Abstract
Background
Based on what is known at this time, pregnant women are at an increased risk of severe illness from COVID-19 compared to nonpregnant women. Additionally, pregnant women with COVID-19 might have an increased risk of adverse pregnancy outcomes. To investigate the effects of coronavirus disease 2019 (COVID-19) on mortality of pregnant and postpartum women, we performed a systematic review of available published literature on pregnancies affected by COVID-19.
Methods
Web of Science, SCOPUS, and MEDLINE- databases were searched for original studies concerning the effect of COVID-19 on mortality of pregnant and postpartum women published by July 10, 2020. Meta-analyses of proportions were used to combine data and report pooled proportions.
Results
117 studies with a total of 11758 pregnant women were included. The age ranged between 15 and 48 years. Most subjects were infected with SARS-CoV-2 in the third trimester. Disease severity was not reported in 1125 subjects. Maternal mortality was 1.3%. In 100% of fatal cases with adequate data, fever alone or with cough was one of the presenting symptoms. Also, dyspnea (58.3%) and myalgia (50%) were the most common symptoms. Sore throat (8.3%) and gastrointestinal symptoms (anorexia, nausea) (8.3%) were rare. The rate of comorbidities was 20% among COVID-19 deaths. The majority of COVID-19-infected women who died had cesarean section (58.3%), 25% had a vaginal delivery, and 16.7% of patients were not full term.
Conclusion
COVID-19 infection in pregnant women was associated with higher rates (and pooled proportions) of cesarean section and mortality. Because new data are continuously being generated and published, the findings of this study can be complete and updated with new researches. The results of this study can guide and improve prenatal counseling of COVID-19-infected pregnant women.
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global public health crisis [1, 2]. The impact of COVID-19 on specific populations, including pregnant women and their newborns, remains mostly unknown and unstudied. There is not sufficient information about the effect of this disease in pregnant women, and most available studies have evaluated the impact of the disease in the general population. Pregnant women are at a higher risk for acquiring viral respiratory infections and severe pneumonia due to physiological changes in their immune and cardiopulmonary systems [1–3].
The observed outcomes have been different from what was seen during the H1N1 pandemic and with influenza outbreaks, all of which resulted in increased mortality in women who were pregnant [4]. Studies in pregnant women during coronavirus outbreaks (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV) show that pregnant women are susceptible to experiencing adverse events such as requiring hospitalization or intensive care unit (ICU) admission, endotracheal intubation, and renal failure [5–7].
Worldwide, there are more than 140 million births every year, and pregnant women are potentially at risk for adverse outcomes of novel coronavirus. Although maternal mortality has been reported in some studies, limited information is available about SARS-CoV-2 infection in critically ill pregnant women hospitalized for COVID-19 [8, 9]. Also, there is a multitude of case reports of infection with SARS-CoV-2 during pregnancy but their small sample size makes it difficult to properly find potential complications [10, 11].
The findings of a study on 8207 SARS-CoV-2-infected pregnant women showed an increased risk for ICU admission and mechanical ventilation compared with nonpregnant women; however, the risk for death was similar [12]. Also, the Centers for Disease Control and Prevention surveillance report from the United States noted that pregnant women were more likely to be admitted to the ICU and receive mechanical ventilation than nonpregnant women. However, after adjusting for age, presence of underlying medical conditions, and race/ethnicity, mortality rate was not increased [12].
Based on data from the early stage of pandemic, it is reassuring that there are low rates of maternal mortality with SARS-CoV-2 [13]. However, more studies are needed to learn more about maternal mortality. This study is aimed at performing a systematic review of available published literature on pregnancies affected by COVID-19 to evaluate the effect of COVID-19 on mortality of pregnant and postpartum women.
2. Methods
2.1. Study Design
This study is a systematic scoping review based on the methodological framework of Arksey and O'Malley [14]. Five stages of the framework they adopted for conducting a scoping study are as follows: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results.
2.2. Research Questions
The main questions of the study included the following. What is the mortality rate of COVID-19 in pregnant and postpartum women, and how many and what type of comorbidities were found in recovered and deceased patients? What were the disease symptoms and the mode of delivery in the maternal deaths?
2.3. Search Strategy and Eligibility Criteria
We searched scientific databases of Web of Science, SCOPUS, and MEDLINE through the interface, for original studies concerning the effect of COVID-19 on maternal death published until July 10, 2020. We designed a comprehensive optimal search strategy consisted of two components related to pregnancy and COVID-19. The complete search strategy is shown in Table 1. Also, the Google Scholar engine was searched for potentially relevant articles. Eligible studies for inclusion in this systematic review were those that met all of the following criteria: (i) the analysis was performed in pregnant and postpartum women affected by COVID-19 (laboratory confirmed and/or clinically diagnosed) and (ii) the study was a full paper with original data and (iii) was written in English. Studies were excluded if they did not provide sufficient information about the patient outcome (survival or death and important related details).
Table 1.
Search strategy.
| Pubmed |
| (Pregnancy [Title/Abstract] OR pregnan∗ [Title/Abstract] OR gestation∗ [Title/Abstract] OR conception [Title/Abstract]) AND (“Novel coronavirus” [Title/Abstract] OR “Novel coronavirus 2019” [Title/Abstract] OR “2019 novel coronavirus” [Title/Abstract] OR “2019 nCoV” [Title/Abstract] OR “Wuhan coronavirus” [Title/Abstract] OR “Wuhan pneumonia” [Title/Abstract] OR covid-19 [Title/Abstract] OR “2019-nCoV” [Title/Abstract] OR “SARS-CoV-2” [Title/Abstract] OR “coronavirus 2019” [Title/Abstract] OR “2019-nCoV”[Title/Abstract]) |
| Web of Science |
| TOPIC: (pregnancy OR pregnan∗ OR gestation∗ OR conception) AND TOPIC: (“Novel coronavirus” OR “Novel coronavirus 2019” OR “2019 novel coronavirus” OR “2019 nCoV” OR “Wuhan coronavirus” OR “Wuhan pneumonia” OR covid-19 OR “2019-nCoV” OR “SARS-CoV-2” OR “coronavirus 2019” OR “2019-nCoV”) |
| SCOPUS |
| (TITLE-ABS-KEY (pregnancy OR pregnan∗ OR gestation∗ OR conception) AND TITLE-ABS-KEY (“Novel coronavirus” OR “Novel coronavirus 2019” OR “2019 novel coronavirus” OR “2019 nCoV” OR “Wuhan coronavirus” OR “Wuhan pneumonia” OR covid-19 OR “2019-nCoV” OR “SARS-CoV-2” OR “coronavirus 2019” OR “2019-nCoV”)) |
2.4. Study Selection
After removing the duplicates, the search output was screened as the first step. The titles and abstracts of the articles were screened by the two authors independently according to the eligibility criteria. Then, in the secondary screening, the full texts of the retrieved articles were reviewed by the same authors. Disagreements were resolved through discussion and consensus.
2.5. Data Extraction
A data collection form was designed by the authors to extract the data of the papers in an integrated way. Characteristics of the studies were extracted, including details of the first author's name, country, sample size, age, gestational age, comorbidities and complications in pregnancy, severity of COVID-19, ICU admission and ventilation need, complications during treatment of COVID-19, and maternal mortality rate. In deceased patients, more information was extracted, including presenting symptoms, mode of delivery, duration of admission to death, and the result of the polymerase chain reaction (PCR) testing of the neonates. Data extraction was performed by two authors independently, and any disagreements were resolved through discussion and team consensus.
2.6. Statistical Analysis
All analyses were conducted by STATA16 (StataCorp, College Station, Texas, USA). The study statistician performed data extraction for primary outcomes. Random effect meta-analyses were applied using restricted maximum likelihood method [15]. The random effect model was used because there may be other unknown, unregistered/unpublished studies to which we could not have access. The between-study heterogeneity was evaluated using the Cochran Q test and Tau-squared, H-squared, and I-squared statistics. Significance results of the test and values higher than 75% for I-squared were considered as substantial heterogeneity while a value of H-squared = 1 indicates perfect homogeneity among the studies [16, 17]. The common effect sizes were calculated as the proportion and rate for binary and count outcomes, respectively, and their 95% confidence intervals (CIs). To assess the publication bias, the funnel plots were drawn. Additionally, Egger's [18] and Begg's [19] tests were conducted. A nonparametric “trim and fill” method of accounting for publication bias was performed and showed that there is no need for a modified effect size [20]. Finally, there were studies that have just one sample in some outcomes that conducted a sensitivity analysis by removing the studies with n = 1 sample.
2.7. Ethical Considerations
The present study complies with all the recommended principles of research ethics. The official approval of the Research Ethics Committee was not obtained for this study because it was a review of the findings of other previously published papers that were available to the public. To comply with the ethical principles, the authors did their best to avoid plagiarism and refused to manipulate the data for personal interests. Respect for the rights of other authors was provided by citing them in the text of the study when information belonging to them was expressed.
3. Results
3.1. Search Results
Figure 1 shows the PRISMA flow chart for study selection. The search strategy retrieved 1348 records and 4 additional records identified through Google Scholar search. After removing 632 duplicates, 720 titles and abstracts were screened. In the second screening, 167 full texts were evaluated and a total of 117 studies were included in the systematic review.
Figure 1.
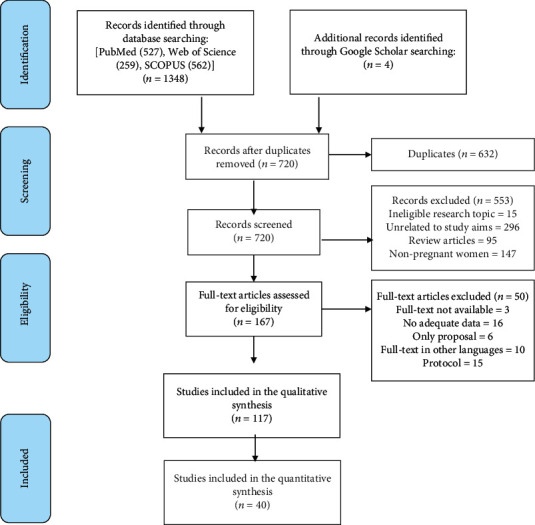
Flow chart of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
3.2. General Characteristics
The characteristics of the included studies are shown in Table 2. A total of 11758 pregnant women entered the review study ranging from 1 to 8207 per study. The age range of patients was between 15 and 48 years. Most subjects were infected with SARS-CoV-2 in the third trimester. Disease severity was not reported in 1125 samples. In the remaining cases, the highest frequency was related to asymptomatic COVID-19 (n = 5466, 51.4%).
Table 2.
Characteristics of included studies.
