Abstract
The abuse of antibiotic growth promoters (AGPs) in feed has led to drug resistance and ecological damage would threaten human health eventually. Natural plants have become a hotspot in the research and application of substituting AGPs because of their advantages of safety, efficiency, and availability. This study was conducted to investigate the effects of Macleaya cordata extract (MCE) in the diet of Xuefeng black-bone chicken on laying performance, egg quality, and serum indices. In this study, 576 birds (47-week-old) were evenly distributed between 4 treatments with 6 replicates of 24 hens each. The control group was fed a basal diet without MCE and the remaining groups received 100, 150, or 200 mg/kg MCE for 84 d. Results revealed that the strength and thickness of the eggshell increased significantly with the dietary addition of MCE (P < 0.05). The serum concentrations of glutathione peroxidase increased in the MCE groups (P < 0.01). Simultaneously, progesterone, follicle stimulating hormone, estradiol as well as serum luteinizing hormone levels also increased with the addition of MCE (P < 0.05). Compared with the control group, supplementation of MCE significantly decreased the tumor necrosis factor-α and interleukin-6 levels (P < 0.01). In summary, it was concluded that diet addition of 200 mg/kg MCE ameliorated egg quality, enhanced anti-oxidation and immune activity, and regulated hormone secretion of Xuefeng black-bone chicken.
Key words: Macleaya cordata extract, Xuefeng black-bone chicken, laying performance, egg quality, anti-oxidation and immune activity
Introduction
It was a common practice for decades to use sub-therapeutic doses of antibiotics in food-animal feeds to prevent animals from diseases and to improve production performance in modern animal husbandry. As a consequence of the increasing concern about the potential for antibiotic-resistant strains of bacteria, the European Commission decided to ban all commonly used feed antibiotics. In the meantime, China is expected to enter the era of non-antibiotic growth promoters in 2021. Concerns over the increasing emergence of antibiotic-resistant bacteria have prompted efforts to develop alternatives to antibiotics, such as Chinese veterinary drugs and natural plant extracts. In addition to the characteristics of plant-derived products of being natural, multi-functional, and low in toxicity, recent studies indicated that natural plant extracts also have positive effects in egg quality and antioxidant capacity of laying hens (Alagawany et al., 2017). With the advantages of being eco-friendly and residue-free, natural plant extract would be an ideal substitution for antibiotics in animal production.
Macleaya cordata is a perennial herb and a traditional Chinese medicine, which is widely distributed in the south of China. In 2004, compounds containing sanguinarine and chelerythrine extracted from M. cordata were registered as feed additives in the European Union. As the main bioactive material, sanguinarine demonstrated physiological effects such as antitumor, immunity enhancement (Kumar and Hazra, 2014), antibacterial (Hamoud et al., 2014), anti-inflammatory (Xue et al., 2017), and insecticidal properties. It was also known that adding M. cordata extract (MCE) to the diet could improve animal performance, immunity, and intestinal health. Previous studies showed that dietary sanguinarine supplementation enhanced serum metabolites and antibodies in growing pigs (Liu et al., 2016a). Indeed, MCE has been added to the diet of cattle, pigs, chickens, and fish in recent years (Vieira et al., 2008). Broiler feeding trials have shown that 20 mg/kg MCE meal significantly increased the weight gain and decreased the feed conversion rate of broilers (Lee et al., 2015).
Consequently, administration of 50 ppm Sangrovit increased body weight and average daily gain, as well as reduced the feed conversion ratio in weaning pigs (Kantas et al., 2015). In addition, dietary MCE demonstrated anti-inflammatory activity, which could improve cow mastitis and reduce the number of somatic cells in milk (Wang et al., 2018). Moreover, MCE played a role in inhibiting bacteria and regulating gut health; antibacterial tests showed that sanguinarine could inhibit Gram-positive bacteria when the minimum inhibitory concentration was 1.6 to 6.3 μg/mL (Obiang-Obounou et al., 2011). For intestinal barrier function, Zhong et al. (2017) and Liu et al. (2016b) reported that dietary sanguinarine concentrations of 1.6 mg/L inhibited the biofilm of Candida albicans, and could significantly inhibit the formation of 72.9% biofilm in growing piglets. All of these indicated that MCE would be an ideal substitute for antibiotics. Therefore, dietary MCE supplementation would have an influence on anti-inflammatory and growth promotion in Xuefeng black-bone chicken.
Xuefeng black-bone chicken, which originated in Hongjiang City, Huaihua City, Hunan Province, is a kind of meat and egg chicken with certain medicinal value, and had been listed in the Chinese national livestock and poultry genetic resources list. The chicken meat and egg products are rich in a variety of essential nutrients, which can not only improve human hemoglobin and red blood cells, but also regulate metabolism and endocrine functions, in agreement with the current considerations of food safety. However, due to the lack of systematic breeding of Xuefeng black-bone chicken, there were some shortcomings, such as low laying performance, rapid decline in laying rate, and high death rate. The market demand for Xuefeng black-bone chicken egg products continued to increase; so it was necessary to expand the scale of Xuefeng black-bone chicken commodity generation. Thus, how to make the best use of the fecundity of Xuefeng black-bone chicken had become an urgent problem to be solved. Previous reports had indicated that MCE had good antibacterial and anti-inflammatory effects in poultry production (Xue et al., 2017).
