Abstract
Taking into account that fructophilic lactic acid bacteria (FLAB) can play an important role in the health of honey bees and can be used as probiotics, phenotypic properties of probiotic interest of Lactobacillus kunkeei (12 strains) and Fructobacillus fructossus bacteria (2 strains), isolated from Apis mellifera gastrointestinal tract, have been studied. We have evaluated survival of tested FLAB in honey bee gut, their susceptibility to antibiotics (ampicillin, erythromycin, tylosin), cell surface hydrophobicity, auto-aggregation ability, co-aggregation with model pathogenic bacteria, biofilm formation capacity, and effect of studied FLAB, added to sucrose syrup bee diet, on longevity of honey bees. The tested FLAB exhibited good gastrointestinal tract tolerance and high antibiotic susceptibility, which are important criteria in the screening of probiotic candidates. It was also found that all FLAB studied have high cell surface hydrophobicity and fulfil next selection criterion for their use as probiotics. Symbionts of A. mellifera showed also auto- and co-aggregation capacities regarded as valuable features for biofilm formation and inhibition of pathogens adhesion to the bee gut cells. Biofilm-development ability is a desired characteristic of probiotic lactic acid bacteria. As indicated by quantitative crystal violet-stained microplate assay and confocal laser scanning microscopy imaging, all studied A. mellifera gut isolates exhibit a biofilm positive phenotype. Moreover, it was also documented, on honey bees kept in cages, that supplementation of A. mellifera sucrose diet with FLAB decreases mortality and improves significantly longevity of honey bees. Presented research showed that A. mellifera FLAB symbionts are good candidates for application as probiotics.
Keywords: Honey bees, Probiotic properties of lactic acid bacteria, Antibiotic resistance, Biofilm formation
1. Introduction
On the health and survival of honey bees significant impact has the composition of the intestinal microflora (Crotti et al., 2013, Gilliam, 1997, Kwong et al., 2017, Kwong and Moran, 2016, Raymann and Moran, 2018). In the maintaining of the proper gut microbial ecosystem particular role play lactic acid bacteria (LAB), which are natural inhabitants of the honey bee gastrointestinal tract (Endo and Salminen, 2013, Forsgren et al., 2010, Pachla et al., 2018, Pattabhiramaiah et al., 2012, Vásquez et al., 2012). Honey bee LAB symbionts have been shown to be responsible for nutrient assimilation by their hosts, triggering immune response of bees, elimination of pathogens, and sustaining microflora homeostasis in the honey bee gut (Asama et al., 2015, Evans and Lopez, 2004, Raymann and Moran, 2018, Vásquez and Olofsson, 2009). It is known that lactic acid bacteria produce antimicrobial substances which eliminate pathogenic microorganisms (Asama et al., 2015, Crotti et al., 2013, Evans and Lopez, 2004, Forsgren et al., 2010, Jack et al., 1995, Pachla et al., 2018, Servin, 2004, Vásquez et al., 2012, Wu et al., 2014). They also ferment lactose as well as other sugars and produce lactic and acetic acids as the end-products which acidify the gastrointestinal tract and inhibit the growth of some harmful bacteria (Carina Audisio et al., 2011, Forsgren et al., 2010, Pachla et al., 2019, 2018). Honey bee are subjected to infection by different parasites, i.e.: bacteria (Paenibacillus larvae, Melissococcus plutonius), fungi (Nosema ceranae, Nosema apis), mites (Varroa destructor), and many different viruses (Chen and Siede, 2007, Dussaubat et al., 2012, Forsgren et al., 2010, Killer et al., 2014, Ptaszyńska et al., 2018, Strauss et al., 2015). The colonization of honey bee alimentary tract by LAB is important feature for their hosts in disease prevention (Raymann and Moran, 2018, Vásquez and Olofsson, 2009). To shape a proper honey bee gut community and improve bee health, supplementation of a pollen substitute diet of the honey bee colonies with honey bee-specific lactic acid bacteria, which colonize a gastrointestinal tract and exhibit beneficial effect on honey bee colonies, is suggested (Kazimierczak-Baryczko and Bozena, 2006, Pătruică et al., 2013, Szymaś et al., 2012). It was documented that lactic acid bacteria, used as a sugar syrup supplements, may not only destroy the pathogens by production of lactic and acetic acids and modulation of the immune response but also by synthesis of hydrogen peroxide and bacteriocins (Butler et al., 2013, Olofsson et al., 2016, Pachla et al., 2018). All these data suggest, that honey bee LAB symbionts, exhibiting therapeutic and antibacterial activity, can be considered as a natural, alternative tool to antibiotics in combating the infection diseases. It is especially important in the era of antibiotics overuse and the rapid spread of antibiotic-resistant infections.
The objective of this paper was to evaluate the potential probiotic properties of lactic acid bacteria isolated from the intestinal tract of Apis mellifera derived from Polish apiary. Parameters such as: survival in honey bee gut, cell surface hydrophobicity, auto- and co-aggregation capacities, ability to form biofilm, and the impact of studied LAB, added to sugar syrup, on health and longevity of honey bees were studied.
2. Materials and methods
2.1. Bacterial strains and culture conditions
In this paper, 12 L. kunkeei strains and 2 F. fructossus ones, previously isolated from a healthy honey bee hives, were aerobically cultured in FYP broth/agar medium or MRS agar medium with 0.5% CaCO3 at 30 °C as described by Pachla et al., (2018). L. acidophilus ATCC4356 (ATCC, Manassas, VA, USA), used as control strain for antibiotic susceptibility analysis of LAB, was cultured in MRS agar/broth medium (Pachla et al., 2018). For co-aggregation assays, the commercially available pathogens such as: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Paenibacillus larvae LMG 9820 were cultured as described earlier (Pachla et al., 2018).
2.2. Survival of lactic acid bacteria in honey bee gut - test in vivo
The analysis of survival of two LAB strains, i.e.: L. kunkeei CH1 and F. fructosus V5 in the gastrointestinal tract of honey bee was performed in two experimental replicates as follows. The one-day-old honey bees, were obtained from the apiary (Puławy, Poland) as described by Ptaszyńska et al., (2016) and kept in laboratory in the wooden cages, 40 bees per each cage, at 30 °C and at humidity of 60%. Bacteria for experiment were grown overnight in FYP broth at 30 °C (Pachla et al., 2018), collected by centrifugation at 5,000 rpm for 15 min, suspended in 0.85% (w/v) sterile saline solution to OD600 ~ 1 (~2x108 bacterial cells ml−1), and such fresh bacterial suspensions were concentrated by centrifugation and added separately to 35% sucrose syrup in the number ~ 1x108 cells per ml of sucrose syrup and used as a feeding for bees for the first 24 h of experiment. For the next 66 h, the bees were fed only sucrose syrup. The control honey bees were fed only 35% sucrose syrup throughout the duration of the experiment. 10 honey bees were removed from the control cages at the beginning of the experiment (0 h) and 24, 48, 72 h after the beginning of the experiment as well as from cages in which bees were fed sucrose syrup supplemented with LAB at 0 h (soon after administration of sucrose syrup with LAB to honey bees) and after 18, 24, 42, 48, 66, 72, and 90 h from the beginning of the experiment. To determine the survival of LAB in the insect's digestive system, the honey bees were anaesthetized by chilling at 4 °C, their guts were removed by pulling the last segment of the abdomen, and the midgut was separated from the rest of gut with a sterile scalpel as described earlier (Pachla et al., 2018). Ten midguts, from both honey bee groups at each time point, were homogenized and serial diluted to determine viable cell count (colony forming units - CFU) of lactic acid bacteria in the bee gut. Serial LAB dilutions were plated on MRS agar medium supplemented with 0.5% CaCO3 (w/v) and plates were incubated at 30 °C in 5% CO2 atmosphere as described earlier (Pachla et al. 2018). The survival of LAB in the honey bee gastrointestinal tract was determined as viable cell number per one midgut at 18, 24, 42, 48, 66, 72, and 90 h of the experiment. The results were analyzed by GraphPad Prism software and presented as CFU per honey bee gut.