| First author's name | Country | n | Age (y) (mean or range) | Gestational age | Comorbidities and complications in gestation | Severity of COVID-19 | ICU need | Ventilation need | Complications during treatment of COVID-19 (n) | Death | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | ||||||||||
| Ahmed, I. [60] | United Kingdom | 1 | 29 | — | — | 1 | Obesity, diabetes, renal tubular acidosis, asthma, vitamin D deficiency | Severe | 1 | 1 | Pulmonary embolism, basilar artery thrombosis | 1 |
| Al-kuraishy, H.M. [84] | Iraq | 1 | 25 | — | — | 1 | None | Nonsevere | — | — | — | — |
| AlZaghal, L.A. [85] | Jordan | 1 | 30 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Alzamora, M.C. [86] | Peru | 1 | 41 | — | — | 1 | Obesity, diabetes | Severe | 1 | 1 | — | — |
| An, P. [21] | China | 3 | 25, 31, 33 | — | — | 3 | Not reported | Nonsevere | — | — | — | |
| Anderson, J. [22] | United States | 1 | 35 | — | 1 | — | Obesity, diabetes, asthma | Severe | 1 | 1 | ARDS | — |
| Bani Hani, D.A. [87] | Jordan | 1 | 29 | — | — | 1 | Not reported | Nonsevere | — | — | — | — |
| Barile, E. [52] | Italy | 1 | 48 | — | 1 | — | Hypertension, obesity, sickle cell trait | Severe | 1 | 1 | ARDS | — |
| Bastug, A. [88] | Turkey | 1 | 20 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Baud, D. [61] | Switzerland | 1 | 28 | — | 1 | — | Obesity | Nonsevere | — | — | — | — |
| Blauvelt, CA [23] | United States | 1 | 34 | — | — | 1 | Obesity, asthma, diabetes | Severe | 1 | 1 | ARDS | — |
| Breslin, N. 1 [24] | United States | 43 | 26.9 | — | — | 43 | Obesity (n = 26), asthma (n = 8), diabetes (n = 3), hypertension (n = 3) | Severe (n = 6) | 2 | — | Renal insufficiency (n = 1) | — |
| Breslin, N 2 [25] | United States | 7 | 27-39 | — | — | 7 | Obesity (n = 5), diabetes (n = 2), asthma (n = 1), hypertension (n = 1), none (n = 4) | Severe (n = 2) | 2 | 1 | Acute kidney injury (n = 1) | — |
| Browne, PC [26] | United States | 1 | 33 | — | 1 | — | Asthma (n = 1) | Nonsevere | — | — | — | — |
| Buonsenso, D. [53] | Italy | 4 | 31, 42, 39, 38 | — | 2 | 2 | None | Severe (n = 1) | 1 | 1 | — | — |
| Cao, D. [89] | China | 10 | 29-35 | — | — | 10 | Diabetes (n = 1), preeclampsia (n = 3), hypothyroidism (n = 1), anemia (n = 1), none (n = 5) | Nonsevere (10) | — | — | — | — |
| Carosso, A. [54] | Italy | 1 | 28 | — | — | 1 | Diabetes | Nonsevere | — | — | — | — |
| Chen, H. [90] | China | 9 | 26-40 | — | — | 9 | Hypertension (n = 1), preeclampsia (n = 1), none (n = 7) | Nonsevere (n = 9) | — | — | — | — |
| Chen, L. [91] | China | 118 | 28-34 | 22 | 21 | 75 | Not reported | Nonsevere (n = 109), severe (N = 9) | 1 | 1 | — | — |
| Chen, R. [122] | China | 17 | NM | — | — | 17 | Anemia (n = 5), hypertension (n = 1), diabetes (n = 2), none (n = 9) | Nonsevere (N = 17) | — | — | — | — |
| Chen, S. [93] | China | 5 | 25-31 | — | — | 5 | Diabetes (n = 2), preeclampsia (n = 1), none (n = 2) | Nonsevere (N = 2) | — | — | — | — |
| Chen, Y. [94] | China | 4 | 23-34 | — | — | 4 | Cholecystitis (n = 1), none (n = 3) | Not reported | 1 | 1 | — | — |
| Chhabra, A. [95] | India | 1 | 28 | — | — | 1 | Obesity, diabetes | Nonsevere | — | — | — | — |
| Cohen, J. [62] | France | 88 | 28-34 | Not extractable | Obesity (n = 15), diabetes (n = 7) | Severe (n = 6) | Not reported | Not reported | — | — | ||
| Collin, J. [63] | Sweden | 13 | 20-35 | Not extractable | Diabetes and obesity (some of the women) | Not reported | 13 | 7 | Not reported | — | ||
| Cooke, W.R. [64] | United Kingdom | 2 | 39, 28 | — | — | 2 | Obesity (n = 1), diabetes (n = 2) | Severe (n = 2) | 2 | 2 | Psychiatric sequelae (n = 2) | — |
| De Socio, GV [55] | Italy | 1 | 33 | — | — | 1 | None | Nonsevere | — | — | — | — |
| De Castro, A. [56] | Italy | 1 | 34 | — | — | 1 | Autoimmune thyroiditis and mitral regurgitation | Severe | Not reported | Not reported | ARDS, endocarditis, cerebral emboli | — |
| Dória, M. [65] | Portugal | 12 | 22-41 | — | — | 12 | Chronic hypertension (n = 1), asthma (n = 1), severe myopia (n = 1), ulcerative colitis and psoriasis (n = 1), severe scoliosis and Behçet's syndrome (n = 1), none (n = 7) | Nonsevere (n = 12) | — | — | — | — |
| Du, Y. [96] | China | 1 | 30 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Ellington, S. [12] | United States | 8207 | 15-44 | Not reported | Diabetes (n = 288), lung disease (n = 409), cardiovascular (n = 262), renal disease (n = 12), liver disease (n = 8), immunocompromised condition (n = 66), neurologic disorders or intellectual disability (n = 17), other (n = 162) | Asymptomatic (n = 5199)a | 120b | 42c | Not reported | 16d | ||
| Fan, C. [97] | China | 2 | 34, 29 | — | — | 2 | None (n = 2) | Nonsevere (n = 2) | — | — | — | — |
| Fassett, M.J. [27] | United States | 17 | 33.2 | — | — | 17 | Known comorbidities (n = 2), none (n = 15) | Asymptomatic (n = 17) | — | — | — | — |
| Ferraiolo, A. [57] | Italy | 1 | 30 | — | — | 1 | None | Asymptomatic | — | — | — | — |
| Fontanella, F. [66] | Netherlands, Ireland | 2 | 39, 29 | — | — | 2 | Obesity (n = 2), diabetes (n = 1), hepatitis B (n = 1) | Nonsevere (n = 2) | — | — | Maternal sepsis (n = 1) | — |
| Forero-Peña, D.A. [98] | Venezuela | 1 | 32 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Fox, N.S. [28] | United States | 33 | 31 | Not extractable | Not reported | Asymptomatic (n = 6), nonsevere (n = 27) | — | — | — | — | ||
| Futterman, I. [29] | United States | 2 | 41, 31 | — | 1 | 1 | None (n = 2) | Not reported | 1 | 1 | DIC (n = 2), ARDS, acute renal failure, sepsis (n = 2) | — |
| Gidlöf, S. [67] | Sweden | 1 | 34 | — | — | 1 | Obesity, diabetes, preeclampsia | Nonsevere | — | — | — | — |
| Goldfarb I.T., [30] | United States | 61 | 25-38 | Obesity (n = 26), asthma (n = 6), diabetes (n = 5) | Not reported | 6 | 4 | Not reported | — | |||
| González Romero, D. [68] | Spain | 1 | 44 | — | — | 1 | None | Severe | 1 | 1 | — | — |
| Govind, A. [69] | United Kingdom | 9 | 18-39 | — | 9 | Diabetes (n = 2), asthma (n = 1), none (n = 6) | Nonsevere (n = 7), severe (n = 2) | 2 | 2 | Not reported | — | |
| Grimminck, K. [70] | Netherlands | 1 | 31 | — | — | 1 | Hypertension, systemic lupus erythematous | Nonsevere | — | — | — | — |
| Gulersen, M. [31] | United States | 65 | 29-35 | — | 65 | Known comorbidity (n = 11, including asthma, chronic hypertension, diabetes, HIV, and autoimmune disorders), none (n = 54) | Asymptomatic (n = 14), nonsevere (n = 44), severe (n = 7) | 5 | Not reported | Not reported | — | |
| Hantoushzadeh, S. [99] | Iran | 9 | Not extractable | — | 9 | Obesity (n = 3), underweight (n = 1), diabetes (n = 1), hypothyroidism (n = 1), none (n = 3) | Severe (n = 9) | 9 | 9 | ARDS (n = 2), cardiopulmonary collapse (n = 2), end organ failure (n = 1), acute renal failure (n = 1), septic shock and DIC (n = 1) | 7 | |
| Hirshberg, A. [32] | United States | 5 | 27-39 | — | 2 | 3 | Obesity (n = 3), hypertension (n = 3), asthma (n = 1), diabetes (n = 1), chronic kidney disease (n = 1) | Severe (n = 5) | 5 | 5 | — | — |
| Hong, L. [33] | United States | 1 | 36 | — | 1 | — | Hypothyroidism, obesity, hyperlipidemia | Severe | 1 | 1 | — | — |
| Huang, W. [100] | China | 8 | 27-33 | — | — | 8 | Anemia (n = 4), preeclampsia (n = 1), none (n = 4) | Nonsevere (n = 5), severe (n = 3) | 3 | 2 | Septic shock, cardiomyopathy, ARDS, MODS (n = 1), HF, RF, (n = 1) | — |
| Iqbal, S. [34] | United States | 1 | 34 | — | — | 1 | Not reported | Nonsevere | — | — | — | — |
| Juusela, A. [35] | United States | 2 | 45, 26 | — | — | 2 | Obesity (n = 2), polycystic ovary syndrome (n = 1) | Not extractable | 1 | 1 | Cardiomyopathy (n = 2) | — |
| Kalafat, E. [101] | Turkey | 1 | 32 | — | — | 1 | Thalassemia | Severe | 1 | 1 | — | — |
| Karami, P. [102] | Iran | 1 | 27 | — | — | 1 | None | Severe | 1 | 1 | MODS | 1 |
| Kayem, G. [71] | France | 617 | Not reported | — | 617 | Obesity (n = 159), asthma (n = 37), diabetes (n = 85), gestational hypertension or preeclampsia (n = 21), chronic hypertension (n = 18) | Nonsevere (n = 582), severe (n = 35) | Not reported | 45 | Not reported | 1 | |
| Kelly, J.C. [36] | United States | 1 | Not reported | — | — | 1 | Obesity | Severe | 1 | 1 | — | — |
| Khan, S. [103] | China | 3 | 28, 33, 27 | — | — | 3 | Not reported | Nonsevere | — | — | — | — |
| Khoury, R. [37] | United States | 241 | 18–47 | — | — | 241 | Not reported | Asymptomatic (n = 102), nonsevere (n = 64), severe (n = 75) | 17 | 9 | Not reported | — |
| Kirtsman, M. [83] | Canada | 1 | 40 | — | — | 1 | Familial neutropenia, diabetes, and frequent bacterial infections | Nonsevere | — | — | — | — |
| Kuhrt, K. [72] | United Kingdom | 1 | 30 | — | — | 1 | Thyroid carcinoma | Nonsevere | — | — | — | — |
| Lang, G. [104] | China | 1 | 30 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Li, N. [105] | China | 16 | 26-37 | — | — | 16 | Diabetes (n = 3), gestational hypertension (3), hypothyroidism (2), preeclampsia (1), chronic hypertension (1), polycystic ovary syndrome (1) | Nonsevere (n = 16) | — | — | — | — |
| Li, Y. [106] | China | 1 | 30 | — | — | 1 | Not reported | Nonsevere | — | — | — | — |
| Liao, X. [107] | China | 1 | 25 | — | — | 1 | Not reported | Nonsevere | — | — | — | — |
| Liu, D. [108] | China | 15 | 23-40 | — | — | 11 | Thalassemia (n = 1), diabetes (n = 1), mitral valve and tricuspid valve replacement (n = 1), none (n = 13) | Nonsevere | — | — | — | — |
| Liu, H. [109] | China | 41 | 22-42 | — | 41 | Diabetes (n = 4), gestational hypertension (n = 3), hepatitis B (n = 1) | Nonsevere | — | — | — | — | |
| Liu, Y. [110] | China | 13 | 22-36 | — | 2 | 11 | Not reported | Severe (n = 1) | 1 | 1 | MODS (n = 1) | — |
| Lokken, E.M. [38] | United States | 46 | 26-34 | 3 | 20 | 23 | Diabetes (n = 3), asthma (n = 4), hypothyroidism (n = 3), hypertension (n = 2), obese (n = 15), underweight (n = 1), Crohn's disease (n = 1), heart valve repair (n = 1), thyroid carcinoma (n = 1), seizure disorder (n = 2) | Asymptomatic (n = 3), nonsevere (n = 36), severe (n = 6) | 1 | — | — | — |
