Abstract
Several anti-nutritional substances are found in plant derivatives for example phytate, that make the nutrients and minerals unavailable to fish, hence leading to poor growth performance. Presence of the anti-nutrient factor such as phytate is a chelated compound and need enzyme for its breakdown and availability of nutrients to improve fish growth. This research work was performed to check the improvement of overall performance of Cyprinus carpio fingerlings by the help of phytase addition in Moringa oleifera by- products based diet. Combination of Moringa seed meal and Moringa leaf meal was utilized as test ingredient to formulate seven test feeds, containing graded levels of phytase (0, 500, 650, 800, 950, 1100 and 1250 FTU kg−1). In feeding trial of 70 days, fingerlings were given feed two times in a day at the rate of 4% of wet weight of their bodies and faeces were collected. According to current results, it was found that growth performance parameters i.e. weight gain; 25 g, specific growth rate; 1.67 and feed conversion ratio; 1.10 were improved to maximum at 950 FTU kg−1. Digestibility of nutrients (crude protein; 73%, crude fat; 71% and gross energy; 67%) and minerals absorption was also maximum (Ca; 70%, Zn; 66%, K; 74%, Mn; 66% and P; 71%) at 950 FTU kg−1. Lowest growth efficiency, nutrient digestibility and mineral absorption were observed in fingerlings fed at control diet (0 FTU kg−1). Results of the current study, proved that 950 FTU kg−1 is the most optimum level of phytase to formulate economical and ecofriendly feed for improved growth of C. carpio fingerlings as it decreases the discharge of minerals and nutrients in water bodies.
Keywords: Moringa oleifera, Phytase, Cyprinus carpio, Performance, Digestibility, Absorption
1. Introduction
The fastest and developing source of animal protein is aquaculture, providing almost half of the entire fish consumed worldwide (Bostock et al., 2010). On account of the rapid human population rise and to fulfill the need of quality food, aquaculture industry has been developed as rapidly growing food producing sector (Ibrahem et al., 2010). In the past, fishmeal (FM) was used as chief source of protein in fish food (Shahzad et al., 2017), because of the presence of large amount of essential nutrients for example amino acids, lipids, growth factors and vitamins, high digestibility and low level of carbohydrate (Zhou et al., 2004, Morales et al., 2014). But, shortage and high cost of FM leads it to a need of using low cost alternate protein sources that are plant derived (Morales et al., 2014). Various plant by-products based diets such as corn gluten (Hussain et al., 2015a, Hussain et al., 2018), sunflower (Rabia et al., 2017), soybean meal (Hussain et al., 2015b, Chu et al., 2016), Moringa oleifera seed meal (Shahzad et al., 2018a) and Moringa oleifera leaf meal (Shahzad et al., 2017) were given to different fish species. One of the economical alternative source of plant protein is Moringa oleifera, commonly recognized as “sohanjana” in Pakistan and easily available in southern Punjab (Hassan et al., 2018). MOLM (M. oleifera leaf meal) was utilized as feed for common carp (Yuangsoi and Masumoto, 2012), Nile tilapia (Afuang et al., 2003, Richter et al., 2003), African catfish (Samkelisiwe and Ngonidzashe, 2014) and Catla catla (Shahzad et al., 2017), because it contains higher protein content ranging from 25 (Makkar and Becker, 1996) to 32% (Soliva et al., 2005). MOSM (M. oleifera seed meal) contains rich amount of protein (32–35%), essential vitamins, amino acids (Hassan et al., 2018) and minerals i.e. P, Na, K, Ca, Zn and Mg etc. (Anjorin et al., 2010).
But major problem in utilization of plant by-product based protein sources such as M. oleifera is the presence of several antinutritional factors (Danwitz et al., 2016). Most important antinutritional factor among these is phytic acid (PA) or phytate (Reddy, 2002). In salt form, phytate comprises 60 to 80% of entire P (phosphorus), chelated form of magnesium, calcium and sodium salts found in plant based diets (Lei et al., 2013). It acts as a lock around P and bounds with other minerals such as Mg, Mn, Cu, Fe and Ca (Denstadli et al., 2006) and decreases their absorption (Hussain et al., 2011a, Dawood et al., 2015, Hussain et al., 2015b). It results in adverse effects on growth performance of monogastric and agastric fishes (NRC, 1993).
Due to all these harmful effects, there is a need of breakdown of phytate-mineral complexes present in plant by-product based diets. Phytic acid cannot be removed easily unless some enzymatic reactions are performed (Vielma et al., 2000). Phytase (PHY) enzyme is very effective to catalyze the hydrolysis of PA and extracts P, making it available for absorption (Kumar et al., 2012a). PHY removes phosphate from PA in a stepwise process (Lei et al., 2013). PHY is thought to be a significant feed additive for agastric and monogastric animals to build the accessibility of P and other fundamental nutrients (Lei and Stahl, 2000). It releases the chelated minerals, leading to their increased absorption (Rabia et al., 2017). Addition of PHY maximizes the bio‐availability of nutrients e.g. P and minimizes its liberation into the aquatic system (Cao et al., 2007a, Kumar et al., 2016, Shahzad et al., 2021). Dietary phytase addition in feed is a useful technique to progress the feed conversion ratio (FCR), absorption of minerals, complete digestion and retention of P in body and consequently decreases the pollution in aquatic bodies (Hussain et al., 2011b, Liu et al., 2013, Hussain et al., 2015b).
Common carp is a local, tropical, freshwater and rapid growing fish (Vong et al., 2003). C. carpio is considered among the highly important cultured species, subsisting in rivers, lakes and reservoirs (Xu et al., 2019). The global estimated yield of common carp in 2014, was 4.16 million tons. The total FM consumption by common carp was estimated to be 374,000 tons in 2014 (FAO, 2014).
Purpose of the current study was to inspect the effectiveness of PHY addition on growth, nutrient digestibility and mineral availability of C. carpio feeding on diet comprising Moringa derivatives based meal. Moreover, it is anticipated that information perceived from this study will be helpful to formulate an ecofriendly and profitable fish feed.
2. Materials and methods
The current experimental work was performed to check the improvement of overall performance of common carp fingerlings, using Moringa derivatives, supplemented with PHY to estimate its impact on growth, nutrient digestibility and mineral absorption. The experiment was carried out in Zoology Department, Division of Science and Technology, University of Education, Lahore.
2.1. Fish and experimental conditions
Common carp fingerlings were procured from Sindwa Fish Hatchery, Head Balloki, Kasur. Before starting experiment, the carps were acclimatized for almost ten days and were kept (fifteen fingerlings in each) in water tanks of V shape with 70 L water holding capacity. During entire period, PHY supplemented moringa derivatives based-feed was given twice a day (Allan and Rowland, 1992). Parameters related to water quality for example temperature, dissolved oxygen and pH were checked regularly and air pump was used for proper supply of oxygen by capillary system. Before starting the feed trial, carps were treated with saline solution (0.5%) for 1–2 min to eliminate the pathogens (Rowland and Ingram, 1991).
2.2. Experimental strategy
Derivatives of moringa plant (combination of MOSM and MOLM) were used as test ingredient and mixture of feed was distributed into seven test feeds, sprayed with varied amounts (0, 500, 650, 800, 950, 1100 and 1250 FTU kg−1) of PHY enzyme. Out of these seven test diets, one control (0 FTU kg−1) and six PHY supplemented test diets were given to seven groups of fingerlings. They were fed at the rate of 4% of live wet body weight for 70 days. Each test and control diet were compared with each other to identify growth, mineral absorption and nutrient digestibility.
2.3. Processing of Moringa derivatives
M. oleifera seeds were purchased from local market of Lahore. Seeds were first dried in air and then de-fatted using press method (Salem and Makkar, 2009). Fresh leaves of moringa were taken from botanical garden of University of Education, Lahore and then washed to make them clean and dirt-free. Then, these leaves were dried under a shady place for nearly six days to protect the vitamins from harm via photo-dynamic oxidation. Dry leaves were detached from the stems to reduce amount of fibre in feed (Madalla et al., 2013). Treated Moringa leaves and seeds were crushed to convert them into powder. The remaining feed components were obtained from market and were ground to pass them through 0.3 mm sieve size. Before the formulation of experimental diets, chemical composition of all diet components was studied by using subsequent standard methods (AOAC, 1995). Firstly, in all experimental diets, 1% Cr2O3 (as an inert marker) was used and mixed with all other components of feed carefully for at least 5–10 min. Then, slow blending of components was done after the addition of 10 to 15% distilled water, to make properly textured dough. Processing of dough was done within the pelleting machine to form pellets (Lovell, 1989). Moringa derivatives were used to prepare six PHY added and one control test diet. whereas, 50 mL distilled water was used to prepare the various concentrations of PHY enzyme (0, 500, 650, 800, 950, 1100 and 1250 FTU kg−1) and were sprayed on every test diet (Robinson et al., 2002). Similar amount of distilled water was also sprayed on control (0 FTU kg−1) diet to maintain the moisture. At the end, all the prepared diets were dried out and then stored at almost 4 °C till use (see Table 1).
