Abstract
Genetic selection and advances in nutrition have improved broiler growth performance. However, meat quality issues have gained preference over increased growth rate. These meat quality issues may be reduced by lowering dietary amino acid (AA) content. In the present study, 5 common commercial broiler strains were fed either a control or an AA-reduced diet. The control diet was formulated to contain the highest digestible AA (lysine, total sulfur AA, and threonine) levels recommended for the 5 strains. The AA-reduced diet was formulated to contain 20% lower levels of these 3 digestible AA than in the control diet. This resulted in a 5 (strains) × 2 (AA levels) factorial arrangement. A total of 1,280 straight run broilers were randomly allocated to 8 replicate blocks. The AA reduction decreased absolute breast weights of 3 strains on day 42 and 2 strains on day 56, and decreased absolute weights of tender, wing, drumstick, and thigh on both day 42 and 56 for all 5 strains. However, the absolute fat pad weight and relative fat pad and thigh weights to BW were increased in the AA reduction treatments on both day 42 and 56. The AA reduction contributed to the lower breast meat pH on both day 42 and 56, which may have been directly related to decreased severe woody breast myopathy (WBM) incidence on day 42 and moderate WBM incidence on day 56. The severity of WBM was positively related to breast weight in all 10 treatments on both day 42 and 56, with the exception of birds in strain 3 on day 56 that were fed the AA-reduced diet. At the same time, AA reduction was more cost-effective when WBM incidence was considered in a theoretical model. In conclusion, WBM severity was associated with higher breast weight in birds of most strains fed either a control or AA-reduced diet. Dietary AA reduction decreased processing yields but decreased WBM incidence, which may be more economical.
Key words: amino acid, broiler, meat quality, strain, woody breast
Introduction
Since 1950, broilers have been successfully selected for rapid growth and high feed efficiency and breast meat yield (Zuidhof et al., 2014). By day 41 of age, a commercial Ross 308 broiler strain from 2005 grew 4 times faster than an unselected strain from 1958. During the same period, the feed conversion ratio (FCR) was reduced by 50%. Pectoralis major (breast fillet) weight has increased by 79% in male broilers and 85% in female broilers (Zuidhof et al., 2014). However, some unintended results, such as poor skeletal, immune, and reproductive system development and breast myopathies, have been concurrently expressed in modern broilers due to genetic selection (Fanatico et al., 2007; Zuidhof et al., 2014; Wen et al., 2017). Modern broilers also exhibit a high incidence of woody breast myopathy (WBM) (Livingston et al., 2019). Pectoralis major muscle of WBM is characterized by a pale appearance, hard texture, polyphasic myodegeneration, and accumulated connective tissue (Sihvo et al., 2014). Meat that exhibits WBM has a higher pH, is lighter, more red and yellow in color, and has higher cooking and drip loss than normal meat (Trocino et al., 2015; Cai et al., 2018; Sun et al., 2018).
Age, sex, and strain can affect the incidence of WBM. Kuttappan et al. (2017) reported that the incidence of moderate and severe WBM in high-breast-yield male broilers increased from 7.9 to 36% and from 2.0 to 15.6% at 42 and 63 d of age, respectively. In addition, male broilers have exhibited a higher incidence of WBM than female broilers. Trocino et al. (2015) reported that WBM incidence on day 46 was 16.3 and 8.0% in male and female broilers, respectively.
The incidence of WBM is positively related to BW, breast weight, and breast yield in Cobb 500 broilers (Cruz et al., 2017). Slowing the growth rate of the Cobb 500 broilers, by decreasing dietary Lys levels, reduced WBM incidence (Cruz et al., 2017). Meloche et al. (2018b) observed that WBM incidence of Yield Plus × Ross 708 male broilers decreased from 81.7 to 51.6% at day 41 of age when dietary digestible Lys decreased by 25% between 15 and 25 d of age. The incidence of severe WBM on day 49 of age has also been shown to decrease from 36.5 to 20.8% when broilers were fed a diet containing 90% of the levels of amino acids (AA) and metabolizable energy recommended by the breeder primary breeder company (Aviagen Inc., Huntsville, AL) between 8 and 49 d of age (Meloche et al., 2018a). Moreover, the decrease in growth rate and dietary nutrients improved the welfare of broilers, including increased mineralization in the tibia, improved walking ability, and decreased footpad lesions (Venalainen et al., 2006; Van Harn et al., 2019). Therefore, slowing the growth of broilers by reducing dietary nutrition should be evaluated as a practical way to control the incidence of WBM.
In the previous companion study, FCR was found to be increased by dietary AA reduction in all 5 strains except for strain 2 between 0 and 55 d. However, if individual periods are considered, FCR increased with dietary AA reduction in all 5 strains at early ages (day 0–41), but was not affected between day 41 and 55 (Zhang et al., 2020). Growth responses to dietary AA reduction varied by different strains and ages. Dietary AA reduction decreased the cost of feed required to reach the same BW in 4 of 5 broiler strains on both day 41 and 55. This was largely due to decreased costs associated with feeding lower AA levels in the diet and because of compensatory growth in the birds fed lower AA diets (Zhang et al., 2020). However, the dietary AA reduction will likely decrease processing weights and WBM incidence, which would influence economic return. Furthermore, these effects on different strains were also unknown. Therefore, in the current trial, dietary AA reduction in 5 different commercial broiler strains was further evaluated for its effects on processing yield, meat quality, and incidence of WBM. In addition, correlations between WBM incidence and severity and meat quality, growth rate, and breast weight were elucidated for different strains that were fed different levels of dietary AA.
Materials and methods
Birds, Diets, and Management
The experiment was conducted in accordance with the principle and specific guidelines of the Institutional Animal Care and Use Committee at Mississippi State University. Eggs from 5 strains of commercial broilers were incubated in a single-stage incubator (ChickMaster, Medina, OH). On the day of hatch, a total of 1,280 (256 birds/strain) chicks were randomly distributed into 10 floor pens in each of 8 replicate blocks (80 total pens) in an environmentally controlled broiler house. The experiment included 10 treatments in a 5 strain × 2 diet factorial arrangement. The 5 broiler strains are currently used for commercial broiler production and differ in their genetic background. The genetic background of strains 1 and 2 is similar, and the genetic background of strains 4 and 5 is similar. In addition, strain 3 shares the same female line as strains 1 and 2. The 2 diets included a control diet and an AA-reduced diet within each feeding phase. In the control diet, the digestible AA (lysine, TSAA, and threonine) were formulated at the highest digestible AA levels recommended for the 5 strains, so that each strain had the potential, from a diet standpoint, to maximize its growth performance. In the AA-reduced diet, the digestible AA (lysine, TSAA, and threonine) were 20% lower than the control diet. Percentages of dietary AA levels, calculated of to recommendation levels (dietary AA level/AA recommendation × 100), in the 5 different strains of broilers are listed in Table 1. In addition, the calculated and measured dietary total AA concentrations are listed in Table 2. The corresponding reduction rates of AA-reduced diets in comparison with the control diets were calculated (Table 2). The reduction rates of total AA (between control and AA-reduced diet) in measured concentrations are in agreement with calculated concentrations. The birds were provided with either the control diet or the AA-reduced diet in each of the 4 feeding phases: starter (day 0–14), grower (day 14–28), finisher (day 28–41), and withdrawal (day 41–56). Details of the bird usage, diets, and their management are provided in the companion study by Zhang et al. (2020).
Table 1.
Percentage of dietary digestible AA levels for the 5 different strains of broilers fed the control diet and AA-reduced diet during starter (day 0–14), grower (day 14–28), finisher (day 28–41), and withdrawal (day 41–56) feeding phases.
| Phases | Nutrients | Strain 1 |
Strain 2 |
Strain 31 |
Strain 4 and 52 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Reduced | Control | Reduced | Control | Reduced | Control | Reduced | ||
| Starter | Dig. Lys, % | 108.5 | 86.8 | 104.9 | 83.9 | 104.1 | 83.3 | 100.0 | 80.0 |
| Dig. TSAA, % | 108.0 | 86.4 | 104.4 | 83.5 | 103.3 | 82.6 | 100.0 | 80.0 | |
| Dig. Thr, % | 111.7 | 89.6 | 108.9 | 87.3 | 107.5 | 86.3 | 100.0 | 80.2 | |
| Grower | Dig. Lys, % | 109.5 | 87.6 | 106.5 | 85.2 | 105.5 | 84.4 | 100.0 | 80.0 |
| Dig. TSAA, % | 108.8 | 87.5 | 106.1 | 85.4 | 104.8 | 84.3 | 100.0 | 80.5 | |
| Dig. Thr, % | 111.6 | 89.9 | 108.5 | 87.3 | 106.9 | 86.1 | 100.0 | 80.5 | |
| Finisher | Dig. Lys, % | 107.4 | 86.3 | 104.1 | 83.7 | 98.1 | 78.8 | 100.0 | 80.4 |
| Dig. TSAA, % | 108.1 | 86.5 | 105.3 | 84.2 | 100.0 | 80.0 | 100.0 | 80.0 | |
| Dig. Thr, % | 104.6 | 83.1 | 101.5 | 80.6 | 98.6 | 78.3 | 100.0 | 79.4 | |
| Withdrawal | Dig. Lys, % | 105.6 | 84.4 | 102.2 | 81.7 | 102.2 | 81.7 | 100.0 | 80.0 |
| Dig. TSAA, % | 105.7 | 84.3 | 102.8 | 81.9 | 101.4 | 80.8 | 100.0 | 79.7 | |
| Dig. Thr, % | 104.9 | 83.6 | 101.6 | 81.0 | 101.6 | 81.0 | 100.0 | 79.7 | |
The nutritional requirements of strain 3 were based on the male line, because there were no specific recommendations for the cross available.