However, the effect of MCE on egg laying performance and egg quality of Xuefeng black-bone chicken had been rarely reported. In this study, we aimed to investigate the effects of supplementation of MCE on laying performance, egg quality, and serum indices of Xuefeng black-bone chicken; moreover, the change of intestinal microflora after adding MCE was investigated in another study (personal communication).
Materials and methods
Animals and Experimental Design
This experiment was conducted in accordance with the Chinese guidelines for animal welfare and with the animal welfare standards of the College of Animal Science and Technology, Hunan Agricultural University. After a 7-day adaptation period, 576 Xuefeng black-bone chicken (47-week-old, white feathers) were divided randomly into 4 groups (6 replicas of 24 hens) and fed for 84 d. Three hens were reared in an individual cage facility with 1 nipple drinker and 1 feeder in a ventilated room (environmental temperature: 20°C–25°C; relative humidity: 65 ± 5%). The hens had ad libitum access to water and were fed optionally twice a day at 6:30 am and 15:30 pm. Moreover, mortality was recorded as it occurred.
Experimental Diets
Diets were formulated to meet or exceed the NRC (1994) nutrient requirements for all nutrients (Table 1). MCE consisted of 7.5% sanguinarine and 92.5% starch.
Table 1.
Composition and nutrition levels of the basal diet (fed basis, %).
| Ingredients | Content (%) | Items | Nutrient levels (%) |
|---|---|---|---|
| Corn | 62.00 | ME (MJ/kg) | 10.85 |
| Soybean meal | 26.00 | CP | 16.14 |
| Limestone | 5.50 | Ca | 2.99 |
| CaHPO4 | 1.00 | Available P | 0.38 |
| Premix | 5.50 | NaCl | 0.37 |
| Total | 100.00 | Lys | 0.95 |
| Met | 0.51 | ||
| Met + Cys | 0.82 |
Each kilogram of diet contains the following: vitamin A, 17,000 IU; vitamin D3, 64,000 IU; vitamin E, 880 IU; vitamin K3, 48 mg; vitamin B1, 48 mg; vitamin B2, 105 mg; vitamin B6, 48 mg; vitamin B12, 0.2 mg; nicotinamide, 380 mg; pantothenic acid, 270 mg; folic acid, 24 mg; biotin, 3.3 mg; Fe, 1,100 mg; Cu, 240 mg; Zn, 1,560 mg; Mn, 1,800 mg; I, 23 mg; and Se, 48 mg.
Sample Collection
To calculate the laying rate and average egg weight, eggs were collected daily by replicate and weighed. At the same time, to calculate the number of qualified eggs, the number of cracked eggs, sand eggs, and soft eggs were recorded. Then, qualified eggs were allowed to hatch to determine the fertilization rate, hatching rate, and healthy chick rate. On the 84th day, 48 hens (2 hens per replicate) were humanely slaughtered after a 12-hour fast (water offered ad libitum) to collect blood, which was collected from the wing vein and centrifuged at 3,000 × g for 10 min to separate the serum, and frozen at −20°C for further analysis. Ninety six eggs (4 eggs per replicate) were randomly selected for egg quality determination on the 56th and 84th day.
Egg Quality
The eggs in each treatment were randomly selected to measure egg quality. Haugh unit (HU) and the yolk index were determined with a digital egg tester (EA-01; ORKA Co. Ltd., Israel). The thickness of the eggshell (without the eggshell membrane) was measured by the average values from 3 different locations (top, middle, and bottom of the egg) by an eggshell thickness tester (NFN-380, FHK Co. Ltd., Japan). Eggshell strength was measured by an eggshell strength tester (EFR-01, ORKA Co. Ltd.).
Serum Indices
Serum total protein levels, albumin, globulin, total cholesterol, triglyceride, urea, glucose (GLU), urea acid (UA), Ca, phosphorus (P), alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase activity, and glutathione peroxidase (GSH-Px) activity, superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were assayed with commercial radioimmunoassay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's guidelines. Serum immunological indices including immunoglobulin A, immunoglobulin M, immunoglobulin G, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) levels were detected by an ELISA kit. The levels of progesterone (PO), follicle stimulating hormone (FSH), estradiol (E2), luteinizing hormone (LH), and parathyroid hormone (PTH) in the serum were measured by radioimmunoassay kits according to the manufacturer's instructions.
Statistical Analysis
Data were statistically analyzed with one-way ANOVA using SPSS 22.0 (SPSS Inc., Chicago, IL). The results were expressed as arithmetic mean and SEM. Differences were considered to be significant at P < 0.05 and highly significant at P < 0.01.
Results
Laying Performance and Reproduction Performance
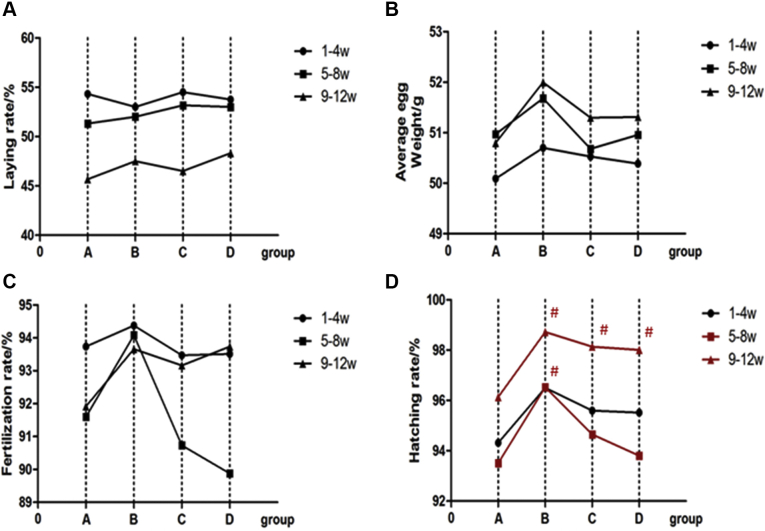
Effects of MCE on laying performance and reproductive performance are presented in Table 2. Dietary MCE tended to increase the hatching rate and the healthy chick rate (P > 0.05). The hatching rate was increased by 2.08, 1.89, and 1.40% (P > 0.05) and the healthy chick rate was increased by 0.50, 0.67, and 1% (P > 0.05) following dietary supplementation with 100, 150, and 200 mg/kg MCE, respectively, in comparison to the control. No significant differences were found in egg production rate, average egg weight, and fertilization rate (P > 0.05).
