Abstract
We report the case of a 25-year-old pregnant woman, parity one, at 34 + 2 weeks of gestation, with a body mass index of 41 kg/m2 but no other comorbidities. There was a family history of COVID-19 among her one-year-old son, husband, brother, father and mother. She was admitted with chest pain and a nasopharyngeal swap positive for COVID-19. Due to the severity of the infection, a multidisciplinary team of anaesthesiologists, intensivists, obstetricians, neonatologists, and infectious disease specialists recommended delivery by caesarean section at 35 + 0 weeks of gestation, with combined spinal and epidural anaesthesia. Three days after delivery, the patient developed severe acute respiratory distress syndrome (ARDS) and was intubated for 25 days. The neonate was observed in the neonatal intensive care unit and no vertical transmission occurred. This case highlights the importance of the timing of delivery, the need for extended postpartum observation and a beneficial effect of inhaled nitric oxide after delivery for women with COVID-19.
Keywords: COVID-19, ARDS, Caesarean section, Case report
Highlights
-
•
Caesarean section in combined spinal and epidural anaesthesia is recommended.
-
•
No vertical transmission of COVID-19 was observed.
-
•
Extended postpartum observation in COVID-19 positive pregnant women is recommended.
-
•
Treatment with iNO can be used as a rescue therapy
1. Introduction
The COVID-19 pandemic is still being intensively investigated. Limited evidence exists to guide clinicians on whether to recommend caesarean section or spontaneous vaginal birth for pregnant women with COVID-19, as its effects on maternal and fetal mortality and morbidity are not known. One review reported that 92% out of 86 pregnant women with COVID-19 were delivered by caesarean section [1] and another study reported a figure of 69% out of 203 women [2]. Evidence regarding COVID-19 in pregnancy and vertical transmission is still lacking and this may contribute to the increase in the caesarean section rate. In a study of 91 neonates, 8,8% were positive for viral RNA or antibodies for SARS-Cov-2 [3].
We present the case of a pregnant patient with confirmed COVID-19 and acute respiratory distress which led to preterm caesarean section and admission to the intensive care unit. We discuss the importance of the timing of delivery, the need for extended postpartum observation and the beneficial effect of inhaled nitric oxide (iNO) after delivery for women with COVID-19.
2. Case Presentation
A 25-year-old pregnant woman, gravida three, parity one and gestational age (GA) 34 + 2 weeks, was admitted with chest pain. Her body mass index (BMI) was 41 kg/m2 but there were no other co-morbidities. Her pregnancy history included one early intrauterine death at GA 6 + 0 and the previous birth of a healthy baby by vaginal delivery at GA 41 + 3 weeks. Prior to admission the patient had had five days of sore throat and coughing followed by fever and myalgia. A COVID-19 nasopharyngeal polymerase chain reaction (PCR) test was positive three days prior to admission.
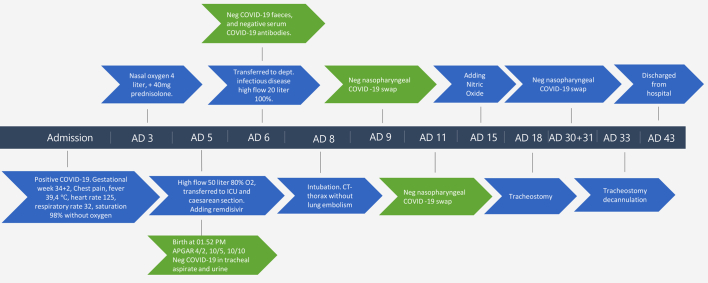
The patient was admitted with fever (39,4 °C), a heart rate of 125, a respiratory rate of 32 with Spo2 98% without oxygen supply, and a blood pressure of 119/78 mmHg (Fig. 1, Table 1). There was no sign of ischemia on an initial electrocardiogram (ECG). Five days after admission she became increasingly hypoxic and was transferred to the intensive care unit. She received high-flow oxygen, 50 l/min with 80% oxygen and intermittent continuous positive airway pressure (CPAP). Daily cardiotocography (CTG) and clinical foetus examination were conducted, and no pathology was observed.