The nutritional requirements of strains 4 and 5 were the same; therefore, the relative percentage was the same in strains 4 and 5.
Table 2.
Calculated and measured total amino acid reduction (%) during starter (day 0–14), grower (day 14–28), finisher (day 28–41), and withdrawal (day 41–56) feeding phases.
| Total amino acid reduction | Starter |
Grower |
Finisher |
Withdrawal |
||||
|---|---|---|---|---|---|---|---|---|
| Calculated1 | Measured2 | Calculated1 | Measured2 | Calculated1 | Measured2 | Calculated1 | Measured2 | |
| Lysin, % | 18.8 | 16.0 | 20.0 | 20.5 | 17.9 | 16.3 | 20.0 | 19.0 |
| TSAA, % | 17.9 | 11.4 | 23.5 | 21.5 | 18.0 | 13.6 | 19.3 | 14.6 |
| Threonine, % | 18.4 | 16.1 | 19.4 | 17.6 | 18.5 | 17.6 | 19.5 | 19.5 |
The concentrations were obtained from the dietary formulation.
The concentrations were analyzed using wet chemical method.
Processing
On day 41 and 55, 4 birds per pen were randomly selected, weighed, and tagged for processing. On day 42 and 56 after 14 h fasting, the birds were processed in a small-scale processing plant on the Mississippi State University Poultry Research Farm. Broilers were hung by their feet in plastic shackles and were electrically stunned by manually placing their heads in a saturated saline bath (11.5 V, <0.5 mA AC to DC for 3 s). The shackle line speed was constant and set so that approximately 22 broilers were stunned per minute. Unilateral neck cutting was manually performed immediately after stunning, and bleeding lasted for 140 s. On completion of exsanguination, the broilers were scalded at 53.3°C for 191 s, picked for 35 s using a rotary drum feather picker (Baader-Johnson, Kansas City, KS), and then mechanically eviscerated. The carcasses and abdominal fat pads were weighed immediately after processing. After harvest, broiler carcasses were stored in ice water in metal (173 cm in length, 85 cm in width, and 68.5 cm in depth) and rubber containers (142 cm in length, 81 cm in width, and 50.8 cm in depth) to mimic the chilling process in a poultry plant. After 4 h of chilling in icy water, the carcasses were divided into breasts (pectoralis major), tenders (pectoralis minor), wings, drumsticks, and thighs and weighed individually. Breast were individually packed in Ziploc bags and stored at 2°C.
Woody Breast Incidence
Breasts were evaluated for WBM incidence 24 h postmortem, in accordance with a procedure described by Tijare et al. (2016). Manual palpation by a single observer was used to quantify degrees of firmness. Firmness was categorized based on a scale of 0 to 3 to indicate different levels of severity of WBM. A score of 0 was indicative of a normal breast where the breast exhibited flexibility throughout. A score of 1 was indicative of a slight incidence of WBM, where breast firmness was restricted to the cranial region. A score of 2 was indicative of a moderate incidence of WBM, where breast firmness was in the cranial and caudal regions but was flexible in the medial portion of the breast. A score of 3 was indicative of severe WBM, where the whole breast was firm and rigid.
Meat Quality
The pH and color of breast samples were measured for breast meat from broilers that were harvested on day 42 and day 56. A pH meter (Model Accumet 61a, Fisher Scientific, Hampton, NH) was used to measure the pH of the breast 24 h postmortem. The pH probe was inserted 2.0 to 2.5 cm below the top of the pectoralis major muscle. The CIE L∗ (lightness) of breast meat was evaluated by using a portable reflected-color spectrophotometer (MiniScan EZ 4500L, HunterLab, Reston, VA) with a 31.8 mm port size, a 10° standard observer and a D65 illuminant. CIE L∗ was measured on the surface of each breast at 3 different locations (cranial, medial, and caudal). At each location, 3 measurements were taken and the average of the 3 measurements was recorded.
Cooking loss and shear force were evaluated in half of the breast meat samples (2 birds/pen, 16 birds/treatment) from broilers that were harvested on day 56. The breast samples that were frozen at −18°C for 6 to 8 wk were thawed overnight at 4°C. Initial weights of the breast samples were recorded, and then they were baked in a preheated oven at 177°C (Viking, Greenwood, MS) to an internal temperature of 77°C. After cooking, broiler breast samples were cooled to room temperature (22 ± 2°C) and their final weights were recorded. Cooking loss of the breast meat samples were reported as a percentage and calculated as [(initial weight - final weight)/(initial weight)] × 100%. For Warner-Bratzler shear force analysis, 6 adjacent 1 cm (width) × 1 cm (thickness) × 2 cm (length) strips were cut from the cranial section of each breast sample, such that the directions of the muscle fibers were parallel. Samples were sheared perpendicular to the muscle fibers using a Warner-Bratzler shear device (Model 3300, Instron, Norwood, MA), and shear force was reported as the maximum force (N) that was required to shear through the sample (Schilling et al., 2012).
Economic Analysis
Normal breast meat price was calculated as the normal market price, which was $0.9459/lb (from USDA AMS Livestock, Poultry & Grain Market News on Friday September 13, 2019) and the price of breast meat exhibiting severe WBM was calculated as half of the price of normal breast meat (personal conversation with Dr. Casey Owens at the Department of Poultry Science, University of Arkansas, Fayetteville, AR). The prices for other parts were also obtained from USDA AMS Livestock, Poultry & Grain Market News on Friday, September 13, 2019. Feed ingredient prices were accessed at the time of feed formulation from https://www.feedstuffs.com/ingredient-market-prices on September 22, 2017. The prices for the diets are available in the article published by Zhang et al. (2020). Briefly, feed costs for starter, grower, finisher, and withdrawal control diets were 264.97, 261.19, 245.16, and 237.74 $/ton, respectively; feed cost for the AA-reduced diets were 240.97, 231.24, 225.02, and 217.71 $/ton, respectively.
Economic marginal return = value of the cut-up parts (skinless and boneless breast, tender, wing, drumstick, and thigh) – cost of feed to produce the cut-up parts.
The value of parts was calculated by breast wt. × $0.9459 + tender wt. × $1.6429 + wing wt. × $1.7522 + drumstick wt. × $0.3063 + thigh wt. × $ 0.4308.
Feed cost on day 41 was calculated by (feed price day 0–14 × FI day 0–14 + feed price day 14–28 × FI day 14–28 + feed price day 28–41 × FI day 28–41)/(average BW of each pen on day 41 × average BW for processing on day 41).
Feed cost on day 55 was calculated by (feed price day 0–14 × FI day 0–14 + feed price day 14–28 × FI day 14–28 + feed price day 28–41 × FI day 28–41 + feed price day 41–55 × FI day 41–55)/(average BW of each pen on day 55 × average BW for processing on day 55).
Statistical Analysis
A randomized complete block design with a 5 (strain) × 2 (diet) factorial arrangement of treatments was used in this study. Diet and strain were designated as fixed effects, and the block was designated as a random effect. A 2-way ANOVA was completed using the PROC GLM procedure of SAS version 9.4 to analyze carcass part weights, pH, color, cooking loss, and economic analysis. The normality of the percentage data was evaluated by using the PROC UNIVARIATE procedure before analysis in SAS. When significant differences (P ≤ 0.05) occurred among treatments, Tukey's honestly significance difference test was conducted to separate treatment means at P ≤ 0.05. The correlation between WBM incidence and BW, breast weight, and meat quality were analyzed using Spearman's rank correlation within each treatment (Meloche et al., 2018a). The incidence of WBM was analyzed using the PROC LOGISTIC procedure and treatment probabilities were generated and separated using the LSMEANS procedure with the ILINK option. It was considered significant at P ≤ 0.05.
Results
The objective of this study was to compare the different processing yield and meat quality responses of various broiler strains to a dietary AA reduction. The results of main effects of strain are not presented in this section or discussed later. The data presented in the tables are only for the readers' reference. Only the strain and diet interaction and dietary AA reduction main effects are presented in this section and are later discussed.
Processing Weight
On day 42, AA reduction lowered absolute weights of live body, carcass, tender, wing, drumstick, and thighs (P ≤ 0.0001; Table 3). However, abdominal fat pad weights were increased by AA reduction (P = 0.016). Absolute breast weights were decreased in strains 3, 4, and 5 (P = 0.008), but were not affected by AA reduction in strains 1 or 2. Carcass, breast, and tender weights relative to BW were decreased by an AA reduction in the diet (P = 0.0008, <0.0001, and 0.043, respectively). However, fat pad and thigh weights relative to BW increased in response to an AA reduction in the diet (P < 0.0001 and P = 0.016, respectively). However, wing and drumstick weights relative to BW were not affected (P > 0.05) by dietary AA treatment.