Table 1.
Chemical and physical ingredients composition (%) of feed.
|
Physical composition |
Chemical composition |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ingredients | Test feed composition | Dry mass (%) | CP (%) | EE (%) | GE (%) | Ash (%) | Crude fiber (%) | Carbohydrates |
| MOSM + MOLM | 36 | 91.24 | 33.31 | 3.91 | 4.02 | 10.07 | 13.56 | 39.15 |
| Fish meal | 17 | 92.39 | 49.31 | 6.99 | 2.23 | 24.66 | 1.29 | 17.75 |
| Wheat flour | 16 | 93.09 | 9.43 | 2.41 | 3.09 | 2.06 | 2.88 | 83.22 |
| Corn Gluten (60%) | 13 | 91.78 | 58.97 | 4.96 | 4.21 | 1.41 | 1.23 | 33.43 |
| Rice polish | 7.5 | 92.2 | 13.02 | 12.76 | 3.03 | 11.17 | 13.06 | 49.99 |
| Fish oil | 6.5 | – | – | – | – | – | – | – |
| PHY level (FTU kg−1) | 0 | – | – | – | – | – | – | – |
| *Mineral Premix | 1 | – | – | – | – | – | – | – |
| **Vitamin Premix | 1 | – | – | – | – | – | – | – |
| Chromic oxide | 1 | – | – | – | – | – | – | – |
| Ascorbic acid | 1 | – | – | – | – | – | – | – |
| Proximate composition (%) of diets with varied amounts of PHY | ||||
|---|---|---|---|---|
| Test feeds | PHY level (FTU kg−1) | CP (%) in feed | EE (%) in feed | GE (Kcal g−1) in feed |
| D–I (Control) | 0 | 31.57±0.37 | 6.16±0.20 | 3.52±0.20 |
| D–II | 500 | 31.56±0.21 | 6.19±0.23 | 3.53±0.22 |
| D–III | 650 | 31.58±0.30 | 6.20±0.18 | 3.50±0.27 |
| D–IV | 800 | 31.59±0.34 | 6.18±0.23 | 3.51±0.29 |
| D–V | 950 | 31.56±0.28 | 6.18±0.22 | 3.50±0.22 |
| D–VI | 1100 | 31.59±0.27 | 6.17±0.19 | 3.51±0.22 |
| D–VII | 1250 | 31.56±0.29 | 6.20±0.24 | 3.53±0.13 |
PHY enzyme was used at cost of wheat flour.
**Vitamin (Vit.) premix kg−1:
Vit. A: 15,000,000 IU Vit. C: 15,000 mg Vit. E:30000 IU.
Vit. B2: 7000 mg Vit. B6: 4000 mg Vit. B12: 40 mg.
Vit. D3: 3,000,000 IU Vit. K3: 8000 mg Ca pantothenate: 12,000 mg.
Nicotinic acid: 60,000 mg Folic acid: 1500 mg.
*Mineral premix kg−1:
Ca: 155 g Na: 45 g P: 135 g Mg: 55 g Cu: 600 mg Co: 40 mg.
Fe: 1000 mg Mn: 2000 mg Zn:3000 mg I: 40 mg Se: 3 mg.
2.4. Feeding procedure and assemblage of sample
C. carpio fingerlings were given prescribed feed two times daily, and after completion of feeding time (two hours), the leftover diet was removed from each tank. The tanks were washed properly to eliminate the particles of feed and were refilled. Faeces were collected from the faecal accumulating tube of every tank by opening the valve-1 and 2 subsequently. Faecal material was collected carefully to lessen the faeces breakage so that nutrients are not leached out in water and were dried in oven at 65 °C for 3 to 4 h and then stored for further analysis.
2.5. Growth study
To study the growth of fingerlings, fifteen carps of average weight (5.65 g fish−1) were kept in each tank and were bulk weighed on periodical basis throughout the experimental period to assess the growth efficiency of C. carpio. Growth parameters for example FCR, weight gain (%) and specific growth rate (SGR) of carps were calculated by using standard formulae (NRC, 1993).
| (1) |
| (2) |
| (3) |
2.6. Chemical analysis of faeces and feed
After 70 days of feeding period, homogenized samples of diet and faeces were dried in oven at 105 °C for almost 12 h and moistness was measured. CP (crude protein) contents were identified by using Micro Kjeldahl Apparatus, whereas EE (ether extract) was analysed through Soxhlet system, by using petroleum ether extraction method. GE (gross energy) was estimated by Oxygen bomb calorimeter. Following formula was used to calculate total carbohydrate contents.
2.7. Assessment of minerals
To estimate the minerals, Atomic Absorption Spectrophotometer, was used according to AOAC (1995) and potassium and sodium were estimated by flame photometer. Contents of marker present in faeces and diets were assessed after oxidation with molybdate reagent (Divakaran et al., 2002) and UV–VIS 2001 Spectrophotometer was used at absorbance of 370 nm. Contents of phosphorous in the diets and faeces were determined with the help of UV/VIS spectrophotometer at absorbance of 720 nm.
2.8. Calculation of ADC (%)
Apparent digestibility coefficient (ADC) of nutrients present in diets was measured by using the following formula (NRC, 1993);
| (4) |
2.9. Statistical analysis
Finally, data of ADC% of nutrients (GE, EE and CP), minerals (Ca, K, Na, Fe, P, Cu, Al, Mg, Mn and Zn) and growth was analyzed by one-way ANOVA test. The differences among all treatments were compared using Tukey’s Honesty Significant Difference Test by considering significant at p < 0.05 (Biswas et al., 2019). The CoStat Computer Package was used to perform statistical analysis.
3. Results
3.1. Growth study of C. carpio
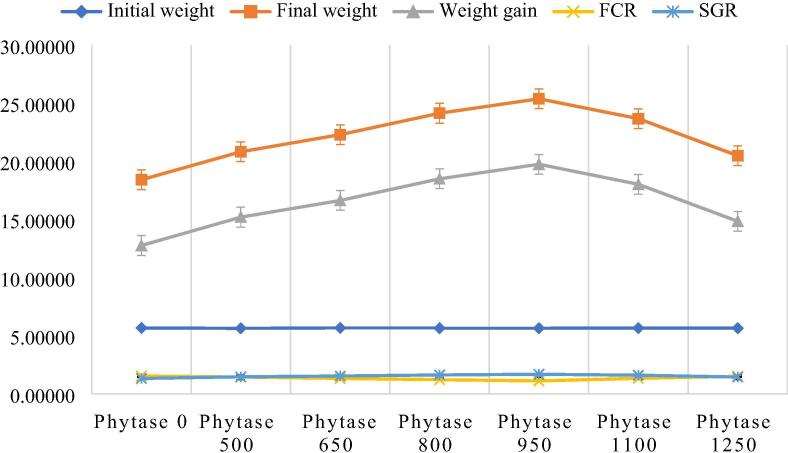
Table 2 presents the mean values of total growth performance i.e. WG (g), WG (%), FCR, SGR and survival rate of C. carpio, feeding on MOSM + MOLM combination based test diets. According to our results, C. carpio that were given 950 FTU kg−1 PHY level based diet, showed maximum weight gain (19 g), WG (350%), SGR (1.67) and minimum FCR (1.10), showing highest conversion of feed into flesh, followed by WG (18 g), WG (327%), SGR (1.61) and second lower FCR (1.20) at 800 FTU kg−1 amount of PHY. These mean values were significantly different (p < 0.05) from the values found on control and other PHY supplemented diets. In contrary, fish feeding on control diet showed significantly least value of weight gain (%) i.e. 225%, SGR (1.31) and highest FCR value (1.52) representing lowest growth rate (Fig. 1).
Table 2.