Fig. 1.
Case progression from admission to discharge.
Table 1.
Maternal laboratory blood values.
| Variable | Reference range | AD | AD 5 | AD 8 | AD 15 | Discharged |
|---|---|---|---|---|---|---|
| Leucocyte count | 3.5–10.0 109/l | 8.1 | 12.3 | 13.1 | 10.8 | 6.3 |
| Haemoglobin | 7.3–9.5 mmol/l | 6.6 | 6.2 | 6.5 | 6.9 | 6.0 |
| Lymphocyte | 1.3–3.50 109/l | 0.95 | 1.09 | 1.22 | 1.09 | 1.39 |
| Platelet count | 165–400 109/l | 338 | 303 | 268 | 631 | 368 |
| Lactate dehydrogenase | 105–205 U/l | 168 | 287 | 634 | 331 | – |
| C-reactive protein | < 8.0 mg/l | 27.6 | 106.1 | 218.2 | 9.6 | 13.0 |
| Alanine aminotransferase | 10–45 U/l | <9 | 23 | 15 | 28 | – |
| Serum urea | 2.6–6.4 mmol/l | 1.5 | 2.0 | – | 28.3 | 2.5 |
| Serum Creatinine | 45–90 μmol/l | 24 | 29 | 36 | 115 | 34 |
| Fibrin D-dimer | < 0.5 mg/l | 4.5 | 2.1 | > 20.0 | 2.5 | – |
| Troponin-I | < 47 ng/l | <3 | <3 | – | <3 | – |
| Creatinine kinase | 50–150 U/l | 20 | 13 | – | 334 | – |
| Creatinine kinase- MB | < 4,0 μg/l | < 0,5 | <0.5 | – | <0.5 | – |
| Interleukin- 6 | < 7.0 ng/l | 15 | 215 | – | – | – |
| CD163 | 0.69–3.86 | 2.62 | 2.84 | – | – | – |
| Ph | 7.37–7.45 | 7.47 | 7.43 | 7.43 | 7.48 | |
| pCO2 | 4.3–5.7 kPa | 3.6 | 3.6 | 4.9 | 7.3 | |
| pO2 | 11.1–14.4 kPa | 12.4 | 8.9 | 11.0 | 7.7 | |
| Base Excess | −3.0–2.0 mmol/l | −4.1 | −5.9 | 0.0 | 14.9 | |
| Bicarbonate | 21.8–26.2 mmol/l | 21.6 | 20.1 | 24.5 | 37.5 |
A couple of hours after admission to the ICU, her SpO2 was >95% on oxygen mask with reservoir. Her condition was assessed to be stable, but an emergency caesarean section on maternal indication was conducted at 35 + 0 weeks of gestation, with combined spinal and epidural (CSE) anaesthesia. Three days later, the patient's need for oxygen increased and therefore she was intubated and a computed tomography (CT) scan of the thorax was obtained; it showed bilateral lung involvement with ground-glass opacities and no central pulmonary embolism. Six days after intubation, her saturation was 80% to 90% on FiO2 0.8 to 1.0 oxygen with PaO2/FiO2 ratio of 15,7 kPa (118 mmHg); therefore iNO was added, starting at 20 ppm. After 24 h, the PaO2/FiO2 had increased to 18.4 kPa (138 mmHg). The patient recovered after a total of 25 days of mechanical ventilation, and she and her new-born were discharged from the hospital in good and stable condition.
The female infant had a birthweight of 2250 g, and cried immediately after birth. The Apgar score was 10 at one minute, but the infant then became apnoeic and hypotonic. At two minutes of age, the heart rate was 60/min with no breathing. Copious secretions were suctioned from the airway, and positive pressure ventilation with positive end expiratory pressure (PEEP) was applied from 1.5 to 3 min. After five minutes, spontaneous breathing was established, and nasal CPAP was applied with a PEEP of 6 cm H2O and FiO2 on 60%. The Apgar score was four at two minutes of age, and 10 at five and 10 min of age.