2.3. Survival of lactic acid bacteria in honey bee guts – test in vitro
For isolation of bee guts, the one-day old honey bees were anaesthetized by chilling at 4 °C and the guts were isolated as described above. For each sample ten midguts were used. The midguts were homogenized, using a sterile tissue grinder pestle, and next, mixed thoroughly (using vortex) with: (I) 10 µl of 30% (w/v) sucrose syrup and 10 µl of 0.6% NaCl containing 107 of fresh lactic acid bacterial cells (L. kunkeei CH1 or F. fructosus V5) prepared as described above and (II) 10 µl of 30% sucrose syrup and 10 µl of 0.6% NaCl. The samples were incubated anaerobically at 30 °C for 24 h. To determine viable cell counts of lactic acid bacteria in the honey bee guts, the serial 10-fold dilutions of homogenized gut material at 0 h (directly after adding bacteria) and 6, 12, and 24 h after adding bacteria were done, plated on MRS agar medium with 0.5% CaCO3 (w/v), incubated at 30 °C in 5% CO2 atmosphere as described earlier (Pachla et al., 2018) for 24–48 h, and the survival rate of lactic acid bacteria was calculated as:
These studies were performed with two replicates. The results of experiment were analyzed by GraphPad Prism software and presented as % survived bacterial cells.
2.4. Determination of antibiotic resistance
Antibiotic susceptibility testing of lactic acid bacteria was conducted for three antibacterial agents: ampicillin as inhibitors of cell wall synthesis as well as erythromycin and tylosin as inhibitors of protein synthesis (EFSA, 2012, Reybroeck et al., 2012). The minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) were determined by the microdilution broth method, with some modifications. First, the antibiotics were dissolved in dimethyl sulfoxide (DMSO), filter-sterilized, and diluted to the concentration 64 µg/ml in FYP medium (Pachla et al., 2018). Subsequently, using the same FYP medium, serial two-fold dilutions of antibiotics, ranging from 64 to 0.0625 ml, were made. The sterile 96-well polystyrene microtitrate plates (Nunc, Denmark) were prepared by adding 200 µl of appropriate dilution of the tested antibiotic per well. Bacterial inocula, from FYP broth cultures incubated at 30 °C for 20 h, were prepared in sterile 0.85% NaCl to a turbidity equivalent of 0.5 McFarland standard, and 2 µl of bacterium inoculum was added to the wells (final bacterium density 1.5 × 106 CFU/ml). DMSO control (DMSO at a final concentration of 10% and inoculum), a positive control (inoculum without antimicrobial agent), and negative control (the tested antibiotics without inoculum) were included in each microplate. The plates were incubated at 30 °C in 5% CO2 for 72 h. The MIC values were determined visually as the lowest concentration of antibiotic, at which bacterial growth was inhibited in comparison to an antibiotic-free control wells. Minimal bactericidal concentration (MBC) was determined by subculturing, onto FYP agar medium, 5 µl of the bacterial culture from each well that showed complete growth inhibition, from the last positive one as well as from the positive control. The plates were incubated at 30 °C in 5% CO2 for 72 h and the MBC value was recorded as the lowest concentration of antibiotic that resulted in a greater than ≥ 99.9% reduction in the number of live bacteria (3 logarithms) compared to positive control. L. acidophilus ATCC4356, sensitive to studied antibiotics, was used as a reference strain. Each experiment was repeated in triplicate.
2.5. Measurement of cell surface hydrophobicity
The LAB cell surface hydrophobicity was determined according to the MATH (microbial adhesion to hydrocarbon) method with some slight modifications using the aliphatic petroleum hydrocarbon n-hexadecane (Sigma, purity ≥ 99%) as the solvent (Vinderola and Reinheimer, 2003). The bacteria were grown in FYP broth for 24 h at 30 °C (Pachla et al., 2018), harvested by centrifugation (5,000 rpm, 15 min, 5 °C), washed twice with PBS buffer (pH 7.4), and suspended in the same PBS solution. The initial absorbance of the bacterial suspension at 560 nm (A0) was adjusted to 1.0 optical unit using spectrophotometer (Biorad, Germany). Three milliliters of cell suspension was dispensed into clean and dry round-bottom test tube and 600 µl of hexadecane was added. The mixture of bacteria and hydrocarbon was vortexed for 2 min, and next, the tube was set aside to rest for 1 h at 30 °C to allow phase separation. The lower aqueous phase was carefully removed with a sterile Pasteur pipette (paying attention not to take the hydrocarbon layer into the pipet) and its absorbance at 560 nm was recorded (At) in quartz measurement cell. Cell surface hydrophobicity in percentage terms (H%) was calculated using the following formula:
The results of two independent experiments, each with two replicates, were reported as mean values ± standard deviations (SD).
2.6. Auto-aggregation assay
The auto-aggregation assay was performed according to Honey Chandran et al., (2018) with some small modifications. Briefly, the bacteria were grown in FYP broth at 30 °C (Pachla et al., 2018), harvested at the stationary phase, collected by centrifugation (3,500 rpm, 15 min, 5 °C), washed twice with PBS solution (pH 7.4), suspended in the same PBS buffer, and adjusted to the OD560 = 1.0 (A0) by measuring the absorbance in spectrophotometer (Biorad, Germany). Bacterial suspension in PBS buffer (4 ml) was vortexed for 10 s, incubated at room temperature for 24 h without disturbing, and absorbance at 560 nm was measured at 0 h, after 1, 2, 4, 6, and 24 h from the beginning of the experiment. The auto-aggregation (Auto-A%) was expressed as the percentage decrease in absorbance after 1, 2, 4, 6, and 24 h relative to that of original suspension as follow:
where, Ao is the initial optical density of bacterial suspension and At represents the absorbance of bacterial suspension after 1, 2, 4, 6, and 24 h from the initial optical density (Nikolic et al., 2010). The data of two independent experiments, each with two replicates, are presented as means ± standard deviation (SD).
2.7. Co-aggregation assay
In the co-aggregation assay (Co-A%), suspensions of lactobacilli and commercially available potentially pathogenic strains: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Paenibacillus larvae LMG 9820 were prepared as described earlier (Pachla et al., 2018). Equal volumes (2 ml) of suspensions of tested LAB strain and pathogenic strain, of the same optical density (OD560 = 1.0), were mixed together in pairs, vortexed for 10 s, and then proceeded as in the case of auto-aggregation. Each strain studied was tested in duplicate. The co-aggregation percentage was calculated using the equation:
where, Ao is the initial optical density of mixture of two bacterial suspensions and At represents the absorbance of mixture of two bacterial suspensions after 1, 2, 4, 6, and 24 h from the initial optical density (Nikolic et al., 2010). The co-aggregation assay was performed two times and data are presented as means ± standard deviation (SD).
2.8. Quantitative determination of biofilm formation
Biofilm formation by studied lactic acid bacterial strains was investigated by the crystal violet-stained microplate assay according to Olofsson et al., (2016) with slight modifications. Briefly, bacteria were grown in FYP broth for 24 h at 30 °C. The absorbance of each bacterial culture was adjusted to 0.6 optical unit at 560 nm, diluted 100-fold in the same medium containing 1% or 5% fructose (w/v) and aliquot of 100 µl of each individual culture, in both media was added into six wells in 96-well polystyrene MicroWell plate (Nunc Sigma-Aldrich). The microplate was covered with a sterile microporous sealing film (AeraSeal catalog no. BS-25) to allow gas exchange and prevent evaporation, and plate was incubated under static conditions at 30 °C for 72 h. Next, the culture supernatants were withdrawn from each well, microplate was air dry, and then, the wells were washed three times with 150 µl of sterile PBS solution (pH 7.4) to remove unbound cells. The bacteria attached to the wall of each well were stained with 100 µl of 0.4% crystal violet (CV) water solution for 30 min. Next, the CV was aspirated and the wells were rinsed three times with sterile water to remove unbound crystal violet. The plate was air-dried and the attachment of bacteria to well walls was quantified by adding 150 µl of 95% ethanol to each dish well. The optical density (OD) was measured at 600 nm with a BioRad Microtiter Plate reader. The experiment was repeated two times for each strain tested.