Table 2.
Effects of MCE on laying performance and reproductive performance of Xuefeng black-bone chicken (1–12 wk).1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| LR (%) | 50.67 | 50.00 | 51.50 | 51.67 | 0.37 | 0.377 |
| ADEW (g) | 50.62 | 51.46 | 50.84 | 50.83 | 0.17 | 0.340 |
| FR (%) | 92.07 | 93.84 | 92.45 | 92.38 | 0.36 | 0.315 |
| HR (%) | 94.31 | 96.31 | 96.13 | 95.65 | 0.30 | 0.066 |
| HCR (%) | 98.83 | 99.33 | 99.50 | 99.83 | 0.15 | 0.093 |
Abbreviations: ADEW, average daily egg weight; FR, fertilization rate; HCR, healthy chick rate; HR, hatching rate; LR, laying rate; MCE, Macleaya cordata extract.
Group means were represented as the mean of the corresponding data from 6 replicates (24 birds per replicate).
As shown in Figure 1, the hatching rate was significantly higher with the 100 mg/kg MCE supplementation, compared with the other groups at 5 to 8 wk, which was 3.13% higher than the control group (P < 0.05). During 9 to 12 wk, the hatching rates was also greatly improved by 2.61, 2.04, and 1.91% with 100, 150, and 200 mg/kg MCE supplementation, respectively (P < 0.05). There were no significant differences in other indicators between these groups.
Figure 1.
Comparison of egg laying performance and reproductive performance of Xuefeng black-bone chicken with different amounts of added MCE (statistics every 4 wk): (A) laying rate; (B) average egg weight; (C) fertilization rate; (D) hatching rate. Note: In the same statistical period (4 wk), # represents significant difference (P < 0.05) compared with group E. E: MCE 0 mg/kg; F: MCE 100 mg/kg; G: MCE 150 mg/kg; H: MCE 200 mg/kg.
Egg Quality
As illustrated in Table 3, at the eighth week of the experiment, as compared with the control group, chickens fed with the 200 mg/kg MCE diet showed the highest eggshell thickness, which was significantly higher than the 0 and 100 mg/kg groups (P < 0.05). The yolk index of the 150 and 200 mg/kg groups which were non-statistically significant increased by 2.33 and 6.98% (P > 0.05), respectively. And there were no significant differences in eggshell strength, egg weight, and HU between these groups (P > 0.05).
Table 3.
Effects of MCE on egg quality of Xuefeng black-bone chicken.1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| Week 8 | ||||||
| Eggshell strength (N/m2) | 3.53 | 3.78 | 3.64 | 3.93 | 0.10 | 0.483 |
| Egg weight (g) | 54.79 | 51.81 | 53.14 | 53.28 | 0.56 | 0.322 |
| Haugh unit | 61.62 | 63.22 | 60.72 | 62.46 | 1.16 | 0.896 |
| Yolk index | 0.43 | 0.42 | 0.44 | 0.46 | 0.01 | 0.080 |
| Eggshell thickness (mm) | 0.33b | 0.33b | 0.34a,b | 0.35a | 0.01 | 0.019 |
| Week 12 | ||||||
| Eggshell strength (N/m2) | 3.36b | 3.63b | 4.06a | 3.68a,b | 0.08 | 0.011 |
| Egg weight (g) | 52.67 | 52.04 | 51.67 | 53.08 | 0.43 | 0.667 |
| Haugh unit | 62.21 | 58.93 | 60.05 | 60.11 | 0.75 | 0.498 |
| Yolk index | 0.37 | 0.37 | 0.38 | 0.38 | 0.01 | 0.319 |
| Eggshell thickness (mm) | 0.31B | 0.33A | 0.33A | 0.33A | 0.01 | 0.006 |
a,bMeans within a row with different superscripts for each factor are significantly different (P < 0.05).
A,BMeans within a row with no common superscripts indicate a highly significant difference (P < 0.01).
Abbreviation: MCE, Macleaya cordata extract.
Group means were represented as the mean of the corresponding data from 6 replicates (4 eggs per replicate).
At the end of the 12th week of the experiment, the eggshell strength of the 150 mg/kg test group was the highest, 4.06 N/m2, which was significantly higher than the 0 and 100 mg/kg groups (P < 0.05). The yolk index, egg weight, and HU did not exhibit significant responses to the dietary treatments (P > 0.05).
Serum Antioxidant Indices
As shown in Table 4, the serum concentrations of GSH-Px were highly significantly increased by 28.37 and 38.49% (P < 0.01) with dietary MCE supplementation of 150 and 200 mg/kg, respectively, compared with the control group. No noticeable difference was detected in the serum concentrations of T-AOC, T-SOD, and MDA between the dietary MCE supplementation groups (P > 0.05).