The neonate's tracheal aspirate and urine were tested for COVID-19 and the results were negative. Blood culture for bacteria was negative. A blood test for COVID-19 antibodies and a faeces test were also negative. A nasopharyngeal swab for COVID-19 on days four and six after birth were negative. Chest X-ray on day one showed radiologic signs of mild respiratory distress syndrome (RDS) (Fig. 2).
Fig. 2.
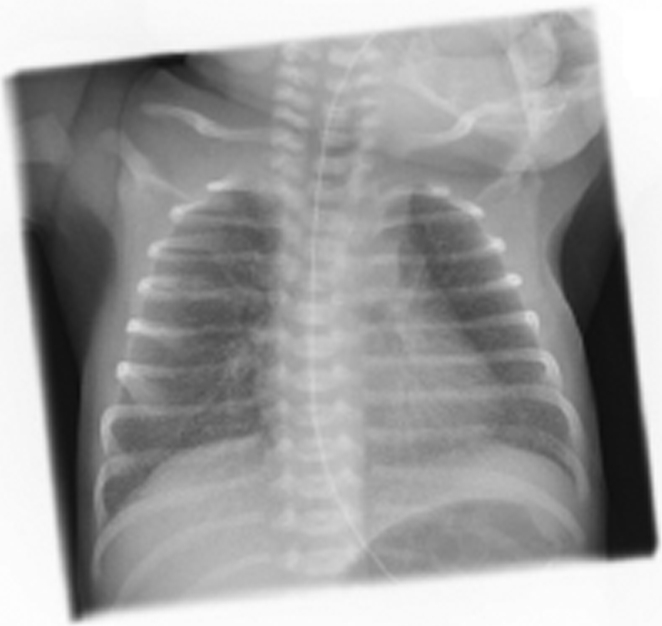
Chest X-ray of the neonate on day one shows radiologic signs of mild respiratory distress syndrome (RDS).
2.1. Treatment
Pressure controlled mechanical ventilation was applied with a lung protective strategy according to current guidelines on ventilation for patients with ARDS including deep sedation, intermittent prone position and administration of neuromuscular blocking agents [[4], [5], [6]]. On admission day 15, continuous iNO at 20 ppm was added due to the increasing oxygen need. The patient was treated with continuous iNO for five days at gradually reducing concentration; nevertheless, the NIH do not recommend routine use [7]. To minimize fetal exposure, the patient was treated with 40 mg prednisolone daily from two days prior to birth until delivery, and switched to dexamethasone 6 mg daily after delivery. Remdesivir was administered just after delivery at 200 mg and thereafter a daily dose of 100 mg and empiric treatment with piperacillin/tazobactam was started. The patient was treated with low molecular weight heparin, 5000 units two times daily, to prevent thromboembolic events.
The new-born baby was treated for mild clinical signs of RDS with CPAP 6 cm H2O and started on empiric treatment with ampicillin and gentamicin (Table 2).
Table 2.
Infant laboratory blood values.
| Variable | Reference | Birth | Day after birth |
|---|---|---|---|
| Leucocyte count | 5.5–19.3 109/l | 16.6 | |
| Haemoglobin | 8.5–14.3 mmol/l | 9.0 | |
| Platelet count | 165–400 109/l | 283 | |
| C-reactive protein | <8.0 mg/l | <4.0 | |
| Ph | 7.37–7.45 | 7.21 | |
| pCO2 | 3.6–5.3 kPa | 7.8 | |
| pO2 | 11.1–14.4 kPa | 4.4 | |
| Base Excess | −4.0–2.0 mmol/l | −4.3 | |
| Bicarbonate | 21.8–26.2 mmol/l | 18.7 | |
| lactate | 0.5–2.5 mmol/l | 2.8 |
3. Discussion
While most pregnant women with COVID-19 have mild symptoms, maternal mortality and morbidity due to COVID-19 are unclear. A recent observational study [8] demonstrated that among 462 pregnant women with COVID-19, 70 (15%) were classified as having a severe infection and 13 were admitted to the ICU (two of whom died). Among the study population the most common comorbidity was obesity (38%).