Table 3.
Processing weights and yields of 5 strains of broilers fed control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than the control diet) on day 42.
| Strain | Diet | Day 42 processing weights (g) |
Day 42 processing yields (% of BW) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | Carcass | Fat pad | Breast | Tender | Wing | Drum-stick | Thigh | Carcass | Fat pad | Breast | Tender | Wing | Drum-stick | Thigh | ||
| Strain 1 | 2,852a,b | 1,987a,b | 43.8a | 548 | 109 | 214b | 269b | 335a,b | 69.6b | 1.55 | 19.2b | 3.84b,c | 7.50b | 9.41b | 11.73b,c | |
| Strain 2 | 2,796b,c | 1,975a,b | 44.3a | 580 | 110 | 210b | 250c | 324b | 70.6a | 1.55 | 20.7a | 3.97a,b | 7.51b | 8.91c | 11.49c | |
| Strain 3 | 2,979a | 2,076a | 44.0a | 523 | 110 | 231a | 289a | 357a | 69.7b | 1.50 | 17.6c | 3.72c,d | 7.77a | 9.68a,b | 11.98a,b | |
| Strain 4 | 2,836a,b | 1,972a,b | 44.4a | 506 | 103 | 217b | 279a,b | 349a | 69.5b | 1.57 | 17.8c | 3.55d | 7.68a,b | 9.81a | 12.29a | |
| Strain 5 | 2,664c | 1,883b | 38.1b | 557 | 109 | 198c | 240c | 312b | 70.6a | 1.46 | 20.8a | 4.11a | 7.46b | 9.01c | 11.74b,c | |
| SEM | 36.7 | 26.4 | 1.25 | 9.26 | 2.37 | 2.65 | 4.34 | 5.68 | 0.22 | 0.041 | 0.21 | 0.051 | 0.058 | 0.091 | 0.107 | |
| Control | 2,943a | 2,071a | 37.7b | 582 | 114a | 224a | 278a | 346a | 70.4a | 1.30b | 19.8a | 3.89a | 7.61 | 9.44 | 11.73b | |
| Reduced | 2,707b | 1,886b | 48.1a | 504 | 103b | 205b | 252b | 325b | 69.7b | 1.75a | 18.7b | 3.79b | 7.56 | 9.29 | 11.97a | |
| SEM | 23.2 | 16.7 | 0.79 | 5.86 | 1.50 | 1.68 | 2.75 | 3.59 | 0.14 | 0.026 | 0.13 | 0.032 | 0.036 | 0.067 | 0.068 | |
| Strain 1 | Control | 2,913 | 2,041 | 38.9 | 566a,b,c | 113 | 221 | 282 | 338 | 70.0 | 1.33 | 19.4 | 3.87 | 7.59 | 9.68 | 11.60 |
| Strain 1 | Reduced | 2,790 | 1,932 | 48.7 | 529c,d,e | 106 | 207 | 255 | 331 | 69.2 | 1.76 | 18.9 | 3.81 | 7.41 | 9.14 | 11.85 |
| Strain 2 | Control | 2,864 | 2,032 | 36.2 | 605a,b | 115 | 217 | 256 | 328 | 70.9 | 1.28 | 21.1 | 4.01 | 7.59 | 8.98 | 11.48 |
| Strain 2 | Reduced | 2,727 | 1,918 | 52.3 | 555b,c,d | 106 | 203 | 243 | 320 | 70.3 | 1.81 | 20.3 | 3.93 | 7.42 | 8.85 | 11.51 |
| Strain 3 | Control | 3,089 | 2,166 | 39.6 | 560a,b,c | 115 | 241 | 303 | 373 | 70.0 | 1.30 | 18.0 | 3.72 | 7.79 | 9.78 | 12.05 |
| Strain 3 | Reduced | 2,868 | 1,986 | 48.4 | 487e,f | 106 | 222 | 275 | 341 | 69.3 | 1.70 | 17.1 | 3.71 | 7.74 | 9.58 | 11.91 |
| Strain 4 | Control | 3,014 | 2,102 | 40.0 | 560a,b,c | 110 | 229 | 294 | 365 | 69.7 | 1.34 | 18.5 | 3.65 | 7.62 | 9.74 | 12.06 |
| Strain 4 | Reduced | 2,657 | 1,842 | 48.8 | 452f | 97.1 | 205 | 263 | 333 | 69.3 | 1.80 | 17.0 | 3.45 | 7.74 | 9.89 | 12.53 |
| Strain 5 | Control | 2,836 | 2,015 | 33.8 | 617a | 118 | 210 | 256 | 325 | 71.1 | 1.23 | 21.7 | 4.17 | 7.44 | 9.03 | 11.46 |
| Strain 5 | Reduced | 2,493 | 1,752 | 42.4 | 497d,e,f | 101 | 186 | 224 | 300 | 70.2 | 1.69 | 19.9 | 4.05 | 7.48 | 8.98 | 12.03 |
| SEM | 51.9 | 37.3 | 1.77 | 13.1 | 3.35 | 3.74 | 6.14 | 8.03 | 0.32 | 0.058 | 0.30 | 0.073 | 0.082 | 0.129 | 0.151 | |
| P-value | Strain | <0.0001 | 0.0001 | 0.003 | <0.0001 | 0.191 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.322 | <0.0001 | <0.0001 | 0.0009 | <0.0001 | <0.0001 |
| Diet | <0.0001 | <0.0001 | 0.016 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.0008 | <0.0001 | <0.0001 | 0.043 | 0.342 | 0.067 | 0.016 | |
| Strain × Diet | 0.072 | 0.099 | 0.172 | 0.008 | 0.562 | 0.454 | 0.594 | 0.339 | 0.924 | 0.808 | 0.107 | 0.790 | 0.290 | 0.121 | 0.122 | |
a–fMeans in a column not sharing a common superscript were different (P < 0.05).
Eight replications/treatment, 4 subsamples/replication/treatment.
On day 56, AA reduction in the diet led to lower absolute live body, carcass, and breast weights in strains 4 and 5 (P = 0.021, 0.011, and 0.011, respectively; Table 4) but not in strains 1, 2, or 3. Dietary AA reduction lowered absolute tender, wing, drumstick, and thigh weights in all 5 strains (P = 0.002, <0.0001, 0.003, and 0.007, respectively). Dietary AA reduction led to increased abdominal fat pad weights (P = 0.016) in birds that were processed on both day 42 and 56. In addition, similar to day 42 processing effects, AA reduction in the diet led to lower carcass, breast, and tender weights relative to BW on day 56 (all P < 0.0001), but led to increased fat pad and thigh weights relative to BW (P = 0.0004 and P = 0.007, respectively).
Table 4.
Processing weights and yields of 5 strains of broilers fed control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than the control diet) on day 56.