Table 4.
Effects of MCE on serum antioxidant indices of Xuefeng black-bone chicken.1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| T-AOC (U/mL) | 7.41 | 7.44 | 6.15 | 6.71 | 0.43 | 0.689 |
| T-SOD (U/mL) | 354.12 | 331.70 | 349.43 | 353.07 | 6.60 | 0.612 |
| GSH-Px (U/mL) | 2,357.01C | 2,359.84C | 3,290.55B | 3,832.09A | 162.65 | <0.01 |
| MDA (nmol/mL) | 11.28 | 11.25 | 10.94 | 10.86 | 0.56 | 0.992 |
A–CMeans within a row with no common superscripts indicate a highly significant difference (P < 0.01).
Abbreviations: GSH-Px, glutathione peroxidase; MCE, Macleaya cordata extract; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase
Group means were represented as the mean of the corresponding data from 6 replicates (2 birds per replicate for serum samples).
Serum Biochemical Indices
Table 5 shows that the serum UA was greatly decreased by 13.87% (P < 0.01) and 24.5% (P > 0.05) with 100 and 150 mg/kg MCE supplementation, respectively, but the 200 mg/kg group was increased by 7.96% (P > 0.05). Compared with the control group, the serum urea level in the 150 mg/kg group was significantly decreased by 59% (P < 0.01); however, in the 200 mg/kg group it was increased by 13.73% (P > 0.05). The GLU content was 12.95 mmol/L following dietary supplementation with 200 mg/kg MCE, which was significantly higher than the 100 and 150 mg/kg groups (P < 0.05), but not significantly different from the control group (P > 0.05). There were no significant differences in other indicators between the groups (P > 0.05).
Table 5.
Effects of MCE on serum biochemical indices of Xuefeng black-bone chicken.1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| TP (g/L) | 51.68 | 52.67 | 54.99 | 51.64 | 0.58 | 0.141 |
| ALB (g/L) | 24.96 | 25.53 | 26.36 | 25.08 | 0.22 | 0.135 |
| GLB (g/L) | 26.72 | 26.26 | 27.64 | 26.55 | 0.42 | 0.677 |
| TC (mmol/L) | 4.25 | 3.87 | 3.77 | 3.51 | 0.15 | 0.384 |
| TG (mmol/L) | 18.28 | 18.09 | 17.96 | 17.14 | 0.29 | 0.539 |
| Urea (mmol/L) | 0.88A | 0.68A,B | 0.36B | 1.02A | 0.07 | <0.01 |
| UA (μmol/L) | 161.83A,B | 139.38C | 151.39B,C | 175.83A | 2.87 | <0.01 |
| GLU (mmol/L) | 12.73a,b | 12.36b | 12.34b | 12.95a | 0.09 | 0.035 |
| AST (U/L) | 168.43 | 176.26 | 170.61 | 188.76 | 3.22 | 0.169 |
| ALT (U/L) | 90.79 | 87.93 | 89.48 | 92.22 | 0.93 | 0.422 |
| ALP (U/L) | 437.68 | 428.76 | 421.54 | 436.82 | 2.70 | 0.112 |
| Ca (mmol/L) | 6.22 | 5.35 | 5.65 | 6.17 | 0.14 | 0.079 |
| P (mmol/L) | 1.24 | 1.27 | 1.39 | 1.24 | 0.04 | 0.549 |
a,bMeans within a row with no common superscripts differ significantly (P < 0.05).
A–CMeans within a row with no common superscripts indicate a highly significant difference (P < 0.01).
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GLB, globulin; GLU, glucose; MCE, Macleaya cordata extract; TC, total cholesterol; TG, triglyceride; TP, total protein; UA, urea acid.
Group means were represented as the mean of the corresponding data from 6 replicates (2 birds per replicate for serum samples).
Serum Immunological Indices
Highly significant differences in serum TNF-α are evident in Table 6, which were decreased by 9.57, 16.60, and 23.31% in the dietary 100, 150, and 200 mg/kg MCE supplementation chickens compared with the control (P < 0.01). And the IL-6 levels were decreased by 6.72, 10.53, and 16.20% with dietary 100, 150, and 200 mg/kg MCE supplementation (P < 0.01), respectively. Serum immunoglobulin A, immunoglobulin M, immunoglobulin G, and IL-6 were not affected by the treatment (P < 0.01).
Table 6.
Effects of MCE on serum immunological indices of Xuefeng black-bone chicken.1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| IgA (g/L) | 2.24 | 2.27 | 2.27 | 2.28 | 0.01 | 0.839 |
| IgM (g/L) | 1.65 | 1.66 | 1.67 | 1.67 | 0.01 | 0.833 |
| IgG (g/L) | 4.20 | 4.24 | 4.27 | 4.29 | 0.02 | 0.438 |
| TNF-α (pg/mL) | 57.18A | 51.71B | 47.69C | 43.85D | 0.69 | <0.01 |
| IL-6 (pg/mL) | 98.64A | 92.01B | 88.25C | 82.66D | 0.87 | <0.01 |
A–DMeans within a row with no common superscripts indicate a highly significant difference (P < 0.01).
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-6, interleukin-6; MCE, Macleaya cordata extract; TNF-α, tumor necrosis factor-α.
Group means were represented as the mean of the corresponding data from 6 replicates (2 birds per replicate for serum samples).