| London, V. [39] | United States | 68 | 25-34 | — | 3 | 65 | None (n = 47), known comorbidities (n = 21, including diabetes (n = 9), chronic hypertension (n = 2), asthma (n = 2), cholestasis (n = 2), preeclampsia (n = 4)) | Asymptomatic (n = 22), symptomatic (n = 46) | Not reported | 1 | Not reported | — |
| Lowe, B. [73] | Australia | 1 | 31 | — | — | 1 | Not reported | Nonsevere | — | — | — | — |
| Lu, D. [111] | China | 1 | 22 | — | — | 1 | None | Asymptomatic | — | — | — | — |
| Lucarelli, E. [40] | United States | 3 | 38, 26, 46 | — | 2 | 1 | Not reported | Severe (n = 3) | 3 | 3 | Acute kidney injury (n = 1) | — |
| Lyra, J. [74] | Portugal | 1 | 35 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Martinelli, I. [58] | Italy | 1 | 17 | — | — | 1 | Obesity | Severe | — | — | Pulmonary embolism | — |
| Martínez-Perez, O. [75] | Spain | 82 | 19-48 | — | 82 | Diabetes (n = 1), preeclampsia (n = 4), asthma (n = 6), hypothyroidism (n = 3), other (n = 20) | Nonsevere (n = 78), severe (n = 4) | 9 | 6 | Sepsis (n = 1) | — | |
| Mehta, H. [41] | United States | 1 | 39 | — | 1 | — | None | Severe | 1 | 1 | ARDS | — |
| Mendoza, M. [76] | Spain | 42 | 26-38 | — | 42 | Diabetes (n = 1) | Nonsevere (n = 34), severe (n = 8) | 8 | Not reported | Preeclampsia-like syndrome (n = 5) | — | |
| Mulvey, J.J. [42] | United States | 5 | 26-40 | — | — | 5 | Polycystic ovary syndrome, iron deficiency anemia (n = 1), hypothyroidism (n = 1), none (n = 3) | Asymptomatic (n = 4), nonsevere (n = 1) | — | — | — | — |
| Naqvi, M. [43] | United States | 1 | 35 | — | 1 | — | Hypertension, diabetes, asthma | Severe | — | — | — | — |
| Nesr, G. [77] | United Kingdom | 2 | 34 | — | 1 | — | Immune thrombocytopenia | Nonsevere | — | — | — | — |
| Panichaya, P. [112] | Thailand | 1 | 43 | — | 1 | — | None | Nonsevere | — | — | — | — |
| Peng, Z. [113] | China | 1 | 25 | — | — | 1 | None | Nonsevere | — | — | — | — |
| Pereira, A. [78] | Spain | 60 | 22-43 | 10 | 16 | 32 | HELLP syndrome (n = 1), preeclampsia (n = 2), DVT (n = 2) | Asymptomatic (n = 15), nonsevere (n = 52), severe (n = 3) | 1 | 2 | Not reported | — |
| Pierce-Williams, RMP [44] | United States | 64 | 33.2 | — | 64 | Cardiac disease (including chronic hypertension, cardiomyopathy) (n = 11), pulmonary pathology (n = 16) | Severe and critical (n = 64) | Not reported | 24 | ARDS (n = 14), cardiac arrest (n = 1) | — | |
| Prabhu, M. [45] | United States | 70 | 30.5 in symptomatic and 31.4 in asymptomatic (med) | — | 70 | Chronic hypertension (n = 3), preeclampsia or gestational hypertension (n = 11), diabetes (n = 10), asthma (n = 6), obesity (n = 12) | Asymptomatic (n = 55), symptomatic (n = 15) | 1 | — | Pulmonary edema (n = 2) | — | |
| Qadri, F. [5] | United States | 16 | 20-40 | — | 16 | Obesity (n = 10) | Nonsevere (n = 16) | — | — | — | — | |
| Qiancheng, X. [114] | China | 28 | 30 | 3 | 1 | 24 | Hypertension (n = 1), diabetes (n = 2), hepatitis B (n = 2), hypothyroidism (n = 1) | Severe (n = 2) | Not reported | Not reported | Not reported | — |
| Rabice, S.R. [46] | United States | 1 | 36 | — | — | 1 | Diabetes, asthma, obesity, preeclampsia | Nonsevere | — | — | Acute pancreatitis | — |
| Rubin, E.S. [47] | United States | 1 | 26 | — | — | 1 | Chronic hypertension | Nonsevere | — | — | — | — |
| San-Juan, R. [79] | Spain | 32 | 32 | 1 | 9 | 22 | None (n = 26), asthma (n = 4), obesity (n = 1), multiple sclerosis (n = 1), diabetes (n = 2) | Severe (n = 18) | 2 | 2 | ARDS (n = 8) | — |
| Savasi, V.M. [59] | Italy | 77 | 15–48 | 4 | 13 | 50e | Known comorbidities (n = 24 including obesity and cardiovascular, autoimmune, endocrine, and metabolic diseases) | Asymptomatic (n = 12), severe (n = 14) | 6 | 6 | — | |
| Schnettler, WT [48] | United States | 1 | 39 | — | — | 1 | Myotonic dystrophy, bicuspid aortic valve, a prior mild cerebrovascular accident | Severe | 1 | 1 | ARDS | — |
| Sentilhes, L. [80] | France | 54 | 19-42 | Not extractable | Obesity (n = 4), asthma (n = 5), chronic hypertension (n = 1), other (n = 4) | Nonsevere (n = 37), severe and critical (n = 17) | 5 | 5 | ARDS (n = 1) | — | ||
| Shojaei, S. [115] | Iran | 1 | 38 | — | 1 | — | None | Severe | 1 | 1 | Cardiac arrest | 1 |
| Silverstein, J.S. [49] | United States | 2 | 17, 34 | — | — | 2 | Obesity (n = 1) | Severe (n = 2) | 2 | 2 | — | — |
| Sinkey, R.G. [50] | United States | 1 | 25 | — | — | 1 | Hypertension, preeclampsia, obesity, anemia | Not reported | Not reported | — | HF, pulmonary edema | — |
| Slayton-Milam, S. [51] | United States | 1 | 27 | — | — | 1 | Not reported | Severe | 1 | 1 | Worsening anemia | — |
| Taghizadieh, A. [116] | Iran | 1 | 33 | — | — | 1 | None | Severe | 1 | 1 | Acute kidney injury | — |
| Takemoto, M.L.S. [117] | Brazil | 978 | 29.5 for recovered, 31.5 for died women | Not reported | Cardiovascular (n = 54), diabetes (n = 89), obesity (n = 44), asthma (n = 23) | Not reported | 207 | 317 | Not reported | 124 | ||
| Tutiya, C.T. [118] | Brazil | 2 | 44, 29 | — | — | 3 | Obesity (n = 2), history of breast cancer (n = 1), hypertension (n = 1) | Severe | 2 | 2 | Pulmonary microthrombi | — |
| Vallejo, V. [8] | United States | 1 | 36 | — | — | 1 | Obesity | Severe | 1 | 1 | MODS | 1 |
| Vibert, F. [81] | France | 1 | 21 | — | 1 | — | None | Severe | 1 | 1 | — | — |
| Vivanti, A.J. [82] | France | 100 | 29–37 | — | 100 | Asthma (n = 9), diabetes (n = 7), hypertension (n = 6) | Severe (n = 10) | 10 | 9 | ARDS (n = 6), transient hepatitis (n = 1) | — | |
| Wang, X. [119] | China | 1 | 28 | — | — | 1 | Not reported | Severe | 1 | Not reported | — | — |
| Wang, Z. [120] | China | 30 | 29.9 | — | — | 30 | Hypertension (n = 5), diabetes (n = 2), hypothyroidism (n = 1), obesity (n = 1) | Nonsevere (n = 30) | Not reported | Not reported | Not reported | — |
| Wu, C. [121] | China | 8 | 26-35 | — | — | 8 | Not reported | Asymptomatic (n = 4), nonsevere (n = 4) | — | — | — | — |
| Wu, X. [122] | China | 23 | 21-37 | 3 | — | 20 | Hypothyroidism (n = 2), hepatitis B (n = 2), hypertension (n = 4), none (n = 15) | Asymptomatic (n = 15), nonsevere (n = 8) | Not reported | Not reported | Not reported | — |
| Wu, Y. [123] | China | 13 | 26-40 | 5 | 3 | 5 | Not reported | Nonsevere | — | — | — | — |
| Xia, H. [124] | China | 1 | 27 | — | — | 1 | Not reported | Not reported | Not reported | — | — | — |
| Xiong, X. [125] | China | 1 | 25 | — | — | 1 | Not reported | Nonsevere | — | — | — | |
| Xu, L. [126] | China | 5 | 23-34 | — | — | 5 | Anemia (n = 2) | Nonsevere (n = 5) | — | — | — | — |
| Yan, J. [127] | China | 116 | 24-41 | 4 | 6 | 106 | Diabetes (n = 8), hypertensive disorders (n = 5) | Nonsevere (n = 108), severe (n = 8) | 8 | 8 | — | — |
| Yang, H. [128] | China | 27 | 22-39 | 4 | — | 23 | Diabetes (n = 3), coagulopathy (n = 3), gestational hypertension (n = 2), hypothyroidism (n = 2), preeclampsia (n = 1), hypoproteinemia (n = 1), hepatitis (n = 2), schistosomiasis (n = 1) | Severe (n = 1) | — | — | — | — |
| Yassa, M. [129] | Turkey | 8 | 19-41 | 3 | 3 | 2 | Not reported | Not reported | 1 | Not reported | — | — |
| Yu, N. [130] | China | 7 | 29-34 | — | — | 7 | Hypothyroidism (n = 1), polycystic ovary (n = 1), none (n = 5) | Not reported | — | — | — | — |
| Zamaniyan, M. [131] | Iran | 1 | 22 | — | — | 1 | Hypothyroidism | Severe | 1 | 1 | ARDS | 1 |
| Zambrano, L.I. [132] | Honduras | 1 | 41 | — | — | 1 | Hypertension, hypothyroidism | Nonsevere | — | — | — | — |
| Zeng, Y. [133] | China | 16 | 25-40 | — | — | 16 | Cardiac disease (n = 2), hypothyroidism (n = 2), thalassemia (n = 1) | Nonsevere (n = 16) | Not reported | — | Not reported | — |
| Zhang, L. [134] | China | 18 | 24-34 | — | — | 16 | Preeclampsia (n = 1), diabetes (n = 3) | Nonsevere (n = 17), severe (n = 1) | Not reported | Not reported | — | — |
ARDS: acute respiratory distress syndrome; MODS: multiple organ dysfunction syndrome; SCIM: septic-induced ischemic cardiomyopathy; HF: heart failure; RF: respiratory failure; DIC: disseminated intravascular coagulation. aData on symptom status were missing for 2852 (35%) pregnant women. bA total of 6079 (74%) pregnant women have missing information for ICU admission and were assumed to have not been admitted to an ICU. cA total of 6351 (77%) pregnant women have missing information for receipt of mechanical ventilation and were assumed to have not received mechanical ventilation. dA total of 3819 (47%) pregnant women have missing information on death and were assumed to have survived. e10 patients were postpartum women.
In terms of the country of origin, the highest frequencies were in China with 34 articles [8, 12, 21–52], the United States with 33 articles [5, 8, 12, 22–51], and Italy with 8 articles [52–59]. 65 studies including 10183 patients were undertaken in the high-income countries [5, 8, 12, 22–83] and 52 studies (n = 1575) in middle-income countries [21, 84–134].
3.3. Outcomes
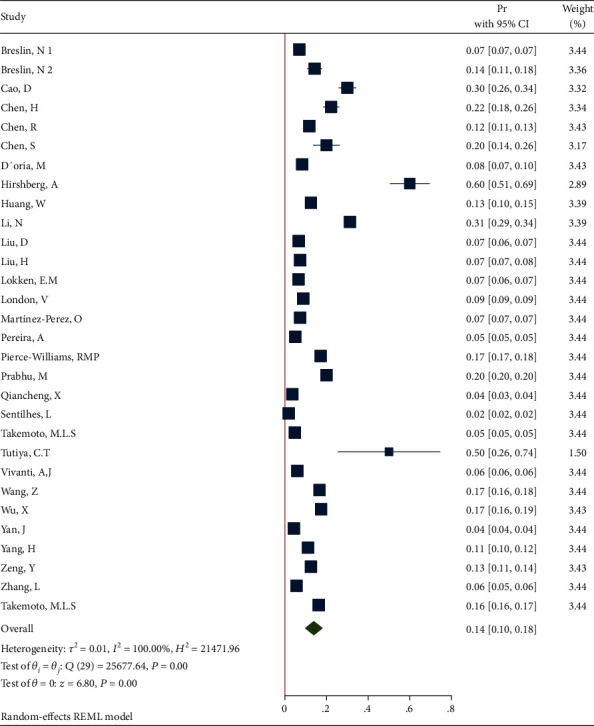
3.3.1. Mortality Rate of Pregnant and Postpartum Women due to COVID-19
In total, there were 153 deaths out of 11758 pregnant and postpartum women affected by COVID-19 (1.30%), of which 19 deceased patients were in high-income countries including the United Kingdom, United States, Italy, Switzerland, France, Sweden, Portugal, Netherlands, Ireland, Spain, Canada, and Australia (mortality rate = 0.19%) and 134 women were in middle-income countries including China, Iran, Iraq, Jordan, Peru, Turkey, India, Venezuela, Thailand, Brazil, and Honduras (mortality rate = 8.51%).
The data on 136 cases of maternal death due to COVID-19 is presented in Table 3. The highest mortality rate was reported in the study of Takemoto et al. in Brazil using the Brazilian Ministry of Health's ARDS Surveillance System. In this study, the authors found 124 deaths in COVID-19-infected pregnant or postpartum women (12.7%) [131].
Table 3.