| Strain | Diet | Day 56 processing weights (g) |
Day 56 processing yields (% of BW) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | Carcass | Fat pad | Breast | Tender | Wing | Drum-stick | Thigh | Carcass | Fat pad | Breast | Tender | Wing | Drum-stick | Thigh | ||
| Strain 1 | 3,845 | 2,764 | 68.8a | 768 | 163a,b | 288b,c | 354b,c | 475b,c | 71.6a,b | 1.82a,b | 19.9b | 4.24a,b | 7.46a,b | 9.16b | 12.27b,c | |
| Strain 2 | 3,759 | 2,723 | 72.1a | 800 | 167a | 273c,d | 335c | 457c | 72.4a | 1.93a | 21.2a | 4.45a | 7.28b | 8.89b | 12.15c | |
| Strain 3 | 4,146 | 2,985 | 71.8a | 800 | 170a | 311a | 396a | 537a | 72.0a | 1.79a,b | 19.3b,c | 4.12b,c | 7.50a | 9.55a | 12.95a | |
| Strain 4 | 3,928 | 2,785 | 70.6a | 720 | 153b | 293b | 380a,b | 502b | 70.7b | 1.83a | 18.3c | 3.89c | 7.47a,b | 9.66a | 12.70a,b | |
| Strain 5 | 3,669 | 2,656 | 56.1b | 791 | 154b | 269d | 332c | 441c | 72.3a | 1.52b | 21.2a | 4.18b | 7.42a,b | 9.15b | 12.08c | |
| SEM | 60.8 | 43.3 | 2.60 | 17.1 | 2.69 | 4.46 | 7.09 | 8.71 | 0.33 | 0.076 | 0.27 | 0.065 | 0.052 | 0.091 | 0.118 | |
| Control | 4,016 | 2,910 | 65.0b | 838 | 172a | 296a | 369a | 493a | 72.5a | 1.65b | 20.8a | 4.31a | 7.39 | 9.21 | 12.28b | |
| Reduced | 3,723 | 2,655 | 70.7a | 714 | 154b | 278b | 350b | 472b | 71.1b | 1.90a | 19.2b | 4.05b | 7.46 | 9.35 | 12.58a | |
| SEM | 38.4 | 27.4 | 1.64 | 10.8 | 1.70 | 2.82 | 4.49 | 5.51 | 0.21 | 0.048 | 0.17 | 0.041 | 0.033 | 0.058 | 0.075 | |
| Strain 1 | Control | 3,978a,b,c | 2,858a,b,c | 65.4 | 824a,b,c | 172 | 294 | 359 | 481 | 71.9 | 1.66 | 20.6 | 4.31 | 7.39 | 9.02 | 12.11 |
| Strain 1 | Reduced | 3,712c,d | 2,669b,c,d | 72.1 | 712c,d,e | 154 | 282 | 350 | 469 | 71.3 | 1.97 | 19.1 | 4.17 | 7.53 | 9.30 | 12.44 |
| Strain 2 | Control | 3,800b,c | 2,783a,b,c | 71.0 | 833a,b | 176 | 277 | 336 | 458 | 73.3 | 1.89 | 21.9 | 4.66 | 7.29 | 8.83 | 12.02 |
| Strain 2 | Reduced | 3,717c,d | 2,662b,c,d | 73.1 | 767b,c,d | 157 | 269 | 333 | 457 | 71.6 | 1.97 | 20.6 | 4.25 | 7.26 | 8.96 | 12.27 |
| Strain 3 | Control | 4,201a | 3,053a | 70.2 | 839a,b | 179 | 317 | 399 | 541 | 72.7 | 1.78 | 20.0 | 4.28 | 7.54 | 9.48 | 12.88 |
| Strain 3 | Reduced | 4,090a,b,c | 2,917a,b | 73.4 | 761b,c,d,e | 161 | 305 | 394 | 533 | 71.3 | 1.80 | 18.6 | 3.96 | 7.47 | 9.62 | 13.02 |
| Strain 4 | Control | 4,130a,b | 2,945a,b | 63.1 | 789b,c,d | 164 | 304 | 398 | 517 | 71.3 | 1.56 | 19.1 | 3.98 | 7.38 | 9.61 | 12.49 |
| Strain 4 | Reduced | 3,725c,d | 2,625c,d | 78.0 | 652e | 141 | 281 | 362 | 487 | 70.1 | 2.10 | 17.5 | 3.80 | 7.56 | 9.70 | 12.91 |
| Strain 5 | Control | 3,969a,b,c | 2,912a,b | 55.1 | 905a | 171 | 286 | 354 | 470 | 73.3 | 1.37 | 22.4 | 4.31 | 7.36 | 9.09 | 11.91 |
| Strain 5 | Reduced | 3,369d | 2,400d | 57.1 | 677d,e | 136 | 251 | 309 | 413 | 71.2 | 1.68 | 20.0 | 4.05 | 7.48 | 9.20 | 12.25 |
| SEM | 86.0 | 61.2 | 3.67 | 24.2 | 3.81 | 6.31 | 10.0 | 12.3 | 0.46 | 0.108 | 0.38 | 0.091 | 0.073 | 0.129 | 0.168 | |
| P-value | Strain | <0.0001 | <0.0001 | 0.0002 | 0.007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.006 | <0.0001 | <0.0001 | 0.027 | <0.0001 | <0.0001 |
| Diet | <0.0001 | <0.0001 | 0.016 | <0.0001 | 0.002 | <0.0001 | 0.003 | 0.007 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | 0.074 | 0.007 | |
| Strain × Diet | 0.021 | 0.011 | 0.367 | 0.011 | 0.122 | 0.201 | 0.122 | 0.170 | 0.561 | 0.131 | 0.596 | 0.588 | 0.588 | 0.948 | 0.927 | |
a–eMeans in a column not sharing a common superscript were different (P < 0.05).
Meat Quality
pH, Lightness, Cook Loss, and Shear Force
On day 42, feeding the AA-reduced diet led to decreased breast meat pH (P = 0.003) and CIE L∗ (P = 0.035) (Table 5). On day 56, AA reduction in the diet also led to decreased breast meat pH (P = 0.007), but CIE L∗ was not affected (P = 0.370). Cooking weight loss was greater for breast meat from broilers that were fed reduced dietary AA (P = 0.044), but the shear force was not affected by dietary AA reduction within each strain (P > 0.05).
Table 5.
The meat quality of 5 strains of broilers fed control or AA-reduced diet on day 42 (pH and lightness) and 56 (pH, lightness, cook loss, and shear force).
| Treatment |
day 42 |
day 56 |
|||||
|---|---|---|---|---|---|---|---|
| Strain | Diet | pH 24 h | L∗1 | pH 24 h | L∗ | Cook loss (%) | Shear force (N) |
| Strain 1 | 5.94a | 64.6 | 5.82b | 63.9 | 26.8 | 17.7 | |
| Strain 2 | 5.89a,b | 65.3 | 5.82b | 64.4 | 26.1 | 16.6 | |
| Strain 3 | 5.83b | 65.3 | 5.80b | 64.6 | 27.5 | 17.8 | |
| Strain 4 | 5.91a | 64.4 | 5.83a,b | 63.7 | 25.8 | 16.1 | |
| Strain 5 | 5.92a | 64.5 | 5.91a | 63.4 | 27.6 | 17.1 | |
| SEM | 0.019 | 0.29 | 0.021 | 0.43 | 1.07 | 0.87 | |
| Control | 5.92a | 65.1a | 5.86a | 64.2 | 25.8b | 17.2 | |
| Reduced | 5.87b | 64.5b | 5.81b | 63.8 | 27.7a | 16.9 | |
| SEM | 0.012 | 0.185 | 0.013 | 0.26 | 0.68 | 0.55 | |
| Strain 1 | Control | 5.95 | 64.6 | 5.84 | 63.6 | 26.2 | 17.7a,b |
| Strain 1 | Reduced | 5.93 | 64.5 | 5.79 | 64.1 | 27.4 | 17.6a,b |
| Strain 2 | Control | 5.90 | 65.6 | 5.83 | 64.6 | 24.8 | 16.0a,b |
| Strain 2 | Reduced | 5.87 | 65.0 | 5.81 | 64.3 | 27.5 | 17.1a,b |
| Strain 3 | Control | 5.85 | 65.9 | 5.82 | 64.5 | 28.1 | 15.6a,b |
| Strain 3 | Reduced | 5.82 | 64.8 | 5.77 | 64.6 | 26.9 | 20.1a |
| Strain 4 | Control | 5.95 | 64.6 | 5.86 | 64.0 | 22.5 | 18.3a,b |
| Strain 4 | Reduced | 5.86 | 64.1 | 5.81 | 63.4 | 29.1 | 13.9b |
| Strain 5 | Control | 5.95 | 64.8 | 5.96 | 64.1 | 27.3 | 18.5a,b |
| Strain 5 | Reduced | 5.89 | 64.3 | 5.87 | 62.8 | 27.9 | 15.7a,b |
| SEM | 0.026 | 0.41 | 0.029 | 0.60 | 1.51 | 1.23 | |
| P-value | Strain | 0.002 | 0.054 | 0.003 | 0.260 | 0.675 | 0.601 |
| Diet | 0.003 | 0.035 | 0.007 | 0.370 | 0.044 | 0.642 | |
| Strain × diet | 0.787 | 0.749 | 0.839 | 0.612 | 0.124 | 0.006 | |
a,bMeans in a column not sharing a common superscript were different (P < 0.05).
L∗ = Lightness.
Woody Breast Myopathy Severity
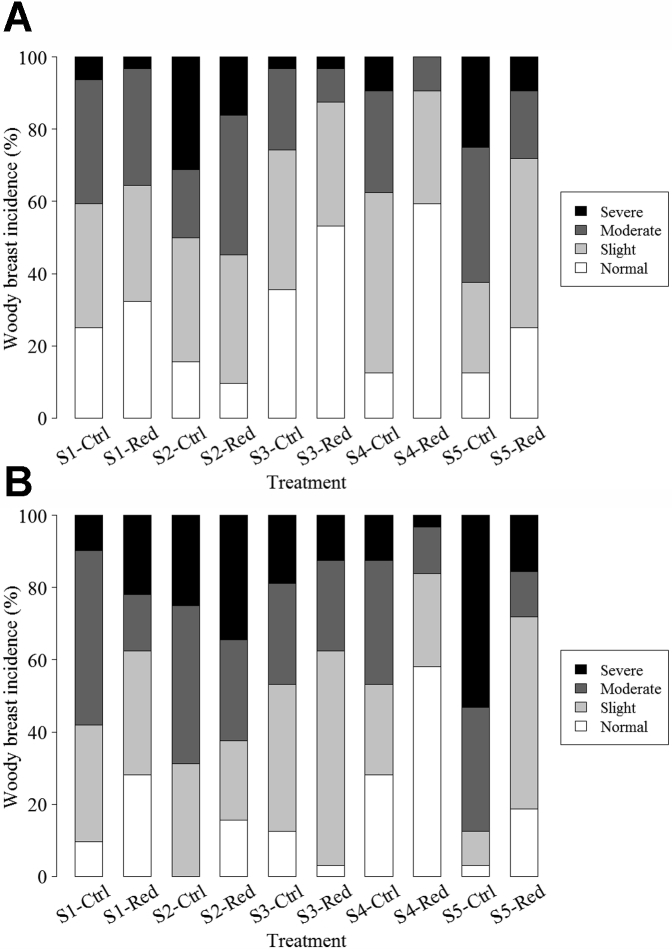
For all strains, AA reduction decreased the probability of birds developing more severe WBM incidence at day 42 of age (P < 0.0001), in which the probability of developing severe WBM decreased from 13 to 6% and moderate WBM decreased from 31 to 19% (Table 6). However, AA reduction only decreased the probabilities of birds developing more severe WBM incidence in strains 4 and 5 on day 56 (P = 0.002), in which the probability of developing severe WBM decreased from 13 to 3% in strain 4 and from 55 to 11% in strain 5, and decreased moderate WBM from 26 to 8% in strain 4. The incidence and severity of WBM in accordance with strain and diet are presented in Figures 1A (day 42) and 1B (day 56).
