Abstract
African swine fever (ASF) is a notifiable contagious disease caused by the African swine fever virus (ASFV), leading to a serious socio-economic impact, constraining pig industry, and affecting food security worldwide. This study aimed to detect and characterize ASFV strains from suspected infected domestic pigs in two South-Kivu province districts of the Democratic Republic of the Congo (DRC). A total of 155 pig samples were screened for viral DNA and sequencing at multiple loci. An infection rate of 5.2% (8/155) was recorded from a total of 155 blood samples with the highest ASFV infection rate of 8% for Uvira (6/75) and mostly in female pigs 5 (7.6%). Most ASF associated clinical signs were redness on the skin and snout at 49% (95% CI: 21–34), followed by the unwillingness of pigs to stand at 29 % (95%, CI: 19–35). Phylogenetic analysis of partial B646L (p72) and the full-length E183 (p54) gene sequences revealed the circulation of genotypes IX and X, which clustered with previously reported viruses in the same region, Uganda, Kenya, and Tanzania. Intragenotypic resolution of the CVR region clustered the viruses into two subgroups: the genotype X strain subgroup (10 repeats, AAAABNAABA) and the genotype IX strain subgroup (11 repeats, AAAAAAAAAAF). This finding provides additional evidence that genetically similar ASFV strains may be circulating within South Kivu province and highlights the need for improved coordination to prevent the spread of the disease in non-infected areas.
Keywords: African swine fever, Democratic republic of Congo, Genotype, Molecular epidemiology, Outbreaks, Pigs
African swine fever; Democratic republic of Congo; Genotype; Molecular epidemiology; Outbreaks; Pigs
1. Introduction
African swine fever is an infectious, hemorrhagic-arboviral disease affecting domestic pig populations with a significant economic impact due to the direct loss of animals with up to 100% mortality rates (Jori and Bastos, 2009; Galindo and Alonso, 2017). It is caused by the African swine fever virus (ASFV), a double-stranded DNA virus belonging to the Asfarvidae family and genus Asfivirus (Alonso et al., 2018). The disease has been reported in more than 26 African countries and is endemic in most of the Sub-Saharan African countries, including the Democratic Republic of Congo (DRC) (Mulumba–Mfumu et al., 2017). Typical signs in infected animals include high fever (40–42 °C), skin hemorrhages, difficulty breathing, unwillingness to stand, and eventually, coma and death within 1–7 days (Sánchez-Vizcaíno et al., 2015). Currently, there is no effective vaccine or antiviral drugs against ASFV infection (Galindo and Alonso, 2017). Therefore, prevention and control strategies rely mainly on early diagnosis, restriction of the live pig movement and their products, strict application of biosecurity measures as well as stamping out infected swine herds (Bellini et al., 2016).
Transmission occurs through the sylvatic, the domestic cycle, or between the sylvatic cycle and domestic pigs. In the sylvatic cycle, the ASF virus spreads between wild pigs (natural reservoir) and ticks (Ornithodoros spp.) (Costard et al., 2013). The domestic cycle involves the transmission of ASFV from one domestic pig to another, or through feeds with infected pig products, without the involvement of sylvatic hosts or arthropod vectors (Costard et al., 2013; Jori et al., 2013). Transmission from the sylvatic cycle to domestic pigs occurs when domestic pigs get infected through tick bites, specifically in most African regions where warthogs are common. The ASFV replicates in the midgut and spreads to the coxal and salivary glands in the ticks. The virus is then transmitted to pigs as the ticks feed.
Currently, there are 24 genotypes that have been identified worldwide based on the nucleotide sequencing of the B646L gene which encodes the major capsid protein p72 (Quembo et al., 2018). The first molecular identification of ASFV in household pig farms in South Kivu with a prevalence of 22.8% involved ASFV genotype IX in 2019 (Bisimwa et al., 2020a). Some of the farmers in this region practice free-range system (Akilimali et al., 2017). This system exposes pigs to various pathogens, including ASFV through contact with neighboring domestic pigs or wild reservoirs. In February 2020, more than 192 suspected ASFV fatality cases were recorded by the Provincial Ministry of Agriculture, Livestock and Fishery (PMALF) in Uvira and Walungu districts of South Kivu, where this study was carried out.
High sequence similarity between ASFV strains from South Kivu, Eastern DRC to those from several eastern African countries (Rwanda, Uganda, Burundi, Tanzania, and Uganda) has been reported (Bisimwa et al., 2020a). This can be attributed to transboundary movement of live pigs and their products between these countries through pig trade. While this is known, continuous identification and characterization of ASFVs responsible for new outbreaks in the study region is important to track the disease's origin.
Therefore, this study aimed to detect and characterize ASFV genetically in domestic pigs suspected to be infected with ASFV from two districts in the South-Kivu province. This is to improve knowledge on the molecular epidemiology of ASFV in the DRC, and provide additional information for the development of control strategies.
2. Materials and methods
2.1. Ethics approval and consent to participate
A consent form that described the aim of the study was signed by farmers willing to participate in the study after translation into local languages. Ethical approval and permission for samples and data collection was issued by the Interdisciplinary Centre for Ethical Research (CIRE) at Université Evangélique en Afrique, Bukavu, DR Congo, (UEA/SGAC/KM 132/2018).