Growth performance of C. carpio given varied amounts of PHY added Moringa derivatives (MOSM and MOLM) mixture- made diets.
| Test diets | PHY levels (FTU kg−1) | Initial weight | FW | WG (g) | WG% | WG /fish /day | Feed intake | FCR | SGR | Survival rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Diet-I (control) | 0 | 5.66 ± 0.31 | 18.41 ± 0.64e | 12.75 ± 0.36e | 225.71 ± 7.90e | 0.18 ± 0.01e | 0.28 ± 0.02b | 1.52 ± 0.06a | 1.31 ± 0.03e | 91.79 ± 3.72 |
| Diet-II | 500 | 5.62 ± 0.25 | 20.82 ± 0.64 cd | 15.19 ± 0.41d | 270.35 ± 6.72d | 0.22 ± 0.01d | 0.31 ± 0.01ab | 1.43 ± 0.01a | 1.45 ± 0.02d | 94.00 ± 5.89 |
| Diet-III | 650 | 5.66 ± 0.19 | 22.29 ± 0.46bc | 16.63 ± 0.28c | 293.69 ± 4.76c | 0.24 ± 0.00c | 0.31 ± 0.01a | 1.31 ± 0.03b | 1.52 ± 0.01c | 95.96 ± 3.51 |
| Diet-IV | 800 | 5.64 ± 0.25 | 24.13 ± 0.72a | 18.49 ± 0.47b | 327.82 ± 6.02b | 0.26 ± 0.01b | 0.32 ± 0.01a | 1.20 ± 0.03c | 1.61 ± 0.02b | 97.92 ± 3.61 |
| Diet-V | 950 | 5.63 ± 0.29 | 25.38 ± 0.97a | 19.74 ± 0.70a | 350.69 ± 7.64a | 0.28 ± 0.01a | 0.31 ± 0.01ab | 1.10 ± 0.02d | 1.67 ± 0.02a | 97.92 ± 3.61 |
| Diet-VI | 1100 | 5.65 ± 0.16 | 23.67 ± 0.50ab | 18.02 ± 0.34b | 318.99 ± 3.31b | 0.26 ± 0.00b | 0.34 ± 0.01a | 1.32 ± 0.03b | 1.59 ± 0.01b | 95.96 ± 3.51 |
| Diet-VII | 1250 | 5.64 ± 0.17 | 20.48 ± 0.42d | 14.84 ± 0.25d | 263.13 ± 3.64d | 0.21 ± 0.00d | 0.32 ± 0.01a | 1.50 ± 0.05a | 1.43 ± 0.01d | 91.79 ± 3.72 |
a-fMeans within column having dissimilar superscripts are quietly different at p < 0.05. Data are means of three replicates with fifteen fingerlings in each.
Fig. 1.
Graphical presentation of growth parameters.
3.2. Nutrients digestibility parameters
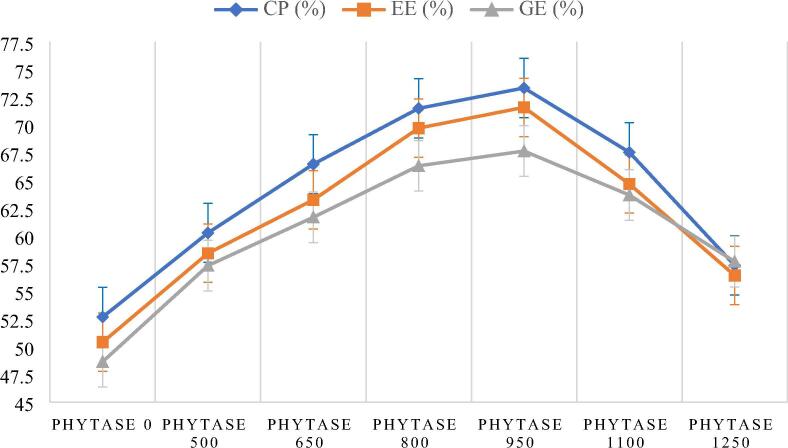
After feeding the fingerlings on iso-caloric and iso-energetic diets for 70 days, it was found that highest ADC (%) of all nutrients i.e. CP (73%), EE (71%) and GE (67%) was observed in fish feeding on D-V with 950 FTU kg−1 of PHY, followed by 800 FTU kg−1 (CP;71%, EE; 69% and GE; 66%). Values of digestibility coefficients of both fish groups (D-V and D-IV) were different (p < 0.05) from control and other PHY containing feeds. Lowest digestibility coefficient of CP (52%), EE (50%) and GE (48%) was found in fish feeding on control (0 FTU kg−1) diet (Fig. 2). It was noticed from the results that values of digestibility coefficient of CP, EE and GE started to increase from 500 to 950 FTU kg−1. It was also noticed that high amounts of PHY in diet did not put any positive impact on digestibility of nutrients as increase in amounts of PHY (1100 and 1250 FTU kg−1) increased the liberation of nutrients in water and started to put negative effect on their digestibility as shown in Table 3.
Fig. 2.
Graphical representation of nutrient digestibility parameters.
Table 3.
Apparent nutrient digestibility (%) of CP, GE and EE in C. carpio feeding on PHY added MOSM + MOLM based diets.
| Test diets | PHY level (FTU kg−1) | CP (%) | EE (%) | GE (%) |
|---|---|---|---|---|
| Diet –I (Control) | 0 | 52.69 ± 0.83e | 50.42 ± 0.78d | 48.66 ± 0.97d |
| Diet –II | 500 | 60.28 ± 0.90c | 58.44 ± 0.94c | 57.33 ± 0.96c |
| Diet –III | 650 | 66.48 ± 0.91b | 63.26 ± 0.98b | 61.69 ± 0.75b |
| Diet –IV | 800 | 71.51 ± 0.94a | 69.73 ± 0.65a | 66.33 ± 0.64a |
| Diet –V | 950 | 73.35 ± 0.89a | 71.60 ± 0.77a | 67.66 ± 0.91a |
| Diet –VI | 1100 | 67.55 ± 0.93b | 64.69 ± 0.86b | 63.69 ± 0.90b |
| Diet –VII | 1250 | 57.35 ± 0.90d | 56.44 ± 0.58c | 57.69 ± 0.98c |
a-fMeans within column having dissimilar superscripts are quietly different at p < 0.05.
Data are means of three replicates with fifteen fingerlings in each.
3.3. Absorption of minerals (Mn, Ca, Zn, K, Na, Al, Fe, P, Cu and Mg)
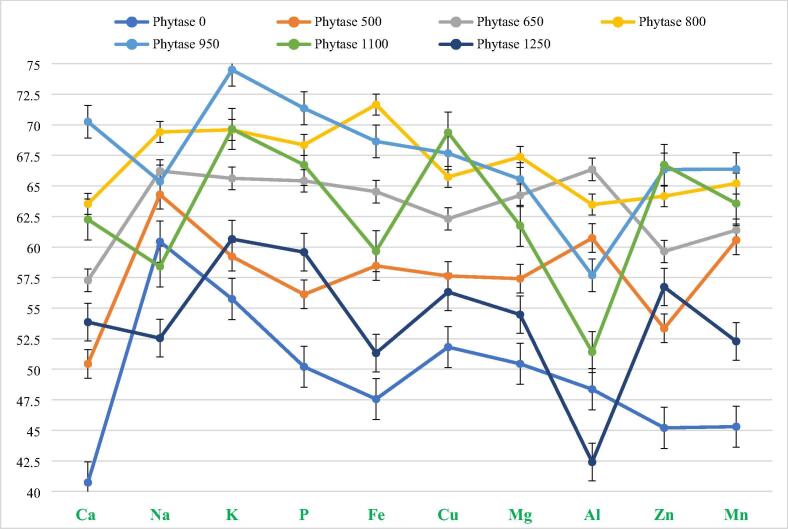
Table 4 shows that amount of all minerals e.g. Mn, Ca, K, Na, Fe, P, Cu, Mg and Zn was almost similar in all diets (control and test). Quantity of Al in diets was hardly detected during analysis, due to its least amount present in diet. It was noticed that faeces of fish fed on different levels of PHY added diets contain different amount of minerals as compared to their respective diets (Table 5). Lowest amount of minerals was present in faeces of fish fed on 950 FTU kg−1 added diet (Diet-V) throughout the research period. Table 6 shows that the absorption of maximum (70, 66, 74, 66 and 71%) amount of minerals (Ca, Zn, K, Mn and P respectively) was observed at 950 FTU kg−1. Highest absorption of few minerals for example Fe, Na and Mg was observed at 800 FTU kg−1 i.e. 71, 69 and 67% respectively. While, lowest absorption was noted at 0 FTU kg−1 (control diet), of maximum minerals such as Ca (40%), K (55%), P (50%), Fe (47%), Cu (51%), Mg (50%), Zn (45%) and Mn (45%). These absorption values were highly significant from those of minerals found at 950 and 800 FTU kg−1 PHY levels based diets. Na and Al showed least absorption value (52% and 42% respectively) at diet-VII (1250 FTU kg−1) instead of control diet (Fig. 3). Results clarified the fact that amount of PHY in diet V (950 FTU kg−1) is sufficient here to discharge the nutrients (CP, EE and GE) and minerals from the phytate complexes and make them available for fish. It decreases nutrient and mineral discharge in water through faeces and ultimately decreases the water pollution.