Table 6.
Woody breast myopathy (WBM) severity probabilities of 5 strains of broilers fed control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than the control diet) on day 42 and 56.
| Treatment |
WBM severity day 42 (probability) |
WBM severity day 56 (probability) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Diet | Normal | Slight | Moderate | Severe | Significance1 | Normal | Slight | Moderate | Severe | Significance1 |
| Strain 1 | 0.26 | 0.41 | 0.24 | 0.09 | b,c | 0.16 | 0.40 | 0.29 | 0.15 | ||
| Strain 2 | 0.13 | 0.32 | 0.36 | 0.19 | a | 0.08 | 0.27 | 0.35 | 0.30 | ||
| Strain 3 | 0.44 | 0.38 | 0.14 | 0.04 | d | 0.15 | 0.38 | 0.30 | 0.17 | ||
| Strain 4 | 0.38 | 0.40 | 0.17 | 0.05 | c,d | 0.36 | 0.42 | 0.16 | 0.06 | ||
| Strain 5 | 0.17 | 0.37 | 0.32 | 0.14 | a,b | 0.08 | 0.29 | 0.36 | 0.27 | ||
| Control | 0.18 | 0.38 | 0.31 | 0.13 | a | 0.10 | 0.30 | 0.35 | 0.25 | ||
| Reduced | 0.35 | 0.40 | 0.19 | 0.06 | b | 0.22 | 0.42 | 0.25 | 0.11 | ||
| Strain 1 | Control | 0.23 | 0.40 | 0.27 | 0.10 | 0.12 | 0.36 | 0.32 | 0.20 | b,c | |
| Strain 1 | Reduced | 0.29 | 0.41 | 0.23 | 0.07 | 0.21 | 0.42 | 0.26 | 0.11 | c | |
| Strain 2 | Control | 0.12 | 0.31 | 0.37 | 0.20 | 0.07 | 0.26 | 0.36 | 0.31 | b | |
| Strain 2 | Reduced | 0.14 | 0.33 | 0.36 | 0.17 | 0.08 | 0.28 | 0.35 | 0.29 | b | |
| Strain 3 | Control | 0.35 | 0.41 | 0.18 | 0.06 | 0.14 | 0.37 | 0.31 | 0.18 | b,c | |
| Strain 3 | Reduced | 0.53 | 0.34 | 0.10 | 0.03 | 0.16 | 0.39 | 0.29 | 0.16 | b,c | |
| Strain 4 | Control | 0.19 | 0.39 | 0.3 | 0.12 | 0.20 | 0.41 | 0.26 | 0.13 | c | |
| Strain 4 | Reduced | 0.60 | 0.30 | 0.08 | 0.02 | 0.56 | 0.33 | 0.08 | 0.03 | d | |
| Strain 5 | Control | 0.10 | 0.28 | 0.38 | 0.24 | 0.03 | 0.13 | 0.29 | 0.55 | a | |
| Strain 5 | Reduced | 0.27 | 0.42 | 0.23 | 0.08 | 0.23 | 0.42 | 0.24 | 0.11 | c | |
| P-value | Strain | <0.0001 | <0.0001 | ||||||||
| Diet | <0.0001 | <0.0001 | |||||||||
| Strain × diet | 0.073 | 0.002 | |||||||||
a–dLeast squares means in a column not sharing a common superscript were different among treatments (P < 0.05); a to d represents the order of woody breast myopathy (WBM) severity probability from the most to least severe.
Figure 1.
Woody breast incidence of broiler breast meat from 5 strains of commercially available broilers that were day 42 (A) and 56 (B) of age and fed a typical control industry diet and a diet being 20% of digestible amino acids (lysine, total sulfur amino acids, and threonine) reduction. Note: S for strain, Ctrl for control diet, Red for reduced diet.
Relationship Between WBM Severity and BW, Breast Weight, and Other Meat Characters
Correlation analysis was conducted between WBM score and BW, breast weight, and meat quality within each treatment group. On day 42, WBM score was positively associated with the BW of strain 1 birds fed the AA-reduced diet (P = 0.003), strain 2 birds fed both diets (P = 0.0004 and < 0.0001), strain 3 and 4 birds fed the control diet (P = 0.012 and 0.007), and strain 5 birds fed both diets (P = 0.043 and 0.0003; Table 7). The scores for WBM were positively associated with breast weight in all 10 treatments on day 42 (all P ≤ 0.037). The WBM scores were also positively associated with breast pH of strain 1, 3, 4, and 5 birds fed the control diet (P = 0.021, 0.0003, 0.044, and 0.018, respectively), and strain 2 birds fed the AA-reduced diet (P = 0.008). The WBM scores were positively associated with meat lightness in strain 2, 4, and 5 birds fed the control diet (P = 0.018, 0.015, and 0.020, respectively), as well as strain 3 and 4 birds that were fed the AA-reduced diet (P = 0.043 and 0.0007, respectively).
Table 7.
The coefficients and P-value of Spearman's rank correlation of WBM score with BW, breast weights, pH, and lightness of 5 strains of broilers fed a control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than control diet) on day 42.
| Treatment |
Diet | BW |
Breast weight |
pH |
L∗ |
||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| Strain 1 | Control | 0.096 | 0.614 | 0.383 | 0.037 | 0.420 | 0.021 | 0.357 | 0.053 |
| Strain 1 | Reduced | 0.515 | 0.003 | 0.764 | <0.0001 | 0.294 | 0.109 | 0.200 | 0.280 |
| Strain 2 | Control | 0.595 | 0.0004 | 0.625 | 0.0002 | 0.110 | 0.555 | 0.421 | 0.018 |
| Strain 2 | Reduced | 0.638 | <0.0001 | 0.711 | <0.0001 | 0.463 | 0.008 | 0.061 | 0.741 |
| Strain 3 | Control | 0.452 | 0.012 | 0.745 | <0.0001 | 0.613 | 0.0003 | 0.245 | 0.192 |
| Strain 3 | Reduced | 0.265 | 0.144 | 0.634 | <0.0001 | 0.121 | 0.509 | 0.360 | 0.043 |
| Strain 4 | Control | 0.478 | 0.007 | 0.517 | 0.003 | 0.365 | 0.044 | 0.433 | 0.015 |
| Strain 4 | Reduced | 0.289 | 0.115 | 0.713 | <0.0001 | 0.019 | 0.921 | 0.574 | 0.0007 |
| Strain 5 | Control | 0.359 | 0.043 | 0.404 | 0.022 | 0.414 | 0.018 | 0.409 | 0.020 |
| Strain 5 | Reduced | 0.602 | 0.0003 | 0.694 | <0.0001 | 0.313 | 0.087 | 0.124 | 0.506 |
Abbreviation: WBM, woody breast myopathy.
On day 56, the relationship between WBM score and BW and breast weights were similar to those on day 42 (Table 8). The WBM scores were positively associated with the BW of strain 1 birds that were fed the AA-reduced diet (P = 0.0006), strain 2 birds fed both diets (P = 0.048 and 0.002), strain 3 birds fed the control diets (P = 0.003), strain 4 birds fed the AA-reduced diet (P = 0.006), and strain 5 birds fed both diets (P = 0.001 and 0.017). There was a positive correlation between WBM and breast weight in all treatments (all P ≤ 0.023), except for strain 3 birds that were fed the AA-reduced diet (P = 0.127). The scores for WBM were positively associated with the pH of breast meat from birds of strain 2 fed both diets (P = 0.001 and 0.022) and strain 5 birds fed an AA-reduced diet (P = 0.005). Meat lightness was positively associated with WBM score in strain 1 and 4 breast meat from birds fed the AA-reduced diet (P = 0.010 and 0.024) and strain 3 birds fed the control diet (P = 0.006). Cooking weight loss and shear force were not associated with the WBM score (P > 0.05).
Table 8.