2.2. Study site
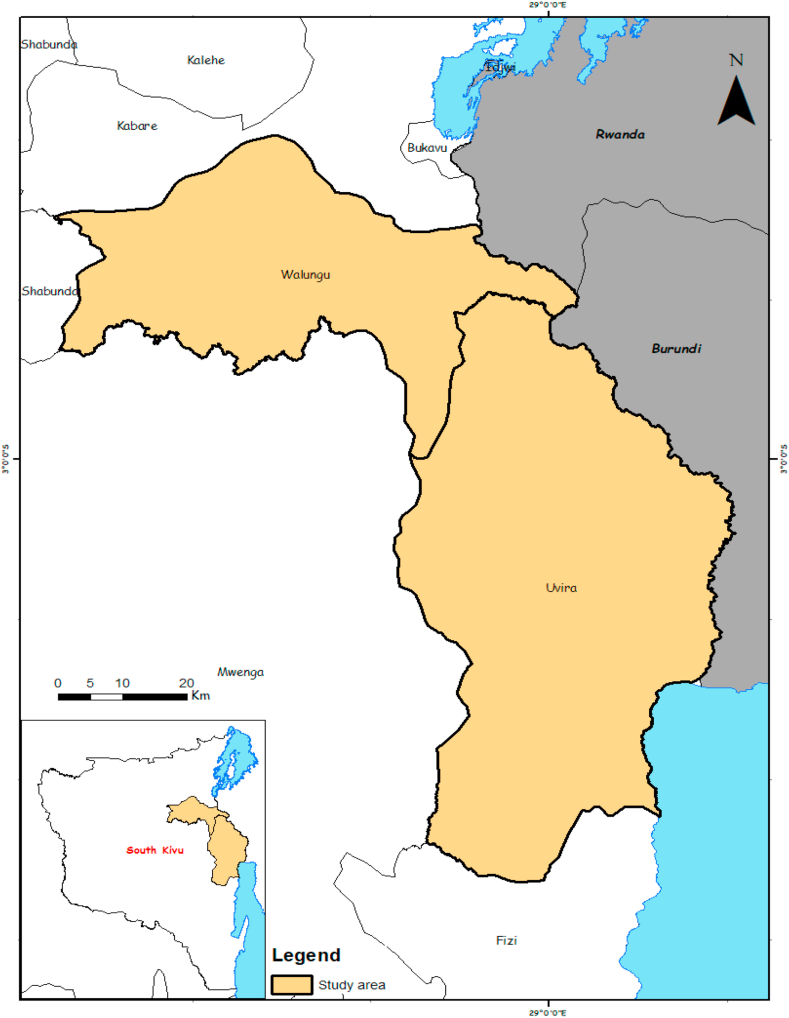
The study was carried out in South Kivu, a province located in the eastern part of the Democratic Republic of Congo. Two districts, namely Uvira and Walungu (Figure 1), were selected for this study following the recent reports by the Ministry of Agriculture, Livestock, and Fishery on the suspected ASFV outbreaks. Walungu lies 1765m above sea level, with an average annual temperature of 18.6 °C. Uvira district is 807m above sea level and receives an average annual temperature and rainfall of 26.0 °C and 981 mm per year, respectively.
Figure 1.
Map of South Kivu showing the study sites (Arc GIS, UEA, Bukavu).
2.3. Sample size determination and sample collection
A cross-sectional study was conducted in pig farms across the Walungu and Uvira districts of South Kivu province to investigate the presence of ASFV and circulating strains. Included were all male and non-pregnant female pigs, excluding those that had less than 3-month litter. The sample size (155) was calculated with a predetermined ASF prevalence of 22% (Bisimwa et al., 2020a) with a 6% precision and a 95% confidence level using the previously sampling method by Dohoo et al. (2009). A total of 3–5ml of whole blood samples were collected aseptically from the jugular vein in vacutainer EDTA tubes (Thomas Scientific, USA) were used to collect about 3–5 ml of blood from pigs. Sampling with the guidance of local veterinarians in both districts was conducted six days after the release of the report by the Ministry of Agriculture Fishery and Livestock on suspected outbreaks.
After collection, all samples were transported unrefrigerated (in cooler box) for three hours from the field to the molecular biology laboratory of Université Evangélique en Afrique (UEA) where they were stored at -20 °C prior to subsequent analysis.
2.4. Collection of farm data and clinical signs
Face to face interviews were conducted with pig farmers using a short questionnaire to elicit information covering the demography and the main clinical signs specific to ASF. The survey involved a total of 120 pig farmers with experience of more than two years in pig farming and having at least two pigs on the farm. Additionally, this was restricted to farmers who had reported suspected ASFV outbreaks in the last two years (2018–2019) on their farm. Moreover, pig owners who had at least one suspected positive case during the sampling period were interviewed. In total, 51 and 69 pig farmers were retained in Walungu and Uvira districts, respectively. In addition, a direct observation on the suspected infected pig was performed, and clinical signs related to ASF were recorded.
2.5. DNA extraction and detection of ASFV by PCR
Viral DNA was extracted from 200 μl aliquots of blood using DNeasy Blood and Tissue Kit (Qiagen, USA) following the manufacturer's instructions. A 0.8% native gel electrophoresis was run, and measurements by NanoDrop™ 2000 (Thermo Fisher, USA) spectrophotometer) were conducted to assess the integrity and the quantity of the genomic DNA, respectively. The confirmation of the presence of the ASFV nucleic acid was done by amplifying a 257bp region of the p72 major capsid protein gene B646L using the diagnostic primers PPA1/PPA2 (Bastos et al., 2003).