Data on 136 cases of maternal death due to COVID-19, details of which were available.
| Case | Study (country) | Maternal age (years), gravida, para, gestational age (weeks) | Comorbidities | Presenting symptoms | Mode of delivery | Duration from admission to death | Polymerase chain reaction testing of the neonate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced maternal age | Obesity | Diabetes | Asthma | Cardiovascular | Other | |||||||
| 1 | Ahmed, I. (United Kingdom) | 29, G2P1, 29 | No | Yes | Yes | Yes | No | Renal disease, vitamin D deficiency | Fever | Cesarean | 15 days from the first admission, 7 days from the second admission | Negative |
| 2 | Karamim, P. (Iran) | 27, G2P1, 30 | No | No | No | No | No | No | Fever, cough, myalgia | Vaginal delivery | 3 days | N/A (stillbirth) |
| 3 | Shojaei, S. (Iran) | 38, G2, Ab1, 23 (twin) | Yes | No | No | No | No | No | Fever, cough, dyspnea | Vaginal delivery | 17 days | N/A (death of both fetuses) |
| 4 | Vallejo, V. (United States) | 36, G5P3Ab1, 37 | Yes | Yes | No | No | No | No | Fever, cough, sore throat | Cesarean | 2 days | Negative |
| 5 | Zamaniyan, M. (United States) | 22, not reported, 32 w | No | No | No | No | No | Hypothyroidism | Fever, cough, dyspnea, myalgia, anorexia, nausea | Cesarean | 19 days | First negative, second test positive 24 hours later |
| 6 | Hantoushzadeh, S. (Iran) | 25-29a, G2P1, 30 | No | No | No | No | No | No | Fever, cough, dyspnea, myalgia | Vaginal delivery | 4 days | N/A (fetal death) |
| 7 | 25-29a, G1P0, 38 | No | Yes | No | No | No | No | Fever, cough, dyspnea, myalgia | Cesarean | 5 days | Negative | |
| 8 | 40-44a, G2P1, 30 | Yes | No | No | No | No | Hypothyroidism | Fever, cough | Cesarean | 6 days | Negative on day of life 1, positive on day of life 7 | |
| 9 | 30-34a, G3P0, 24 | No | No | No | No | No | No | Fever, cough, dyspnea, myalgia | Undelivered | 8 days | N/A (fetal death) | |
| 10 | 30-34a, G2P1, 36 | No | No | Yes | No | No | No | Fever, cough | Cesarean | 10 days | Negative | |
| 11 | 35-39a, G2P0, 24 | Yes | No | No | No | No | No | Fever, cough, dyspnea, myalgia | Undelivered | 22 days | N/A (fetal death) | |
| 12 | 45-49a, G2P1, 28 | Yes | No | No | No | No | Underweight | Fever, cough, dyspnea | Cesarean | 18 days | Negative | |
| 13 to 136 | Takemoto, M.L.S. (Brazil)b | 31.5 (mean), no data about gravida or gestational age was available | Not reported | 13c | 22d | 5e | 13f | Not reported | Not reported | Not reported | Not reported | Not reported |
aMaternal age was gated in inclusive 5-year blocks (patient identification). b74 were pregnant and 50 were postpartum women. cMissing/unknown (%) = 50.8. dMissing/unknown (%) = 47.6. eMissing/unknown (%) = 56.5. fMissing/unknown (%) = 35.5.
3.3.2. Presenting Symptoms of COVID-19 in Pregnant and Postpartum Women Who Died of COVID-19
In all of the fatal cases with adequate data, fever alone or with cough was one of the presenting symptoms. After them, dyspnea (58.3%) and myalgia (50%) were the most common symptoms, respectively. Sore throat (8.3%) and gastrointestinal symptoms (anorexia, nausea) (8.3%) were rare.
3.3.3. Comorbidity Rate in Pregnant and Postpartum Women Who Died of COVID-19
The comorbidity rate in women who died from COVID-19 was 20% (Table 3). In total, 41.7% of the deceased patients were 35 years or older (advanced maternal age), 31.1% had diabetes, 21.9% were obese, 14.1% had cardiovascular disease (essential hypertension, gestational hypertension, preeclampsia, HELLP syndrome, and heart problems), and 9.1% had a history of asthma.
3.3.4. Meta-Analysis Results for Morbidities in Patients Who Died of COVID-19
The effect size in five studies for obesity was 0.47 (95% CI: 0.04 to 0.90, P value = 0.03), for diabetes was 0.29 (95% CI: -0.08 to 0.65, P value = 0.12), for asthma was 0.41 (95% CI: -0.06 to 0.88, P value = 0.09), for cardiovascular disease was 0.03 (95% CI: -0.03 to 0.10, P value = 0.32), for advanced maternal age was 0.61 (95% CI: 0.13 to 1.08, P value = 0.01), and for all comorbidity rate was 14.02 (95% CI: -7.59 to 35.63, P value = 0.20), based on a random effect model, with significant heterogeneity between studies (I2 = 100.0% or about 100.0%, H2 > 1 and PQ < 0.001 for all effect sizes). Figure 2 shows the forest plot of individual effect sizes within each study. Assessment for bias by Egger's and Begg's tests showed no significant small-study effects (all P > 0.05). Further visual inspection of the funnel plot suggested a slight degree of publication bias (Figure 3).
Figure 2.
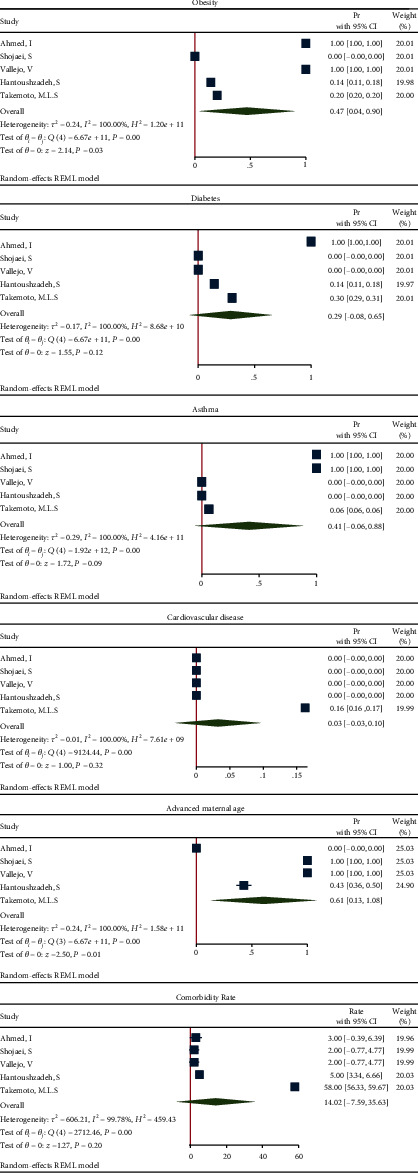
Forest plot of individual effect size for components and all comorbidities.
Figure 3.
Funnel plot of log relative risks vs. the standard error for components and all comorbidities.
3.3.5. Meta-Analysis Results for Obesity
(1) Obesity in All Pregnant and Postpartum Women Affected by COVID-19. The proportion from 33 studies was 0.69 (95% CI: 0.56 to 0.82, P value < 0.001) based on a random effect model, with significant heterogeneity between studies (τ2 = 0.14, I2 = 100.0%, H2 = 1.28e + 11, Q(df = 32) = 9.68e + 11, PQ < 0.001). Figure 4 shows the forest plot of individual effect sizes within each study. Assessment for bias by Egger's and Begg's tests showed no significant small-study effects (P = 0.308 > 0.05 and P = 0.054 > 0.05, respectively). Further visual inspection of the funnel plot suggests a slight degree of publication bias (Figure 5).
Figure 4.
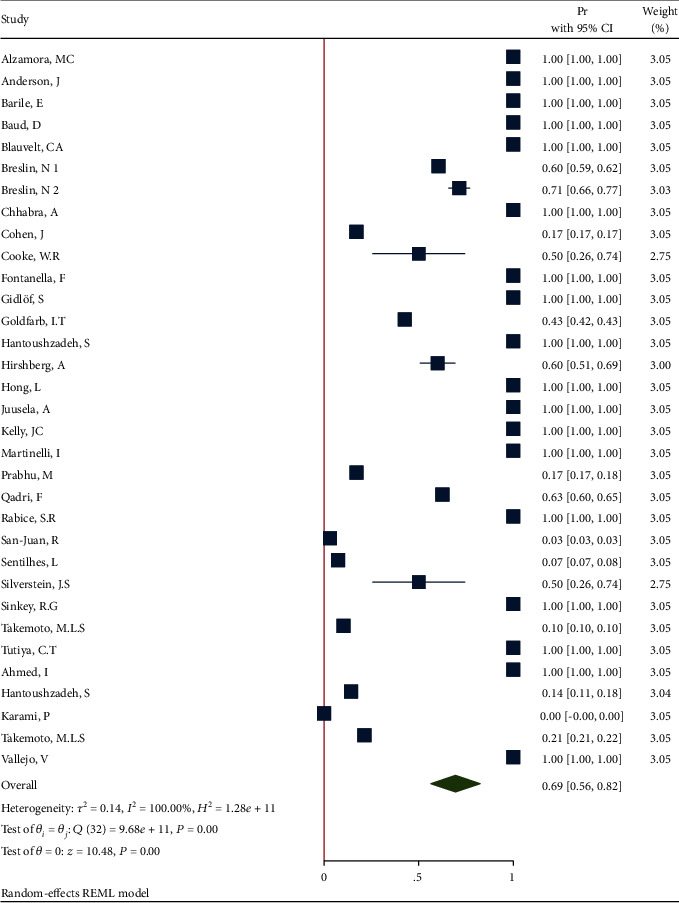
Forest plot of individual effect size for obesity.
Figure 5.
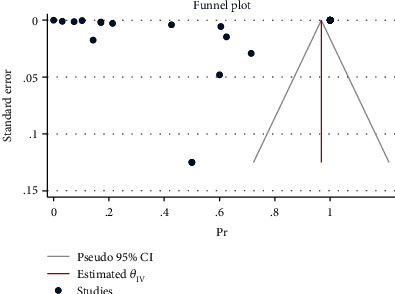
Funnel plot of log relative risks vs. the standard error for obesity.
(2) Sensitivity Analysis after Deleting the Studies with n=1. Removing these studies resulted in an effect size Pr = 0.49 (95% CI: 0.33 to 0.66, P value < 0.001), with a significant heterogeneity (τ2 = 0.12, I2 = 100.0%, H2 = 8.72e + 10, Q(df = 17) = 1.07e + 07, PQ < 0.001). Figure 6 shows the forest plot of individual effect sizes within each study.
Figure 6.
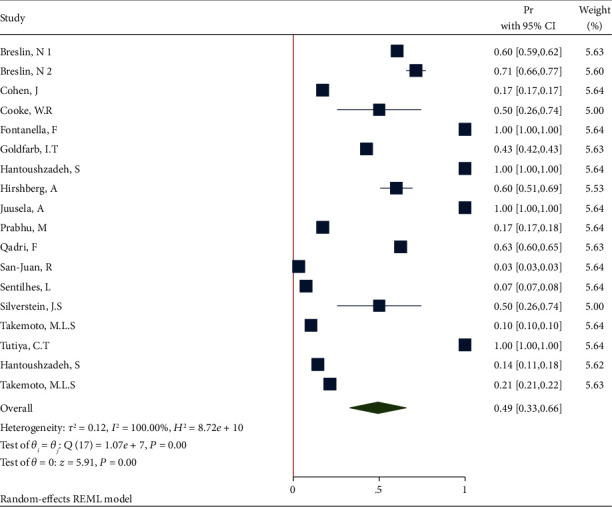
Forest plot of individual effect size for obesity after removing some studies.
(3) Subgroup Analysis of Obesity in Recovered and Dead Pregnant and Postpartum Women. The forest plot of the individual effect size of predetermined subgroup analysis by dead/recovered is presented in Figure 7. The results indicate higher proportion of outcome in the recovered subgroup (Pr = 0.53, 95%CI = 0.36 to 0.71), than in the deceased subgroup (Pr = 0.18, 95%CI = 0.11 to 0.25). Therefore, the test showed a significant difference between the subgroups (Q(df = 1) = 13.58, PQ < 0.001). Additionally, the heterogeneity did not reduce in all subgroups (I2 = 93.65% and I2 = 100% in the recovered and deceased subgroups, respectively).
Figure 7.
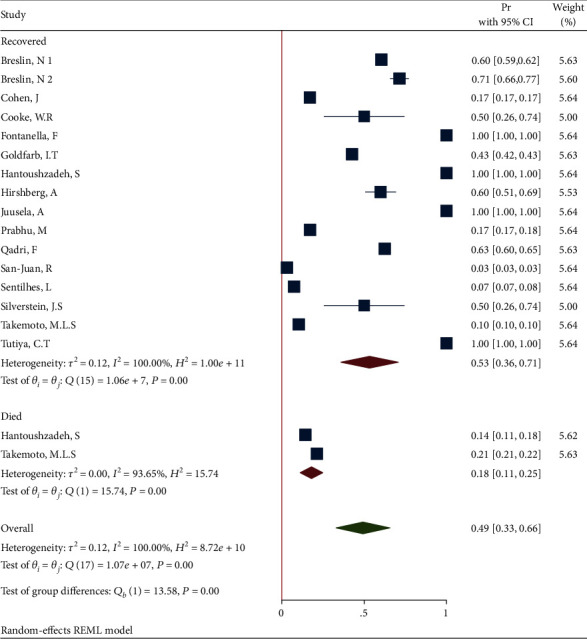
Forest plot of individual effect size for obesity by subgroups.
3.3.6. Meta-Analysis Results for Diabetes (Pregestational or Gestational)
(1) Diabetes in All Pregnant and Postpartum Women Affected by COVID-19. The proportion from 38 studies was 0.38 (95% CI: 0.25 to 0.51, P value < 0.001) based on a random effect model, with significant heterogeneity between studies (τ2 = 0.15, I2 = 100.0%, H2 = 4.62e + 10, Q(df = 37) = 5.95e + 7, PQ < 0.001). Figure 8 shows the forest plot of individual effect sizes within each study. Assessment for bias by Egger's and Begg's tests showed no significant small-study effects (P = 0.969 > 0.05 and P = 0.339 > 0.05, respectively). Further visual inspection of the funnel plot suggested a slight degree of publication bias (Figure 9).