The coefficients and P-value of Spearman's rank correlation of WBM score with BW, breast weights, and meat quality of 5 strains of broilers fed a control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than control diet) on day 56.
| Treatment |
Diet | BW |
Breast weight |
pH |
L∗ |
Cook loss |
Shear force |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| Strain 1 | Control | 0.308 | 0.098 | 0.413 | 0.023 | 0.105 | 0.580 | 0.249 | 0.185 | 0.069 | 0.814 | 0.106 | 0.717 |
| Strain 1 | Reduced | 0.589 | 0.0006 | 0.719 | <0.0001 | 0.162 | 0.393 | 0.464 | 0.010 | −0.349 | 0.202 | 0.238 | 0.394 |
| Strain 2 | Control | 0.353 | 0.048 | 0.523 | 0.002 | 0.545 | 0.001 | 0.073 | 0.695 | −0.196 | 0.468 | 0.051 | 0.850 |
| Strain 2 | Reduced | 0.525 | 0.002 | 0.819 | <0.0001 | 0.409 | 0.022 | 0.349 | 0.055 | 0.251 | 0.367 | 0.403 | 0.136 |
| Strain 3 | Control | 0.507 | 0.003 | 0.631 | 0.0001 | 0.206 | 0.258 | 0.473 | 0.006 | 0.329 | 0.213 | 0.343 | 0.194 |
| Strain 3 | Reduced | 0.066 | 0.721 | 0.276 | 0.127 | 0.247 | 0.174 | −0.146 | 0.426 | −0.182 | 0.501 | 0.419 | 0.106 |
| Strain 4 | Control | 0.289 | 0.115 | 0.410 | 0.022 | 0.198 | 0.285 | 0.035 | 0.850 | −0.433 | 0.107 | −0.087 | 0.757 |
| Strain 4 | Reduced | 0.487 | 0.006 | 0.598 | 0.0004 | −0.030 | 0.871 | 0.406 | 0.024 | −0.272 | 0.326 | 0.245 | 0.380 |
| Strain 5 | Control | 0.552 | 0.001 | 0.666 | <0.0001 | 0.130 | 0.485 | 0.036 | 0.847 | 0.104 | 0.702 | −0.211 | 0.434 |
| Strain 5 | Reduced | 0.418 | 0.017 | 0.615 | 0.0002 | 0.485 | 0.005 | 0.336 | 0.065 | 0.033 | 0.903 | 0.492 | 0.053 |
Abbreviation: WBM, woody breast myopathy.
WBM in Different BW Range
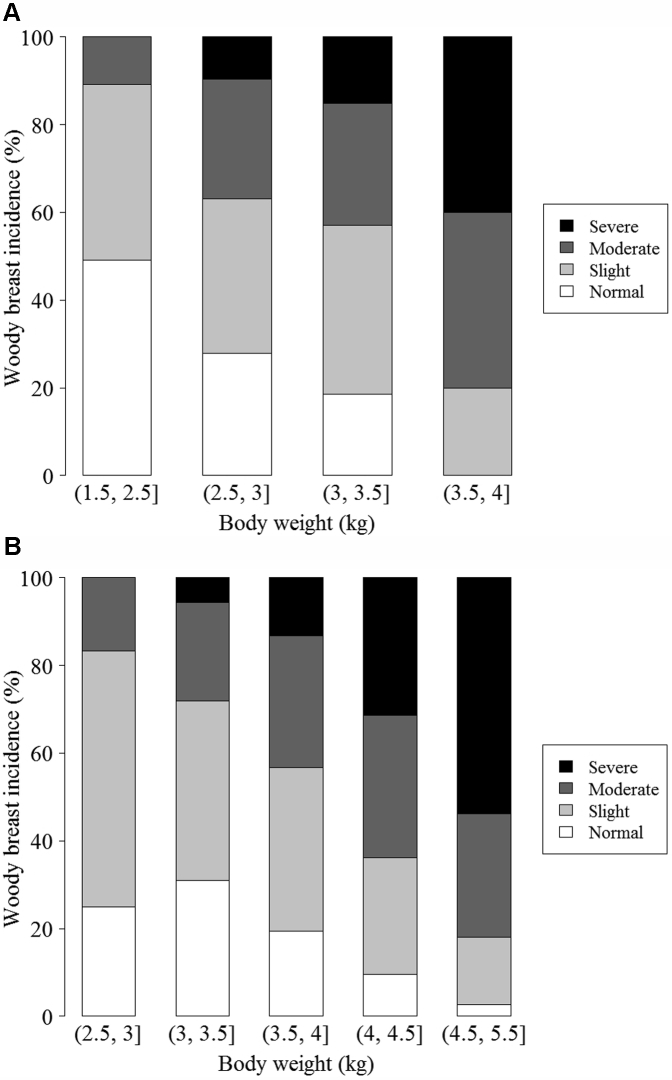
Birds from all the treatment groups were pooled into different BW ranges and exhibited in Figure 2 for visualizing the relationship between BW and WBM. No statistical analysis was performed in Figure 2. The statistical correlation analysis between BW and WBM is listed in Tables 7 and 8. The figure indicates that the percentage of WBM incidence and severity tended to increase as BW increased.
Figure 2.
Woody breast incidence of broiler breast meat in 5 different weight ranges across all treatments on day 42 (A) and 56 (B). Note: Body weights (a, b] on the x axis of each graph means a < BW ≤ b.
Economic Return: Cost of Feed With or Without WBM Considered
On day 42, if WBM was not considered, that is, WBM was sold at the same price as normal breast meat, dietary AA reduction did not affect economic return: feed cost ratio (Table 9). However, if unacceptable breast meat (severe WBM) was sold separately, dietary AA reduction increased economic return to feed cost ratio on day 42 and day 56 (P = 0.015 and 0.026, respectively).
Table 9.
Economic marginal return to cost of feed ratio of 5 strains of broilers fed control or amino acid–reduced diet (with digestible lysine, TSAA, and threonine being 20% lower than the control diet), with or without woody breast loss considered.
| Treatment |
Economic return: feed cost on day 42 processing |
Economic return: feed cost on day 56 processing |
|||
|---|---|---|---|---|---|
| Strain | Diet | WB was not considered2 | Severe WB considered3 | WB was not considered2 | Severe WB considered3 |
| Strain 1 | 1.695b | 1.662 | 1.372a | 1.284 | |
| Strain 2 | 1.746a,b | 1.597 | 1.355a,b | 1.187 | |
| Strain 3 | 1.617b | 1.599 | 1.365a,b | 1.283 | |
| Strain 4 | 1.617b | 1.590 | 1.239b | 1.196 | |
| Strain 5 | 1.837a | 1.726 | 1.412a | 1.207 | |
| SEM1 | 0.0337 | 0.0400 | 0.0327 | 0.0312 | |
| Control | 1.684 | 1.590b | 1.335 | 1.200b | |
| Reduced | 1.721 | 1.680a | 1.362 | 1.263a | |
| SEM | 0.0213 | 0.0253 | 0.0207 | 0.0197 | |
| Strain 1 | Control | 1.650 | 1.609 | 1.391a,b,c | 1.335 |
| Strain 1 | Reduced | 1.740 | 1.716 | 1.352a,b,c | 1.234 |
| Strain 2 | Control | 1.725 | 1.529 | 1.253b,c | 1.117 |
| Strain 2 | Reduced | 1.767 | 1.666 | 1.458a,b | 1.257 |
| Strain 3 | Control | 1.605 | 1.587 | 1.341a,b,c | 1.238 |
| Strain 3 | Reduced | 1.629 | 1.611 | 1.389a,b,c | 1.328 |
| Strain 4 | Control | 1.607 | 1.552 | 1.216c | 1.149 |
| Strain 4 | Reduced | 1.627 | 1.627 | 1.261a,b,c | 1.243 |
| Strain 5 | Control | 1.833 | 1.674 | 1.476a | 1.161 |
| Strain 5 | Reduced | 1.842 | 1.778 | 1.349a,b,c | 1.254 |
| SEM | 0.0476 | 0.0566 | 0.0463 | 0.0441 | |
| P-value | Strain | <0.0001 | 0.084 | 0.006 | 0.062 |
| Diet | 0.226 | 0.015 | 0.367 | 0.026 | |
| Strain × diet | 0.924 | 0.884 | 0.012 | 0.070 | |
a–cMeans in a column not sharing a common superscript were different (P < 0.05).
The value of parts was calculated by breast wt. × $0.9459 + tender wt. × $1.6429 + wing wt. × $1.7522 + drumstick wt. × $0.3063 + thigh wt. × $ 0.4308.
Feed cost on day 41 was calculated by (feed price day 0–14 × FI day 0–14 + feed price day 14–28 × FI day 14–28 + feed price day 28–41 × FI day 28–41)/average BW of each pen on day 41 × average BW for processing on day 41.
Feed cost on day 55 was calculated by (feed price day 0–14 × FI day 0–14 + feed price day 14–28 × FI day 14–28 + feed price day 28–41 × FI day 28–41 + feed price day 41–55 × FI day 41–55)/average BW of each pen on day 55 × average BW for processing on day 55.
The prices for the diets are listed in Table 1.
n = 16, 40, and 8, respectively, for treatments of strain, diet, and interaction of strain and diet.
Economic marginal return = value of the cut-up parts (skinless and boneless breast, tender, wing, drumstick, and thigh) – cost of feed to produce the cut-up parts.
The value of breast with severe woody breast myopathy was calculated at half price of normal breast price of $0.9459.
Discussion
Processing Yield
For birds selected for processing, dietary AA reduction decreased the BW of those in strains 3, 4, and 5 on day 41 and strains 4 and 5 on day 55. These results reflected the effects observed for the whole pen BW (Zhang et al., 2020). Furthermore, the BW of birds selected for processing (calculated by dividing total bird weight by the number of birds selected for processing) also responded to dietary AA reduction as did the BW of whole pens (calculated by dividing whole pen weight by the number of birds in each pen) (Zhang et al., 2020). This indicates that the birds for processing were successfully randomized (i.e., the birds selected for processing are representative of the whole pens).