2.9. Analysis of biofilms by confocal laser scanning microscope (CLSM)
24-h bacterial cultures in FYP liquid medium were equilibrated to OD600 = 0.6, diluted 100-fold in the same medium with 5% fructose, and 100 µl of each bacterial culture was added to six wells in 96-well polystyrene MicroWell plate (Nunc Sigma-Aldrich) and biofilms were grown according to method described by Janczarek et al., (2015) with some modification. Microplates were incubated under static aerobic conditions at 30 °C for 48 h. Subsequently, the culture supernatants were discarded from the wells, each well was washed gently three times with 200 µl of 0.85% NaCl to remove planktonic cells, and biofilms were subsequently air dried for 30 min and stained in darkness for 30 min with 5 µM Syto-9 and 30 µM propidium iodide (PI) in 0.85% NaCl (LIVE/DEAD BacLight stain kit (Invitrogen) (Janczarek et al., 2015). Next, the dyes were removed from the wells, the wells were washed three times with 0.85% NaCl, and biofilm structures were analyzed using the Confocal Laser Scanning Microscope LSM 5 PASCAL (Carl Zeiss, Germany). The images of biofilms were recorded using the fluorescence microscope Axiovert 200 M with the scanning head LSM 5 PASCAL (Carl Zeiss, Germany). The biofilm thickness was recorded using AIM 4.2 software (Carl Zeiss) in a multifaceted laser scan mode. The surface area occupied by the biofilm was measured at a magnification of 200x. To establish the ratios of live/dead cells in the biofilm, formed by each individual strain growing in FYP medium with 5% sucrose, two independent sets of images, separately for green - Syto-9 (live cells) and red - propidium iodide (dead cells) fluorescences, were collected from six wells for each strain. The Live/Dead bacteria ratio was based on 3 images from each biofilm. The images were recorded using the AxioVision 4.8 (Carl Zeiss) software and the ratio of live/dead cells was determined by multichannel fluorescence technique using AxioCam HR3 camera and 470 nm and 546 nm filters for the green and red channels, respectively. The ratio of live to dead cells and the percentage of the area covered by biofilm were calculated using ImageJ 1.43e software (Wayne Rasband, NIH, USA) (Beyenal et al., 2004, Ploux et al., 2007). The experiments were performed in duplicate and repeated twice.
2.10. The survival of honey bees fed sucrose syrup supplemented with lactic acid bacteria - the cage experiment
A collection of 1-day-old honey bees, Apis mellifera carnica, was obtained from apiary (Puławy, Poland) and kept in wooden cages (40 bees/cage) as described earlier (Ptaszyńska et al., 2016). The honey bees were fed for seven days with a fresh, daily prepared 35% sucrose syrup (w/v) supplemented with: (T1) (treatment 1) - L. kunkeei CH1 in the number of ~ 2x108 cells per ml of sucrose syrup, (T2) - F. fructosus V5 in the number of ~ 2x108 cells per ml of sucrose syrup, (T3) − 12 studied L. kunkeei strains, each in the number of ~ 1.7x107 cells per ml of sucrose syrup, (T4) - F. fructosus VIII1 and F. fructosus V5 strains - each in the number of ~ 1 x108 cells per ml of sucrose syrup, (T5) − 12 studied L. kunkeei strains and two F. fructosus strains – each in the number of ~ 1.4x107 per ml of sucrose syrup. Control honey bees (T6) were fed 35% sucrose syrup without lactic acid bacteria. Bacterial cultures, for use as a bee food supplements, were prepared daily by culturing bacteria in FYP broth at 30 °C as described by Pachla et al., (2018). In total, for each of six honey bee treatments 120 honey bees were used. The mortality of bees, fed sucrose syrup supplemented with lactic acid bacteria and sucrose syrup without lactic acid bacteria, was scored every second day during 24 days of experiment. This experiment was repeated two times. The obtained results were statistically analyzed by SAS software (2002–2003) using the ANOVA (a group and variant effects were the experimental factors) and the Tukey’s honestly significant difference (HSD) test (SAS Institute 2002–2003).
We want to emphasize that cage experiments on the survival of honey bees fed sucrose syrup supplemented with LAB was carried out and was supervised by assoc. prof. Aneta Ptaszyńska. The experiment was performed in the Institute of Biological Sciences of Maria Curie-Skłodowska University as part of the Innovation Incubator project entitled: “The probiotic preparation based on bacterial strains of Lactobacillus and Fructobacillus genera isolated from the digestive tract of honeybees”. The results obtained in the framework of this experiment are subject to all legal regulations.
3. Results
Two selected strains, i.e.: L. kunkeei CH1 and F. fructosus V5, able to survive in 35% sugar syrup and resistant to pH 3.5–11 (Pachla et al., 2018), in studies in vivo with honey bees fed for 24 h 35% sugar syrup containing lactic acid bacteria and next, for 66 h only sugar syrup, tolerated well the changes in pH throughout the honey bee intestinal tract and survived well in honey bee guts. The number of viable lactic acid bacteria in the midgut remained constant for the first 48 h of the experiment. In the subsequent 42 h, the number of live L. kunkeei CH1 and F. fructosus V5 bacteria decreased (Table 1). At the beginning of this experiment (0 h) the LAB were not found in the midgut of honey bees. The lactic acid bacteria were also not found in the midgut of honey bees fed only sucrose syrup (control group) for the entire duration of experiment (0, 24, 48, 72 h) (data not presented).
Table 1.
Survival of LAB in A. mellifera midgut (CFU/midgut) – test in vivo1.
| Strain | 18 h | 24 h | 42 h | 48 h | 66 h | 72 h | 90 h |
|---|---|---|---|---|---|---|---|
| CH1a | 5.03x105 ± 1.00x105 | 6.50x105 ± 5.57x104 | 5.67x105 ± 3.51x104 | 4.47x105 ± 4.04x104 | 5.27x104 ± 7.02x103 | 2.93x104 ± 4.51x103 | 1.73x104 ± 3.06x103 |
| V5b | 3.45x105 ± 5.89x104 | 5.47x105 ± 5.03x104 | 3.10x105 ± 5.57x104 | 4.07x105 ± 3.06x104 | 5.43x104 ± 6.51x103 | 1.70x104 ± 9.54x103 | 1.23x104 ± 3.21x103 |
Values are means ± SD;
L. kunkeei;
F. fructosus
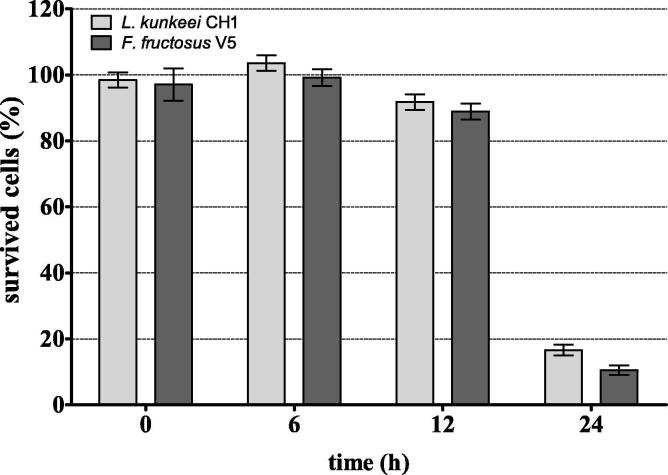
Temporary persistence of L. kunkeei CH1 and F. fructosus V5 bacteria, in the honey bee midgut content, was also found in our in vitro studies (Fig. 1). Bacteria examined survived well in the midgut content, without significant reduction of their number for the first 12 h after adding of lactobacilli. In the subsequent 12 h, the survival rate of L. kunkeei CH1 and F. fructosus V5 strains decreased dramatically to ~ 17% and ~ 10%, respectively. It must be emphasized that the midgut contents of honey bees from control group (fed sugar syrup without LAB) were free from lactic acid bacteria throughout the duration of experiment (0–24 h) (data not presented).
