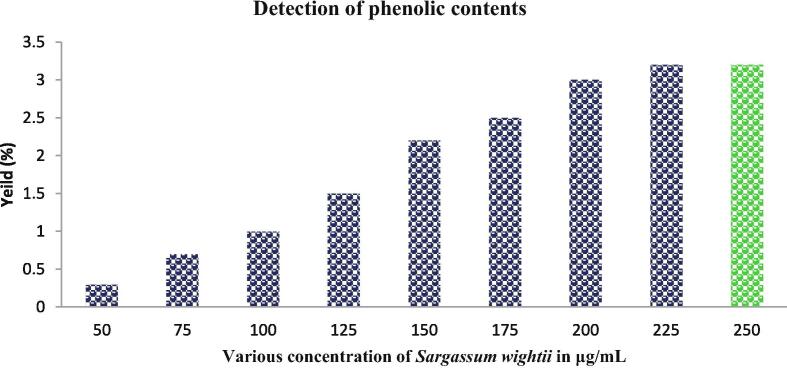
3.3. Detection of phenolic content of Sargassum wightii crude extract
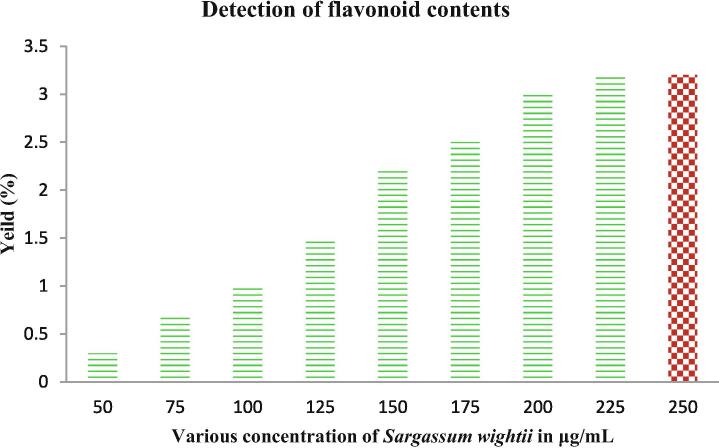
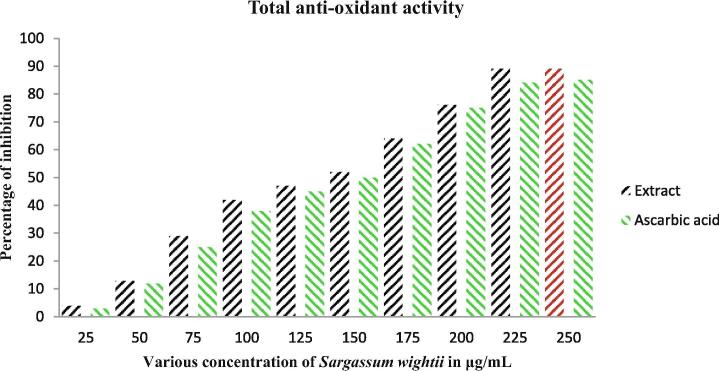
Our result was shown with rich content of phenolic and flavonoid in the crude extract of Sargassum wightii after compared with respective control of gallic acid and rutin correlation. The available phenolic and flavonoid properties of the extract were given for both in 25–250 μg/mL concentration (Fig. 3). This concentration was very low for Sargassum wightii when compared with other seaweed extracts that reported previously. In addition the respective controls of the gallic acid and rutin was exhibited more phenols and flavonoids and it correlated highly for Sargassum wightiiI only. Additionally, the total sugar and protein level was too low in this study compared with phenol and flavonoid (Fig. 4). The polysaccharide rich seaweed extract has rich phenols and flavonoids contents as well as low level of proteins and sugars (Maneesh and Chakraborty, 2017a, Maneesh and Chakraborty, 2017b). Interestingly, Sivanandhan et al. (2015) stated that the phenol and flavonoid content of the seaweed extract has more anti-oxidant activity.
Fig. 3.
Available phenolic content of Sargassum wightii crude extract by folin-ciocalteu’s method of inviro study.
Fig. 4.
Presence of flavonoid content in the Sargassum wightii crude extract by colorimetric assay.