Table 4.
Examined composition of minerals in a diet, containing mixture of MOSM + MOLM.
| Test diets | PHY level | Ca | Na | K | P | Fe | Cu | Mg | Al | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet-I (Control) | 0 | 0.86 ± 0.08 | 0.017 ± 0.002 | 1.28 ± 0.07 | 2.08 ± 0.08 | 0.051 ± 0.007 | 0.0059 ± 0.0008 | 0.0094 ± 0.0007 | 0.00050 ± 0.00008 | 0.039 ± 0.008 | 0.021 ± 0.007 |
| Diet-II | 500 | 0.86 ± 0.06 | 0.016 ± 0.002 | 1.28 ± 0.08 | 2.08 ± 0.07 | 0.051 ± 0.007 | 0.0059 ± 0.0009 | 0.0093 ± 0.0006 | 0.00051 ± 0.00004 | 0.038 ± 0.003 | 0.020 ± 0.009 |
| Diet-III | 650 | 0.85 ± 0.05 | 0.018 ± 0.002 | 1.28 ± 0.05 | 2.08 ± 0.10 | 0.051 ± 0.008 | 0.0058 ± 0.0006 | 0.0094 ± 0.0007 | 0.00052 ± 0.00007 | 0.038 ± 0.003 | 0.021 ± 0.008 |
| Diet-IV | 800 | 0.85 ± 0.06 | 0.017 ± 0.004 | 1.28 ± 0.05 | 2.09 ± 0.08 | 0.050 ± 0.010 | 0.0059 ± 0.0008 | 0.0093 ± 0.0008 | 0.00052 ± 0.00007 | 0.039 ± 0.008 | 0.022 ± 0.006 |
| Diet-V | 950 | 0.85 ± 0.09 | 0.017 ± 0.004 | 1.27 ± 0.08 | 2.08 ± 0.05 | 0.050 ± 0.008 | 0.0059 ± 0.0007 | 0.0094 ± 0.0009 | 0.00052 ± 0.00010 | 0.039 ± 0.006 | 0.021 ± 0.004 |
| Diet-VI | 1100 | 0.87 ± 0.06 | 0.018 ± 0.003 | 1.29 ± 0.09 | 2.08 ± 0.09 | 0.050 ± 0.006 | 0.0060 ± 0.0007 | 0.0093 ± 0.0007 | 0.00051 ± 0.00009 | 0.040 ± 0.004 | 0.022 ± 0.003 |
| Diet-VII | 1250 | 0.87 ± 0.08 | 0.017 ± 0.005 | 1.28 ± 0.05 | 2.07 ± 0.07 | 0.052 ± 0.009 | 0.0059 ± 0.0009 | 0.0095 ± 0.0007 | 0.00050 ± 0.00005 | 0.040 ± 0.008 | 0.022 ± 0.006 |
Data are means of three replicates with fifteen fingerlings in each.
Table 5.
Examined composition of minerals in C. carpio faeces fed on diet containing mixture of MOSM + MOLM.
| Test feeds | PHY levels | Ca | Na | K | P | Fe | Cu | Mg | Al | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet-I (Control) | 0 | 0.55 ± 0.05a | 0.007 ± 0.001a | 0.62 ± 0.02a | 1.13 ± 0.06a | 0.029 ± 0.004a | 0.0031 ± 0.0005a | 0.0051 ± 0.0003a | 0.00028 ± 0.00005ab | 0.023 ± 0.004a | 0.012 ± 0.004a |
| Diet-II | 500 | 0.47 ± 0.04b | 0.006 ± 0.000a | 0.57 ± 0.04a | 0.99 ± 0.02b | 0.023 ± 0.002ab | 0.0027 ± 0.0005ab | 0.0043 ± 0.0003abc | 0.00022 ± 0.00002b | 0.019 ± 0.001ab | 0.009 ± 0.004a |
| Diet-III | 650 | 0.40 ± 0.03bc | 0.007 ± 0.001a | 0.48 ± 0.02b | 0.79 ± 0.04c | 0.020 ± 0.003ab | 0.0024 ± 0.0003ab | 0.0037 ± 0.0002 cd | 0.00019 ± 0.00003b | 0.017 ± 0.001ab | 0.009 ± 0.003a |
| Diet-IV | 800 | 0.34 ± 0.03 cd | 0.006 ± 0.001a | 0.43 ± 0.01bc | 0.72 ± 0.04 cd | 0.015 ± 0.004b | 0.0022 ± 0.0003ab | 0.0033 ± 0.0004d | 0.00021 ± 0.00003b | 0.015 ± 0.003ab | 0.008 ± 0.002a |
| Diet-V | 950 | 0.28 ± 0.02d | 0.006 ± 0.001a | 0.36 ± 0.01c | 0.66 ± 0.05d | 0.017 ± 0.003b | 0.0021 ± 0.0002ab | 0.0036 ± 0.0004 cd | 0.00024 ± 0.00004ab | 0.015 ± 0.003b | 0.008 ± 0.002a |
| Diet-VI | 1100 | 0.36 ± 0.02 cd | 0.008 ± 0.001a | 0.43 ± 0.04bc | 0.76 ± 0.03 cd | 0.022 ± 0.003ab | 0.0020 ± 0.0003b | 0.0039 ± 0.0003bcd | 0.00027 ± 0.00004ab | 0.015 ± 0.002b | 0.009 ± 0.001a |
| Diet-VII | 1250 | 0.44 ± 0.04bc | 0.009 ± 0.002a | 0.55 ± 0.02a | 0.92 ± 0.02b | 0.028 ± 0.005a | 0.0028 ± 0.0004ab | 0.0047 ± 0.0003ab | 0.00032 ± 0.00002a | 0.019 ± 0.003ab | 0.011 ± 0.003a |
a-fMeans within column having dissimilar superscripts are quietly different at p < 0.05.
Data are means of three replicates with fifteen fingerlings in each
Table 6.
Examined minerals absorption in C. carpio feeding on diet containing mixture of MOSM + MOLM.
| Test feeds | PHY levels | Ca | Na | K | P | Fe | Cu | Mg | Al | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet-I (Control) | 0 | 40.73 ± 0.90e | 60.43 ± 0.98c | 55.75 ± 0.92e | 50.20 ± 0.92f | 47.56 ± 0.85f | 51.81 ± 0.90e | 50.44 ± 0.90f | 48.36 ± 0.96f | 45.20 ± 0.55f | 45.30 ± 0.90f |
| Diet-II | 500 | 50.44 ± 0.63d | 64.29 ± 0.82b | 59.22 ± 0.88d | 56.12 ± 0.33e | 58.46 ± 0.93d | 57.63 ± 0.93d | 57.41 ± 0.82d | 60.73 ± 0.88c | 53.34 ± 0.75e | 60.56 ± 0.82d |
| Diet-III | 650 | 57.27 ± 0.88c | 66.22 ± 0.96b | 65.62 ± 0.62c | 65.41 ± 0.79c | 64.53 ± 0.90c | 62.31 ± 0.96c | 64.23 ± 0.98b | 66.35 ± 0.81a | 59.64 ± 0.78c | 61.38 ± 0.67 cd |
| Diet-IV | 800 | 63.53 ± 0.90b | 69.41 ± 0.87a | 69.59 ± 0.91b | 68.35 ± 0.99b | 71.65 ± 0.95a | 65.73 ± 0.86b | 67.37 ± 0.90a | 63.47 ± 0.59b | 64.16 ± 1.08b | 65.20 ± 0.76ab |
| Diet-V | 950 | 70.25 ± 0.99a | 65.36 ± 0.71b | 74.50 ± 0.71a | 71.35 ± 0.86a | 68.64 ± 0.87b | 67.67 ± 0.85ab | 65.55 ± 0.82ab | 57.69 ± 0.79d | 66.34 ± 0.86ab | 66.37 ± 0.79a |
| Diet-VI | 1100 | 62.25 ± 0.92b | 58.41 ± 0.72c | 69.66 ± 0.80b | 66.72 ± 0.97bc | 59.66 ± 0.90d | 69.37 ± 0.87a | 61.74 ± 0.81c | 51.41 ± 0.82e | 66.72 ± 0.95a | 63.56 ± 0.96bc |
| Diet-VII | 1250 | 53.85 ± 0.84c | 52.54 ± 0.98d | 60.65 ± 0.95d | 59.58 ± 0.93d | 51.32 ± 0.96e | 56.32 ± 0.87d | 54.47 ± 0.83e | 42.40 ± 0.94 g | 56.73 ± 0.88d | 52.28 ± 0.84e |
a-fMeans within column having dissimilar superscripts are quietly different at p < 0.05.