Serum Hormone Indices
As shown in Table 7, as compared with the control group, chickens fed with the 100, 150, and 200 mg/kg MCE diet showed higher FSH contents, which were significantly increased by 11.00, 18.48, and 26.26% (P < 0.01). Compared with the control group, E2 in the 100, 150, and 200 mg/kg groups were significantly increased by 11.45, 20.81, and 27.50%, respectively (P < 0.01). Serum E2 levels were significantly increased by 11.45, 20.81, and 27.50% in the 100, 150, and 200 mg/kg MCE groups (P < 0.01), compared with the control group. Serum LH levels of the 100, 150, and 200 mg/kg MCE diet were significantly higher than the control group, which were increased by 10.62, 17.93, and 26.68% (P < 0.01). The content of PTH reduced in the groups fed with the 100, 150, and 200 mg/kg MCE meal by 7.68, 11.88, and 5.45% compared with the control group (P < 0.01). Compared with the control group, the levels of PO in groups 100, 150, and 200 mg/kg were increased (P < 0.05), which were increased by 6.25, 24.31, and 27.95%, respectively.
Table 7.
Effects of MCE on serum hormone indices of Xuefeng black-bone chicken.1
| Item | Groups (MCE mg/kg) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| PO (ng/mL) | 1.65b | 1.76b | 2.17a,b | 2.78a | 0.16 | 0.045 |
| FSH (mIU/mL) | 22.89D | 25.07C | 28.08B | 31.04A | 0.46 | <0.01 |
| E2 (pg/mL) | 23.75D | 26.82C | 29.99B | 32.76A | 0.53 | <0.01 |
| LH (mIU/mL) | 28.19D | 31.54C | 34.35B | 38.45A | 0.61 | <0.01 |
| PTH (pg/mL) | 129.25B | 119.32C | 113.89D | 135.88A | 0.98 | <0.01 |
a,bMeans within a row with no common superscripts differ significantly (P < 0.05).
A–DMeans within a row with no common superscripts indicate a highly significant difference (P < 0.01).
Abbreviations: FSH, follicle stimulating hormone; E2, estradiol; LH, luteinizing hormone; MCE, Macleaya cordata extract; PO, progesterone; PTH, parathyroid hormone.
Group means were represented as the mean of the corresponding data from 6 replicates (2 birds per replicate for serum samples).
Discussion
Sanguinarine isolated from M. cordata is a compound with various biological activities (Gu et al., 2015), which has been used as a feed additive in swine and poultry production in recent years (Vieira et al., 2008; Pellikaan et al., 2010). Several investigators have reported that MCE diets could ameliorate production performance, improve gut health and body immunity, and promote growth (Bojjireddy et al., 2013; Khadem et al., 2014). Besides, sanguinarine is the major active ingredient of M. cordata, which has been found to have anti-inflammatory activity (Niu et al., 2012), inhibit the activation of NF-κB, and regulate inflammatory response (Wullaert et al., 2011). Gradually, it evoked attention as a substitute of antibiotics (Kim et al., 2012). Although sanguinarine is poisonous, an average daily oral dose of alkaloids of up to 5 mg/kg animal body weight has been proven safe (Kosina et al., 2004). In our study, we assessed the effects of additional dosages of MCE of 0, 100, 150, or 200 mg/kg feed for every treatment on laying performance, egg quality, and serum indices of Xuefeng black-bone chicken, respectively.
Laying Performance
Laying performance, in the final analysis, was determined by the development of follicles in the ovary and gonadotropins; for example, FSH and LH played a particularly important role in the course of follicular development and ovulation (Long et al., 2017). The present study found that dietary 200 mg/kg MCE supplementation increased the concentrations of FSH, P, and E2. The laying rate of hens fed the 200 mg/kg MCE diet increased significantly at 9 to 12 wk, indicating that the MCE diet played a role and helped to increase the laying rate. However, at this stage of 9 to 12 wk, the egg laying rate decreased, which might be related to the increase in age. Our current study showed that 100 mg/kg MCE supplementation affected the hatching rate, which was significantly higher than the other 3 groups during 5 to 8 and 9 to 12 wk. Besides, the hatching rate and healthy chick rate tended to increase during the whole period of the experiment, but we failed to observe increases in the laying rate, average egg weight, and fertilization rate with MCE supplementation diets. As a result, further studies are needed to confirm if the addition of MCE has a correlation with laying performance of meat breeder chickens.
Egg Quality
Eggshell quality is an important parameter in the poultry industry; it is affected by many factors, such as disease, nutrition, heat stress, and age (Akyurek and Okur, 2009). The strength and thickness of the eggshell have been found to be independent of the egg quality (De Ketelaere et al., 2002). On the other hand, HU is the standard for quantifying interior egg quality; it is generally believed that the larger the value, the better the egg quality. The results of this experiment showed that on the 84th day, dietary MCE levels at 200 mg/kg increased the eggshell strength and eggshell thickness, but did not affect HU and other egg quality characteristics. To our knowledge, there are no available studies on the effects of MCE on egg quality in hens. Besides, serum PTH levels increased following dietary supplementation with 200 mg/kg MCE. PTH is secreted by parathyroid master cells and regulated by the negative feedback of Ca ions in the extracellular fluid, which could promote bone turnover and mobilize bone Ca into blood. Ca and phosphorus are very important for eggshell quality,eggshell thickness, and strength; these are mainly affected by genetic factors and Ca and phosphorus metabolism. However, dietary MCE did not affect the levels of Ca and phosphorus in the serum. In contrast to earlier studies (Park et al., 2017), it was found that eggshell quality had little correlation with blood Ca level. In addition, we found that the level of P in serum increased significantly with MCE diets, which is secreted by the ovarian corpus luteum and played an important role in egg laying. The improvement of P increased the thickness and strength of eggshells and stimulated eggshell calcification (Pollock and Orosz, 2002); MCE diets might improve the egg quality by hormone regulation.