Fig. 1.
Survival rate (%) of LAB in A. mellifera midguts – test in vitro. The values are means and error bars represent standard deviations.
The another question posed in this research concerned the resistance of the probiotic candidates to three antibiotics used in beekeeping practice, i.e. ampicillin, erythromycin, and tylosin (Reybroeck et al., 2012).
In this study, 14 fructophilic lactic acid bacteria, originating from A. mellifera guts, were tested for susceptibility to ampicillin, erythromycin, and tylosin by using serial two-fold dilution method to estimate the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of antibiotics (Tejchman et al., 2017). Additionally, the mode of drug action, i.e. bacteriostatic (MBC/MIC > 4) or bactericidal one (MBC/MIC ≤ 4) was determined (Mogana et al., 2020). Overall, 12 L. kunkeei and 2F. fructosus strains showed the high susceptibility to all three antibiotics used with MIC of 0.25–1, 0.25–05, 0.5–4 µg/ml for ampicillin, erythromycin, and tylosin, respectively (Table 2). All three drugs used exhibited bactericidal activity against most studied honey bee endosymbionts (MBC/MIC = 1–4) and bacteriostatic effect of ampicillin and erythromycin against L. kunkeei CH2 and L. kunkeei CH1 strains, respectively (MBC/MIC = 16) (Table 2).
Table 2.
Antibiotic susceptibility of LAB to ampicillin, erythromycin, and tylosin.
| Antibiotic | Strain |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| III1a | VI1a | VI3a | VI4a | VI6a | VII4a | CH1a | CH2a | CH3a | Z1a | Z3a | Z5a | V5b | VIII1b |
L. acidophilus ATCC 4356 |
||
|
Ampicillin |
MIC (ml) | 0,25 | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 1 | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 0,25 |
| MBC (ml) | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 | 1 | 8 | 1 | 1 | 1 | 0,5 | 0,5 | 0,5 | 0,5 | |
| MBC/MIC | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 16 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | |
| Erythromycin | MIC (ml) | 0,5 | 0,25 | 0,25 | 0,5 | 0,25 | 0,25 | 0,5 | 0,25 | 0,5 | 0,5 | 0,5 | 0,25 | 0,25 | 0,5 | 0,25 |
| MBC (ml) | 2 | 1 | 0,5 | 1 | 0,5 | 0,5 | 8 | 1 | 2 | 2 | 2 | 0,5 | 0,5 | 0,5 | 0,5 | |
| MBC/MIC | 4 | 4 | 2 | 2 | 2 | 2 | 16 | 4 | 4 | 4 | 4 | 2 | 2 | 1 | 2 | |
| Tylosin | MIC (ml) | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 4 | 2 | 2 | 0,5 | 2 | 2 | 1 |
| MBC (ml) | 4 | 2 | 2 | 4 | 2 | 2 | 4 | 2 | 8 | 4 | 4 | 2 | 4 | 4 | 2 | |
| MBC/MIC | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | |
MIC - minimum inhibitory concentration; MBC - minimum bactericidal concentration
L. kunkeei;
F. fructosus;
The lactic acid bacteria were further evaluated for cell surface hydrophobicity associated with bacterial adhesion capacity to biotic and abiotic surfaces (Doyle, 2000, Goulter et al., 2009, Janashia et al., 2016). Bacterial cell surface hydrophobicity was determined according to simple MATH method based on bacterial adherence to the n-hexadecane (alkane hydrocarbon) (Rosenberg, 1984, Vinderola and Reinheimer, 2003). The cell hydrophobicity of the 13 strains ranged from 84% to 99%. Only one strain (L. kunkeei VI) showed lower hydrophobic cell surface, i.e. 77% (Fig. 2).
Fig. 2.
Hydrophobicity (%) of L. kunkeei (III1, VI1, VI3, VI4, VI6, VII4, CH1, CH2, CH3, Z1, Z3, Z5) and F. fructosus (V5, VIII1) strains to n-hexadecane. The values are means and error bars represent standard deviations.
Other preliminary criterion used for selection of lactic acid bacteria with probiotic potential was their ability to form multicellular aggregates composed of the same strain cells (auto-aggregation, self-aggregation) or the cells of genetically different strains (co-aggregation) (Honey Chandran and Keerthi, 2018, Nikolic et al., 2010). The tested A. mellifera gut isolates, i.e. six strains representing L. kunkeii and two classified as F. fructosus showed ability to auto-aggregation. The LAB self-aggregation increased as a function of time and was the highest at 24 h of bacteria incubation at room temperature (18–20 °C) in PBS, i.e. ranged between 24.3 and 37.8% in the case of L. kunkeii strains and between 42.2 and 46.5% in the case of F. fructosus bacteria (Table 3). The co-aggregation capacities of A. mellifera symbionts with potential pathogenic strains increased also along with the incubation time and achieved the highest values after 24 h of these bacteria mixture incubation in PBS at room temperature. The co-aggregation efficiency was dependent not only on lactic acid bacterial strain but was also dependent on potential pathogenic strain. It was especially high between: F. fructosus V5 × E. coli (79.7%), F. fructosus VIII1 × E. coli (77.9%), F. fructosus V5 × P. larvae (75.4%) after 24 h of bacteria incubation at room temperature in PBS, particularly low between all studied lactic acid bacteria and K. pneumoniae, and low between L. kunkeii Z3 and E.coli after 24 h of experiment (Fig. 3).
Table 3.
Auto-aggregation (%) of LAB strains presented as a function of time1.
| Strain | 1 h | 2 h | 4 h | 6 h | 24 h |
|---|---|---|---|---|---|
| VI1a | 1,1 ± 0,40 | 2,6 ± 0,62 | 4,9 ± 0,67 | 6,3 ± 0,76 | 24,3 ± 3,86 |
| VI3a | 2,7 ± 0,72 | 6,7 ± 0,45 | 9,2 ± 0,80 | 10,0 ± 0,30 | 30,0 ± 1,45 |
| VII4a | 1,9 ± 0,57 | 3,5 ± 0,82 | 6,2 ± 1,48 | 7,0 ± 1,05 | 31,1 ± 2,67 |
| CH1a | 0,4 ± 0,53 | 2,6 ± 0,57 | 4,5 ± 0,70 | 6,0 ± 0,42 | 36,4 ± 1,57 |
| CH3a | 1,8 ± 0,40 | 2,3 ± 0,30 | 5,1 ± 1,21 | 7,3 ± 0,83 | 33,1 ± 2,95 |
| Z3a | 0,4 ± 0,51 | 2,9 ± 0,36 | 5,1 ± 0,40 | 5,6 ± 0,55 | 37,8 ± 2,11 |
| V5b | 0,7 ± 1,21 | 9,1 ± 0,85 | 17,6 ± 0,66 | 21,2 ± 1,05 | 42,2 ± 1,31 |
| VIII1b | 9,2 ± 1,17 | 22,0 ± 1,05 | 28,4 ± 1,13 | 31,1 ± 0,39 | 46,5 ± 1,73 |
Values are means ± SD;
L. kunkeei;
F. fructosus
Fig. 3.
Co-aggregation (%) of LAB strains: (A) L. kunkeei VI1, (B) L. kunkeei VI3, (C) L. kunkeei VII4, (D) L. kunkeei CH1, (E) L. kunkeei CH3, (F) L. kunkeei Z3, (G) F. fructosus V5, (H) F. fructosus VIII1 with E. coli, K. pneumoniae and P. larvae presented as a function of time. The values are means and error bars represent standard deviations.