The timing of caesarean section for pregnant women with COVID-19 is controversial. In this case the patient's condition rapidly worsened, with respiratory failure, and with obesity as a known risk factor caesarean section was decided on maternal indication. The decision was based on several theoretical considerations. Firstly, the patient's condition was suitable for CSE rather than general anaesthesia, which is also recommended by others [9]. In this case, CSE anaesthesia was applied to avoid the risk of airway management, in case of insufficient spinal anaesthesia, and to avoid the need for opioids for postoperative pain management. Secondly, there is a theoretical assumption that a caesarean section may improve the patient's lung functional residual capacity. Retrospectively, a caesarean section in our case was inevitable due to the disease duration and development. However, a study did question if surgery itself increases mortality and pulmonary complications for patients with preoperative COVID-19 [10]. For the infant, preterm delivery likely contributed to the development of respiratory distress and the need for supportive treatment and tube feeding.
The use of iNO in COVID-19-related ARDS is controversial; consequently, the NIH do not recommend routine use of iNO [7], due to lack of evidence. A study did question the benefit of iNO in COVID-19-related ARDS. In 8 out of 20 patients with COVID-19-related ARDS, an increase in PaO2/FiO2 ratio in response to iNO was seen. This was lower than was seen for ARDS not related to COVID-19, which was 10 out of 13 [11]. On the other hand, a recent publication found a positive effect of iNO in 23 out of 35 patients with COVID-19-related ARDS [12]. Due to the small number of published studies, larger randomized trials are required to clarify whether iNO has a beneficial effect in COVID-19-related ARDS.
This case demonstrates the importance of timing of the caesarean section, as it made CSE anaesthesia possible. This case clarifies the importance of extended postpartum observation of COVID-19 positive women and a beneficial effect of iNO as a rescue therapy.
Acknowledgments
Contributors
S Paramanathan drafted the paper and is the lead author.
KJ Kyng contributed to planning and the critical revision of the paper.
AL Laursen contributed to planning and the critical revision of the paper.
LD Jensen contributed to planning and the critical revision of the paper.
AM Grejs contributed to planning and the critical revision of the paper.
D Jain contributed to planning and the critical revision of the paper.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
No funding from an external source supported the publication of this case report.
Patient Consent
Consent was obtained from the patient after she regained capacity.
Provenance and Peer Review
This case report was peer reviewed.
References
- 1.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020;99:823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debrabandere M.L., Farabaugh D.C., Giordano C. Vol. 1. 2020. A Review on Mode of Delivery During COVID-19 between December 2019 and April 2020. [DOI] [PubMed] [Google Scholar]
- 3.Caspi Or, Smart Michael J., Noland R.B. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and mandarin on the novel coronavirus COVID. Ann. Oncol. 2020:19–21. [Google Scholar]
- 4.Griffiths M.J.D., McAuley D.F., Perkins G.D., Barrett N., Blackwood B., Boyle A. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019;6 doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrucci N., Iacovelli W. 2004. Distress Syndrome (Review) [DOI] [Google Scholar]
- 6.Blot F., Marty A., Chtara K., Bouzidi H., Stoclin A. Vol. 345. 2012. With Acute Respiratory Failure; pp. 568–573. [PubMed] [Google Scholar]
- 7.NIH . Vol. 2020. 2019. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; p. 130. Disponible en: https://covid19treatmentguidelines.nih.gov/. Nih. [PubMed] [Google Scholar]
- 8.Blitz M.J., Rochelson B., Minkoff H., Meirowitz N., Prasannan L., London V. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am. J. Obstet. Gynecol. 2020;223:595–599. doi: 10.1016/j.ajog.2020.06.020. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer M.E., Chiware R., Pancaro C. Neuraxial procedures in COVID-19–positive parturients: a review of current reports. Anesth. Analg. 2020;131 doi: 10.1213/ANE.0000000000004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhangu A. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longobardo A., Montanari C., Shulman R., Benhalim S., Singer M., Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br. J. Anaesth. 2021;126:e44–e46. doi: 10.1016/j.bja.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfield B., McFadyen C., Briar C., Bleakley C., Vlachou A., Baldwin M. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br. J. Anaesth. 2021;126:e72–e75. doi: 10.1016/j.bja.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]