Serum Antioxidant Indices
In order to assess the antioxidant capacity of MCE, several antioxidant parameters such as T-AOC, SOD, GSH-Px, and MDA in serum were monitored. It has been reported that the T-AOC, GSH-Px, and SOD of serum increased in MCE-supplemented piglets (Chen et al., 2019). GSH-Px is an important peroxidase that is widely present in the body, which catalyzes the reduction reaction of lipid peroxides caused by reduced glutathione to protect the cell membrane (Johnson et al., 2003). SOD is an active protease containing metal elements, which protects cells from superoxide-free radicals by degrading superoxide radicals into hydrogen peroxide (Hao et al., 2015). The T-AOC levels represented T-AOC, reflecting non-enzymatic antioxidant defense systems (Momeni and Eskandari, 2017). MDA is one of the most commonly used indexes of lipid peroxidation, indicating cell membrane damage caused by the increase of free radical formation (Niedernhofer et al., 2003). Our current study showed that dietary MCE supplementation increased the level of GSH-Px in the serum. In our latest study, we reported that dietary MCE significantly increased the activity of catalase and GSH-Px, and reduced the content of MDA (Qin et al., 2006; Guan et al., 2019). It was found that sanguinarine could weaken the activity of the nicotinamide adenine dinucleotide phosphate enzyme, which indicates that sanguinarine is an inhibitor of enzymes rather than a scavenger of reactive oxygen species. Liu et al. (2015) confirmed that sanguinarine could inhibit the activity of nicotinamide adenine dinucleotide phosphate oxidase 2 and the generation of reactive oxygen species in H9c2 cardiac cells. Thus, it has been indicated that dietary MCE could reduce oxidative stress in vivo.
Serum Biochemical Indices
The serum biochemical index is an internal microscopic reflection of the physiological function of the animal body, indirectly reflecting nutrient metabolism, and changes in organ functions and nutrient deposition (Wang et al., 2009). Generally, UA is a product of purine derivatives, which is reabsorbed and excreted in the proximal tubule by a voltage-sensitive urate channel and a urate-anion exchange mechanism (Siu et al., 2006). Vaziri et al. (1995) reported that there was a decrease in the urinary excretion of UA and an increase in plasma UA in rats with renal disease. Therefore, the UA content is considered to be an indicator for testing the kidney function of birds, which could directly reflect the level of protein catabolism in the body. The present study found that dietary MCE supplementation with 100 and 150 mg/kg decreased the concentrations of serum UA, indicating that MCE had no adverse effect on the kidney, and could reduce the catabolism of proteins except at high doses. In addition, dietary MCE supplementation with 100 and 150 mg/kg decreased the serum urea by 22.73 and 59.09%. Urea is not only the end product of body protein, but is also one of the main indexes of renal function, reflecting the sensitive index of early glomerular injury and the decrease of glomerular filtration function. Lower content of serum urea is related with a higher utilization rate of nitrogen (Coma et al., 1995). The decrease in serum urea showed that the addition of low-dose MCE improved the nitrogen utilization rate. In our study, dietary MCE supplementation had no significant influences on blood GLU content, while dietary 200 mg/kg MCE supplementation was the most effective in improving GLU content, which was significantly higher than dietary 100 and 150 mg/kg MCE, indicating that the addition of high-dose MCE may promote the digestion and absorption of carbohydrates in the diet.
Serum Immunological Indices
In this experiment, we also measured the content of inflammatory factors in the serum. IL-6 participates in the development of inflammation by enhancing other inflammatory cytokines (Webel et al., 1997). TNF-α is a pro-inflammatory cytokine that plays different physiological roles and could induce apoptosis of intestinal epithelial cells (van Dullemen et al., 1995). Previous studies have shown that macrophages increased in the colonic mucosa of patients with inflammatory diseases, and the expressions of inflammatory factors TNF-α, IL-1, and IL-6 also increased (Rogler et al., 1998). In this study, MCE supplementation reduced the content of IL-6 and TNF-α in the serum; this suggests that the MCE diet could inhibit inflammation. More research on this is needed in the future.
Serum Hormone Indices
Gonadotropins, for example FSH and LH, play a particularly important role in the course of follicular development and ovulation (Mendez-Herrera et al., 1998). Accordingly, there is a certain relationship between egg production and reproductive hormone content in laying hens. In this study, with the increase in the supplementation of MCE diets, the levels of FSH, E2, LH, and PO in serum increased significantly in a concentration-dependent manner. Improvement of FSH and LH levels might increase the number of germ cells, stimulate follicular growth and maturation, and secrete PO in follicles. It has been determined that steroids E2 and PO promote the growth and differentiation of reproductive organs and regulate steroid production, promote ovarian granulosa cell proliferation, and maintain general development of ovarian follicles (Drummond and Findlay, 1999; Faria et al., 2010). Furthermore, the hatching rates were significantly increased with MCE supplementation in the 9 to 12-week trials, indicating that the MCE diets might affect the reproductive performance of Xuefeng black-bone chicken by increasing the level of endocrine hormones. So far, there are a few reports on the effect of MCE on animal reproductive hormones; the underlying mechanism of MCE on the reproductive performance of Xuefeng black-bone chicken needs further study.