The total volume for all reactions was 25 μl containing 1.25 μM of each primer, One Taq 2xMasterMix with Standard buffer (New England Biolabs), which contains 1.8mM MgCl2, 0.2mMdNTPs, 25 units/ml One Taq DNA polymerase, and 20 mMTris-Hcl. MgCl2 (2.5mM) was added to the reaction setup. The DNA amplification was performed at 95 °C for 5 min as initial denaturation, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 62 °C for 1 min, and extension at 72 °C for 1 min. These cycles were followed by an extension step of 72 °C for 7 minutes. Amplification products were loaded on a 1.5% agarose gel stained with GelRed® (Biotium, San Francisco Bay) and run in an electrophoresis chamber at 60 V/cm for 40 min. The gel was visualized using a Standard UV trans-illuminator (Cleaver Scientific, U.K.).
2.6. Genotyping and nucleotide sequencing
Identification of ASFV genotype(s) circulating in the positive pig samples was done by setting up three separate PCR assays targeting three polymorphic loci p72, p54, and CVR. The p72 genotyping classification was done through amplification of a 478bp region within the C-terminal region of the B646L with p72- U/D primer set. Complete E183L gene (676bp) and B602L CVR (358bp) were amplified using primer sets PPA722/PPA89 and CVR-FL1 & CVR–FL2 pairs, respectively. The primer sequences used for both diagnosis and genotyping, their nucleotide sequences, and sources are presented in Table 1.
Table 1.
Primer sequences used for detection and genetic characterization of ASFV.
| Primer name | Oligonucleotide sequences 5′ to 3′ | Amplicon size (bp) | Reference |
|---|---|---|---|
| PPA1/PPA2 | F- AGTTATGGGAAACCCGACCC | 257 | (Bastos et al., 2003) |
| R- CCCTGAATCGGAGCATCCT | |||
| P72_D/P72_U | F- GGCACAAGTTCGGACATGT | 478 | (Bastos et al., 2003) |
| R- GTACTGTAACGCAGCACAG | |||
| PPA722/PPA89 | F- CGAAGTGCATGTAATAAACGTC | 676 | (Gallardo et al., 2009) |
| R- TGTAATTTCATTGCGCCACAAC | |||
| CVR–FL1/CVR–FL2 | F- TCGGCCTGAAGCTCATTAG | 358 | (Mwiine et al., 2019) |
| R- CAGGAAACTAATGATGTTCC |
Following the PCR amplification, amplicons of the expected size were purified using QIAquick PCR purification Kit (Qiagen, Germany) as per the manufacturer's instructions and sequenced under the Sanger sequencing platform. The resulting nucleotide sequences were trimmed to remove poor quality reads using CLC main Workbench (Qiagen) tool, and both forward and reverse reads were used to assemble a consensus sequence using CLC Main Work Bench version 7.8.1 software. The sequences generated from this study were further compared with those from previous sequencing projects in the same region. ASFV reference strains were retrieved from the GenBank database by BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple alignments were carried out using the Clustal W implemented in MEGA X (Version 10.2.2.) software. The output sequence alignments were subsequently used for phylogenetic analysis using Mega X. A comparison topology tree was generated by the maximum likelihood method using the Kimura-2-parameter model (Kumar et al., 2016) with 1000 replications. All the sequences generated from this study have been submitted to the GenBank under accession number MW082572- MW082576 for p72 and MW082577- MW082581 for p54.
2.7. Statistical analysis
A descriptive and univariate statistical analysis was performed using CDC Epi-info™ version 7. The statistical significance of the association between positive pigs with clinical signs was performed for discrete variables using Fisher's exact test at 95% confidence level.
3. Results
3.1. Detection of ASFV infection in the study sites and clinical signs associated with infection
In this study, a total of 155 swine blood samples collected from domestic pigs were tested for the presence of ASF viral genome using primers p72- U/D. A total of 8 samples from 155 samples analyzed, (8; 5.2%) tested positive for ASFV. The highest number of infected pigs was found in Uvira (6; 8%) with the highest infection recorded in female pigs (5; 7.6%) (Table 2). Interestingly, 137 (88.4%) of the sampled pigs showed clinical signs related to ASFV.
Table 2.
Proportion of ASFV in suspected infected pigs from Uvira and Walungu districts, South Kivu province.
| Variables | Modality | Number tested | Clinically infected | PCR positive n (%) |
|---|---|---|---|---|
| Districts | Uvira | 75 | 64 (85.3) | 6 (8) |
| Walungu | 80 | 73 (91.2) | 2 (2.5) | |
| Sex | Female | 89 | 78 (87.6) | 3 (3.4) |
| Male | 66 | 59 (89.4) | 5 (7.6) | |
| Total | 155 | 137(88.4) | 8(5.2) | |
The most frequent clinical signs associated with ASFV reported by farmers included redness on the skin and snout (49%; 95% CI: 21–34), followed by the unwillingness of pigs to stand (29 %; 95 % CI: 19–35), while the least common clinical sign was the presence of high fever (5 %; 95 % CI: 5–14) (Figure 2). However, the statistical analysis revealed that redness on the skin and snout was the only ASF related sign observed to be significantly (p < 0.05) associated with ASFV positivity.