Data are means of three replicates with fifteen fingerlings in each.
Fig. 3.
Graphical representation of minerals absorption.
4. Discussion
Phytic acid present in plant meal based diets binds and reduces the absorption of nutrients and minerals that are required to support the normal growth of fish (Kumar et al., 2012a, Kumar et al., 2012b). The enzymatic activity of PHY breaks down these antinutritional factors into simple and easily absorbed nutrients (Sokrab et al., 2012). Maximum growth performance in terms of WG (19 g), FCR (1.10) and SGR (1.67) was estimated in C. carpio at 950 FTU kg−1 of PHY, followed by 800 FTU kg−1. Like above mentioned results, Hussain et al., 2017, Shahzad et al., 2018b found highest growth performance in C. catla when fed on a PHY supplemented (900 FTU kg−1) moringa by-product based diet in comparison of control and other PHY supplemented diets. Maas et al. (2019) observed improved growth performance in Nile tilapia at 1000 FTU kg−1 supplemented in sunflower meal based diet. Hassan et al., 2009, Hassaan et al., 2013, Rachmawati and Samidjan, 2016 also studied higher growth performance in Chanos chanos, O. niloticus and Penaeus monodon respectively, by using PHY (1000 FTU kg−1) in SBM (soybean meal) based diet. In a growth study performed by Rachmawati and Samidjan, (2017), all growth parameters were improved at PHY levels ranging from 943 to1100. Slightly different results from our conclusions were found by Hussain et al., 2015c, Hussain et al., 2011c) and Baruah et al. (2007a) in rohu at 750 FTU kg−1. Biswas et al. (2019) detailed that addition of PHY upto 2000 FTU kg−1 level in SPC (soy protein concentrate) made diet caused betterment in FMW (final mean weight) of Pagrus major. Olusola and Nwanna (2014) reported higher growth rate in O. niloticus and C. gariepinus, fed on SBM containing 8000 FTU kg−1. In contrast to our results, PHY did not put any positive impact on growth parameters of fish such as, in research work of Xue, 2014, Qiu and Davis, 2016, PHY caused no significant differences in growth of Nile tilapia and Litopenaeus vannamei respectively. These alterations in effect of PHY and results may be connected to various factors for example varying PHY levels, types of diet ingredients used, feed processing methods, presence or absence of stomach, stomach pH, fish species and processes utilized for drying of feed (Wang et al., 2009). Therefore, PHY should always be added in diet in view of all these factors (Cao et al., 2007b). In current research, it was explained that 950 FTU kg−1 is best PHY level for C. carpio to increase the digestibility of CP, EE and GE. Similar to these results, Shahzad et al. (2018a; 2020) found highest digestibility of crude protein and fat in C. catla fed on 900 FTU kg−1 in MOSM and M. oleifera by-products based diets respectively. They also noticed that further increment in PHY dose (upto the level of 1500 FTU kg−1) does not increase the digestibility of CP, EE and GE. Similarly, Maas et al. (2018) observed highest CP digestibility in Nile tilapia, and maximum value of fat digestibility (80%) was observed in rohu by Bano and Afzal (2017) when given SBM containing 1000 FTU kg−1. Little different results were shown in research work of Thanh et al., 2017, Hussain et al., 2011b, Hussain et al., 2011c, Hussain et al., 2015d), who observed maximum CP digestibility (85%) in Tra catfish and EE digestibility coefficient n L. rohita respectively, at 750 FTU kg−1 of PHY. Better digestibility of protein and fat (85%) was identified by Nwanna et al. (2008) in Colossoma macropomum fish when given feed with 4000 FTU kg−1 of PHY. Different to our results, significant changes were found in nutrients digestibility e.g. in research work of Danwitz et al., 2016, Wang et al., 2009, who observed decreased protein and lipid digestibility on feeding PHY supplemented diet to Clarias gariepinus and O. mykiss respectively than the control diet. The possible reasons for variations in outcomes of protein and fat digestibility are considered to be the dissimilar sources of enzyme PHY, feed components, stomach pH, methods of feed processing or unlike species of fish (Liu et al., 2013). ADC of GE also increased to its maximum (71%) at 950 FTU kg−1 amount of PHY, followed by digestibility co-efficient (69%) at 800 FTU kg−1. Hussain et al. (2017) reported maximum GE (74%) at 900 FTU kg−1 in C. catla, that is very close to our results. While, lowest value of GE (53%) was found at diet with 0 FTU kg−1. Apparent GE digestibility was also observed better at 1000 FTU kg−1, in Cirrhinus mrigala by Hussain et al. (2014). Similar results of GE digestibility were observed at slightly different amount of PHY (750 FTU kg−1) in L. rohita fed on cottonseed meal (Hussain et al., 2015c) and canola meal based diet (Hussain et al., 2015d). Highly different results related to GE digestibility coefficient (64.30) were observed by Nwanna and Olusola, (2014) at 8000 FTU kg−1 in Nile tilapia. The contradictory results to our work were shown by Lanari et al. (1998) who didn’t assess any optimistic response in rainbow trout fed on PHY supplemented SBM based diet. The contradictions and variations in results are considered to occur because of the type and source of PHY enzyme used, numerous nutritional factors, along with the source and quantity of PA (Selle et al., 2000) and protein sources in fish diets (Sugiura et al., 2001). In current experiment, it was found that 950 FTU kg−1 is most suitable amount of PHY for C. carpio to increase the absorption of minerals like Ca, Zn, K, Mn and P. Moreover, increase in absorption (%) of minerals initiated from 500 to 950 FTU kg−1, while increase in level of PHY (1100 and 1250 FTU kg−1) did not increase the absorption coefficient of minerals (Shahzad et al., 2020). Value of minerals absorption was found to be lowest at control diet. Close to the outcomes of current study, Shahzad et al. (2018a and b) found higher absorption and bioavailability of minerals in C. catla fed on 900 FTU kg−1 added in MOSM and mixture of MOLM and MOSM based diets, respectively. Similarly, Maas et al., 2018, Rabia et al., 2017 observed highest absorption of few minerals in Nile tilapia and L. rohita respectively at 1000 FTU kg−1. Little different results were shown in research work of Hussain et al., 2016, Marjan et al., 2014. They observed highest digestibility of minerals in L. rohita when given feed containing 750 FTU kg−1 of PHY. More different from our results were those of Qiu and Davis, (2016). They noticed improved digestibility of P and retention of Cu at 500 and 2000 FTU kg−1 in Litopenaeus vannamei. High levels of PHY i.e. 1000, 2000 and 4000 FTU kg−1 gave enhanced P digestibility, bone minerals and reduced faecal P in Clarias gariepinus (Olugbenga et al., 2017). In contrast to present findings, Nwanna et al. (2005) observed better Ca deposition and fewer P discharge at 8000 FTU kg−1 in C. gariepinus. Contrarily, negative impacts were also observed regarding minerals absorption e.g. in research work of Xue (2014). They observed no difference in retention of minerals excluding Zinc on feeding PHY supplemented SPC based diet to Nile tilapia in comparison of the control diet. In the light of above-mentioned results, we can argue that the possible explanations for dissimilarities in results of minerals retention and absorption may be the quantity, quality (Baruah et al., 2007b, Dersjant-Li et al., 2015) and dissimilar sources of PHY, feed components, methods of diet formulation or changed fish species (Liu et al., 2013).