Conclusion
In conclusion, the present study demonstrated that dietary supplementation with 200 mg/kg MCE in Xuefeng black-bone chicken improved the antioxidant capacity and immunity level, regulated hormone secretion, and ameliorated egg quality.
Acknowledgments
This study was funded by National Natural Science Foundation of China (32072711) and start-up funds from Hunan Agricultural University.
Disclosures
There authors have no conflicts of interest to declare.
References
- Akyurek H., Okur A.A. Effect of Storage time, temperature and hen age on egg quality in free-Range layer hens. J. Anim. Vet. Adv. 2009;8:1953–1958. [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Saeed M., Arain M.A., Bhutto Z.A., Fazlani S.A., Brohi S.A., Arif M. Effect of some phytogenic additives as dietary supplements on performance, egg quality, serum biochemical parameters and oxidative status in laying hens. Indian J. Anim. Sci. 2017;87:900–905. [Google Scholar]
- Bojjireddy N., Sinha R.K., Panda D., Subrahmanyam G. Sanguinarine suppresses IgE induced inflammatory responses through inhibition of type II PtdIns 47kinase(s) Arch. Biochem. Biophys. 2013;537:192–197. doi: 10.1016/j.abb.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Chen J., Kang B., Yao K., Fu C., Zhao Y. Effects of dietary Macleaya cordata extract on growth performance, immune responses, antioxidant capacity, and intestinal development in weaned piglets. J. Appl. Anim. Res. 2019;47:349–356. [Google Scholar]
- Coma J., Carrion D., Zimmerman D.R. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J. Appl. Anim. Res. 1995;73:472–481. doi: 10.2527/1995.732472x. [DOI] [PubMed] [Google Scholar]
- De Ketelaere B., Govaerts T., Coucke P., Dewil E., Visscher J., Decuypere E., De Baerdemaeker J. Measuring the eggshell strength of 6 different genetic strains of laying hens: techniques and comparisons. Br. Poult. Sci. 2002;43:238–244. doi: 10.1080/00071660120121454. [DOI] [PubMed] [Google Scholar]
- Drummond A.E., Findlay J.K. The role of estrogen in folliculogenesis. Mol. Cell. Endocrinol. 1999;151:57–64. doi: 10.1016/s0303-7207(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Faria T.d.S., Brasil F.d.B., Sampaio F.J.B., Ramos C.d.F. Effects of maternal undernutrition during lactation on estrogen and androgen receptor expressions in rat ovary at puberty. Nutrition. 2010;26:993–999. doi: 10.1016/j.nut.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Gu S., Yang X.-C., Xiang X.-Y., Wu Y., Zhang Y., Yan X.-Y., Xue Y.-N., Sun L.-K., Shao G.-G. Sanguinarine-induced apoptosis in lung adenocarcinoma cells is dependent on reactive oxygen species production and endoplasmic reticulum stress. Oncol. Rep. 2015;34:913–919. doi: 10.3892/or.2015.4054. [DOI] [PubMed] [Google Scholar]
- Guan G., Ding S., Yin Y., Duraipandiyan V., Al-Dhabi N.A., Liu G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci. China Life Sci. 2019;62:1019–1027. doi: 10.1007/s11427-018-9494-6. [DOI] [PubMed] [Google Scholar]
- Hamoud R., Reichling J., Wink M. Synergistic antimicrobial activity of combinations of sanguinarine and EDTA with vancomycin against multidrug resistant bacteria. Drug Metab. Lett. 2014;8:119–128. doi: 10.2174/187231280802150212100742. [DOI] [PubMed] [Google Scholar]
- Hao R., Li Q., Zhao J., Li H., Wang W., Gao J. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest Sci. 2015;178:237–242. [Google Scholar]
- Johnson R.J., Kang D.-H., Feig D., Kivlighn S., Kanellis J., Watanabe S., Tuttle K.R., Rodriguez-Iturbe B., Herrera-Acosta J., Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- Kantas D., Papatsiros V.G., Tassis P.D., Athanasiou L.V., Tzika E.D. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim. Sci. J. 2015;86:92–98. doi: 10.1111/asj.12240. [DOI] [PubMed] [Google Scholar]
- Khadem A., Soler L., Everaert N., Niewold T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory propertiese. Br. J. Nutr. 2014;112:1110–1118. doi: 10.1017/S0007114514001871. [DOI] [PubMed] [Google Scholar]
- Kim J.C., Hansen C.F., Mullan B.P., Pluske J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012;173:3–16. [Google Scholar]
- Kosina P., Walterova D., Ulrichova J., Lichnovsky V., Stiborova M., Rydlova H., Vicar J., Krecman V., Brabec M.J., Simanek V. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem. Toxicol. 2004;42:85–91. doi: 10.1016/j.fct.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kumar G.S., Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. Rsc Adv. 2014;4:56518–56531. [Google Scholar]
- Lee K.-W., Kim J.-S., Oh S.-T., Kang C.-W., An B.-K. Effects of dietary sanguinarine on growth performance, relative organ weight, Cecal microflora, serum cholesterol level and meat quality in broiler chickens. J. Poult. Sci. 2015;52:15–22. [Google Scholar]
- Liu G., Aguilar Y.M., Zhang L., Ren W., Chen S., Guan G., Xiong X., Liao P., Li T., Huang R., Yang H.S., Park I., Kim S.W., Yin Y. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs. J. Anim. Sci. 2016;94:75–78. [Google Scholar]