3.4. Antioxidant and DPPH scavenging activity
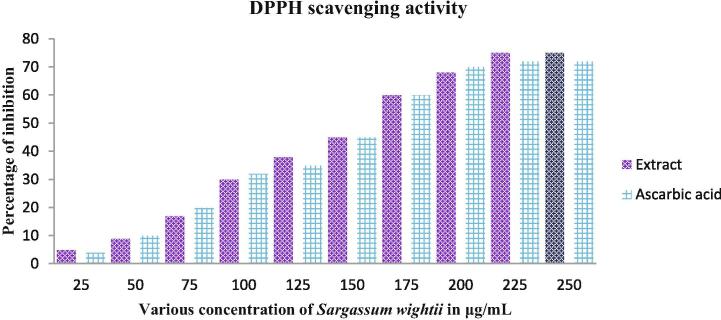
Anti-oxidant activity of Sargassum wightii crude extract was shown with excellent result, and it also more correlated with previous phenol and flavonoid content result. Because, the polysaccharides rich phenols and flavonoids seaweed extract exhibited more biological properties including anti-oxidant, anti-cancer and anti-microbial. In our study, the control result of gallic acid and rutin almost closely related to extract result at the concentration of 200 μg/mL (Fig. 5). Both the gallic acid and rutin was more correlated with extract each other. Therefore, our result was suggested that the anti-oxidant capacity was very high in crude extract of Sargassum wightii. Further, the supportive result of DPPH scavenging assay of Sargassum wightii crude extract was also very effective at 200 μg/mL concentration. Interestingly, the DPPH result was also much closed to control result and suggested the plant mediated anti-oxidant activity was useful for other biological studies (Fig. 6). In result, the extract may influence the scavenging activity at the same 200 μg/mL concentration by the activation of phenol and flavonoid regulatory genes (Vijayanand et al., 2014). The similar result was reported recently by Immanuel et al. (2012) and seaweed based anti-oxidant activity was detected more compared to other plant and microbial extract (Maneesh and Chakraborty (2018)). Also, the phenol, flavonoid rich seaweed extract has more anti-oxidant activity and extended to intracellular damage and high level of ROS generation in infected pathogens (Matías et al., 2020).
Fig. 5.
Detection of anti-oxidant activity of Sargassum wightii crude extract by phosphor molybdenum method.
Fig. 6.
Invitro DPPH scavenging assay of Sargassum wightii crude extract.
3.5. Minimum inhibition concentration
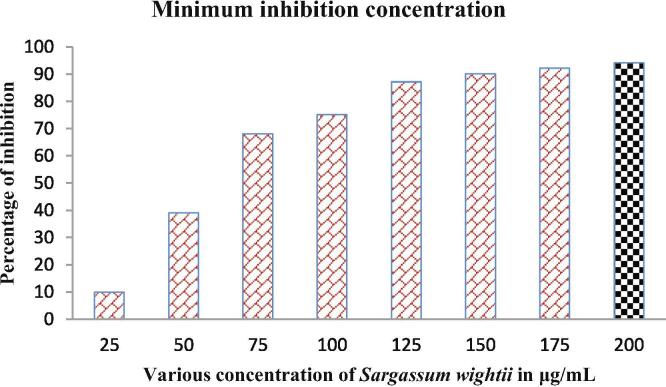
The turbidity based antimicrobial efficiency of Sargassum wightii crude extract was exhibited excellent minimum inhibition concentration at very lowest concentration of 200 µg/mL. In this concentration, the turbidity was looking very low and its originality was changed gradually at increasing concentration. Additionally, the inhibition level was very high at low concentration. Based on the triplicate calculation, the inhibition percentage was 84% at the 200 µg/mL wells and it confirmed that the Sargassum wightii crude extract was more sufficient against P. aeruginosa. The result was suggested that the Sargassum wightii extract was may inhibit the P. aeruginosa culture at concentration dependent mode. Initially, the turbidity was more in 200 µg/mL wells compared with initial well (Fig. 7). Also, the control culture was shown with clear both color after 24 h also. Previously, MIC was one of the excellent method for detect the efficiency of plant and seaweed extract. Recently, Rajivgandhi et al., 2018 agreed our result and the seaweed extract have more virulence deactivation activity. The result was most accordance with previous report of Xinjun et al., 2020, and exopolyscharides, peptidoglycan and theichoic acid of bacterial pathogen was more sensitive to seaweed extract (Maruthupandy et al., 2020). Finally, the 96-well plate result was confirmed that the crude extract of Sargassum wightii was potential anti-bacterial agent against P. aeruginosa at very lowest concentration and it can be used for further invitro studies in future. Recently, the researchers were reported that the seaweed extract of Sargassum wightii and Halimeda gracillis has excellent anti-biofilm activity at decreased concentration (Suganya et al., 2019). In addition, the antioxidative and phytochemical compounds were reported from seaweed of Sargassum wightii (Maneesh and Chakraborty, 2017a, Maneesh and Chakraborty, 2017b; J. Marimuthu et al., 2012, Johnson et al., 2019).