In this paper the biofilm formation ability of lactic acid bacteria isolated from intestinal tract of healthy honey bees and structure of biofilms formed by them were determined in experiments in vitro. Bacteria were grown in polystyrene microtiter plates at 30 °C without shaking as described in materials and methods (2.8., 2.9.). The ability of LAB to biofilm formation was evaluated by quantitative technique using crystal violet to stain bacteria (Christensen et al., 1985) and qualitative method using for visualization of bacteria, analysis of their viability and investigation of the structure of biofilm formed by them, two fluorescent dyes, i.e. SYTO-9 to identify live bacterial cells with intact DNA and propidium iodide to stain dead cells with damaged membrane (Boulos et al., 1999).
The ability to form biofilm was assayed first by measuring the optical density of crystal violet stained bacteria adsorbed to hydrophobic surface of microtiter plates (Fig. 4). Mass of biofilm formed by LAB, measured by the absorbance at 600 nm, was similar in all studied strains grown in FYP medium with 1 or 5% of fructose but bacterial cell density was twice higher in biofilms formed by LAB grown in broth with 5% than in biofilms of bacteria grown in FYP medium with 1% fructose. Based on the biomass of biofilm formed by studied lactic acid bacteria (OD600 nm) L. kunkeii and F. fructosus honey bee gut symbionts were classified as strongly adherent to substratum, according to Christensen et al., (1985).
Fig. 4.
Biofilm formation of L. kunkeei (III1, VI1, VI3, VI4, VI6, VII4, CH1, CH2, CH3, Z1, Z3, Z5) and F. fructosus (V5, VIII1) strains on FYP medium with 1% (light grey bars) or 5% (dark grey bars) fructose, quantified by crystal violet assay. The values are means and error bars represent standard deviations.
In the next experiment, biofilms formed by five L. kunkeii and two F. fructosus honey bee endosymbionts were visualized by confocal laser scanning microscopy (CLSM) using LIVE/DEAD stains, specific for live or dead cells, respectively (Boulos et al., 1999). Observation of 48 h old biofilms by confocal microscopy reviled their early step formation, mainly with one-two layers of cell aggregates joined to polystyrene surface of microtiter plate, in some places biofilms were composed of three or more layers of bacterial cell aggregates (Supplementary Figure 1). The size of biofilm was measured as the area colonized by bacterial aggregates and the height of aggregated bacteria (Table 4). Some differences between bacteria in surface covered with biofilm were noted; with minimum of surface coated with biofilm of 40.89% and maximum comprising 76.39% of plate substratum. The differences in the height of the biofilm formed by studied bacteria were also noted and the mean and the maximum biofilm heights are presented in Table 4.
Table 4.
The parameters of biofilms formed by LAB1.
| Strain | VI3a | VII4a | CH1a | CH3a | Z3a | V5b | VIII1b |
|---|---|---|---|---|---|---|---|
| Ratio of live/dead cells | 25.29 ± 1,78 | 22.63 ± 5.69 | 20.57 ± 1.99 | 21.06 ± 4.21 | 18.69 ± 2.87 | 0.59 ± 0.47 | 0.28 ± 0.22 |
| Depth of biofilm – average thickness (µm) | 13.30 ± 1.99 | 14.08 ± 1.21 | 16.62 ± 0.92 | 11.14 ± 1.54 | 15.64 ± 0.95 | 11.73 ± 1.19 | 11.93 ± 0.93 |
| Depth of biofilm – maximum thickness (µm) | 14.86 ± 0.78 | 17.40 ± 0.63 | 17.21 ± 0.19 | 12.71 ± 0.47 | 17.40 ± 0.37 | 13.69 ± 0.33 | 13.49 ± 0.96 |
| Area covered by biofilm (%) | 48.30 ± 4.09 | 47.58 ± 7.19 | 76.39 ± 4.53 | 60.66 ± 3.91 | 51.18 ± 2.55 | 40.89 ± 2.93 | 61.95 ± 4.80 |
Values are means ± SD;
L. kunkeei;
F. fructosus
Viability of lactic acid bacteria in biofilm formed in polystyrene microplates was assessed by CLSM using LIVE/DEAD staining of bacteria. Green fluorescence pointed out that bacteria were viable, red one was indicator of dead cells. The percentage of live cells in biofilms formed by L. kunkeii bacteria was very high (94.92–96.20%) compared to that in the biofilm created by F. fructosus strains (22.11 – 36.93%) (Table 4).
The last question posed in this paper concerned the effect of A. mellifera endosymbiotic LAB on the longevity and survival rate of healthy honey bees kept in cages, in laboratory conditions. Supplementation of the honey bee diet with lactic acid bacteria, naturally present in the bee gut, promoted the honey bee survival and significantly decreased the bee mortality compared to the mortality of control bees fed sugar syrup without LAB (Fig. 5). The honey bees fed 35% sucrose syrup without LAB exhibited a high mortality (80% ± 0.48%) after 24 days of experiment. The mortality percentage of honey bees, fed sugar syrup containing lactic acid bacteria, ranged from 51.7% to 56.7%. The especially low honey bee mortality was found when bees were fed sugar diet containing L. kunkeei CH1 strain (51.7%).
Fig. 5.
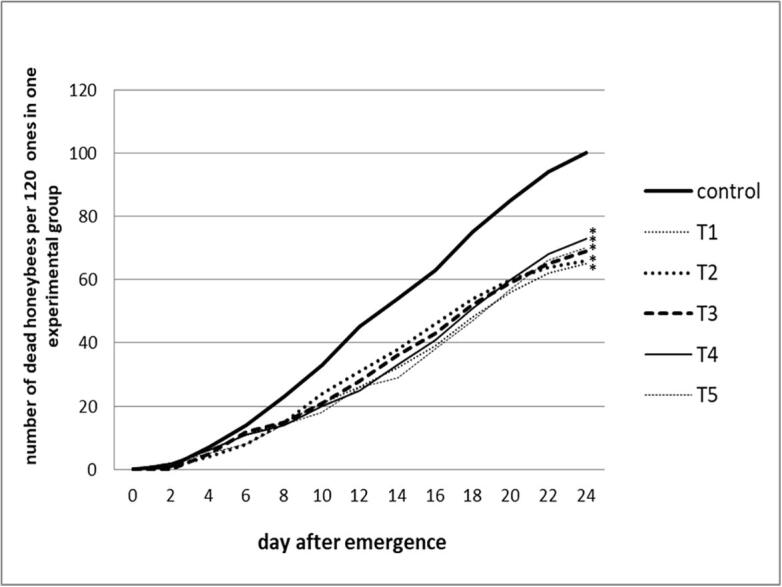
Mortality od honey bees fed sucrose syrup supplemented with LAB – cage experiment. Honey bee groups treated with: (T1) - L. kunkeei CH1; (T2) - F. fructosus V5; (T3) − 12 studied L. kunkeei strains; (T4) - F. fructosus VIII1 and F. fructosus V5 strains; (T5) − 12 studied L. kunkeei strains and two F. fructosus strains. Control - sucrose syrup without lactic acid bacteria. Asterisks (*) indicates a statistically significant differences compared to control group (p < 0.05).
4. Discussion
One of the most important global problem in keeping of honey bees, the key pollinators of agricultural and horticultural crops, is spreading of pathogens in honey bee colonies (Crenna et al., 2017, Klein et al., 2007). The high density of honey bees within the colony, the trophallaxis among members of a community, and feeding of the honey bee larvae by the nurse bees favor horizontal transmission of the diseases among honey bee individuals (Chen et al., 2006, Forfert et al., 2015). For these reasons, the use of probiotics, as alternative to antibiotics, in preventing and combating diseases in honey bees, is of particular importance. Probiotics are defined as live, safe microorganisms, which positively affect a health and promote a longevity of their hosts (AFRC and FULLER, 1989, FAO/WHO, 2002). It was found that probiotic bacteria function better, when they are used as supplements in the diet of organisms from which they were originally isolated (Ptaszyńska et al., 2016, Saarela et al., 2000). In the present study, fructophilic lactic acid bacteria isolated from the intestinal tract of healthy Polish honey bees (Pachla et al., 2018) were assessed for the functional probiotic properties and beneficial effects on the health and the longevity of honey bees.