5. Conclusion
Existing research work was led for 70 days to explore the nutritional benefits of seed meal and leaf meal of moringa plant and PHY enzyme supplemented in diet at varied levels (0, 500, 650, 800, 950, 1100 and 1250 FTU kg−1). Impact of addition of enzyme PHY was checked on growth parameters, nutrients digestibility and mineral absorption of C. carpio. It was concluded that all growth parameters for example FW, WG(g), WG (%), FCR and SGR, digestibility of nutrients (CP, EE and GE) and absorption of some minerals such as Ca, Zn, K, Mn and P were improved at 950 FTU kg−1 level of PHY in comparison of the control diet. So, this research provides sufficient evidences about positive effects of PHY regarding break down of phytate complexes, releasing minerals for absorption and improving overall performance of C. carpio fingerlings. Addition of PHY increases retention of nutrients in body, reducing water pollution and helps to formulate healthy and cost- effective fish feed than the expensive FM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This is the original paper submitted to well reputed and one of the most reviewed Journal “Saudi Journal of Biological Sciences.
Peer review under responsibility of King Saud University.
References
- Afuang W., Siddhuraju P., Becker K. Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of Moringa (Moringa oleifera Lam.) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L.) Aqua. Res. 2003;34:1147–1159. doi: 10.1046/j.1365-2109.2003.00920.x. [DOI] [Google Scholar]
- Allan G.L., Rowland S.J. Development of an experimental diet for silver perch (Bidyanus bidyanus) Austasia Aqua. 1992;6:39–40. [Google Scholar]
- Anjorin T.S., Ikokoh P., Okolo S. Mineral composition of Moringa oleifera leaves, pods and seeds from two regions in Abuja. Nigeria. Int. J. Agri. Bio. 2010;12:431–434. http://www.fspublishers.org/.../22.pdf [Google Scholar]
- AOAC. (Association of Official Analytical Chemists). 1995. Official methods of analysis.15th Ed. Association of Official Analytical chemists, Washington, D.C. USA., p. 1094.
- Bano N., Afzal M. Synchronized effect of citric acid and phytase supplementation on growth performance and nutrient digestibility of Labeo rohita. Aqua. Nutr. 2017;24:786–792. doi: 10.1111/anu.12607. [DOI] [Google Scholar]
- Baruah K., Pal A.K., Sahu N.P., Debnath D., Nourozitallab P., Sorgeloos P. Microbial phytase supplementation in rohu, Labeo rohita, diets enhances growth performance and nutrient digestibility. J. World Aqua. Soc. 2007;38:129–137. doi: 10.1111/j.1749-7345.2006.00081.x. [DOI] [Google Scholar]
- Baruah K., Sahu N.P., Pal A.K., Jain K.K., Debnath D., Mukherjee S.C. Dietary microbial phytase and citric acid synergistically enhances nutrient digestibility and growth performance of Labeo rohita (Hamilton) juveniles at sub-optimal protein level. Aqua. Res. 2007;38:109–120. doi: 10.1111/j.1365-2109.2006.01624.x. [DOI] [Google Scholar]
- Biswas A., Araki H., Sakata T., Nakamori T., Takii K. Optimum fish meal replacement by soy protein concentrate from soymilk and phytase supplementation in diet of red sea bream, Pagrus major. Aquaculture. 2019;506:51–59. doi: 10.1016/j.aquaculture.2019.03.023. [DOI] [Google Scholar]
- Bostock, J., McAndrew, B., Richards, R., Jauncey, K., Telfer, T., Lorenzen, K., Little, D., Ross, L., Handisyde, N., Gatward, L., Corner, R., 2010. Aquaculture: global status and trends. Philosophical Transactions of the Royal Society B: Biol. Sci. 365, 2897–2912. https://doi.org/10.1098/rstb.2010.0170. [DOI] [PMC free article] [PubMed]
- Cao L., Wang W., Yang C., Yang Y., Diana J., Yakupitiyage A., Luo Z., Li D. Application of microbial phytase in fish feed. Enzyme and Microbial Technol. 2007;40:497–507. doi: 10.1016/j.enzmictec.2007.01.007. [DOI] [Google Scholar]
- Cao L., Yang Y., Wang W.M., Yakupitiyage A., Yuan D.R., Diana J.S. Effect of pre-treatment with microbial phytase on phosphorus utilization and growth performance of Nile Tilapia (Oreochromis niloticus) Aqua. Nutr. 2007;14:99–109. doi: 10.1111/j.1365-2095.2007.00508.x. [DOI] [Google Scholar]
- Chu Z.J., Yu D.H., Dong G.F., Gong S.Y. Partial replacement of fish meal by soybean meal with or without methionine and phytase supplement in diets for juvenile Chinese sucker, Myxocyprinus asiaticus. Aqua. Nutr. 2016;22:989–996. doi: 10.1111/anu.12318. [DOI] [Google Scholar]
- Danwitz A.V., Bussel C.G.J.V., Klatt S.F., Schulz C. Dietary phytase supplementation in rapeseed protein based diets influences growth performance, digestibility and nutrient utilization in turbot (Psetta maxima) Aquaculture. 2016;450:405–411. doi: 10.1016/j.aquaculture.2015.07.026. [DOI] [Google Scholar]
- Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. BioMed Res. Int. 2015;2015:1–11. doi: 10.1155/2015/514196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denstadli V., Skrede A., Krogdahl Å., Sahlstrøm S., Storebakken T. Feed intake, growth, feed conversion, digestibility, enzyme activities and intestinal structure in Atlantic salmon (Salmo salar L.) fed graded levels of phytic acid. Aquaculture. 2006;256:365–376. doi: 10.1016/j.aquaculture.2006.02.021. [DOI] [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., Partridge G. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food and Agri. 2015;95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaran S., Obaldo L.G., Forster I.P. Note on the methods for determination of chromic oxide in shrimp feeds. J. Agri. Food Chem. 2002;50:464–467. doi: 10.1021/jf011112s. [DOI] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the United Nations (FAO . Italy; Rome: 2014. Fisheries and Aquaculture Department. [Google Scholar]
- Hassaan M.S., Soltan M.A., Agouz H.M., Badr A.M. Influences of calcium/phosphorus ratio on supplemental microbial phytase efficiency for Nile tilapia (Oreochromis niloticus) The Egyptian J. Aquatic Res. 2013;39:205–213. doi: 10.1016/j.ejar.2013.09.001. [DOI] [Google Scholar]
- Hassan S.K.U., Khalique A., Pasha T.N., Sahota A.W. Effect of dried Moringa oleifera leaves on growth performance and immune response of broilers. Journal of Animal and Plant Sciences. 2018;28(6):1579–1583. [Google Scholar]
- Hassan S., Altaff K., Satyanarayana T. Use of soybean meal supplemented with cell bound phytase for replacement of fish meal in the diet of juvenile milkfish, Chanos chanos. Pak. J. Nutr. 2009;8:341–344. doi: 10.3923/pjn.2009.341.344. [DOI] [Google Scholar]
- Hussain S.M., Nisar A., Farhat J., Arshad J., Nosheen A., Majid H., Ahmad S., Arsalan M.Z.H., Riaz D., Shahzad M.M. Effects of citric acid and phytase supplementation on nutrient digestibility and growth performance of Cirrhinus mrigala fingerlings fed on corn gluten (30%) meal-based diets. Int. J. Biosci. 2015;6:82–91. doi: 10.12692/ijb/6.7.82-91. [DOI] [Google Scholar]
- Hussain S.M., Afzal M., Javid A., Hussain A.I., Qasim A., Mustafa I., Chatha S.A.S., Shah S.Z.H., Hussain M., Ullah M.I. Efficacy of phytase supplementation on growth performance and mineral digestibility of Labeo rohita fingerlings fed on cottonseed meal-based diet. Pak. J. Zool. 2015;47:699–709. [Google Scholar]
- Hussain S.M., Afzal M., Rana S.A., Javid A., Hussain M. Impact of phytase supplementation on nutrient digestibility for Labeo rohita fingerlings fed on sunflower meal-based diets. Pak. J. Life Soc. Sci. 2011;9:85–90. [Google Scholar]
- Hussain, S.M., Afzal, M., Nasir, S., Javid, A., Azmat, H., Makhdoom, S.M., Shah, S.Z.H., Hussain, M., Mustafa, I., Iqbal, M., 2015d. Role of phytase supplementation in improving nutrient digestibility and growth performance for Labeo rohita fingerlings fed on canola meal-based diet. J. Applied Ani. Res. 45, 15–21. https://doi.org/10.1080/09712119.2015.1091331.