As expected, dietary AA reduction decreased the tenders, wings, drumsticks, and thighs weights of birds belonging to all the strains on day 42 and day 56 (Tables 3 and 4). A large number of previous literatures have addressed the importance of dietary AA concentrations on the growth of broilers. However, in the present study, the effects of AA reduction on breast weight were different among the different strains examined. For breast weight on day 42, strains 3, 4, and 5 were more sensitive to an AA reduction than were strains 1 and 2. In addition, birds belonging to strains 4 and 5 were more sensitive to an AA reduction in their diets than were birds belonging to strains 1, 2, and 3 on day 56. The different responses among strains are likely due to differences in the genetic backgrounds and age of the broilers. Strains 1 and 2 share similar genetic backgrounds, which are different from that of strains 4 and 5. Moreover, the AA requirements of strains 1 and 2 were lower than that of strains 4 and 5 (Table 1). As a result, strains 1 and 2 were probably not affected as much by the same dietary AA reduction as strains 4 and 5. In addition, older birds are less susceptible to the effects of a dietary AA reduction, which is related to the changes in their nutritional requirements. Older birds have a lower dietary AA requirement than younger birds, because a bird's AA requirement decreases as their age increases (Rostagno et al., 2011; Cemin et al., 2017; Cobb-Vantress, 2018; Aviagen, 2019). As a result, the effects of dietary AA reduction on breast weight were less evident in older broilers.
Previous studies have found that fat pad weight was influenced by dietary energy levels rather than dietary fat levels when broilers were fed isocaloric diets (Griffiths et al., 1977). In the present study, the AA-reduced diet had a lower concentration of dietary fat than the control diet, but the energy level (AME) was the same in the 2 diets. The absolute and relative fat pad weights were not decreased in broilers fed the AA-reduced diet on both day 42 and 56, when most other carcass parts experienced decreases in absolute and relative weights. Meloche et al. (2018b) reported that absolute weights of the breast decreased, and fat pad increased at day 40 when digestible lysine was reduced by 25% between 15 and 25 d of age. The decrease in breast weight and increase in fat pad weight might be due to an imbalance in AA concentration in the diet. It has been reported that the rate of protein synthesis and deposition in the breast muscle decreased when dietary lysine was deficient in the broiler diet (Tesseraud et al., 1996). Unbalanced dietary AA is used as an energy source or is excreted out of the body as uric acid, instead of being deposited in the body as lean tissue. By contrast, Kidd et al. (1998) found that the breast meat weight increased and fat pad yield decreased at the age of day 50 when broilers were fed a diet with a 20% higher level of lysine between 1 and 18 d of age. Even though breast and tender relative weights to BW were decreased by AA reduction on both day 42 and 56, thigh weights relative to BW were increased by AA reduction on both day 42 and 56. This increase in relative thigh weight is probably attributable to the fact that BW decreased more than thigh weight because relative thigh weight was calculated by dividing thigh weight by BW. Relative wing and drumstick weights were not affected by an AA reduction on day 42 or 56. These results suggest that different body parts respond differently to a dietary AA reduction, with breast meat yield being impacted most by a reduction in AA levels in the diet.
Meat Quality and Woody Breast
The dietary AA reduction led to a lower breast meat pH on both day 42 and 56 and to a darker meat color on day 42 (Table 5). At the same time, AA reduction increased the percentages of normal breast and incidences of slight WBM and decreased the percentages of moderate and the incidences of severe WBM (Table 6, Figure 1A). As mentioned earlier, Meloche et al. (2018a,b) reported that lowering dietary energy and AA by 10% between day 8 and 49 after hatch or lowering digestible lysine from day 15 to 25 by 25% decreased WBM incidence in Yield Plus × Ross 708 male broilers. Therefore, both the present and previous studies suggest that lowering dietary AA is a potential method by which to decrease WBM incidence and lessen the negative economic and meat quality effects associated with meat exhibiting WBM.
On day 56, AA reduction did not affect the incidence of WBM within strains 1, 2, or 3 (P > 0.05) but led to less WBM incidence within strains 4 and 5 (P < 0.05) (Table 6, Figure 1B). Feeding a AA-reduced diet decreased (P < 0.05) the percentage of moderate and severe WBM from 45 to 11% for strain 4 and from 84 to 35% for strain 5 (Table 7), as well as decreased the body and breast weights of strains 4 and 5 when compared with the birds fed a control diet (Table 4). These results indicate that strains 4 and 5 are more susceptible to the dietary AA reduction than strains 1, 2, and 3. These different responses of WBM incidence among strains were consistent with the breast weight results. As mentioned earlier, different genetic backgrounds and nutritional requirements might lead to different responses in WBM incidence among strains. The sex of broilers could be another potential influential factor because male broilers were more likely to develop WBM than female broilers (Trocino et al., 2015). In the present study, we used both male and female birds and randomly assigned male and female birds in each pen. Male and female birds may respond to AA reduction differently because male birds generally have a higher nutrient requirement than female birds (Rostagno et al., 2011). The individual responses of male and female bird may be studied in the future to elucidate the mechanisms underlying the effects of sex on WBM development.
In the present study, AA reduction in the diet decreased the incidence of severe (unacceptable) WBM on day 42 and concurrently decreased the pH of breast meat. Correlation analysis also showed that the pH of the breast meat from 42 day-old broilers was positively related to WBM severity when the control diet was fed. The exception to this was in strain 2 when those birds were fed the AA-reduced diet. The positive relationship may be due to the fact that the pH of the WBM meat was generally higher than normal breast meat (Cai et al., 2018). The increased pH is likely caused by the decreased concentration of glycogen in the WBM meat (Abasht et al., 2016). The pH of breast meat is related to the amount of glycogen, which could be broken down into lactic acid which subsequently causes a lower postmortem pH (Mir et al., 2017). The WBM meat had lighter color than the normal meat (60.0 vs. 56.4) (Cai et al., 2018), and this was observed in our study for some treatment groups (Tables 7 and 8). In this study, CIE L ∗ (lightness) of the breast meat was positively correlated to WBM severity in broilers that were fed the control diet on day 42, with the exception of strain 3. Interestingly, breast meat lightness in strain 3 was positively related to WBM severity when birds were fed the AA-reduced diet.
On day 56, WBM severity was only positively associated with pH in strain 2 birds that were fed both diets and in strain 5 birds that were fed the AA-reduced diet. Severity of WBM was positively associated with breast meat lightness in fewer treatment groups as well. The positive relationship of WBM severity with breast meat pH and lightness may diminish with broiler age because older birds exhibit a reduced response to dietary AA reduction.
Dietary AA reduction led to increased cooking loss of breast meat on day 56. A possible explanation for the increase in cooking loss may be due to a decrease in pH of breast meat (Santiago, 2002; Ke, 2006). Qiao et al. (2001) reported that breast meat with a lower pH had a lower moisture content, which would contribute to an increase in cooking weight loss. Even though significant, the higher cooking weight loss in breast meat from birds fed the AA-reduced diet does not contribute to a decrease in meat quality. This can occur because the pH of the meat is closer to the isoelectric point (4.7–5.1) of the myofibrillar proteins, which leads to a slight decrease in their water-holding capacity (Offer and Knight, 1988).
Woody Breast and Growth
Correlation analysis showed that both BW and WBM severity were positively related in most treatment groups at 42 and 56 d of age (Tables 7 and 8). When the birds were grouped by BW, heavier birds had a higher WBM incidence on day 42 and 56 (Figure 2). These positive relationships were consistent with a previous study showing that WBM incidence linearly increased as BW, breast weight, and breast yield increased on day 35, and when digestible lysine ranged from 0.77 to 1.17% from 12 to 28 d of age (Cruz et al., 2017). However, Cruz et al. (2017) also reported that WBM incidence exhibited a quadratic relationship (increased then decreased) with BW and breast weight on day 42, and when digestible lysine ranged from 0.68 to 1.08% from day 28 to 42. In the present study, positive relationships between BW and WBM severity occurred for strain 1 broilers fed the control diet and strain 3 broilers fed the AA-reduced diet at 42 and 56 d of age. This lack of correlations suggests that WBM may be alleviated by genetic selection and by manipulating the nutrient compositions of the diet.
In addition, the positive relationship between WBM severity and breast meat weight was stronger than that between WBM incidence and BW. In Tables 7 and 8, more treatments exhibited higher correlation coefficient values between WBM severity and breast weight than that between WBM and BW on day 42 and 56. Higher breast weights were shown to be associated with a higher WBM severity in all treatment groups on day 42 and 56. The only exception to this was strain 3 birds fed AA-reduced diets on day 56. Griffin et al. (2018) reported that the incidence of severe WBM increased with increased breast thickness and yield, but did not increase with increasing BW. Correlation differences might be due to different responses in breast weight and BW to the same dietary change. Cruz et al. (2017) reported that the levels of dietary lysine required to achieve maximum broiler BW, breast weight, carcass weight, and breast yield were different. As a result, BW and breast weight may respond differently to the same dietary AA reduction and, therefore, correlate differently with WBM score. In the last couple of decades, breeder companies have focused more on increasing breast meat yield than that of other meat parts (Tavarez and Solis de los Santos, 2016), which may be another explanation why there were stronger relationships between WBM and breast weight than that between WBM and whole BW.