Figure 8.
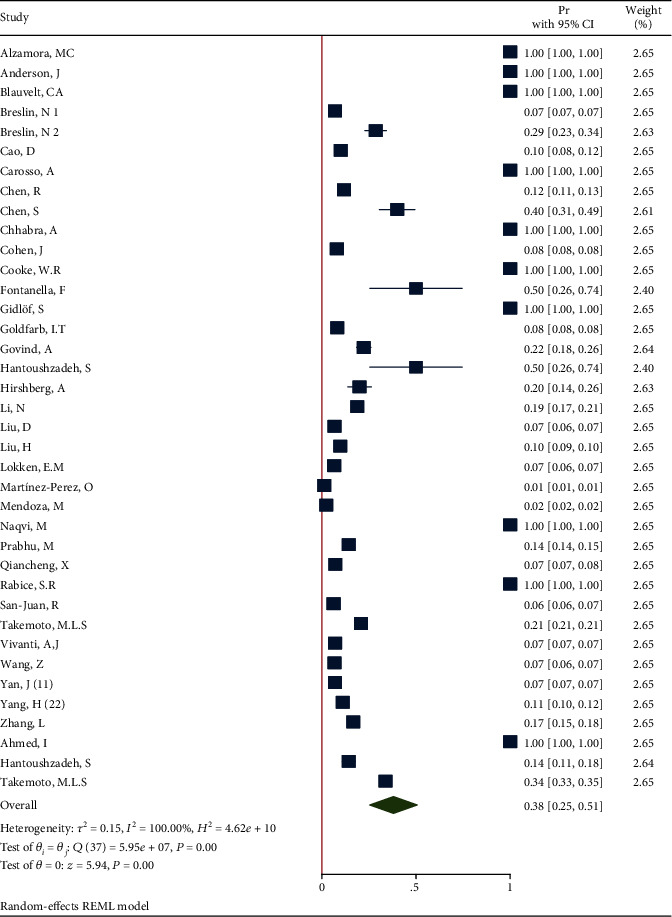
Forest plot of individual effect size for diabetes.
Figure 9.
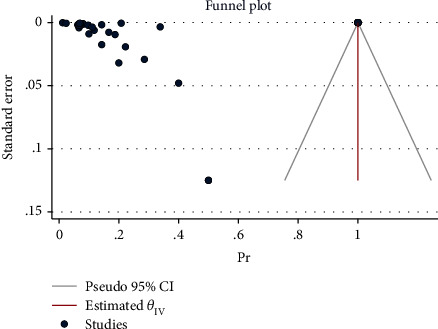
Funnel plot of log relative risks vs. the standard error for diabetes.
(2) Sensitivity Analysis after Deleting the Studies with n=1. Removing these studies resulted in an effect size Pr = 0.18 (95% CI: 0.11 to 0.25, P value < 0.001), with a significant heterogeneity (τ2 = 0.04, I2 = 100.0%, H2 = 1.76 + 5, Q(df = 27) = 5.95e + 07, PQ < 0.001). Figure 10 shows the forest plot of individual effect sizes within each study.
Figure 10.
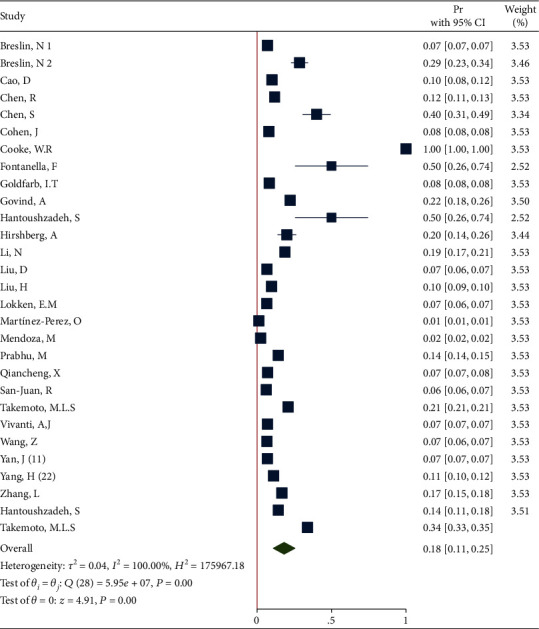
Forest plot of individual effect size for obesity after removing some studies.
(3) Subgroup Analysis of Diabetes in Recovered and Dead Pregnant and Postpartum Women. The forest plot of the individual effect size of predetermined subgroup analysis by dead/recovered is presented in Figure 11. The results indicate lower proportion of outcome in the recovered subgroup (Pr = 0.18, 95%CI = 0.10 to 0.25), than in the dead subgroup (Pr = 0.18, 95%CI = 0.11 to 0.25), so that the test showed a nonsignificant difference between the subgroups (Q(df = 1) = 0.37, PQ = 0.54 > 0.05). Additionally, the heterogeneity did not reduce in all subgroups (I2 = 100.0% and I2 = 99.17% in the recovered and dead subgroups, respectively).
Figure 11.
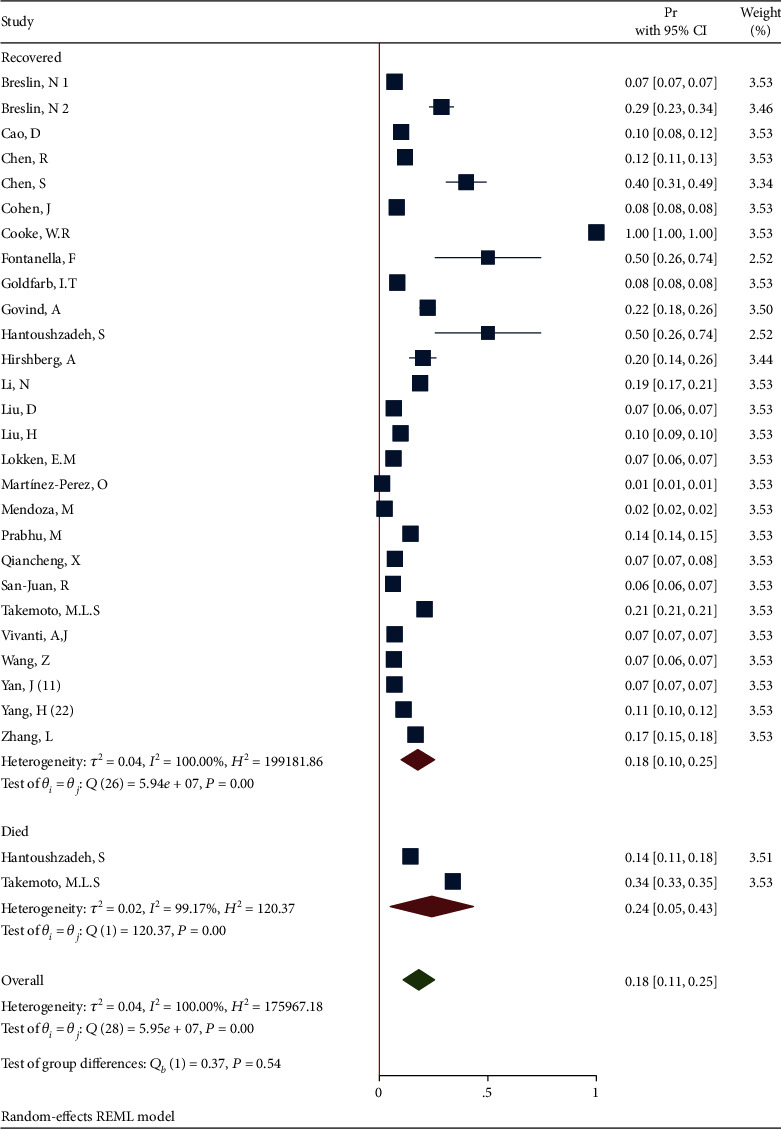
Forest plot of individual effect size for obesity by subgroups.
3.3.7. Meta-Analysis Results for Cardiovascular Diseases
(1) Cardiovascular Diseases in All Pregnant and Postpartum Women Affected by COVID-19. The proportion from 40 studies was 0.36 (95% CI: 0.24 to 0.48, P value < 0.001) based on a random effect model, with significant heterogeneity between studies (τ2 = 0.15, I2 = 100.0%, H2 = 3.52e + 10, Q(df = 39) = 3.42e + 8, PQ < 0.001). Figure 12 shows the forest plot of individual effect sizes within each study. Assessment for bias by Egger's and Begg's tests showed no significant small-study effects (P = 0.942 > 0.05 and P = 0.129 > 0.05, respectively). Further visual inspection of the funnel plot suggested a slight degree of publication bias (Figure 13).
Figure 12.
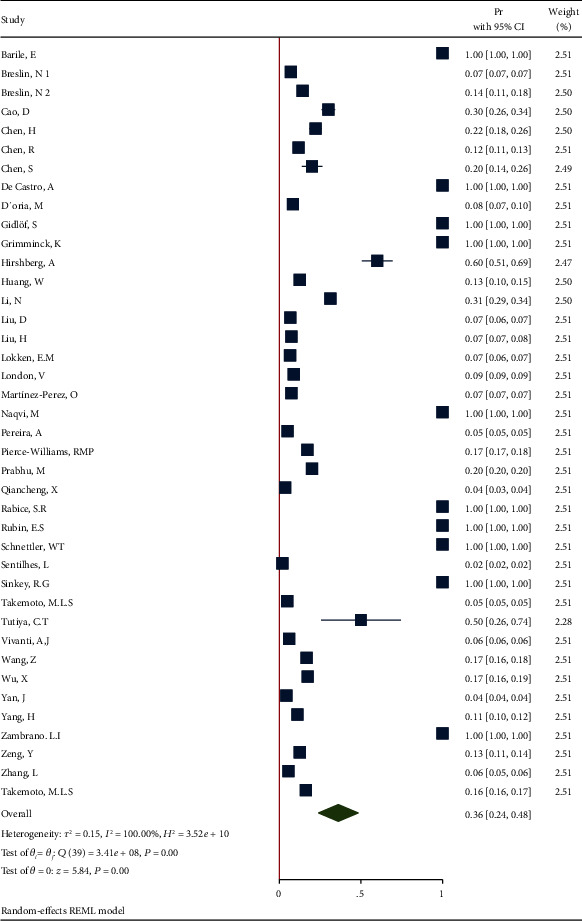
Forest plot of individual effect size for CVD.
Figure 13.
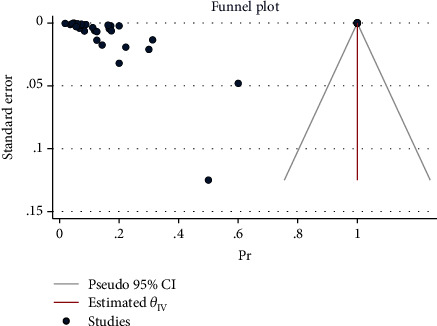
Funnel plot of log relative risks vs. the standard error for CVD.
(2) Sensitivity Analysis after Deleting the Studies with n=1. Removing these studies resulted in an effect size Pr = 0.14 (95% CI: 0.10 to 0.18, P value < 0.001), with a significant heterogeneity (τ2 = 0.01, I2 = 100.0%, H2 = 2.15e + 4, Q(df = 29) = 2.57e + 04, PQ < 0.001). Figure 14 shows the forest plot of individual effect sizes within each study.
Figure 14.
Forest plot of individual effect size for obesity after removing some studies.
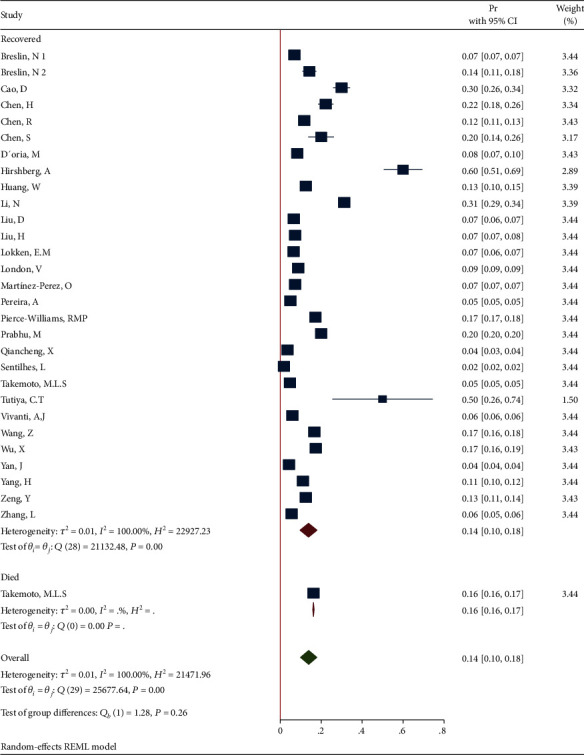
(3) Subgroup Analysis of Cardiovascular Diseases in Recovered and Dead Pregnant and Postpartum Women. The forest plot of the individual effect size of predetermined subgroup analysis by dead/recovered is presented in Figure 15. The results indicate lower proportion of outcome in the recovered subgroup (Pr = 0.14, 95%CI = 0.10 to 0.18), than in the dead subgroup (Pr = 0.16, 95%CI = 0.16 to 0.17), so that the test showed a nonsignificant difference between the subgroups (Q(df = 1) = 1.28, PQ = 0.26 > 0.05). Additionally, the heterogeneity did not reduce in all subgroup especially (I2 = 100.0% and I2 = noncomputable in the recovered and dead subgroups, respectively).