Important features of probiotic bacteria, used as a honey bee food supplement, is their ability to survive a passage through the bee intestinal tract (Gaggìa et al., 2018, Kumar and Kumar, 2015, Zuo et al., 2016). Studied L. kunkeei bacteria, which are resistant to low pH and dominant members of midgut of summer honey bees, were expected to survive well in the bee intestinal tract after their reintroduction (Pachla et al., 2018). It is worth underlining, as some studies showed, that probiotic bacteria usually disappear from the intestinal tract within a couple of weeks after discontinuation of probiotic administration and that “permanent colonization of gut is seldom, if ever occurs” (Alander et al., 1999, Duncan, 2013).
It is known that the usage of antibiotics in beekeeping is prohibited in the EU Member States, with zero tolerance for the presence of antibiotics in honey (Reybroeck et al., 2012). However, some EU Member States (Belgium, France), as well as UK, Switzerland, USA, Canada, India, Argentina have established limits for antibiotics presence in honey and accepted the use of some antibiotics for treatment American and European foulbrood, and nosemosis in honey bee colonies (Reybroeck et al., 2012).
According to the updated guidance document of European Food Safety Authority (EFSA) (EFSA 2012), obligate heterofermentative L. kunkeei strains studied were defined as susceptible to ampicillin and erythromycin based on the cut-off values allowing to distinguish bacteria with antibiotic acquired resistance from antibiotic susceptible ones. The data from the serial two-fold dilution experiment showed that the growth of studied lactobacilli was inhibited at the minimum concentrations of ampicillin and erythromycin, equal or lower than the cut-off values for these two drugs established by EFSA (≤2 µg/ml for ampicillin and ≤ 1 µg/ml for erythromycin) (Table 2) (EFSA 2012). In EFSA guidance document (EFSA 2012) there is no information on tylosin cut-off values for heterofermentative lactobacilli but MIC values of these bacteria allow to categorize them as tylosin susceptible. The high antibiotic susceptibility of all studied fructophilic lactic acid bacteria recommends these microbes for using as a honeybee food supplements and points also to a detrimental role of these antibiotics for honey bee by the depletion of lactic acid bacteria number in the gut, which in turn leads to higher risk of bacterial and fungal diseases (Daisley et al., 2020, Forsgren et al., 2010, Honey Chandran and Keerthi, 2018).
Recently, many researchers have shown that hydrophobic surface of bacterial cells play an essential role in bacteria aggregation, adhesion to the host cells, and is important cell property for the screening of probiotic bacteria (Doyle, 2000, Feng et al., 2017, Krasowska and Sigler, 2014, Nikolic et al., 2010, Rosenberg and Kjelleberg, 1986, Schiffer et al., 2019, Tuo et al., 2013). A. mellifera FLAB studied showed high cell surface hydrophobicity, that is, fulfil the next selection criterion for their use as probiotics. These bacteria exhibited also auto- and co-aggregation capacities regarded as valuable features for biofilm formation and inhibition of pathogens adhesion to the bee gut cells (Table 3, Fig. 3). The auto- aggregation and co-aggregation abilities of lactic acid bacteria, together with their antimicrobial activities and LAB adherence ability to the intestinal cells are desirable probiotic features in the removal of pathogens from the epithelium of honey bee gastrointestinal tract and all these features should be taken into account in the selection of potential probiotic lactic acid bacteria (FAO, 2001).
Interestingly, bacterial surface hydrophobicity together with aggregation are considered to be beneficial properties for biofilm formation. Biofilm, is defined as microbial community enclosed in a self-synthesized extracellular polymeric substances (EPS) composed of polysaccharides, proteins, and DNA and attached to biotic or abiotic surfaces (Costerton et al., 1995). Its development starts with the attachment of planktonic bacteria to a surface followed by cell division and formation of a complex three-dimensional biofilm structure composed of bacterial microcolonies embedded in EPS and separated by channels (Stanley and Lazazzera, 2004). The biofilm formation capacity of LAB promotes the colonization of the gut of their hosts and prevents the adhesion of pathogens to intestinal tract by their competitive exclusion (Berríos et al., 2018, Faten et al., 2016, Gómez et al., 2016, Jalilsood et al., 2015, Tuo et al., 2013, Woo and Ahn, 2013, Wu et al., 2013).
The biofilm-forming ability of FLAB was documented by using quantitative test described by Christensen et al., (1985) and biofilm architecture was revealed by CLSM using LIVE/DEAD stains (Fig. 4; Table 4; Supplementary Figure 1). It is worth to pointing out that ability of lactic acid bacteria to form biofilm is considered to be important and desirable feature for selection of probiotic strains (Berríos et al., 2018, Saarela et al., 2000, Shokri et al., 2018) and therefore, studied honey bee gut endosymbionts were further studied for their health beneficial function and possibility to use them as A. mellifera feed supplements.
A positive impact of lactic acid bacteria on the longevity and mortality of honey bees, fed sucrose syrup supplemented with LAB, was supported in cage experiment carried out in laboratory conditions (Fig. 5). The positive effect of the gut-dwelling lactic acid bacteria on the survival of honey bees may be associated with improving gut microbial homeostasis, elimination of intestinal pathogens, stimulation of immune system, and with their participation in the host food digestion process (Evans and Lopez, 2004, Janashia et al., 2016, Olofsson and Vásquez, 2008, Servin, 2004, Vásquez et al., 2012).
Additionally, in order to be sure about the health beneficial function of examined honey bee gut endosymbionts and possibility to use them as A. mellifera feed supplements further studies will be undertaken in beekeeping practice.
