Version Changes
Revised. Amendments from Version 1
Two minor additions have been made to the discussion in response to reviewers’ comments. First, we included standing height in our regression models, but have added a note in the discussion acknowledging that sitting height and thoracic height are more closely related to lung function. We included a sensitivity analysis using sitting height, but thoracic height was not available. Second, we have added a suggestion that sex-differential effects of HHIP SNPs may be cell type-specific.
Abstract
Background: Lung function is highly heritable and differs between the sexes throughout life. However, little is known about sex-differential genetic effects on lung function. We aimed to conduct the first genome-wide genotype-by-sex interaction study on lung function to identify genetic effects that differ between males and females.
Methods: We tested for interactions between 7,745,864 variants and sex on spirometry-based measures of lung function in UK Biobank (N=303,612), and sought replication in 75,696 independent individuals from the SpiroMeta consortium.
Results: Five independent single-nucleotide polymorphisms (SNPs) showed genome-wide significant (P<5x10 -8) interactions with sex on lung function, and 21 showed suggestive interactions (P<1x10 -6). The strongest signal, from rs7697189 (chr4:145436894) on forced expiratory volume in 1 second (FEV 1) (P=3.15x10 -15), was replicated (P=0.016) in SpiroMeta. The C allele increased FEV 1 more in males (untransformed FEV 1 β=0.028 [SE 0.0022] litres) than females (β=0.009 [SE 0.0014] litres), and this effect was not accounted for by differential effects on height, smoking or pubertal age. rs7697189 resides upstream of the hedgehog-interacting protein ( HHIP) gene and was previously associated with lung function and HHIP lung expression. We found HHIP expression was significantly different between the sexes (P=6.90x10 -6), but we could not detect sex differential effects of rs7697189 on expression.
Conclusions: We identified a novel genotype-by-sex interaction at a putative enhancer region upstream of the HHIP gene. Establishing the mechanism by which HHIP SNPs have different effects on lung function in males and females will be important for our understanding of lung health and diseases in both sexes.
Keywords: genome-wide interaction study, lung function, sex, HHIP, expression
Introduction
Measures of lung function, including forced expiratory volume in 1 second (FEV 1) and forced vital capacity (FVC), are used to determine diagnosis and severity of chronic obstructive pulmonary disease (COPD). COPD refers to a group of complex lung disorders characterised by irreversible (and usually progressive) airway obstruction, and is projected to be the third leading cause of death globally in 2020 1. The major risk factor for COPD is smoking, but other environmental and genetic factors have been identified.
Physiological lung development and function differ throughout life between males and females 2. It is known that sex hormones can influence these processes but the mechanisms are not well understood 3, 4. The incidence and presentation of lung diseases such as COPD also exhibit sexual dimorphism. Traditionally viewed as a disease of older males, COPD has been increasing in prevalence amongst females over the last two decades. It has been reported that females are more vulnerable to environmental risk factors for COPD and are over-represented amongst sufferers of early-onset severe COPD 5, 6. Females are also more likely to present with small airway disease whereas males are more likely to develop emphysematous phenotype. Moreover, females report more frequent and/or severe exacerbations of respiratory symptoms than males and higher levels of dyspnoea and cough 5.
In a recent paper, 279 genetic loci were reported as associated with lung function traits, but these only explain a small proportion of the heritability 7. One possible source of hidden heritability is the interaction between genetic factors and biological sex on lung function traits. A genome-wide genotype-by-sex interaction study in three studies comprising 6260 COPD cases and 5269 smoking controls found a putative sex-specific risk factor for COPD in the CELSR1 gene, a region not previously implicated in COPD or lung function 8. However, having sufficient statistical power to reproducibly detect genotype-by-sex interactions requires much larger sample sizes. Statistical power can also be enhanced by using quantitative lung function traits as outcomes instead of COPD diagnoses, but we are not aware of any genome-wide genotype-by-sex interaction studies on lung function traits. Understanding the role of sex in lung function and COPD will be important for developing therapeutics that work for both males and females 9.
In this study, we tested for an interaction effect of 7,745,864 variants and sex on FEV 1, FEV 1/FVC, FVC and PEF in 303,612 individuals from the UK Biobank resource. We sought replication of our findings in 75,696 independent individuals from the SpiroMeta consortium. To our knowledge this is the first genome-wide sex-by-genotype interaction study on lung function traits, and the largest sex-by-genotype interaction study to focus on COPD-related outcomes.