Figure 2.
Clinical signs related to ASFV observed on pigs during sample collection. Percentages of observed clinical signs are indicated by numbers.
3.2. Nucleotide sequence analysis of ASFV of the B646L (p72) and E183L (p54) genes
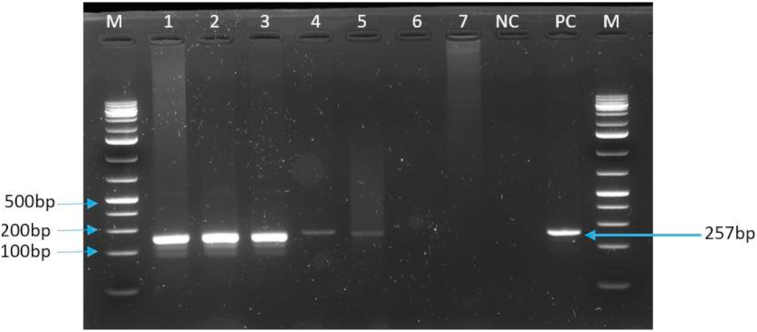
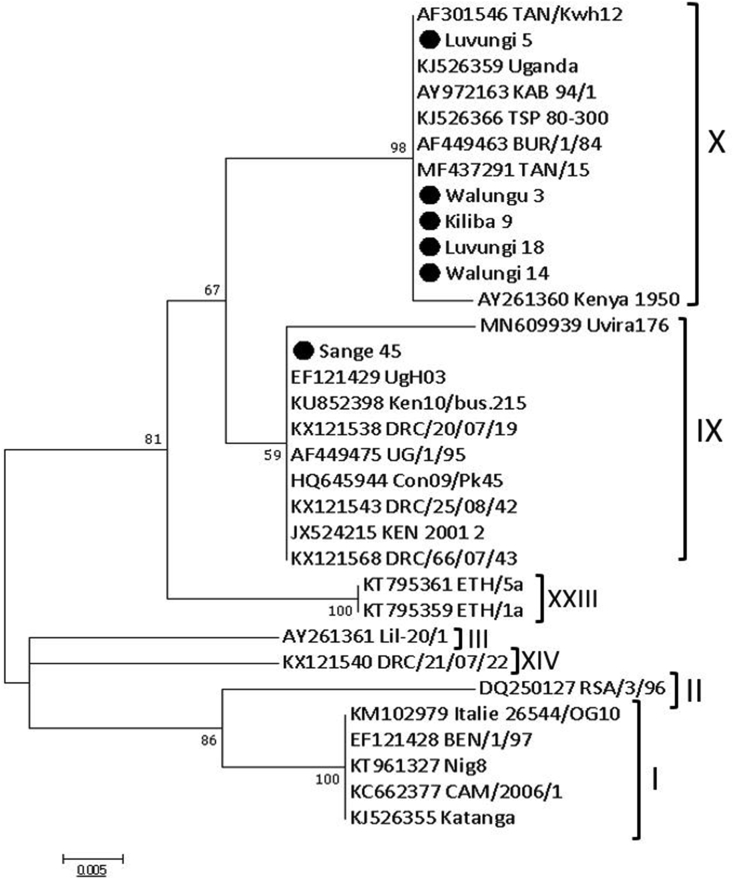
Positive samples showed clear amplicons with the expected size (257bp) by conventional PCR using diagnostic primers PPA1/PPA2 (Figure 3) and were subsequently sequenced. Of the 8 ASFV positive samples, only six were sequenced and trimmed successfully for the B646L (p72) gene, and 5 for the E183L (p54) gene. Sequences that failed the quality filters were disregarded in the subsequent downstream analysis. Sequence alignment of the p72 and p54 nucleotide sequences showed 100% identity in the Uvira and Walungu samples. The phylogenetic analysis revealed that the ASFV from this study belonged to p72 genotypes IX and X viruses. Additionally, these strains were clustered with some strains from previous studies within the same region as well as those from the neighboring countries including Uganda, Kenya, and Tanzania (Figure 4). This is the second report of genotype X virus in the Democratic Republic of Congo. Genotype X virus was the most prevalent and is circulating in both districts, while genotype IX and X viruses are co-circulating in Uvira district.
Figure 3.
PCR amplification of the B646L gene encoding p72 protein. The PCR products were resolved in a 1.5% agarose gel. Lane M: 1Kb plus (Thermo Fisher Scientific) Molecular weight DNA marker; Lanes 1,2,3,4, and 5 are selected positive samples with an approximately 257bp band size, Lane 6 and 7 are selected negative samples. Line NC is the negative control, and lane PC is a positive control.
Figure 4.
Phylogenetic relationships of p72 genotypes. The evolutionary history was inferred by the maximum likelihood method based on the Kimura 2-parameter model (Kumar et al., 2016). The phylogeny was inferred following 1,000 bootstrap replications, and the node values show percentage bootstrap support. Strain names such as Kiliba 9, Luvungi 5, Luvungi 18 and Sange 45 are viruses from Uvira districts while Walungu 3 and Walungu 14 are strains from Walungu district. The scale bar indicates nucleotide substitutions per site. Dark-spot circles (plain circle •) represent sequences from this study.