Fig. 7.
Minimum inhibition concentration of Sargassum wightii crude extract against P. aeruginosa.
4. Conclusion
Based on the result, we have concluded that the crude extract of Sargassum wightii has more phytochemical derivatives, bioactive compounds and some hydrocarbons. Also, it has very rich polysaccharides content, exhibited more phenol and flavonoid content with increasing concentration. Also, the anti-oxidant activity of Sargassum wightii was indicated that the extract has an excellent anti-oxidant activity at 200 μg/mL concentration. Further, the antimicrobial activity of Sargassum wightii extract against P. aeruginosa was confirmed at 200 μg/mL concentration. Finally, the result was confirmed that the Sargassum wightii as an excellent anti-oxidant and antimicrobial agent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, ‘‘Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURG-1442-073.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Govindan Ramachandran, Email: ramachandhiranmicro@gmail.com.
Natesan Manoharan, Email: biomano21@yahoo.com.
References
- Angela A., Montserrat S., Amadeu M.V.M.S., Rosa F. Anti-inflammatory drugs in the marine environment: Bioconcentration, metabolism and sub-lethal effects in marine, Bivalves. Environ. Pollut. 2020;263:114442. doi: 10.1016/j.envpol.2020.114442. [DOI] [PubMed] [Google Scholar]
- Anjana A., Nazeer Ahamed K.F.H., Ravichandiran V., Sumithra M., Anbu J. Anticancer activity of Sargassum wightii Greville on Dalton’s ascitic lymphoma. Chin. J. Nat. Med. 2014;12:0114–0120. doi: 10.1016/S1875-5364(14)60018-2. [DOI] [PubMed] [Google Scholar]
- Antonisamy J.M., Essakimuthu P., Narayanan J., Anantham B., Joy R., Tharmaraj J.M., Arumugam S. Phytochemical characterization of brown seaweed Sargassum wightii. Asian Pacif. J. Trop. Dis. 2012:S109–S113. [Google Scholar]
- Bibi S.J., Mohamad Fawzi M. Essential oils from 9 exotic and endemic medicinal plants from Mauritius shows in vitro antibacterial and antibiotic potentiating activities. South African J. Bot. 2020;132:355–362. [Google Scholar]
- Cai Y., Xu Y., Zhao Y., Zhou K., Ma X. A spatial-temporal approach for corrosion prediction in time-varying marine environment using acoustic emission technique in the marine environment. J. Loss Prevent. Process Indust. 2020;66:104161. [Google Scholar]
- Cyril R., Lakshmanan R., Thiyagarajan A. In vitro bioactivity and phytochemical analysis of two marine macro-algae. J. Coast. Life Med. 2017;5:427–432. [Google Scholar]
- Danjie Z., Ramachandran G., Ramzi A.M., Nasir A.S., Riaz U., Omer M.A., Rajivgandhi G., Manoharan N. Biosynthesized silver nanoparticles using Caulerpa taxifolia against A549 lung cancer cell line through cytotoxicity effect/morphological damage. Saudi J. Biolog. Sci. 2020;27:3421–3427. doi: 10.1016/j.sjbs.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepkumar V., Srinivasa Rao M., Misra S., Sugnana Kumari S. Evaluation of different solvent extracts of Sargassum wightii (brown algae) for its antifungal efficacy against silkworm pathogens. J. Entomol. Zoolog. Stud. 2018;6:1125–1130. [Google Scholar]
- Dussossoy E., Brat P., Bony E., Boudard F., Poucheret P., Mertz C., Giaimis J. Chracterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni jiuce (Morinda citrifolia L.) J. Ethnopharmacol. 2011;133:108–115. doi: 10.1016/j.jep.2010.08.063. [DOI] [PubMed] [Google Scholar]
- El-Din S.M., El-Ahwany A.M.D. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea) J. Taibah Univ. Sci. 2016;10:471–484. [Google Scholar]
- Hanjabam M.D., Kumar A., Tejpal C.S., Elavarasan K., Kishore P., Ashok Kumar K. Isolation of crude fucoidan from Sargassum witghtii using conventional and ultrasonication assisted methods. Bioact. Carbohyd. Diet. Fibre. 2019;20:100200. [Google Scholar]