Figure 15.
Forest plot of individual effect size for obesity by subgroups.
3.3.8. Mode of Delivery in Pregnant and Postpartum Women Who Died of COVID-19
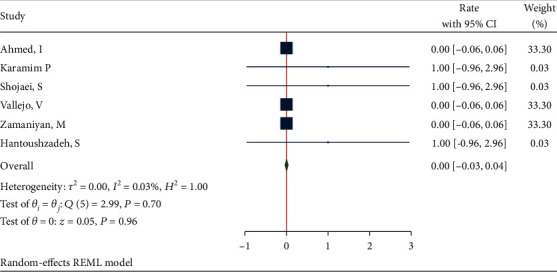
The mode of delivery in deceased cases with sufficient data (n = 12) was as follows: 58.3% had cesarean section, 25% had vaginal delivery, and 16.7% were not full term. Meta-analysis for mode of delivery in the fatal cases showed that the proportion from 6 studies was 0.00 (95% CI: -0.03 to 0.04, P value = 0.96) based on a random effect model, with nonsignificant heterogeneity between studies (τ2 = 0.00, I2 = 0.03%, H2 = 1, Q(df = 5) = 2.99, PQ = 0.70). Figure 16 shows the forest plot of individual effect sizes within each study.
Figure 16.
Forest plot of individual effect size for delivery type.
Assessment for bias by Egger's and Begg's tests showed no significant small-study effects (P = 0.084 > 0.05 and P = 0.181 > 0.05, respectively). Further visual inspection of the funnel plot suggested a slight degree of publication bias (Figure 17).
Figure 17.
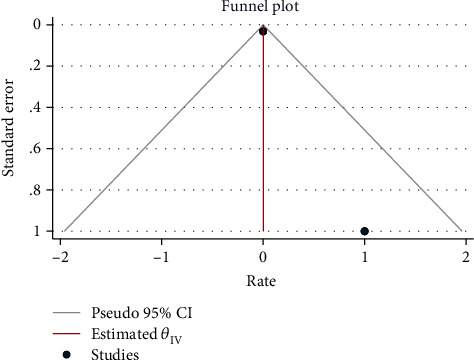
Funnel plot of log relative risks vs. the standard error for delivery type.
3.3.9. Other Findings
Duration from admission to death was between 2 and 22 days [85, 124]. The most common complication during treatment of COVID-19 in pregnant and postpartum women was acute respiratory distress syndrome (ARDS). In fatal cases, PCR testing of the neonate was not indicated in 41.6% of cases (stillbirth, undelivered). In 41.6%, it was negative, and in 16.7% of the cases, the result of the initial test was negative and the second test was positive [124, 134].
4. Discussion
In this study, we systematically investigated 117 published reports involving 11758 pregnant women from the high- and middle-income countries assessing the effect of COVID-19 on the risk of mortality.
In this systematic review, the mortality rate of COVID-19 in pregnant and postpartum women was 1.30% and the rate of severe pneumonia was reported from 0 to 14%. The majority of the patients were admitted to the ICU, and the maternal death was consistent with reported outcomes from other severe viral lower respiratory tract infections [5, 135–140]. Unlike the current study, in some studies, the mortality rate of COVID-19-infected pregnant women was not higher than nonpregnant women of reproductive age [13, 58, 141]. The absence of deaths was explained by the younger age pregnant women who were infected because it has been shown that the mortality rate in COVID-19 patients is high in older individuals and those patients with at least one comorbidity [127]. Also, the number of cases in these studies was relatively small and all women were in their third trimester of pregnancy and most of them gave birth earlier than seven days after diagnosis of the disease. Hence, this clinical manifestation-to-delivery time may be too short to affect pregnancies [142]. Additionally, this discrepancy may be due to the data available at the time of publication in our study. The pregnancy-related immunological changes may be one of the causes of maternal vulnerability to COVID-19, but this did not significantly affect the response against SARS-CoV-2 [142]. In addition, maternal mortality rates were lower in high-income compared with low-income countries. In this systematic review, most of the studies were from China. It is possible that our study reported a higher mortality rate than other studies because our sample size was larger. Similar to this finding, it was shown that the incidence of maternal mortality rate in middle-income countries seems at least six times higher than that in high-income countries [142]. These findings indicate the weaknesses of maternity services in low-income countries. In addition, major barriers for a more equitable delivery of critical care in low-income countries may be an important factor, such that in Brazil—a middle-income country—only 72% of COVID-19-infected pregnant or postpartum women with COVID-19 were admitted to the ICU and 15% of them did not receive ventilation support [131]. In Mexico, only two out of seven deaths had been admitted to the ICU and received invasive respiratory assistance [143].
Viral pneumonia is one of the leading causes of pregnancy deaths worldwide [12]. The symptoms of pneumonia in pregnant women are not different from others [144]. Maternal deaths due to cardiopulmonary complications, sometimes with multiorgan failure, have been reported in the previous literatures [9, 31, 102, 145]. In one study, pregnant women with SARS-CoV-2 infection in their second or third trimester of pregnancy died due to cardiopulmonary complications [9].
In all of the fatal cases, fever alone or with cough, dyspnea, and myalgia were the most common symptoms, respectively. Sore throat and gastrointestinal symptoms were rare. In accordance with this finding in another systematic review, the most common symptoms at presentation were fever, cough, dyspnea/shortness of breath, fatigue, and myalgia [146]. Data from nonpregnant adults have described the most common presenting symptoms of COVID-19 as fever, cough, and dyspnea [147, 148]. In contrast with our review, in some other systematic reviews, the symptoms were significantly different with fever and cough occurring more than myalgia as well as dyspnea and fatigue occurring only in approximately one-sixth of symptomatic pregnant women [3, 149–153].
Another finding in this study was the high prevalence of maternal comorbidities. The comorbidity rate in deceased women was 20%, and most of the pregnant women show biochemical evidence of inflammation, mainly lymphopenia. However, in one study, nearly half of all patients (46%) had no baseline comorbidities [31]. In another study, approximately one out of every three women with SARS-CoV-2 infection had a comorbid condition, but no maternal deaths secondary to COVID-19 were reported [13]. High comorbidity and lack of maternal mortality may be due to the younger age of mothers. While similar to Khalil et al.'s study, maternal mortality in our study was higher due to advanced maternal age (35 years of age or older) [148] which makes management of comorbidities challenging. Also, these comorbidities per se could cause maternal deaths.
Advanced maternal age (age > 35) was the most prevalent comorbidity; other comorbidities included diabetes, obesity, cardiovascular disease (essential hypertension, gestational hypertension, preeclampsia, HELLP syndrome, and heart problems), and history of asthma, respectively. These comorbidities suggest that maternal morbidity is not different from nonpregnant women of reproductive age. In one study, obesity and pulmonary conditions such as asthma and obstructive sleep apnea (OSA) were the most common comorbidities. Also, maternal ICU admission was one of the other outcomes. Also, pregnant and postpartum women with COVID-19 admitted to the ICUs are at risk for maternal death, which may occur even in the absence of substantial baseline comorbidities [31, 148]. Another comorbidity was antiviral drug use [146, 148]; while these two causes were not found in our study, the comorbidities could indirectly lead to patients' ICU admission and administration of antiviral drugs. In contrast to our study, another study showed none of the pregnant patients had preexisting comorbidities, such as hypertension, cardiovascular disease, and asthma [9, 146].
Based on the results, the majority of deliveries in pregnant women with SARS COVID-19 were cesarean section. Similar to this result, COVID-19 infection was associated with a relatively higher cesarean delivery in other studies [10, 31, 146]. Also, in another systematic review, the rate of cesarean delivery was higher than in our study because more than 90% of cesarean sections were from China (306/332). Some articles from China have shown SARS-CoV-2 infection as an indication for cesarean delivery [154–156], thereby justifying such a difference in the rate of cesarean section. In contrast to this finding, in Ferrazzi et al.'s study, 57% of women delivered vaginally and elective cesarean sections were performed in 43% of cases. Dyspnea or other COVID-19-related symptoms resulted in 23.8% cesarean sections among COVID-19-infected patients [157]. In the study by Khalil et al., the rate of cesarean section in mothers with COVID-19 was less than in our study. This difference can be explained by the fact that in the study by Khalil et al., the rate of comorbidity was higher than our study (32.5% vs. 20%), and this issue could be the reason for the cesarean section rate reduction.
5. Limitation
There are no data available for the first and early second trimester of pregnancy infections. Other limitation is the retrospective design (especially reports and case series) of the study. Also, we have only included studies which are reported in the English language. The strengths of this study are large number of studies, relatively high sample size, and the inclusion of studies from different countries.
6. Conclusion
COVID-19 infection was associated with higher rates (and pooled proportions) of cesarean section in pregnant women and their mortality. Based on the results of this study, COVID-19 cannot be considered as an indication for caesarian delivery. Therefore, the timing and mode of delivery should be individualized based on obstetrical indication and maternal situation. The findings of this study can be a guide to prenatal enhanced counseling for pregnant women with COVID-19.
Acknowledgments
Thanks are due for the financial support, guidance, and advice from the “Clinical Research Development Unit of Baqiyatallah Hospital.”
Contributor Information
Amir Vahedian-Azimi, Email: amirvahedian63@gmail.com.
Amirhossein Sahebkar, Email: amir_saheb2000@yahoo.com.
Data Availability
Data are available from the first and corresponding authors upon a reasonable request.
Conflicts of Interest
The authors report no conflict of interest.