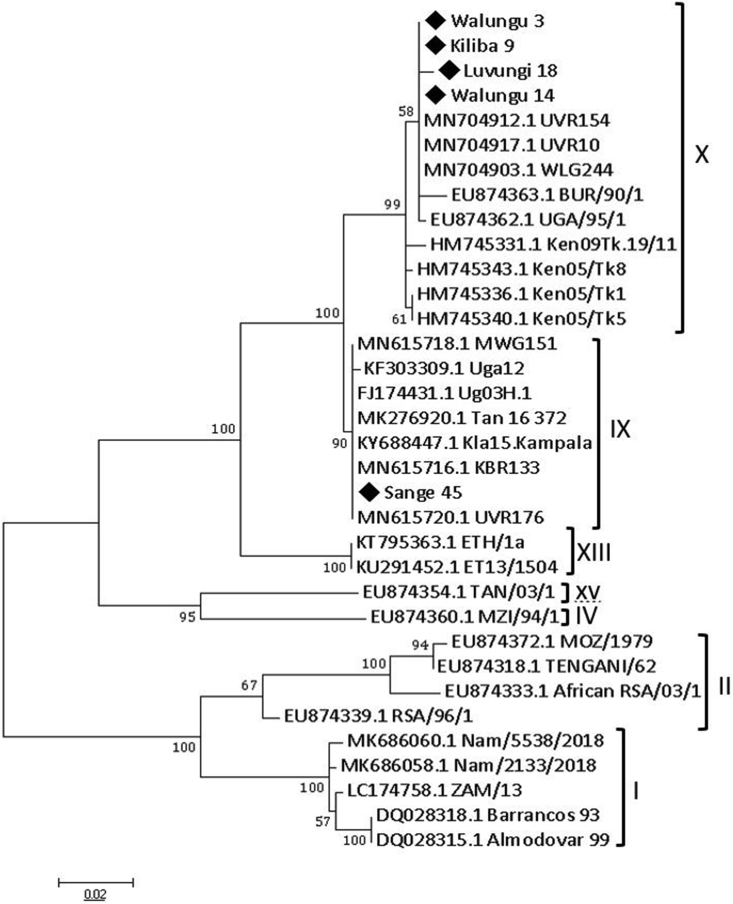
Reads (676bp in length) were obtained from amplification and sequencing of E183L (p54) gene in 5 strains. Clusters from the alignment of these reads with 31 p54 ASFV sequences retrieved from GenBank confirmed the classification of South Kivu ASFV strains to subgroups IX and X as seen in p72 genotyping results (Figure 5).
Figure 5.
Genetic relationship between ASFV strains analyzed in this study and previously identified ASFV genotypes according to nucleotide sequencing of the p54 gene fragment represented by the Maximum likelihood tree. Strain names such as Kiliba 9, Luvungi 18, and Sange 45 are viruses from Uvira districts while Walungu 3 and Walungu 14 are strains from Walungu district. Numbers at nodes represent the percentage of 1000 bootstrap replicates. The scale bar indicates the number of nucleotide substitutions per site. The p54 sequences from this study are marked by black diamond (◆).
3.3. Sequence analysis of ASFV of the B602L (Central variable region) gene
The predicted amino acid sequences of the CVR nucleotide sequences from 5 (3 Uvira, and 2 Walungu samples), and specific characteristics based on the known ASFV tetrameric amino acid repeats within the CVR as previously reported by Nix et al. (2006) were confirmed.
Analysis of CVR features of the B602L gene revealed that four viruses (p72 genotype X) had ten repeats AAAABNAABA, consistent with the CVR previously characterized in the same study area. Only one sequence belonging to genotype IX contained 12 unique patterns of tetramer AAAAAAAAAAF not present in the other strains characterized. The sequence repeats corresponded to variant CAST (A), CADT (B), NVDT (N), and CANT) (F) (Table 3). The two CVR signatures identified differed from the genotype X strains from Burundi (BUR 84/1, BUR84/2), Uganda (Ug95/3), and Tanzania (TAN13/Moshi) (Nix et al., 2006; Misinzo et al., 2011).
Table 3.
The amino acid sequence of the tetrameric repeats of the central variable region (CVR).