- Hussain S.M., Afzal M., Nasir S., Javid A., Makhdoom S.M., Jabeen F., Azmat H., Hussain M., Shah S.Z.H. Efficacy of phytase supplementation in improving mineral digestibility in Labeo rohita fingerlings fed on canola meal-based diets. Iranian J. Fisheries Sci. 2016;15:645–661. [Google Scholar]
- Hussain S.M., Shahzad M.M., Afzal M., Javid A., Mubarik M.S., Shah S.Z.H., Hussain M., Ahmad S., Arsalan M.Z.U.H., Manzoor R., Riaz D. Efficacy of phytase enzyme for increasing mineral digestibility of Cirrhinus mrigala fingerlings fed on soybean meal-based diet. Pak. J. Zool. 2015;47:1807–1816. [Google Scholar]
- Hussain S.M., Shahzad M.M., Afzal M., Jabeen F., Nasir S., Afzal A., Javid A., Ahmad S., Arsalan M.Z.U.H., Riaz D., Khichi T.A.A., Ahmad A.W., Furqan M. Growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed on soybean meal-based diet supplemented by phytase. Int. J. Biosci. 2014;5:212–221. doi: 10.12692/ijb/5.12.212-221. [DOI] [Google Scholar]
- Hussain S.M., Shahzad M.M., Aslam N., Javid A., Hussain A.I., Hussain M., Arsalan M.Z.U.H. Use of phytase at graded levels for improving nutrient digestibility, growth and hematology of Catla catla fingerlings fed Moringa oleifera seed meal (MOSM) based diet. Indian J. Fisheries. 2017;64:48–57. doi: 10.21077/ijf.2017.64.2.62418-08. [DOI] [Google Scholar]
- Hussain S.M., Ahmad N., Javid A., Shahzad M.M., Hussain M., Arsalan M.Z.U.H. Effects of phytase and citric acid supplemented corn gluten (30%) meal-based diets on the mineral digestibility of Cirrhinus mrigala fingerlings. Turkish J. Fisheries Aquatic Sci. 2018;18:501–507. doi: 10.4194/1303-2712-v18_4_01. [DOI] [Google Scholar]
- Hussain S.M., Rana S.A., Afzal M., Shahid M. Efficacy of phytase supplementation on mineral digestibility in Labeo rohita fingerlings fed on corn gluten meal (30%) based diets. Pak. J. Agri. Sci. 2011;48:237–241. [Google Scholar]
- Hussain S., Afzal M., Rana S.A., Javid A., Iqbal M. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int. J. Agri. Bio. 2011;13:916–922. [Google Scholar]
- Ibrahem M.D., Fathi M., Mesalhy S., El-Aty A.A. Effect of dietary supplementation of inulin and vitamin C on the growth, hematology, innate immunity and resistance of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2010;29:241–246. doi: 10.1016/j.fsi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Kumar N., Sharma R., Tripathi G., Kumar K., Dalvi R.S., Krishna G. Cellular metabolic, stress, and histological response on exposure to acute toxicity of endosulfan in Tilapia (Oreochromis mossambicus) Environ. Toxicol. 2016;31:106–115. doi: 10.1002/tox.22026. [DOI] [PubMed] [Google Scholar]
- Kumar V., Barman D., Kumar K., Mandal S.C., De C.E. Anti-nutritional factors in plant feedstuffs used in aquafeeds. World Aqua. 2012;43:64–68. [Google Scholar]
- Kumar V., Sinha K., Makkar H.P.S., Boeck G.D., Becker K. Phytate and phytase in fish nutrition. J. Ani. Physiol. Ani. Nutr. (Berl) 2012;96:335–364. doi: 10.1111/j.1439-0396.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Lanari, D., D'agaro, E., Turri, C., 1998. Use of nonlinear regression to evaluate the effects of phytase enzyme treatment of plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 161, 345–356.
- Lei X.G., Stahl C.H. Nutritional benefits of phytase and dietary determinants of its efficacy. J. Applied Ani. Res. 2000;17:97–112. doi: 10.1080/09712119.2000.9706294. [DOI] [Google Scholar]
- Lei X.G., Weaver J.D., Mullaney E., Ullah A.H., Azain M.J. Phytase, a new life for an “old” enzyme. Ann. Rev. Ani. Biosci. 2013;1:283–309. doi: 10.1146/annurev-animal-031412-103717. [DOI] [PubMed] [Google Scholar]
- Liu L.W., Su J.M., Zhang T., Liang X.Z., Luo Y.L. Apparent digestibility of nutrients in grass carp diet supplemented with graded levels of phytase using pre-treatment and spraying methods. Aqua. Nutr. 2013;19:91–99. doi: 10.1111/j.1365-2095.2012.00942.x. [DOI] [Google Scholar]
- Lovell T. Nutrition and Feeding of Fish. 1989;Vol. 260 doi: 10.1007/978-1-4757-1174-5_6. [DOI] [Google Scholar]
- Maas R.M., Verdegem M.C., Dersjant-Li Y., Schrama J.W. The effect of phytase, xylanase and their combination on growth performance and nutrient utilization in Nile tilapia. Aquaculture. 2018;487:7–14. doi: 10.1016/j.aquaculture.2017.12.040. [DOI] [Google Scholar]
- Madalla N., Agbo N.W., Jauncey K. Evaluation of aqueous extracted moringa leaf meal as a protein source for Nile tilapia Juveniles. Tanzania J. Agri. Sci. 2013;12:53–64. [Google Scholar]
- Makkar H.A., Becker K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Ani. Feed Sci. Technol. 1996;63:211–228. doi: 10.1016/s0377-8401(96)01023-1. [DOI] [Google Scholar]
- Marjan S., Afzal M., Shah S.Z.H., Hussain S.M., Mubarik M.S. Role of phytase supplementation in improving mineral digestibility of dry bread meal based diet fed to Labeo rohita fingerlings. Pak. J. Life Soc. Sci. 2014;12:150–154. [Google Scholar]
- Morales G.A., Marquez L., Rodriganez M.S.D., Bermudez L., Robles R., Moyano F.J. Effect of phytase supplementation of a plant-based diet on phosphorus and nitrogen bioavailability in sea bream (Sparus aurata) Aqua. Nutr. 2014;20:172–182. doi: 10.1111/anu.12063. [DOI] [Google Scholar]
- NRC (National Research Council). (1993). Nutrient requirements of fish. Washington, DC, Nat. Acad. Press, p. 114. https://doi.org/10.17226/2115.