- Immanuel G., Sivagnanavelmurugan M., Marudhupandi T., Radhakrishnan S., Palavesama A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab) Fish & Shellfish Immunol. 2012;32:551–564. doi: 10.1016/j.fsi.2012.01.003. [DOI] [PubMed] [Google Scholar]
- M. Johnson, S. Asha Kanimozhi, T. Renisheya J. J. Malar, T. Shibila, P.R. Freitas, S.R. Tintino, I.R.A. Menezes, J.G.M. da Costa, H.D.M. Coutinho, 2019. The antioxidative effects of bioactive products from Sargassum polycystum C. Agardh and Sargassum duplicatum J. Agardh against inflammation and other pathological issues, Complement. Therap. Med. 46, 19–23. [DOI] [PubMed]
- Kumar S., Sahoo D. A comprehensive analysis of alginate content and biochemical composition of leftover pulp from brown seaweed Sargassum wightii. Algal Res. 2017;23:233–239. [Google Scholar]
- Kumar S., Sahoo D., Levine I. Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res. 2015;9:117–125. [Google Scholar]
- Lin L., Mao X., Sun Y., Rajivgandhi G., Cui H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019;292:21–30. doi: 10.1016/j.ijfoodmicro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Maneesh A., Chakraborty K. Previously undescribed fridooleanenes and oxygenated labdanes from the brown seaweed Sargassum wightii and their protein tyrosine phosphatase-1B inhibitory activity. Phytochem. 2017;144:19–32. doi: 10.1016/j.phytochem.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Maneesh A., Chakraborty K. Unprecedented antioxidative and anti-inflammatory aryl polyketides from the brown seaweed Sargassum wightii. Food Res. Int. 2017;100:640–649. doi: 10.1016/j.foodres.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Maneesh A., Chakraborty K. Previously undescribed antioxidative O-heterocyclic angiotensin converting enzyme inhibitors from the intertidal seaweed Sargassum wightii as potential antihypertensives. Food Res. Internat. 2018;113:474–486. doi: 10.1016/j.foodres.2018.07.035. [DOI] [PubMed] [Google Scholar]
- Marimuthu J., Petchiammal A., Janakiraman E., Babu N., Renisheya A., Jeba J., Tharmaraj M., Arumugam S. Phytochemical characterization of brown seaweed Sargassum wightii. Asian Pacif. J. Trop. Dis. 2012;2:S109–S113. [Google Scholar]
- Marudhupandi T., Thangappan T. Ajith Kumar, Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Current Pharmaceut. J. 2013;2:156–158. [Google Scholar]
- Maruthupandy M., Rajivgandhi G., Kadaikunnan S., Veeramani T., Alharbi N.S., Muneeswaran T., Khaled M., Wen Jun. J., Alanzi F., K Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P aeruginosa and K. pneumonia. Carbohyd. Polym. 2020;230:115646. doi: 10.1016/j.carbpol.2019.115646. [DOI] [PubMed] [Google Scholar]
- Matías A.R., María B.V., Alfonsina E.A., Héctor R.J. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020;36:100651. [Google Scholar]
- Mehdinezhad N., Ghannadi A., Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res. Pharm. Sci. 2016;11:243–249. [PMC free article] [PubMed] [Google Scholar]
- Naiyf S.A., Sami A.A., Ramachandran G., Chenthis Kanisha C., Shine K., Jamal M.K., Khalid F.A., Rajivgandhi G., Manoharan N. Screening of anti-oxidant and anti-bacterial metabolites from brown algae Turbinaria ornata for inhibits the multi-drug resistant P. aeruginosa. J. King Saud Univ. – Sci. 2020;32:3447–3453. [Google Scholar]
- Putri T., Arsianti A., Aya P., Subroto M., Lesmana E. Phytochemical analysis and antioxidant activity of marine algae Eucheuma Sp. AIP Conf. Proc. 2019;2092:030016. [Google Scholar]
- Rajivgandhi, G., Ramachandran, G., Maruthupandy, M., Saravanakumar Subramaniyan, Manoharan, N., Viji, R., 2018. Antibacterial Effect of Endophytic Actinomycetes from 2/8 Marine Algae against Multi Drug Resistant Gram Negative Bacteria. Examines Mar Biol Oceanogr. 1(4). EIMBO.000522.2018.