| Stains | Year | Location | CVR amino acid sequence | No. of TRS | P72 genotype | Reference |
|---|---|---|---|---|---|---|
| Luvungi 18 | 2020 | Uvira | AAAABNAABA | 10 | X | This study |
| Walungu 3 | 2020 | Walungu | AAAABNAABA | 10 | X | This study |
| Kiliba 9 | 2020 | Uvira | AAAABNAABA | 10 | X | This study |
| Walungu 14 | 2020 | Walungu | AAAABNAABA | 10 | X | This study |
| Sange 45 | 2020 | Uvira | AAAAAAAAAAF | 11 | IX | This study |
| Fizi-121 | 2019 | Fizi | AABNAABA | 8 | X | (Bisimwa et al., 2020b) |
| Kabare-30 | 2019 | Kabare | AABNAABA | 8 | X | (Bisimwa et. Al, 2020b) |
| Kalehe-11 | 2019 | Kalehe | AABNAABA | 8 | X | (Bisimwa et al., 2020b) |
| Mwenga-336 | 2019 | Mwenga | AABNAABA | 8 | X | (Bisimwa et al., 2020b) |
| Uvira-50 | 2019 | Uvira | AAAABNAABA | 10 | X | (Bisimwa et al., 2020b) |
| Uvira-53 | 2019 | Uvira | AAAABNAABA | 10 | X | (Bisimwa et al., 2020b) |
| rc65/11/4 | 2011 | Nsele | AAAAAABNABNBTDBNAAAAAAAAF | 25 | I | (Mulumba–Mfumu et al., 2017) |
| drc65/11/3 | 2011 | Nsele | AAAAAAAF | 8 | I | (Mulumba–Mfumu et al., 2017) |
| drc86/10/2 | 2010 | Ngafula | AAAAAAAAAAAF | 12 | IX | (Mulumba–Mfumu et al., 2017) |
| drc35/08/20 | 2008 | Boende | AAAABNABBNABBAABBNABNABA | 24c | IX | (Mulumba–Mfumu et al., 2017) |
| rc21/07/p22 | 2007 | Kipushi | AVVOVAVVNBVOV | 13 | XIV | (Mulumba–Mfumu et al., 2017) |
| drc75/05/1 | 2005 | Maniema | AAAAAAAAF | 9 | I | (Mulumba–Mfumu et al., 2017) |
| Uganda 95/3 | 1995 | Uganda | AABNBABA | 8 | X | (Mulumba–Mfumu et al., 2017) |
| Tanzania/13Moshi∗ | 2013 | Tanzania | BNBBNBNNA | 9 | X | (Misinzo et., 2011) |
| Burundi 84/1∗ | 1984 | Burundi | AAAAAAABA | 9 | X | (Nix et al., 2006) |
| Burundi 84/2∗ | 1984 | Burundi | AAAAAAABA | 9 | X | (Nix et al., 2006) |
Key: A (CAST); B (CADT), N (NVDT) and F (CANT). ∗indicates strains obtained from the GenBank and used as a reference for comparison. CVR, central variable region. TRS, tetrameric repeat sequence. The sequences from this study are highlighted in bold text.
4. Discussion
African swine fever is endemic in DRC. It constitutes a significant challenge to pig production in South Kivu province, characterized by regular outbreaks throughout the year (Akilimali et al., 2017; Bisimwa et al., 2020a). Continous characterization of ASFV isolates responsible for outbreaks is vital in improving knowledge on the epidemiological patterns of the disease that may help in the design of appropriate disease control strategies.
The present study aimed to detect and characterize ASFV from domestic pigs in Uvira and Walungu districts of the South Kivu province, in which suspected hemorrhagic disease outbreaks were reported in February 2020, using molecular biology tools.
Our study confirmed the presence of ASF in both Uvira and Walungu districts of South Kivu province. The proportion of 5.2% obtained in this study is almost similar to 6.6% recently reported in South Kivu (Bisimwa et al., 2020b). These findings suggest that outbreaks could be declining, and only a low number of pigs sampled were found to be infected. This is possibly due to the adoption of various biosecurity practices by farmers in the study area coupled with their decision to sell their stocks when the disease was reported.
A higher rate of infection was observed in Uvira (8%) compared to Walungu (2.5%). This is in closer agreement with the findings from our previous study, where a higher infection rate was registered in Uvira district (13.2%) when compared to Walungu (8.9%) (Bisimwa et al., 2020b).
This difference could be further attributed to the ASFV strains circulating in the region. In contrast to our previous findings on the circulation of genotype X in clinically infected pigs is that both genotype IX and X are co-circulating (Bisimwa et al., 2020a).
The results from our study show that a greater number of farmers were knowledgeable about ASF and associated clinical symptoms. Nevertheless, farmers failed to report suspected cases to the relevant government ministry for fear of losses as a consequence of the imposition of quarantine or stamping out. Such practices may be among the common key drivers for the rapid spread of the disease to previously unaffected areas. Similar practices have been reported in studies conducted in Uganda and Nigeria (Chenais et al., 2015; Abwage et al., 2015). High fever was not regularly observed in all the ASFV-infected pigs. This can be missed due to lack of routine use of thermometers by the majority of farmers as well as the difficulty to achieve good observation especially in the free-range farming system. At the farm level, this can sometimes be confusing and can delay an accurate diagnostic in the field.
Phylogenetic analysis based on the B646L (p72) and E183L (p54) genes clustered four of the South Kivu strains identified in this study into genotypes X and one strain into genotype IX but none of the individual samples yielded more than one genotype. These findings corroborate with previous studies characterizing ASFV and found the circulation of both genotype IX and X in South Kivu province (Bisimwa et al., 2020a, 2020b). In addition, these results are in agreement with a previous study carried out in Mozambique where co-circulation of two p72 genotypes in the same outbreak was reported (Bastos et al., 2004).
The genotype IX reported from this study was clustered with viruses causing outbreaks in other provinces of DRC (Mulumba–Mfumu et al., 2017). This suggests that this genotype is circulating throughout the country. In addition, the South Kivu strains clustered with viruses circulating in countries neighboring South Kivu, such as Burundi, Kenya, Tanzania, and Uganda. These illustrate the implication of neighboring countries in the spread of the African swine fever (Gallardo et al., 2011; Atuhaire et al., 2013). This observation is in agreement with a recent study conducted in Uganda where the role of cross-border pig movements was reported and linked to the occurrence of ASF outbreaks in the neighboring countries (Atuhaire et al., 2014).