References
- 1.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker A. How does COVID-19 affect pregnancy and postpartum care? June 2020, https://www.tmc.edu/news/2020/06/how-does-covid-19-affect-pregnancy-and-postpartum-care/
- 5.Qadri F., Mariona F. Pregnancy affected by SARS-CoV-2 infection: a flash report from Michigan. The Journal of Maternal-Fetal & Neonatal Medicine. 2020:1–3. doi: 10.1080/14767058.2020.1765334. [DOI] [PubMed] [Google Scholar]
- 6.Wong S. F., Chow K. M., Leung T. N., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. American Journal of Obstetrics and Gynecology. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ksiazek T. G., Erdman D., Goldsmith C. S., et al. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348(20):1953–1966. doi: 10.1056/nejmoa030781. [DOI] [PubMed] [Google Scholar]
- 8.Vallejo V., Ilagan J. G. A postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstetrics & Gynecology. 2020;136(1):52–55. doi: 10.1097/AOG.0000000000003950. [DOI] [PubMed] [Google Scholar]
- 9.Hantoushzadeh S., Shamshirsaz A. A., Aleyasin A., et al. Maternal death due to COVID-19. American Journal of Obstetrics and Gynecology. 2020;223(1):109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. American Journal of Obstetrics & Gynecology MFM. 2020;2(2, article 100107) doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boelig R. C., Manuck T., Oliver E. A., et al. Labor and delivery guidance for COVID-19. American Journal of Obstetrics & Gynecology MFM. 2020;2(2) doi: 10.1016/j.ajogmf.2020.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellington S., Strid P., Tong V. T., et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. Morbidity and Mortality Weekly Report. 2020;69(25):769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huntley B. J., Huntley E. S., Di Mascio D., Chen T., Berghella V., Chauhan S. P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) Infection. Obstetrics & Gynecology. 2020;136(2):303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 14.Arksey H., O'Malley L. Scoping studies: towards a methodological framework. International Journal of social Research Methodology. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 15.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Hardy R. J., Thompson S. G. A likelihood approach to meta-analysis with random effects. Statistics in Medicine. 1996;15(6):619–629. doi: 10.1002/(SICI)1097-0258(19960330)15:6<619::AID-SIM188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J. P., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 20.Harbord R. M., Egger M., Sterne J. A. C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 21.An P., Wood B. J., Li W., Zhang M., Ye Y. Postpartum exacerbation of antenatal COVID-19 pneumonia in 3 women. CMAJ. 2020;192(22):E603–E6E6. doi: 10.1503/cmaj.200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson J., Schauer J., Bryant S., Graves C. R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Reports in Women's Health. 2020;27 doi: 10.1016/j.crwh.2020.e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blauvelt C. A., Chiu C., Donovan A. L., et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19) Obstetrics & Gynecology. 2020;136(1):46–51. doi: 10.1097/aog.0000000000003949. [DOI] [PubMed] [Google Scholar]
- 24.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American Journal of Obstetrics & Gynecology MFM. 2020;2(2):p. 100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslin N., Baptiste C., Miller R., et al. Coronavirus disease 2019 in pregnancy: early lessons. American Journal of Obstetrics & Gynecology MFM. 2020;2(2):p. 100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne P. C., Linfert J. B., Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. American Journal of Perinatology. 2020;37(8):866–868. doi: 10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassett M. J., Lurvey L. D., Yasumura L., et al. Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization. American Journal of Perinatology. 2020;37(11):1110–1114. doi: 10.1055/s-0040-1714060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox N. S., Melka S. COVID-19 in pregnant women: case series from one large New York City obstetrical practice. American Journal of Perinatology. 2020;37(10):1002–1004. doi: 10.1055/s-0040-1712529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Futterman I., Toaff M., Navi L., Clare C. A. Covid-19 and hellp: overlapping clinical pictures in two gravid patients. American Journal of Perinatology Reports. 2020;10(2):E179–e182. doi: 10.1055/s-0040-1712978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldfarb I. T., Clapp M. A., Soffer M. D., et al. Prevalence and severity of coronavirus disease 2019 (COVID-19) illness in symptomatic pregnant and postpartum women stratified by Hispanic ethnicity. Obstetrics and Gynecology. 2020;136(2):300–302. doi: 10.1097/aog.0000000000004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulersen M., Blitz M. J., Rochelson B., Nimaroff M., Shan W., Bornstein E. Clinical implications of SARS-CoV-2 infection in the viable preterm period. American Journal of Perinatology. 2020;37(11):1077–1083. doi: 10.1055/s-0040-1713851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirshberg A., Kern-Goldberger A. R., Levine L. D., et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. American Journal of Obstetrics and Gynecology. 2020;223(2):286–290. doi: 10.1016/j.ajog.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong L., Smith N., Keerthy M., et al. Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Reports in Women's Health. 2020;27:p. e00217. doi: 10.1016/j.crwh.2020.e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal S. N., Overcash R., Mokhtari N., et al. An uncomplicated delivery in a patient with Covid-19 in the United States. New England Journal of Medicine. 2020;382(16):p. e34. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juusela A., Nazir M., Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. American Journal of Obstetrics & Gynecology MFM. 2020;3(2, article 100113) doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly J. C., Dombrowksi M., O'Neil-Callahan M., Kernberg A. S., Frolova A. I., Stout M. J. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. American Journal of Obstetrics & Gynecology MFM. 2020;2(3, article 100130) doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury R., Bernstein P. S., Debolt C., et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstetrics and Gynecology. 2020;136(2):273–282. doi: 10.1097/AOG.0000000000004025. Epub 2020/06/20. eng. [DOI] [PubMed] [Google Scholar]
- 38.Lokken E. M., Walker C. L., Delaney S., et al. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington state. American Journal of Obstetrics and Gynecology. 2020;223(6):911.e1–911.e14. doi: 10.1016/j.ajog.2020.05.031. Epub 2020/05/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.London V., McLaren R., Jr., Atallah F., et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. American Journal of Perinatology. 2020;37(10):991–994. doi: 10.1055/s-0040-1712164. Epub 2020/05/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucarelli E., Behn C., Lashley S., Smok D., Benito C., Oyelese Y. Mechanical ventilation in pregnancy due to COVID-19: a cohort of three cases. American Journal of Perinatology. 2020;37(10):1066–1069. doi: 10.1055/s-0040-1713664. Epub 2020/06/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta H., Ivanovic S., Cronin A., VanBrunt L., Mistry N. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Reports in Women's Health. 2020;27, article e00220 doi: 10.1016/j.crwh.2020.e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulvey J. J., Magro C. M., Ma L. X., Nuovo G. J., Baergen R. N. A mechanistic analysis placental intravascular thrombus formation in COVID-19 patients. Annals of Diagnostic Pathology. 2020;46:p. 151529. doi: 10.1016/j.anndiagpath.2020.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naqvi M., Zakowski P., Glucksman L., Smithson S., Burwick R. M. Tocilizumab and remdesivir in a pregnant patient with coronavirus disease 2019 (COVID-19) Obstetrics and Gynecology. 2020;136(5):1025–1029. doi: 10.1097/AOG.0000000000004050. Epub 2020/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 44.RAM P.-W., Burd J., Felder L., et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. American Journal of Obstetrics & Gynecology MFM. 2020;2(3, article 100134) doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prabhu M., Cagino K., Matthews K. C., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG : An International Journal of Obstetrics and Gynaecology. 2020;127(12):1548–1556. doi: 10.1111/1471-0528.16403. Epub 2020/07/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabice S. R., Altshuler P. C., Bovet C., Sullivan C., Gagnon A. J. COVID-19 infection presenting as pancreatitis in a pregnant woman: a case report. Case Reports in Women's Health. 2020;27, article e00228 doi: 10.1016/j.crwh.2020.e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubin E. S., Sansone S. A., Hirshberg A., Clement E. G., Srinivas S. K. Detection of COVID-19 in a vulvar lesion. American Journal of Perinatology. 2020;37(11):1183–1184. doi: 10.1055/s-0040-1713665. Epub 2020/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnettler W. T., Al Ahwel Y., Suhag A. Severe acute respiratory distress syndrome in coronavirus disease 2019-infected pregnancy: obstetric and intensive care considerations. American Journal of Obstetrics & Gynecology MFM. 2020;2(3, article 100120) doi: 10.1016/j.ajogmf.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverstein J. S., Limaye M. A., Brubaker S. G., et al. Acute respiratory decompensation requiring intubation in pregnant women with SARS-CoV-2 (COVID-19) American Journal of Perinatology Reports. 2020;10(2):E169–e175. doi: 10.1055/s-0040-1712925. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinkey R. G., Rajapreyar I., Robbins L. S., et al. Heart failure with preserved ejection fraction in a postpartum patient with superimposed preeclampsia and COVID-19. American Journal of Perinatology Reports. 2020;10(2):E165–e168. doi: 10.1055/s-0040-1712926. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slayton-Milam S., Sheffels S., Chan D., Alkinj B. Induction of labor in an intubated patient with coronavirus disease 2019 (COVID-19) Obstetrics and Gynecology. 2020;136(5):962–964. doi: 10.1097/AOG.0000000000004044. Epub 2020/06/27. eng. [DOI] [PubMed] [Google Scholar]
- 52.Barile L., Cerrano M., Locatelli A., Puppo A., Signorile A. F., Barzaghi N. Prone ventilation in a 27 week pregnant woman with COVID-19 severe ards. Signa Vitae. 2020;16(1):199–202. [Google Scholar]
- 53.Buonsenso D., Raffaelli F., Tamburrini E., et al. Clinical role of lung ultrasound for diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound in Obstetrics & Gynecology. 2020;56(1):106–109. doi: 10.1002/uog.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carosso A., Cosma S., Borella F., et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020;249:98–99. doi: 10.1016/j.ejogrb.2020.04.023. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Socio G. V., Malincarne L., Arena S., et al. Delivery in asymptomatic Italian woman with SARS-CoV-2 infection. Mediterranean Journal of Hematology and Infectious Diseases. 2020;12(1, article e2020033) doi: 10.4084/MJHID.2020.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Castro A., Abu-Hishmeh M., El Husseini I., Paul L. Haemophilus parainfluenzae endocarditis with multiple cerebral emboli in a pregnant woman with coronavirus. IDCases. 2019;18, article e00593 doi: 10.1016/j.idcr.2019.e00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferraiolo A., Barra F., Kratochwila C., et al. Report of positive placental swabs for sars-cov-2 in an asymptomatic pregnant woman with covid-19. Medicina. 2020;56(6):p. 306. doi: 10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinelli I., Ferrazzi E., Ciavarella A., et al. Pulmonary embolism in a young pregnant woman with COVID-19. Thrombosis Research. 2020;191:36–37. doi: 10.1016/j.thromres.2020.04.022. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savasi V. M., Parisi F., Patanè L., et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstetrics and Gynecology. 2020;136(2):252–258. doi: 10.1097/AOG.0000000000003979. Epub 2020/05/21. eng. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed I., Azhar A., Eltaweel N., Tan B. K. First COVID‐19 maternal mortality in the UK associated with thrombotic complications. British Journal of Haematology. 2020;190(1):e37–e38. doi: 10.1111/bjh.16849. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baud D., Greub G., Favre G., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA - Journal of the American Medical Association. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen J., Vignaux O., Jacquemard F. Covid-19 in pregnant women: general data from a French National Survey. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;251:267–268. doi: 10.1016/j.ejogrb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collin J., Byström E., Carnahan A., Ahrne M. Public Health Agency of Sweden’s brief report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstetricia et Gynecologica Scandinavica. 2020;99(7):819–822. doi: 10.1111/aogs.13901. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooke W. R., Billett A., Gleeson S., et al. SARS-CoV-2 infection in very preterm pregnancy: experiences from two cases. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;250:259–260. doi: 10.1016/j.ejogrb.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dória M., Peixinho C., Laranjo M., Mesquita Varejão A., Silva P. T. Covid-19 during pregnancy: a case series from an universally tested population from the north of Portugal. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;250(10):261–262. doi: 10.1016/j.ejogrb.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontanella F., Hannes S., Keating N., et al. COVID-19 infection during the third trimester of pregnancy: current clinical dilemmas. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;251:268–271. doi: 10.1016/j.ejogrb.2020.05.053. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gidlöf S., Savchenko J., Brune T., Josefsson H. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstetricia et Gynecologica Scandinavica. 2020;99(7):948–949. doi: 10.1111/aogs.13862. [DOI] [PubMed] [Google Scholar]
- 68.González Romero D., Ocampo Pérez J., González Bautista L., Santana-Cabrera L. Pregnancy and perinatal outcome of a woman with COVID-19 infection. Revista Clínica Española. 2020;220(8):533–534. doi: 10.1016/j.rce.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Govind A., Essien S., Karthikeyan A., et al. Re: Novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020;251:272–274. doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grimminck K., Santegoets L. A. M., Siemens F. C., Fraaij P. L. A., Reiss I. K. M., Schoenmakers S. No evidence of vertical transmission of SARS-CoV-2 after induction of labour in an immune-suppressed SARS-CoV-2-positive patient. BMJ Case Reports. 2020;13(6, article e235581) doi: 10.1136/bcr-2020-235581. Epub 2020/07/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kayem G., Lecarpentier E., Deruelle P., et al. A snapshot of the Covid-19 pandemic among pregnant women in France. Journal of Gynecology Obstetrics and Human Reproduction. 2020;49(7, article 101826) doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhrt K., McMicking J., Nanda S., Nelson-Piercy C., Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by COVID-19, without vertical transmission to the babies. American Journal of Obstetrics & Gynecology MFM. 2020;2(3, article 100135) doi: 10.1016/j.ajogmf.2020.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lowe B., Bopp B. COVID-19 vaginal delivery – a case report. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2020;60(3):465–466. doi: 10.1111/ajo.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyra J., Valente R., Rosário M., Guimarães M. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Médica Portuguesa. 2020;33(6, article 13883):429–431. doi: 10.20344/amp.13883. [DOI] [PubMed] [Google Scholar]