- Maas R.M., Verdegem M.C., Schrama J.W. Effect of non-starch polysaccharide composition and enzyme supplementation on growth performance and nutrient digestibility in Nile tilapia (Oreochromis niloticus) Aqua. Nutr. 2019;25:622–632. [Google Scholar]
- Nwanna L.C., Olusola S.E. Effect of supplemental phytase on phosphorus digestibility and mineral composition in Nile tilapia (Oreochromis niloticus) Int. J. Aqua. 2014;4:89–95. doi: 10.5376/ija.2014.04.0015. [DOI] [Google Scholar]
- Nwanna L.C., Fagbenro O.A., Adeyo A.O. Effects of different treatments of dietary soybean meal and phytase on the growth and mineral deposition in African catfish Clarias gariepinus. J. Ani. Vet. Adv. 2005;4:980–987. [Google Scholar]
- Nwanna L., Oishi C., Pereira-Filho M. Use of phytase to improve the digestibility of alternative feed ingredients by Amazon tambaqui, Colossoma macropomum. Sci. Asia. 2008;34:353–360. doi: 10.2306/scienceasia1513-1874.2008.34.353. [DOI] [Google Scholar]
- Olugbenga O., Falaye A.E., Kareem O.K. Effect of phytase supplementation on the growth, mineral composition and phosphorus digestibility of African catfish (Clarias gariepinus) juveniles. Ani. Res. Int. 2017;14:2741–2750. [Google Scholar]
- Olusola S.E., Nwanna L.C. Growth performance of Nile tilapia (Oreochromis niloticus) fed processed soybean meal-based diets supplemented with phytase. Int. J. Aqua. 2014;4:48–54. doi: 10.5376/ija.2014.04.0008. [DOI] [Google Scholar]
- Qiu X., Davis D.A. Effects of dietary phytase supplementation on growth performance and apparent digestibility coefficients of Pacific white shrimp Litopenaeus vannamei. Aqua. Nutr. 2016;23:942–951. doi: 10.1111/anu.12462. [DOI] [Google Scholar]
- Rabia S., Afzal M., Shah S.Z.H. Nutrient digestibility performance by rohu (Labeo rohita) juveniles fed acidified and phytase pre-treated sunflower meal-based diet. J. Applied Ani. Res. 2017;45:331–335. doi: 10.1080/09712119.2016.1190731. [DOI] [Google Scholar]
- Rachmawati D., Samidjan I. Effect of phytase enzyme on growth boost in the artificial feed made of plant protein to shorten production time of giant tiger prawn [Penaeus monodon, (Fabricus 1798)] Aquatic Procedia. 2016;7:46–53. doi: 10.1016/j.aqpro.2016.07.006. [DOI] [Google Scholar]
- Rachmawati D., Samidjan I. Performance efficiency of feed utilization, relative growth rate, and survival rate of common carp (Cyprinus carpio) through the addition of phytase in the feed. In IOP Conference Series: Earth. Environ. Sci. 2017;137 doi: 10.1088/1755-1315/137/1/012027. [DOI] [Google Scholar]
- Reddy N.R. Occurrence, distribution, content, and dietary intake of phytate. Food Phytates. 2002;25–51 doi: 10.1201/9781420014419.ch3. [DOI] [Google Scholar]
- Richter N., Siddhuraju P., Becker K. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.) Aquaculture. 2003;217:599–611. doi: 10.1016/s0044-8486(02)00497-0. [DOI] [Google Scholar]
- Robinson E.H., Li M.H., Manning B.B. Comparison of microbial phytase and dicalcium phosphate for growth and bone mineralization of pond-raised channel catfish, (Ictalurus punctatus) J. Appl. Aqua. 2002;12:81–88. doi: 10.1300/j028v12n03_08. [DOI] [Google Scholar]
- Rowland, S.J., Ingram, B.A., 1991. Diseases of Australian native fishes. In: Fisheries Bulletin 4 NSW Fisheries, Sydney, NSW, Australia.
- Salem H.B., Makkar H.P.S. Defatted Moringa oleifera seed meal as a feed additive for sheep. Ani. Feed Sci. Technol. 2009;150:27–33. doi: 10.1016/j.anifeedsci.2008.07.007. [DOI] [Google Scholar]
- Samkelisiwe H.N., Ngonidzashe M.A.G. Replacing fishmeal with kikuyu grass and moringa leaves: effects on growth, protein digestibility, histological and haematological parameters in Clarias gariepinus. Turkish J. Fisheries Aquatic Sci. 2014;14:795–806. doi: 10.4194/1303-2712-v14_3_22. [DOI] [Google Scholar]
- Selle P.H., Ravindran V., Caldwell A., Bryden W.L. Phytate and phytase: consequences for protein utilization. Nutr. Res. Reviews. 2000;13:255–278. doi: 10.1079/095442200108729098. [DOI] [PubMed] [Google Scholar]
- Shahzad M.M., Hussain S.M., Jabeen F., Hussain A.I., Javid A., Asrar M., Arsalan M.Z.U.H. Improvement in mineral digestibility and whole body composition of Catla catla fingerlings fed phytase supplemented MOSM based diet. Pak. J. Zool. 2018;50:1909–1920. doi: 10.17582/journal.pjz/2018.50.5.1909.1920. [DOI] [Google Scholar]
- Shahzad M.M., Hussain S.M., Javid A., Hussain M. Role of phytase supplementation in improving growth parameters and mineral digestibility of Catla catla fingerlings fed moringa by-products based test diet. Turkish J. Fisheries Aquatic Sci. 2018;18:557–566. doi: 10.4194/1303-2712-v18_4_07. [DOI] [Google Scholar]
- Shahzad M.M., Hussain S.M., Jabeen F., Hussain A.I., Ahmad S., Ashraf A., Arsalan M.Z.U.H. Effect of phytase supplementation on mineral digestibility to Catla catla fingerlings fed Moringa oleifera leaf meal-based test diets. Punjab Univ. J. Zool. 2017;32:65–73. [Google Scholar]
- Shahzad M.M., Rafique T., Hussain S.M., Hussain Z., Zahoor M.Y., Hussain M., Rehman R.A., Ahmad N., Liaquat I., Bashir S. Effect of phytase supplemented Moringa by-products based diets on the performance of Oreochromis niloticus fingerlings. J. Anim. Plant Sci. 2021;31(1):288–295. doi: 10.36899/japs.2021.1.0216. [DOI] [Google Scholar]
- Shahzad M.M., Hussain S.M., Hussain M., Tariq M., Ahmed N., Furqan M., Khalid F., Rafique T. Improvement in overall performance of Catla catla fingerlings fed phytase included low cost plant by products-based diet. Saudi J. Biol. Sci. 2020;27(8):2089–2096. doi: 10.1016/j.sjbs.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokrab A.M., Ahmed I.A.M., Babiker E.E. Effect of germination on antinutritional factors, total and extractable minerals of high and low phytate corn (Zea mays L.) genotypes. J. Saudi Soc. Agri. Sci. 2012;11:123–128. doi: 10.1016/j.jssas.2012.02.002. [DOI] [Google Scholar]
- Soliva C.R., Kreuzer M., Foidl N., Foidl G., Machmuller A., Hess H.D. Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Ani. Feed Sci. Technol. 2005;118:47–62. doi: 10.1016/j.anifeedsci.2004.10.005. [DOI] [Google Scholar]
- Sugiura S.H., Gabaudan J., Dong F.M., Hardy R.W. Dietary microbial phytase supplementation and the utilization of phosphorus, trace minerals and protein by rainbow trout [Oncorhynchus mykiss (Walbaum)] fed soybean meal-based diets. Aqua. Res. 2001;32:583–592. doi: 10.1046/j.1365-2109.2001.00581.x. [DOI] [Google Scholar]
- Thanh H.L., Binh V.T.T., Poonperm W., Ader P. The use of phytase and acidifier supplementation on growth and feed utilization of Tra catfish (Pangasianodon Hypophthalmus) Universal J. Agri. Res. 2017;5:202–208. doi: 10.13189/ujar.2017.050304. [DOI] [Google Scholar]
- Vielma J., Makinen T., Ekholm P., Koskela J. Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout (Oncorhynchus mykiss) and algal availability of phosphorus load. Aquaculture. 2000;183:349–362. doi: 10.1016/s0044-8486(99)00299-9. [DOI] [Google Scholar]
- Vong Q.P., Chan K.M., Cheng C.H. Quantification of common carp (Cyprinus carpio) IGF-I and IGF-II mRNA by real-time PCR: differential regulation of expression by GH. J. Endocrinology. 2003;178:513–521. doi: 10.1677/joe.0.1780513. [DOI] [PubMed] [Google Scholar]
- Wang F., Yang Y.H., Han Z.Z., Dong H.W., Yang C.H., Zou Z.Y. Effects of phytase pretreatment of soybean meal and phytase-sprayed in diets on growth, apparent digestibility coefficient and nutrient excretion of rainbow trout (Oncorhynchus mykiss Walbaum) Aqua. Int. 2009;17:143–157. doi: 10.1007/s10499-008-9187-5. [DOI] [Google Scholar]
- Xu M., Wang T., Wang J., Wan W., Wang Z., Guan D., Sun H. An evaluation of mixed plant protein in the diet of Yellow River carp (Cyprinus carpio): growth, body composition, biochemical parameters, and growth hormone/insulin-like growth factor 1. Fish Physiol. Biochem. 2019;45:1331–1342. doi: 10.1007/s10695-019-00641-6. [DOI] [PubMed] [Google Scholar]
- Xue Y. The effect of dietary phytase supplementation and incubation in soy protein concentrate based diet fed to Nile tilapia. Norwegian Univ. Life Sci. 2014 [Google Scholar]
- Yuangsoi B., Masumoto T. Replacing moringa leaf (Moringa oleifera) partially by protein replacement in soybean meal of fancy carp (Cyprinus carpio) Songklanakarin J. Sci. Technol. 2012;34:479–485. [Google Scholar]
- Zhou Q.C., Tan B.P., Mai K.S., Liu Y.J. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture. 2004;241:441–451. doi: 10.1016/j.aquaculture.2004.08.044. [DOI] [Google Scholar]