The analysis of B602L (CVR) gene revealed the presence of four different CVR variants based on the number of tetrameric amino acid repeat sequences (TRS). All the genotypes X had ten tetramers. These strains were identical to viruses from clinical pigs in South Kivu in 2019 (Bisimwa et al., 2020b), also suggesting that this virus may be circulating between districts within the same province. In contrast, genotype IX was characterized by 12 tetramers distinct from other strains in the current study and previous study (Bisimwa et al., 2020a) carried out in the same study region. They were, however, identical to the strains identified in some regions in DRC, as previously reported (Mulumba–Mfumu et al., 2017). The variation of repeats in both number and sequence depends on the virus genotype and individual virus strains (Irusta et al., 1996; Achenbach et al., 2017). The ASFV strains found in the current study agreed with those reported in the previous study within the same area and neighboring regions to South Kivu province. This indicates that there may be a constant circulation and re-introduction of ASFV strains in the study region, probably due to the movement of pigs or pig products between regions in DRC to South Kivu or from a country bordering South Kivu (Rwanda, Burundi, and Tanzania) for various reasons, mostly trade.
5. Conclusion
This study confirmed two genetically distinct genotypes of ASFV in symptomatic pigs. These genotypes were responsible for outbreaks in South Kivu in 2019. Evolutionary relationship analysis revealed that ASFV strains from this study clustered closely with those from other parts of DRC, in Burundi, Tanzania, Uganda, and Kenya. This may probably be due to the uncontrolled movement of pigs from one area to another. Therefore, the continuous characterization of viruses responsible for outbreaks in South Kivu and other regions in DRC is recommended to assess the genetic changes in genotypes and track their origin. A further study targeting more samples in different provinces of DRC is also needed to elucidate any additional ASFV genotypes that may be circulating in DRC. Equally, more sensitive diagnostics tools including real-time PCR is needed to eliminate false-negative results as it was not in the scope of the current study.
Declarations
Author contribution statement
Patrick N. Bisimwa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lionel K. Ishara: Conceived and designed the experiments; Performed the experiments.
Dieudonné S. Wasso, Fabrice Bantuzeko, Ahadi B. Bwihangane: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ronald Tonui: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by The University Research Award (Faculty Research Supports 2018-2020), Department of Animal Science and Production, Université Evangélique en Afrique (UEA) DR Congo.
Data availability statement
Data will be made available on request.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Our sincere appreciation goes to the Provincial Ministry of Agriculture, Livestock and Fishery of South Kivu for facilitating identification and access to the farms for blood sample collection.
References
- Abwage S.A., Umaru G.A., Musa Y.B., Adamu Z., Akensire U.A., Njobdi A.B., Bello O.A. Detection of African swine fever virus (ASFV) antibodies in pigs in Taraba state north east Nigeria. Sokoto J. Vet. Sci. 2015;13:2. [Google Scholar]
- Achenbach J.E., Gallardo C., Nieto-Pelegrín E., Rivera-Arroyo B., Degefa-Negi T., Arias M., Jenberie S., Mulisa D.D., Gizaw D., Gelaye E., Chibssa T.R., Belaye A., Loitsch A., Forsa M., Yami M., Diallo A., Soler A., Lamien C.E., Sánchez-Vizcaíno J.M. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound. Emerg. Dis. 2017;64:1393–1404. doi: 10.1111/tbed.12511. [DOI] [PubMed] [Google Scholar]
- Akilimali K.I., Wasso D.S., Baenyi P., Bajope J.P. Caractérisation des systèmes de production porcine de petits exploitants dans trois zones agro-écologiques du Sud-Kivu en République Démocratique du Congo. J. Appl. Biosci. 2017;120:12086–12097. [Google Scholar]
- Alonso C., Borca M., Dixon L., Revilla Y., Rodriguez F., Escribano J.M. ICTV report consortium. ICTV virus taxonomy profile. Asfarviridae. J. Gen. Virol. 2018;99:613–614. doi: 10.1099/jgv.0.001049. [DOI] [PubMed] [Google Scholar]
- Atuhaire D.K., Afayoa M., Ochwo S., Mwesigwa S., Mwiine F.N., Okuni J.B., Mukani W.O., Ojok L.M. Prevalence of African swine fever virus in apparently healthy domestic pigs in Uganda. BMC Vet. Res. 2013;9:263. doi: 10.1186/1746-6148-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atuhaire D.K., Afayoa M., Ochwo S., Mwesigwa S., Okuni J.B., Olaho-Mukani W., Ojok L. Molecular characterization and phylogenetic study of African swine fever virus isolates from recent outbreaks in Uganda (2010-2013) Virol. J. 2014;10:247. doi: 10.1186/1743-422X-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.D., Penrith M.L., Cruciere C., Edrich J.L., Hutchings G., Roger F., Couacy-Hymann E., Thomson R. Genotyping field strains of African swine fever virus by partial p72 gene characterization. Arch. Virol. 