- Rajivgandhi G.N., Maruthupandy M., Li J.L., Dong L., Alharbi N.S., Kadaikunnan S., Khaled J.M., Alanzi K.F., Li W.J. Photocatalytic reduction and anti-bacterial activity of biosynthesized silver nanoparticles against multi drug resistant Staphylococcus saprophyticus BDUMS 5 (MN310601) Mat. Sci. Engin. C. 2020;114:111024. doi: 10.1016/j.msec.2020.111024. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G.N., Ramachandran G., Maruthupandy M., Manoharan N., Alharbid N.S., Kadaikunnan S., Khaled J.M., Almanaa T.N., Li W.J. Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumoniae. Colloid. Surf. A. 2020;600:124830. [Google Scholar]
- Ramachandran G., Rajivgandhi G., Maruthupandy M., Manoharan N. Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens. Biocat. Agricult. Biotechnol. 2019;17:750–757. [Google Scholar]
- Raman R.P., Parthiban S., Karthikeyan S., Muthuraman M.S., Sivasubramaniyan A. Antimicrobial and anti-inflammatory studies on Sargassum wightii extracts. Int. J. Pharm. Pharm. Sci. 2014;6:611–614. [Google Scholar]
- Ramani R., Parthiban S., Meenakshi Sundaram K.S. Antimicrobial and anti-inflammatory studies on Sargassum Wightii extracts. Int. J. Pharm. And Pharmaceut. Sci. 2014;6:0975–1491. [Google Scholar]
- Sastry V.M.V.S., Rao G.R.K. Dioctyl phthalate, and antibacterial compound from the marine brown alga —Sargassum wightii. J. Appl. Phycol. 1995;7:185–186. [Google Scholar]
- Sivanandhan G., Arunachalam C., Selvaraj N., Alharbi A., Sulaiman Y.P., Lim A. Ganapathi. Expression of important pathway genes involved in with anolides biosynthesis in hairy root culture of Withania somnifera upon treatment with Gracilaria edulis and Sargassum wightii. Plant Physiol. Biochem. 2015;91:61–64. doi: 10.1016/j.plaphy.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Suganya S., Ishwarya R., Jayakumar R., Govindarajan M., Alharbi N.S., Kadaikunnan S., Khaled J.M., Al-anbr M.N., Vaseeharan B. New insecticides and antimicrobials derived from Sargassum wightii and Halimeda gracillis seaweeds: Toxicity against mosquito vectors and antibiofilm activity against microbial pathogens. South Afr. J. Bot. 2019;125:466–480. [Google Scholar]
- Vijayanand N., Sivasangari Ramya S., Rathinavel S. Potential of liquid extracts of Sargassum wightii on growth, biochemical and yield parameters of cluster bean plant. Asian Pacif. J. Reproduct. 2014;3:150–155. [Google Scholar]
- Xinjun Y., Govindan Nadar R., Govindan R., Naiyf S.A., Shine K., Jamal M.K., Taghreed N.A., Natesan M., Rajan, Viji Preparative HPLC fraction of Hibiscus rosa-sinensis essential oil against biofilm forming Klebsiella pneumonia. Saudi J. Biolog. Sci. 2020;27:2853–2862. doi: 10.1016/j.sjbs.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Zhou Y., Zhou Y., Pan T., Sun L., Liu D. Localized corrosion induced damage monitoring of large-scale RC piles. Construct. Build. Mat. 2020;243:118270. [Google Scholar]
Further Reading
- Anastasia G., Paraskevopoulos S., Matsiori S. Public perceptions of the marine environment and behavioral intentions to preserve it: The case of three coastal cities in Greece. Marine Policy. 2020;111:103727. [Google Scholar]
- K. Murugan, M. Roni, C. Panneerselvam, A.T. Aziz, U. Suresh, R. Rajaganesh, R. Aruliah, J.A. Mahyoub, S. Trivedi, H. Rehman, H.A.N. Al-Aoh, S. Kumar, A. Higuchi, B. Vaseeharan, H. Wei, S. Senthil Nathan, A. Canale, G. Benelli, Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera, Physiolog. Mol. Plant Pathol., 101, January 2018, 202–213.