- Liu G., Guan G., Fang J., Martinez Y., Chen S., Bin P., Duraipandiyan V., Gong T., Tossou M.C.B., Al-Dhabi N.A., Yin Y. Macleaya cordata extract decreased Diarrhea Score and enhanced intestinal barrier function in growing piglets. Biomed. Res. Int. 2016;2016:1069585. doi: 10.1155/2016/1069585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jiao R., Ma Z.-G., Liu W., Wu Q.-Q., Yang Z., Li F.-F., Yuan Y., Bian Z.-Y., Tang Q.-Z. Sanguinarine inhibits angiotensin II-induced apoptosis in H9c2 cardiac cells via restoring reactive oxygen species-mediated decreases in the mitochondrial membrane potential. Mol. Med. Rep. 2015;12:3400–3408. doi: 10.3892/mmr.2015.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Wu S.G., Yuan F., Zhang H.J., Wang J., Qi G.H. Effects of dietary octacosanol supplementation on laying performance, egg quality, serum hormone levels, and expression of genes related to the reproductive axis in laying hens. Poult. Sci. 2017;96:894–903. doi: 10.3382/ps/pew316. [DOI] [PubMed] [Google Scholar]
- Mendez-Herrera M.C., Tamez L., Candido A., Reyes-Esparza J.A., Pedernera E. Follicle stimulating hormone increases somatic and germ cell number in the ovary during chick embryo development. Gen. Comp. Endocrinol. 1998;111:207–215. doi: 10.1006/gcen.1998.7108. [DOI] [PubMed] [Google Scholar]
- Momeni H.R., Eskandari N. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Exp. Toxicol. Pathol. 2017;69:93–97. doi: 10.1016/j.etp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Niedernhofer L.J., Daniels J.S., Rouzer C.A., Greene R.E., Marnett L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Niu X., Fan T., Li W., Xing W., Huang H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur. J. Pharmacol. 2012;689:262–269. doi: 10.1016/j.ejphar.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Obiang-Obounou B.W., Kang O.-H., Choi J.-G., Keum J.-H., Kim S.-B., Mun S.-H., Shin D.-W., Kim K.W., Park C.-B., Kim Y.-G., Han S.-H., Kwon D.-Y. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011;36:277–283. doi: 10.2131/jts.36.277. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Park J.H., Jeong J.S., Lee S.I., Kim I.H. Influence of dietary Particle size and Sources of calcium and vitamin D-3 on production performance, egg quality and blood calcium concentration of ISA Brown laying hens. Anim. Nutr. Feed Technol. 2017;17:1–12. [Google Scholar]
- Pellikaan W.F., Andres-Elias N., Durand A., Bongers L.J.G.M., van Laar-van Schuppen S., Torrallardona D. Effect of carob bean gum, spray dried porcine plasma and sanguinarine on fermentation activity in the gut of weanling pigs. Livest Sci. 2010;133:164–168. [Google Scholar]
- Pollock C.G., Orosz S.E. Avian reproductive anatomy, physiology and endocrinology. Vet. Clin. N. Am-small. 2002;5:441–474. doi: 10.1016/s1094-9194(02)00010-5. [DOI] [PubMed] [Google Scholar]
- Qin F., Patel R., Yan C., Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic. Bio Med. 2006;40:236–246. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P.A., Scholmerich J., Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Siu Y.-P., Leung K.-T., Tong M.K.-H., Kwan T.-H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- van Dullemen H.M., van Deventer S.J., Hommes D.W., Bijl H.A., Jansen J., Tytgat G.N., Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- Vaziri N.D., Freel R.W., Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J. Am. Soc. Nephrol. 1995;6:1313–1317. doi: 10.1681/ASN.V641313. [DOI] [PubMed] [Google Scholar]
- Vieira S.L., Oyarzabal O.A., Freitas D.M., Berres J., Pena J.E.M., Torres C.A., Coneglian J.L.B. Performance of broilers fed diets supplemented with sanguinarine-Like alkaloids and organic acids. J. Appl. Poult. Res. 2008;17:128–133. [Google Scholar]
- Wang W., Dolan L.C., von Alvensleben S., Morlacchini M., Fusconi G. Safety of standardized Macleaya cordata extract in an eighty-four-day dietary study in dairy cows. J. Anim. Physiol. Anim. Nutr. (Berl). 2018;102:E61–E68. doi: 10.1111/jpn.12702. [DOI] [PubMed] [Google Scholar]
- Wang J.P., Yoo J.S., Kim H.J., Lee J.H., Kim I.H. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 2009;125:298–303. [Google Scholar]
- Webel D.M., Finck B.N., Baker D.H., Johnson R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997;75:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- Wullaert A., Bonnet M.C., Pasparakis M. NF-kappa B in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.D., Wu S.B., Choct M., Pastor A., Steiner T., Swick R.A. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017;96:3581–3585. doi: 10.3382/ps/pex164. [DOI] [PubMed] [Google Scholar]
- Zhong H., Hu D.D., Hu G.H., Su J., Bi S., Zhang Z.E., Wang Z., Zhan R.L., Xu Z., Jiang Y.Y., Wang Y. Activity of Sanguinarine against Candida albicans Biofilms. Antimicrob. Antimicrob. Agents. Chemother. 2017;61:e02259–e02316. doi: 10.1128/AAC.02259-16. [DOI] [PMC free article] [PubMed] [Google Scholar]