2003;148:693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- Bastos A.D.S., Penrith M.L., Macome F., Pintoe F., Thomson G.R. Co-circulation of two genetically distinct viruses in an outbreak of African swine fever in Mozambique: noevidence for individual co-infection. Vet. Microbiol. 2004;103:169–182. doi: 10.1016/j.vetmic.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bellini S., Rutili D., Guberti V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016;58:82. doi: 10.1186/s13028-016-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisimwa N.P., Machuka M.E., Dedan G., Banswe G., Amimo O.J., Ongus R.J., Masembe C., Bishop P.R., Steinaa L., Djikeng A., Pelle R. Evidence for the presence of African swine fever virus in apparently healthy pigs in South-Kivu Province of the Democratic Republic of Congo. Vet. Microbiol. 2020;240:108521. doi: 10.1016/j.vetmic.2019.108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisimwa N.P., Ongus J.R., Tiambo C.K., Machuka E.M., Bisimwa E.B., Steinaa L., Pelle R. First detection of African swine fever (ASF) virus genotype X and serogroup 7 in symptomatic pigs in the Democratic Republic of Congo. Virol. J. 2020;17:135. doi: 10.1186/s12985-020-01398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenais E., Boqvist S., Sternberg-Lewerin S., Emanuelson U., Ouma E., Dione M., Aliro T., Crafoord F., Masembe C., Stahl K. Knowledge, attitudes and practices related to African swine fever within smallholder pig production in northern Uganda. Transbound. Emerg. Dis. 2015;64(1):101–115. doi: 10.1111/tbed.12347. [DOI] [PubMed] [Google Scholar]
- Costard S., Mur L., Lubroth J., Sanchez-Vizcaino J.M., Pfeiffer D.U. Epidemiology of African swine fever virus. Virus Res. 2013;173(1):191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Dohoo I.R., Martin S.W., Stryhn H. second ed. VER Inc.; Charlottetown: 2009. Veterinary Epidemiologic Research; p. 610. [Google Scholar]
- Galindo I., Alonso C. African swine fever virus: a review. Viruses. 2017;9:103. doi: 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo C., Mwaengo D.M., Macharia J.M., Arias M., Taracha E.A., Soler A., Okoth E., Martín E., Kasiti J., Bishop R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Gene. 2009;38(1):85–95. doi: 10.1007/s11262-008-0293-2. [DOI] [PubMed] [Google Scholar]
- Gallardo C., Okoth E., Pelayo V., Anchuelo R., Martın E., Simon A., Llorente A., Nieto R., Soler A., Martin R., Arias M., Bishop R.P. African swine fever viruses with two different genotypes, both of which occur in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site. J. Gen. Virol. 2011;92:432–444. doi: 10.1099/vir.0.025874-0. [DOI] [PubMed] [Google Scholar]
- Irusta P.M., Borca M.V., Kutish G.F., Lu Z., Caler E., Carrillo C., Rock D.L. Amino acid tandem repeats within a late viral gene define the central variable region of African swine fever virus. Virology. 1996;220(1):20–27. doi: 10.1006/viro.1996.0281. [DOI] [PubMed] [Google Scholar]
- Jori F., Bastos A.D.S. Role of wild suids in the epidemiology of African swine fever. EcoHealth. 2009;6:296–310. doi: 10.1007/s10393-009-0248-7. [DOI] [PubMed] [Google Scholar]
- Jori F., Vial L., Penrith M.L., Perez-Sanchez R., Etter E., Albina E., Michaud V., Roger F. Review of the sylvatic cycle of 73 African swine fever in sub-Saharan Africa and the Indian Ocean. Virus Res. 2013;173(1):212–227. doi: 10.1016/j.virusres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. Molecular evolutionary genetic analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misinzo G., Magambo J., Masambu J., Yongolo M.G., Van, Doorsselaere J., Nauwynck H.J. Genetic characterization of African swine fever viruses from a 2008 outbreak in Tanzania. Transbound. Emerg. Dis. 2011;58(1):86–92. doi: 10.1111/j.1865-1682.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Mulumba–Mfumu L.K., Achenbach E.J., Mauldin M.R., Dixon L.K., Tshilenge C.G., Thiry E., Moreno N., Blanco E., Saegerman C., Lamien C.E., Diallo A. Genetic assessment of african swine fever isolates involved in outbreaks in the Democratic Republic of Congo between 2005 and 2012 reveals Co-circulation of p72 genotypes I, IX and XIV, including 19 variants. Viruses. 2017;9(2):31. doi: 10.3390/v9020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwiine F.N., Nkamwesiga J., Ndekezi C., Ochwo S. Molecular characterization of African swine fever viruses from outbreaks in Peri-Urban Kampala, Uganda. Adv. Virol. 2019;8 doi: 10.1155/2019/1463245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix R.J., Gallardo C., Hutchings G., Blanco E., Dixon L.K. Molecular epidemiology of African swine fever virus studied by analysis of four variable genome regions. Arch. Virol. 2006;151:2475–2494. doi: 10.1007/s00705-006-0794-z. [DOI] [PubMed] [Google Scholar]
- Quembo C.J., Jori F., Vosloo W., Heath L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2018;65(2):420–431. doi: 10.1111/tbed.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Vizcaíno J., Mur L., Gomez-Villamandos J., Carrasco L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015;152(1):9–21. doi: 10.1016/j.jcpa.2014.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.