5. Conclusion
The studies presented in this paper indicate that tested lactic acid bacteria, isolated from intestinal tract of honey bees, have a great probiotic potential for using them as A. mellifera food supplements but further investigations on A. mellifera colonies in apiaries are necessary to confirm the honey bee health-promoting properties of these bacteria. Indispensable are also studies supporting that lactic acid bacteria survive and retain functionality during manufacturing of probiotics under industrial conditions and during storage as probiotic products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.12.040.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- AFRC, Fuller, R., 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66, 365–378. https://doi.org/10.1111/j.1365-2672.1989.tb05105.x [PubMed]
- Alander M., Satokari R., Korpela R., Saxelin M., Vilpponen-Salmela T., Mattila-Sandholm T., Von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 1999;65:351–354. doi: 10.1128/aem.65.1.351-354.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asama T., Arima T.H., Gomi T., Keishi T., Tani H., Kimura Y., Tatefuji T., Hashimoto K. Lactobacillus kunkeei YB38 from honeybee products enhances IgA production in healthy adults. J. Appl. Microbiol. 2015;119:818–826. doi: 10.1111/jam.12889. [DOI] [PubMed] [Google Scholar]
- Berríos P., Fuentes J.A., Salas D., Carreño A., Aldea P., Fernández F., Trombert A.N. Inhibitory effect of biofilm-forming Lactobacillus kunkeei strains against virulent Pseudomonas aeruginosa in vitro and in honeycomb moth (Galleria mellonella) infection model. Benef. Microbes. 2018;9:257–268. doi: 10.3920/BM2017.0048. [DOI] [PubMed] [Google Scholar]
- Beyenal H., Lewandowski Z., Harkin G. Quantifying biofilm structure: Facts and fiction. Biofouling. 2004 doi: 10.1080/0892701042000191628. [DOI] [PubMed] [Google Scholar]
- Boulos L., Prévost M., Barbeau B., Coallier J., Desjardins R. LIVE/DEAD(®) BacLight(TM): Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods. 1999;37:77–86. doi: 10.1016/S0167-7012(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Butler È., Alsterfjord M., Olofsson T.C., Karlsson C., Malmström J., Vásquez A. Proteins of novel lactic acid bacteria from Apis mellifera mellifera: An insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol. 2013;13 doi: 10.1186/1471-2180-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carina Audisio M., Torres M.J., Sabaté D.C., Ibarguren C., Apella M.C. Properties of different lactic acid bacteria isolated from Apis mellifera L. bee-gut. Microbiol. Res. 2011;166:1–13. doi: 10.1016/j.micres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Chen Y., Evans J., Feldlaufer M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006;92:152–159. doi: 10.1016/j.jip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Chen, Y.P., Siede, R., 2007. Honey Bee Viruses. Adv. Virus Res. https://doi.org/10.1016/S0065-3527(07)70002-7 [DOI] [PubMed]
- Christensen G.D., Simpson W.A., Younger J.J., Baddour L.M., Barrett F.F., Melton D.M., Beachey E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J.W., Lewandowski Z., Caldwell D.E., Korber D.R., Lappin-Scott H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Crenna E., Sala S., Polce C., Collina E. Pollinators in life cycle assessment: towards a framework for impact assessment. J. Clean. Prod. 2017;140:525–536. doi: 10.1016/j.jclepro.2016.02.058. [DOI] [Google Scholar]
- Crotti E., Sansonno L., Prosdocimi E.M., Vacchini V., Hamdi C., Cherif A., Gonella E., Marzorati M., Balloi A. Microbial symbionts of honeybees: A promising tool to improve honeybee health. N. Biotechnol. 2013;30:716–722. doi: 10.1016/j.nbt.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Daisley B.A., Pitek A.P., Chmiel J.A., Al K.F., Chernyshova A.M., Faragalla K.M., Burton J.P., Thompson G.J., Reid G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2020;14:476–491. doi: 10.1038/s41396-019-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R.J. Contribution of the hydrophobic effect to microbial infection. Microbes Infect. 2000 doi: 10.1016/S1286-4579(00)00328-2. [DOI] [PubMed] [Google Scholar]
- Duncan, B., 2013. Prebiotics, Probiotics, and Health Promotion:: An Overview, in: Watson, R., Preedy, V. (Eds.), Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. Elsevier Inc., pp. 449–463. https://doi.org/10.1016/B978-0-12-397154-8.00005-1
- Dussaubat C., Brunet J.L., Higes M., Colbourne J.K., Lopez J., Choi J.H., Martín-Hernández R., Botías C., Cousin M., McDonnell C., Bonnet M., Belzunces L.P., Moritz R.F.A., Le Conte Y., Alaux C. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10. https://doi.org/10.2903/j.efsa.2012.2740
- Endo A., Salminen S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013;36:444–448. doi: 10.1016/j.syapm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Evans J.D., Lopez D.L. Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae) J. Econ. Entomol. 2004;97:752–756. doi: 10.1093/jee/97.3.752. [DOI] [PubMed] [Google Scholar]
- FAO/WHO, 2002. Food and Agriculture Organization: FAO/WHO Working Group report on drafting guidelines for the evaluation of probiotics in food. FAO Food Nutr. Pap. 85 11.
- FAO, 2001. Probiotics in food: Health and nutritional properties and guidelines for evaluation. Food Nutr. Pap. 85, 71.
- Faten K., Hamida K., Soumya E.A., Saad I.S.K., Hasna M., Hassan L., Moktar H. Lactobacillus plantarum: Effect of a protective biofilm on the surface of olives during storage. Brazilian J. Microbiol. 2016;47:202–209. doi: 10.1016/j.bjm.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Qiao L., Liu R., Yao H., Gao C. Potential probiotic properties of lactic acid bacteria isolated from the intestinal mucosa of healthy piglets. Ann. Microbiol. 2017;67:239–253. doi: 10.1007/s13213-017-1254-6. [DOI] [Google Scholar]
- Forfert N., Natsopoulou M.E., Frey E., Rosenkranz P., Paxton R.J., Moritz R.F.A. Parasites and pathogens of the honeybee (Apis mellifera) and their influence on inter-colonial transmission. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E., Olofsson T.C., Vásquez A., Fries I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie. 2010;41:99–108. doi: 10.1051/apido/2009065. [DOI] [Google Scholar]
- Gaggìa F., Baffoni L., Alberoni D. Probiotics for Honeybees’ health. Probiotics and Prebiotics in Animal Health and Food Safety. 2018:219–245. doi: 10.1007/978-3-319-71950-4_9. [DOI] [Google Scholar]
- Gilliam, M., 1997. Identification and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiol. Lett. https://doi.org/10.1016/S0378-1097(97)00337-6
- Gómez N.C., Ramiro J.M.P., Quecan B.X.V., de Melo Franco B.D.G. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157: H7 biofilms formation. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulter, R.M., Gentle, I.R., Dykes, G.A., 2009. Issues in determining factors influencing bacterial attachment: A review using the attachment of Escherichia coli to abiotic surfaces as an example. Lett. Appl. Microbiol. https://doi.org/10.1111/j.1472-765X.2009.02591.x [DOI] [PubMed]
- Honey Chandran C., Keerthi T.R. Probiotic potency of Lactobacillus plantarum KX519413 and KX519414 isolated from honey bee gut. FEMS Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fnx285. [DOI] [PubMed] [Google Scholar]
- Jack R.W., Tagg J.R., Ray B. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 1995;59:171–200. doi: 10.1128/mmbr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilsood T., Baradaran A., Song A.A.L., Foo H.L., Mustafa S., Saad W.Z., Yusoff K., Rahim R.A. Inhibition of pathogenic and spoilage bacteria by a novel biofilm-forming Lactobacillus isolate: A potential host for the expression of heterologous proteins. Microb. Cell Fact. 2015;14 doi: 10.1186/s12934-015-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janashia I., Choiset Y., Rabesona H., Hwanhlem N., Bakuradze N., Chanishvili N., Haertlé T. Protection of honeybee Apis mellifera by its endogenous and exogenous lactic flora against bacterial infections. Ann. Agrar. Sci. 2016;14:177–181. doi: 10.1016/j.aasci.2016.07.002. [DOI] [Google Scholar]
- Janczarek M., Rachwał K., Cieśla J., Ginalska G., Bieganowski A. Production of exopolysaccharide by Rhizobium leguminosarum bv. trifolii and its role in bacterial attachment and surface properties. Plant Soil. 2015;388:211–227. doi: 10.1007/s11104-014-2320-5. [DOI] [Google Scholar]