Economic Return
The incidence of WBM is positively related to growth performance. However, Bodle et al. (2018) reported that a decrease in WBM incidence at day 45 was not accompanied by a decrease in BW or breast weight when dietary AA was reduced by 15% between day 13 and 24. The different responses between studies may be due to differences in the timing and levels of AA reduction. In this study, broilers were continually fed an AA-reduced diet from day 0 to 56, which might have lowered the compensatory growth of broilers. The birds could have exhibited higher compensatory growth if the dietary AA in the later feeding phases was at the recommended level rather than being reduced. However, in the previous study, broilers were fed an AA-reduced diet only during day 13–24 and were then fed a normal diet between day 24 and 45, during which broilers can exhibit compensatory growth (Bodle et al., 2018). These differences may also be due to a 20% reduction in digestible AA (lysine, TSAA, and threonine) in comparison with a 15% reduction in the previous study (Bodle et al., 2018).
Severe WBM affected breast meat would be downgraded and sold at a lower price, which would cause economic loss to the broiler industry (Zanetti et al., 2018). In the present study, the decreased price of WBM meat was considered in the economic analysis. Even with some degree of reduction in growth performance as a result of an AA reduction in the diets of the birds in the present study, the ratio of marginal return to feed cost was increased with dietary AA reduction when severe WBM incidence was considered in the calculation at both day 42 and 56. Feed cost: BW was decreased in broilers fed an AA-reduced diet in the companion study (Zhang et al., 2020). It is necessary to point out that the economic analysis in the present study did not consider all factors related to commercial broiler production. For example, the increased duration of the growth period in response to a lower dietary AA level and the subsequent reduction in processing volume associated with decreased BW and carcass weight will reduce overall broiler meat production and market availability. An economic analysis in which all these factors are accounted for in future studies will provide more accurate and complete estimations of cost, return, and production volume for the broiler industry.
Conclusion
Although dietary AA reduction decreased processing yields of broilers, the effects varied among different strains and ages of broilers. Positive associations between WBM severity and BW or breast weight exist in most strains either fed a control or an AA-reduced diet. Because of the lower price of feed and lower WBM incidence in AA-reduced treatment groups, even after accounting for some processing weight loss, dietary AA reduction still resulted in a marginal increase in economic return in a theoretical model. Therefore, dietary AA reduction is a potential strategy to control WBM without negatively influencing economic return.
Acknowledgments
This material is based on the work that was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2017-67017-26473 and Hatch project under accession number MIS-329250.
Disclosures
The authors declare no conflicts of interest.
References
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11:e0153750. doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen . Aviagen Group; Huntsville, AL: 2019. Ross Broiler Nutrition Specifications. [Google Scholar]
- Bodle B., Alvarado C., Shirley R., Mercier Y., Lee J. Evaluation of different dietary alterations in their ability to mitigate the incidence and severity of woody breast and white striping in commercial male broilers. Poult. Sci. 2018;97:3298–3310. doi: 10.3382/ps/pey166. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y., Nair M., Suman S., Beach C., Guyton M., Schilling M. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Cemin H.S., Vieira S.L., Stefanello C., Kipper M., Kindlein L., Helmbrecht A. Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed. PLoS One. 2017;12:e0179665. doi: 10.1371/journal.pone.0179665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb-Vantress . COBB-VANTRESS.COM; Siloam Springs, AR: 2018. Cobb Broiler Performance & Nutrition Supplement. [Google Scholar]
- Cruz R.F., Vieira S.L., Kindlein L., Kipper M., Cemin H.S., Rauber S.M. Occurrence of white striping and wooden breast in broilers fed grower and finisher diets with increasing lysine levels. Poult. Sci. 2017;96:501–510. doi: 10.3382/ps/pew310. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Griffiths L., Leeson S., Summers J.D. Influence of energy system and level of various fat sources on performance and carcass composition of broilers. Poult. Sci. 1977;56:1018–1026. [Google Scholar]
- Ke S. Univ. Massachusetts Amherst; Amherst, MA: 2006. Effect of pH and Salts on Tenderness and Water-Holding Capacity of Muscle Foods. PhD Diss. [Google Scholar]
- Kidd M.T., Kerr B.J., Halpin K.M., McWard G.W., Quarles C.L. Lysine levels in starter and grower-finisher diets affect broiler performance and carcass traits. J. Appl. Poult. Res. 1998;7:351–358. [Google Scholar]
- Kuttappan V.A., Owens C.M., Coon C., Hargis B.M., Vazquez-Anon M. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017;96:3005–3009. doi: 10.3382/ps/pex072. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Landon C., Barnes H.J., Brake J. White striping and wooden breast myopathies of broiler breast muscle is affected by time-limited feeding, genetic background, and egg storage. Poult. Sci. 2019;98:217–226. doi: 10.3382/ps/pey333. [DOI] [PubMed] [Google Scholar]
- Meloche K., Fancher B., Emmerson D., Bilgili S., Dozier W., III. Effects of reduced dietary energy and amino acid density on Pectoralis major myopathies in broiler chickens at 36 and 49 days of age1. Poult. Sci. 2018;97:1794–1807. doi: 10.3382/ps/pex454. [DOI] [PubMed] [Google Scholar]
- Meloche K.J., Dozier W.A., III, Brandebourg T.D., Starkey J.D. Skeletal muscle growth characteristics and myogenic stem cell activity in broiler chickens affected by wooden breast. Poult. Sci. 2018;97:4401–4414. doi: 10.3382/ps/pey287. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer G., Knight P. The structural basis of water-holding in meat; Part 2: drip Losses. In: Lawrie R., editor. Developments in Meat Science 4. Elsevier; Oxford: 1988. pp. 173–241. [Google Scholar]
- Qiao M., Fletcher D., Smith D., Northcutt J. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Rostagno H.R., Albino L.F.T., Donzele J.L., Gomes P.C., de Olveira R.F., Lopes D.C., Firiera A.S., Barreto S.L., Euclides R.F. 3rd ed. Universidade Federal de Viçosa, Departamento de Zootecnia; Vicosa, MG, Brazil: 2011. Brazilian Tables for Poultry and Swine: Composition of Feedstuffs and Nutritional Requirements. [Google Scholar]
- Santiago H.L. Virginia Tech; Blacksburg, VA: 2002. Biological, Nutritional, and Processing Factors Affecting Breast Meat Quality of Broilers. PhD Diss. [Google Scholar]
- Schilling M.W., Radhakrishnan V., Vizzier-Thaxton Y., Christensen K., Joseph, Williams P.J.B., Schmidt T.B. The effects of low atmosphere stunning and deboning time on broiler breast meat quality. Poult. Sci. 2012;91:3214–3222. doi: 10.3382/ps.2012-02266. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Sun X., Koltes D.A., Coon C.N., Chen K., Owens C.M. Instrumental compression force and meat attribute changes in woody broiler breast fillets during short-term storage. Poult. Sci. 2018;97:2600–2606. doi: 10.3382/ps/pey107. [DOI] [PubMed] [Google Scholar]
- Tavarez M.A., Solis de los Santos F. Impact of genetics and breeding on broiler production performance: a look into the past, present, and future of the industry. Anim. Front. 2016;6:37–41. [Google Scholar]
- Tesseraud S., Peresson R., Lopes J., Chagneau A. Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Br. J. Nutr. 1996;75:853–865. doi: 10.1079/bjn19960191. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Trocino A., Piccirillo A., Birolo M., Radaelli G., Bertotto D., Filiou E., Petracci M., Xiccato G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- Van Harn J., Dijkslag M., Van Krimpen M. Effect of low protein diets supplemented with free amino acids on growth performance, slaughter yield, litter quality, and footpad lesions of male broilers. Poult. Sci. 2019;98:4868–4877. doi: 10.3382/ps/pez229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venalainen E., Valaja J., Jalava T. Effects of dietary metabolisable energy, calcium and phosphorus on bone mineralisation, leg weakness and performance of broiler chickens. Br. Poult. Sci. 2006;47:301–310. doi: 10.1080/00071660600741776. [DOI] [PubMed] [Google Scholar]
- Wen C., Jiang X., Ding L., Wang T., Zhou Y. Effects of dietary methionine on growth performance, meat quality and oxidative status of breast muscle in fast-and slow-growing broilers. Poult. Sci. 2017;96:1707–1714. doi: 10.3382/ps/pew432. [DOI] [PubMed] [Google Scholar]
- Zanetti M.A., Tedesco D.C., Schneider T., Teixeira S.T.F., Daroit L., Pilotto F., Dickel E.L., Santos S.P., dos Santos L.R. Economic losses associated with wooden breast and white striping in broilers. Semina: Ciências Agrárias. 2018;39:887–892. [Google Scholar]
- Zhang B., Zhang X., Schilling M.W., Tabler G.T., Peebles E.D., Zhai W. Effects of broiler genetic strain and dietary amino acid reduction on (Part I): growth performance and internal organ development. Poult. Sci. 2020;99:3266–3279. doi: 10.1016/j.psj.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]