- 75.Martínez-Perez O., Vouga M., Cruz Melguizo S., et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA - Journal of the American Medical Association. 2020;324(3):296–299. doi: 10.1001/jama.2020.10125. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mendoza M., Garcia-Ruiz I., Maiz N., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG: An International Journal of Obstetrics & Gynaecology. 2020;127(11):1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nesr G., Garnett C., Bailey C., Arami S. Immune thrombocytopenia flare with mild COVID-19 infection in pregnancy: a case report. British Journal of Haematology. 2020;190(3):e146–e148. doi: 10.1111/bjh.16928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2020;99(7):839–847. doi: 10.1111/aogs.13921. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.San-Juan R., Barbero P., Fernández-Ruiz M., et al. Incidence and clinical profiles of COVID-19 pneumonia in pregnant women: a single-centre cohort study from Spain. EClinicalMedicine. 2020;23:p. 100407. doi: 10.1016/j.eclinm.2020.100407. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sentilhes L., De Marcillac F., Jouffrieau C., et al. COVID-19 in pregnancy was associated with maternal morbidity and preterm birth. American Journal of Obstetrics and Gynecology. 2020;223(6):914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vibert F., Kretz M., Thuet V., et al. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;250:257–258. doi: 10.1016/j.ejogrb.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vivanti A. J., Mattern J., Vauloup-Fellous C., et al. Retrospective description of pregnant women infected with severe acute respiratory syndrome coronavirus 2, France. Emerging Infectious Diseases. 2020;26(9):2069–2076. doi: 10.3201/eid2609.202144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirtsman M., Diambomba Y., Poutanen S. M., et al. Probable congenital sars-cov-2 infection in a neonate born to a woman with active sars-cov-2 infection. CMAJ. 2020;192(24):E647–E650. doi: 10.1503/cmaj.200821. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Kuraishy H., Al-Maiahy T., Al-Gareeb A., Musa R., Ali Z. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pacific Journal of Reproduction. 2020;9(3):156–158. [Google Scholar]
- 85.AlZaghal L. A., AlZaghal N., Alomari S. O., Obeidat N., Obeidat B., Hayajneh W. A. Multidisciplinary team management and cesarean delivery for a Jordanian woman infected with SARS-COV-2: a case report. Case Reports in Women's Health. 2020;27, article e00212 doi: 10.1016/j.crwh.2020.e00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alzamora M. C., Paredes T., Caceres D., Webb C. M., Valdez L. M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. American Journal of Perinatology. 2020;37(8):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bani Hani D. A., Alsharaydeh I., Bataineh A. M., et al. Successful anesthetic management in cesarean section for pregnant woman with COVID-19. The American Journal of Case Reports. 2020;21, article e925512 doi: 10.12659/AJCR.925512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bastug A., Hanifehnezhad A., Tayman C., et al. Virolactia in an asymptomatic mother with COVID-19. Breastfeeding Medicine. 2020;15(8):488–491. doi: 10.1089/bfm.2020.0161. [DOI] [PubMed] [Google Scholar]
- 89.Cao D., Yin H., Chen J., et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. International Journal of Infectious Diseases. 2020;95:294–300. doi: 10.1016/j.ijid.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H. J., Guo J. J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen L., Li Q., Zheng D., et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. New England Journal of Medicine. 2020;382(25):p. e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen R., Zhang Y., Huang L., Cheng B. H., Xia Z. Y., Meng Q. T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing cesarean delivery: a case series of 17 patients. Canadian Journal of Anesthesia. 2020;67(6):655–663. doi: 10.1007/s12630-020-01630-7. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. Journal of Medical Virology. 2020;92(9):1556–1561. doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Peng H., Wang L., et al. Infants born to mothers with a new coronavirus (COVID-19) Frontiers in Pediatrics. 2020;8:p. 104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chhabra A., Rao T., Kumar M., Singh Y., Subramaniam R. Anaesthetic management of a COVID-19 parturient for caesarean section - case report and lessons learnt. Indian Journal of Anaesthesia. 2020;64(14):S141–S143. doi: 10.4103/ija.IJA_509_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du Y., Wang L., Wu G., Lei X. M., Li W., Lv J. R. Anesthesia and protection in an emergency cesarean section for pregnant woman infected with a novel coronavirus: case report and literature review. Journal of Anesthesia. 2020;34(4):613–618. doi: 10.1007/s00540-020-02796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan C., Lei D., Fang C., et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clinical Infectious Diseases. 2020;17, article ciaa226 doi: 10.1093/cid/ciaa226. [DOI] [Google Scholar]
- 98.Forero-Peña D. A., Rodríguez M. I., Flora-Noda D. M., et al. The first pregnant woman with COVID-19 in Venezuela: pre-symptomatic transmission. Travel Medicine and Infectious Disease. 2020;25, article 101805 doi: 10.1016/j.tmaid.2020.101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hantoushzadeh S., Shamshirsaz A. A., Aleyasin A., et al. Maternal death due to COVID-19 disease. American Journal of Obstetrics and Gynecology. 2020;223(1):109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang W., Zhao Z., He Z., et al. Unfavorable outcomes in pregnant patients with COVID-19 outside Wuhan, China. Journal of Infection. 2020;81(2):e99–e101. doi: 10.1016/j.jinf.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalafat E., Yaprak E., Cinar G., et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound in Obstetrics & Gynecology. 2020;55(6):835–837. doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 102.Karami P., Naghavi M., Feyzi A., et al. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Medicine and Infectious Disease. 2020;(article 101665) doi: 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan S., Peng L. Y., Siddique R., et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infection Control & Hospital Epidemiology. 2020;41(6):748–750. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lang G. J., Zhao H. Can SARS-CoV-2-infected women breastfeed after viral clearance? Journal of Zhejiang University. Science. B. 2020;21(5):405–407. doi: 10.1631/jzus.B2000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li N., Han L., Peng M., et al. Maternal and neonatal outcomes of pregnant women with Coronavirus Disease 2019 (COVID-19) pneumonia: a case-control study. Clinical Infectious Diseases. 2020;71(16):2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Capobianco G., Saderi L., Aliberti S., et al. COVID-19 in pregnant women: a systematic review and meta-analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;252:543–558. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liao X. G., Yang H., Kong J. F., Yang H. B. Chest CT findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Medical Journal. 2020;37(4):226–228. doi: 10.4274/balkanmedj.galenos.2020.2020.3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu D., Li L., Wu X., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR. American Journal of Roentgenology. 2020;215:W17–W18. doi: 10.2214/AJR.20.23247. [DOI] [PubMed] [Google Scholar]
- 109.Liu H. H., Liu F., Li J. N., Zhang T. T., Wang D. B., Lan W. S. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. Journal of Infection. 2020;80(5):E7–E13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. The Journal of Infection. 2020;4 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu D. W., Sang L., Du S. H., Li T., Chang Y. G., Yang X. A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. Journal of Medical Virology. 2020;92(9):1660–1664. doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panichaya P., Thaweerat W., Uthaisan J. Prolonged viral persistence in COVID-19 second trimester pregnant patient. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2020;50:p. 263. doi: 10.1016/j.ejogrb.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng Z., Wang J., Mo Y., et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. Journal of Infection and Public Health. 2020;13(5):818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiancheng X., Jian S., Lingling P., et al. Coronavirus disease 2019 in pregnancy. International Journal of Infectious Diseases. 2020;95:376–383. doi: 10.1016/j.ijid.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shojaei S., Kouchek M., Miri M. M., et al. Twin pregnant woman with COVID-19: a case report. Journal of Cellular & Molecular Anesthesia. 2020;5(1):43–46. [Google Scholar]
- 116.Taghizadieh A., Mikaeili H., Ahmadi M., Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respiratory Medicine Case Reports. 2020;30:p. 101090. doi: 10.1016/j.rmcr.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takemoto M. L. S., Menezes M. O., Andreucci C. B., et al. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. International Journal of Gynaecology and Obstetrics. 2020;151(1):154–156. doi: 10.1002/ijgo.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tutiya C. T., Siaulys M. M., Kondo M. M., et al. Possible formation of pulmonary microthrombi in the early puerperium of pregnant women critically ill with COVID-19: two case reports. Case Reports in Women's Health. 2020;27, article e00237 doi: 10.1016/j.crwh.2020.e00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 Novel coronavirus in a pregnant woman with preterm delivery. Clinical Infectious Diseases. 2020;71(15):844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z., Wang Z., Xiong G. Clinical characteristics and laboratory results of pregnant women with COVID-19 in Wuhan, China. International Journal of Gynecology & Obstetrics. 2020;150(3):312–317. doi: 10.1002/ijgo.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu C., Yang W., Wu X., et al. Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women. Virologica Sinica. 2020;35(3):305–310. doi: 10.1007/s12250-020-00227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu X. Q., Sun R. H., Chen J. P., Xie Y. L., Zhang S. T., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. International Journal of Gynecology & Obstetrics. 2020;150(1):58–63. doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Y., Liu C., Dong L., et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG: An International Journal of Obstetrics & Gynaecology. 2020;127(9):1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. British Journal of Anaesthesia. 2020;124(5):e216–e218. doi: 10.1016/j.bja.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong X., Wei H., Zhang Z., et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID‐19. Journal of Medical Virology. 2020;92(9):1657–1659. doi: 10.1002/jmv.25857. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu L., Yang Q., Shi H., et al. Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Scientific Bulletin. 2020;65(18):1537–1542. doi: 10.1016/j.scib.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan J., Guo J., Fan C., et al. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. American Journal of Obstetrics and Gynecology. 2020;23(20):p. 30462. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang H., Hu B., Zhan S., Yang L. Y., Xiong G. Effects of SARS-CoV-2 infection on pregnant women and their infants: a retrospective study in Wuhan, China. Archives of Pathology & Laboratory Medicine. 2020;18(10):p. 2020. doi: 10.5858/arpa.2020-0232-SA. [DOI] [PubMed] [Google Scholar]
- 129.Yassa M., Birol P., Mutlu A. M., Tekin A. B., Sandal K., Tug N. Lung ultrasound can influence the clinical treatment of pregnant women with COVID-19. Journal of Ultrasound in Medicine. 2020;1 doi: 10.1002/jum.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu N., Li W., Kang Q., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. The Lancet Infectious Diseases. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zamaniyan M., Ebadi A., Aghajanpoor S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenatal Diagnosis. 2020;40(13):1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zambrano L. I., Fuentes-Barahona I. C., Bejarano-Torres D. A., et al. A pregnant woman with COVID-19 in Central America. Travel Medicine and Infectious Disease. 2020;36, article 101639 doi: 10.1016/j.tmaid.2020.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng Y., Lin L., Yan Q., et al. Update on clinical outcomes of women with COVID-19 during pregnancy. International Journal of Gynecology & Obstetrics. 2020;150(2):264–266. doi: 10.1002/ijgo.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang L., Dong L., Ming L., et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy and Childbirth. 2020;20(1):1–7. doi: 10.1186/s12884-020-03026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rasmussen S., Jamieson D., Uyeki T. Effects of influenza on pregnant women and infants. Obstetric Anesthesia Digest. 2013;33(4):208–209. doi: 10.1097/01.aoa.0000436327.13983.3c. [DOI] [PubMed] [Google Scholar]
- 136.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infectious Diseases. 2016;16(1):1–4. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aagaard-Tillery K., Silver R., Dalton J. Normal pregnancy. Seminars in Fetal & Neonatal Medicine. 2006;11:279–295. doi: 10.1016/j.siny.2006.04.003. Immunology of the Female Genital Tract. 2002;8:12. [DOI] [PubMed] [Google Scholar]
- 138.Littauer E. Q., Skountzou I. Hormonal regulation of physiology, innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Frontiers in Immunology. 2018;9:p. 2455. doi: 10.3389/fimmu.2018.02455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mosby L. G., Rasmussen S. A., Jamieson D. J. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. American Journal of Obstetrics and Gynecology. 2011;205(1):10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 140.Berghella V. Coronavirus disease 2019 (COVID-19): pregnancy issues. 2020. UpToDate Internet, last updated: Feb 15, 2021.
- 141.Muhidin S., Moghadam Z. B., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV; a systematic review. Archives of Academic Emergency Medicine. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 142.Menezes M. O., Takemoto M. L. S., Nakamura‐Pereira M., et al. Risk factors for adverse outcomes among pregnant and postpartum women with acute respiratory distress syndrome due to COVID-19 in Brazil. International Journal of Gynecology & Obstetrics. 2020;151(3):415–423. doi: 10.1002/ijgo.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lumbreras‐Marquez M. I., Campos‐Zamora M., Lizaola‐Diaz de Leon H., Farber M. K. Maternal mortality from COVID-19 in Mexico. International Journal of Gynecology & Obstetrics. 2020;150(2):266–267. doi: 10.1002/ijgo.13250. [DOI] [PubMed] [Google Scholar]
- 144.Panahi L., Amiri M., Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Archives of Academic Emergency Medicine. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 145.Knight M., Bunch K., Vousden N., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Juan J., Gil M., Rong Z., Zhang Y., Yang H., Poon L. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound in Obstetrics & Gynecology. 2020;56(1):15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guan W. J., Ni Z. Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khalil A., Kalafat E., Benlioglu C., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25, article 100446 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cao M., Zhang D., Wang Y., et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. MedRxiv; 2020. [Google Scholar]
- 150.Parazzini F., Bortolus R., Mauri P. A., Favilli A., Gerli S., Ferrazzi E. Delivery in pregnant women infected with SARS-CoV-2: A fast review. International Journal of Gynecology & Obstetrics. 2020;150(1):41–46. doi: 10.1002/ijgo.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wen R., Sun Y., Xing Q.-S. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. Journal of Microbiology, Immunology and Infection. 2020;53(3):499–500. doi: 10.1016/j.jmii.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong L., Tian J., He S., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. Journal of the American Medical Association. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng H., Xu C., Fan J., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. Journal of the American Medical Association. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murad M. H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu H., Wang L., Fang C., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Translational Pediatrics. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu J., Liao X., Qian S., et al. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerging Infectious Diseases. 2020;26(6):1320–1323. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferrazzi E., Frigerio L., Savasi V., et al. Mode of delivery and clinical findings in COVID-19 infected pregnant women in Northern Italy. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3562464. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the first and corresponding authors upon a reasonable request.