- Kazimierczak-Baryczko M., Bozena S. Improvement of the composition of pollen substitute hor Honeybee (Apis mellifera L.), through implementation of probiotic preparations. J. Apic. Sci. 2006;50:15–23. [Google Scholar]
- Killer J., Dubná S., Sedláček I., Švec P. Lactobacillus apis sp. nov., from the stomach of honeybees (Apis mellifera), having an in vitro inhibitory effect on the causative agents of American and European foulbrood. Int. J. Syst. Evol. Microbiol. 2014;64:152–157. doi: 10.1099/ijs.0.053033-0. [DOI] [PubMed] [Google Scholar]
- Klein, A.M., Vaissière, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T., 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. https://doi.org/10.1098/rspb.2006.3721 [DOI] [PMC free article] [PubMed]
- Krasowska, A., Sigler, K., 2014. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. https://doi.org/10.3389/fcimb.2014.00112 [DOI] [PMC free article] [PubMed]
- Kumar A., Kumar D. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe. 2015;33:117–123. doi: 10.1016/j.anaerobe.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Kwong W.K., Mancenido A.L., Moran N.A. Immune system stimulation by the native gut microbiota of honey bees. R. Soc. Open Sci. 2017;4 doi: 10.1098/rsos.170003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, W.K., Moran, N.A., 2016. Gut microbial communities of social bees. Nat. Rev. Microbiol. https://doi.org/10.1038/nrmicro.2016.43 [DOI] [PMC free article] [PubMed]
- Mogana R., Adhikari A., Tzar M.N., Ramliza R., Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium Patentinervium miq. Against bacterial clinical isolates. BMC Complement. Med. Ther. 2020;20:55. doi: 10.1186/s12906-020-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M., Jovcic B., Kojic M., Topisirovic L. Surface properties of Lactobacillus and Leuconostoc isolates from homemade cheeses showing auto-aggregation ability. Eur. Food Res. Technol. 2010;231:925–931. doi: 10.1007/s00217-010-1344-1. [DOI] [Google Scholar]
- Olofsson T.C., Butler È., Markowicz P., Lindholm C., Larsson L., Vásquez A. Lactic acid bacterial symbionts in honeybees - an unknown key to honey’s antimicrobial and therapeutic activities. Int. Wound J. 2016;13:668–679. doi: 10.1111/iwj.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson T.C., Vásquez A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008;57:356–363. doi: 10.1007/s00284-008-9202-0. [DOI] [PubMed] [Google Scholar]
- Pachla A., Ptaszyńska A.A., Wicha M., Oleńska E., Małek W. Fascinating fructophilic lactic acid bacteria associated with various fructose-rich niches. Ann. Univ. Mariae Curie-Sklodowska, Sect. C – Biol. 2019;72:41. [Google Scholar]
- Pachla, A., Wicha, M., Ptaszyńska, A.A., Borsuk, G., –Trokenheim, Ł.Ł., Małek, W., 2018. The molecular and phenotypic characterization of fructophilic lactic acid bacteria isolated from the guts of Apis mellifera L. derived from a Polish apiary. J. Appl. Genet. 59, 503–514. https://doi.org/10.1007/s13353-018-0467-0 [DOI] [PubMed]
- Pătruică S., Dumitrescu G., Popescu R., Filimon N.M. The effect of prebiotic and probiotic products used in feed to stimulate the bee colony (Apis mellifera) on intestines of working bees. J. Food. Agric. Environ. 2013;11:2461–2464. [Google Scholar]
- Pattabhiramaiah, M., Reddy, M.S., Brueckner, D., 2012. Detection of novel probiotic bacterium Lactobacillus spp. in the workers of Indian honeybee, Apis cerana indica, Agris On-line Papers in Economics and Informatics. https://doi.org/10.6088/ijes.00202030002
- Ploux L., Beckendorff S., Nardin M., Neunlist S. Quantitative and morphological analysis of biofilm formation on self-assembled monolayers. Colloids Surfaces B Biointerfaces. 2007;57:174–181. doi: 10.1016/j.colsurfb.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Ptaszyńska A.A., Borsuk G., Zdybicka-Barabas A., Cytryńska M., Małek W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol. Res. 2016;115:397–406. doi: 10.1007/s00436-015-4761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptaszyńska A.A., Gancarz M., Hurd P.J., Borsuk G., Wiącek D., Nawrocka A., Strachecka A., Załuski D., Paleolog J. Changes in the bioelement content of summer and winter western honeybees (Apis mellifera) induced by Nosema ceranae infection. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann, K., Moran, N.A., 2018. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. https://doi.org/10.1016/j.cois.2018.02.012 [DOI] [PMC free article] [PubMed]
- Reybroeck, W., Daeseleire, E., De Brabander, H.F., Herman, L., 2012. Antimicrobials in beekeeping. Vet. Microbiol. https://doi.org/10.1016/j.vetmic.2012.01.012 [DOI] [PubMed]
- Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol. Lett. 1984;22:289–295. doi: 10.1111/j.1574-6968.1984.tb00743.x. [DOI] [Google Scholar]
- Rosenberg M., Kjelleberg S. Hydrophobic Interactions: Role in Bacterial Adhesion. In: Marshall K.C., editor. Advances in Microbial Ecology. Springer; US, Boston, MA: 1986. pp. 353–393. [DOI] [Google Scholar]
- Saarela M., Mogensen G., Fondén R., Mättö J., Mattila-Sandholm T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000 doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Schiffer C., Hilgarth M., Ehrmann M., Vogel R.F. Bap and cell surface hydrophobicity are important factors in Staphylococcus xylosus biofilm formation. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin, A.L., 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. https://doi.org/10.1016/j.femsre.2004.01.003 [DOI] [PubMed]
- Shokri D., Khorasgani M.R., Mohkam M., Fatemi S.M., Ghasemi Y., Taheri-Kafrani A. The Inhibition Effect of Lactobacilli Against Growth and Biofilm Formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins. 2018;10:34–42. doi: 10.1007/s12602-017-9267-9. [DOI] [PubMed] [Google Scholar]
- Stanley, N.R., Lazazzera, B.A., 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. https://doi.org/10.1111/j.1365-2958.2004.04036.x [DOI] [PubMed]
- Strauss U., Pirk C.W.W., Crewe R.M., Human H., Dietemann V. Impact of Varroa destructor on honeybee (Apis mellifera scutellata) colony development in South Africa. Exp. Appl. Acarol. 2015;65:89–106. doi: 10.1007/s10493-014-9842-7. [DOI] [PubMed] [Google Scholar]
- Szymaś B., Łangowska A., Kazimierczak-Baryczko M. Obraz histologiczny jelita środkowego pszczół (Apis mellifera L.) żywionych namiastkami pyłku kwiatowego wzbogaconymi probiotykami. J. Apic. Sci. 2012;56:5–12. doi: 10.2478/v10289-012-0001-2. [DOI] [Google Scholar]
- Tejchman W., Korona-Glowniak I., Malm A., Zylewski M., Suder P. Antibacterial properties of 5-substituted derivatives of rhodanine-3-carboxyalkyl acids. Med. Chem. Res. 2017;26:1316–1324. doi: 10.1007/s00044-017-1852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Y., Yu H., Ai L., Wu Z., Guo B., Chen W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013;96:4252–4257. doi: 10.3168/jds.2013-6547. [DOI] [PubMed] [Google Scholar]
- Vásquez A., Forsgren E., Fries I., Paxton R.J., Flaberg E., Szekely L., Olofsson T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez A., Olofsson T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009;48:189–195. doi: 10.3896/IBRA.1.48.3.07. [DOI] [Google Scholar]
- Vinderola C.G., Reinheimer J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003;36:895–904. doi: 10.1016/S0963-9969(03)00098-X. [DOI] [Google Scholar]
- Woo J., Ahn J. Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Lett. Appl. Microbiol. 2013;56:307–313. doi: 10.1111/lam.12051. [DOI] [PubMed] [Google Scholar]
- Wu M., Sugimura Y., Iwata K., Takaya N., Takamatsu D., Kobayashi M., Taylor D., Kimura K., Yoshiyama M. Inhibitory effect of gut bacteria from the Japanese honey bee, Apis cerana japonica, against Melissococcus plutonius, the causal agent of European foulbrood disease. J. Insect Sci. 2014;14 doi: 10.1093/jis/14.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Sugimura Y., Taylor D., Yoshiyama M. Honeybee Gastrointestinal Bacteria for Novel and Sustainable Disease Control Strategies. J. Dev. Sustain. Agric. 2013;8:85–90. doi: 10.11178/jdsa.8.85. [DOI] [Google Scholar]
- Zuo F., Yu R., Feng X., Chen L., Zeng Z., Khaskheli G.B., Ma H., Chen S. Characterization and in vitro properties of potential probiotic Bifidobacterium strains isolated from breast-fed infant feces. Ann. Microbiol. 2016;66:1027–1037. doi: 10.1007/s13213-015-1187-x. [DOI] [Google Scholar]