Results
We tested 7,745,864 genome-wide variants with minor allele frequency (MAF) ≥ 0.01 and imputation quality scores ≥ 0.3 for genotype-by-sex interactions on lung function in 303,612 unrelated individuals of European ancestry from UK Biobank. Five independent signals were identified showing genome-wide significant (P<5 x 10 -8) interaction with sex on at least one of four lung function traits (FEV 1, FEV 1/FVC, FVC, and PEF) with a further 21 SNPs showing suggestive significance (P<1 x 10 -6) ( Table 1; Figure S1, Extended data 10). The top three genome-wide significant signals had been previously reported for association with lung function: rs7697189 near the gene encoding hedgehog-interacting protein ( HHIP) (interaction P = 3.15 x 10 -15), rs9403386 near the gene encoding Adhesion G Protein-Coupled Receptor G6 ( ADGRG6, previously known as GPR126) (interaction P = 4.56 x 10 -9), and rs162185 downstream of the gene encoding transcription factor 21 ( TCF21) (interaction P = 4.87 x 10 -9) 11– 16. This may, in part, reflect greater power to detect interactions with variants with strong main effects on lung function. Only rs355079 (interaction P = 8.84 x 10 -7) showed significant effects in opposite directions in males compared to females.
Table 1. Association between top SNPs and lung function in males and females, and genotype-by-sex interaction results.
| SNP (nearest
gene) and coordinates |
Test/
other allele |
Trait | Lung function UK Biobank
males |
Lung function UK Biobank
females |
Sex interaction in UK
Biobank |
Sex interaction in
SpiroMeta |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | Beta (SE) | P | MAF | Beta (SE) | P | Beta (SE) | P | Beta (SE) | P | |||
| rs7697189 (HHIP)
4:145436894 |
C/G | FEV 1 | 0.390 | 0.052 (0.004) | 2.13E-33 | 0.392 | 0.013 (0.003) | 1.16E-05 | -0.040 (0.005) | 3.15E-15 | -0.025 (0.01) | 0.016 |
| rs9403386 (ADGRG6)
6:142764073 |
C/A | FEV 1/FVC | 0.031 | 0.214 (0.012) | 4.48E-75 | 0.031 | 0.128 (0.009) | 2.16E-43 | -0.086 (0.015) | 4.56E-09 | -0.035 (0.032) | 0.281 |
| rs162185 (TCF21)
6:134226147 |
C/T | PEF | 0.411 | -0.038 (0.004) | 1.35E-18 | 0.410 | -0.009 (0.003) | 0.002 | 0.030 (0.005) | 4.87E-09 | 0.022 (0.0139) | 0.083 |
| rs6480592 (CHST3)
10:73764509 |
C/T | PEF | 0.398 | -0.021 (0.004) | 1.66E-06 | 0.400 | 0.007 (0.003) | 0.011 | 0.028 (0.005) | 2.85E-08 | 0.003 (0.012) | 0.808 |
| rs111893604 (ZSCAN10)
16:3141104 |
G/T | FEV 1 | 0.059 | 0.040 (0.009) | 1.70E-05 | 0.059 | -0.020 (0.006) | 0.002 | -0.060 (0.011) | 4.04E-08 | 0.006 (0.026) | 0.827 |
| rs72694266
(RP11-907D1.1) 14:97578576 |
A/C | PEF | 0.077 | -0.044 (0.008) | 2.69E-07 | 0.078 | 0.008 (0.006) | 0.145 | 0.053 (0.010) | 6.31E-08 | -0.049 (0.027) | 0.066 |
| rs72781459
10:10247676 |
C/T | PEF | 0.096 | 0.031 (0.007) | 3.44E-05 | 0.097 | -0.012 (0.005) | 0.014 | -0.046 (0.009) | 1.08E-07 | 0.007 (0.021) | 0.729 |
| rs74316059
(RP11-649A16.1) 3:146983325 |
T/C | FEV 1/FVC | 0.042 | 0.049 (0.010) | 2.52E-06 | 0.043 | -0.018 (0.008) | 0.029 | -0.068 (0.013) | 2.38E-07 | -0.031 (0.028) | 0.269 |
| rs55789572 (EIF2S2/RALY) 20:32687822 | A/C | FEV 1 | 0.022 | 0.041 (0.015) | 0.006 | 0.022 | -0.047 (0.010) | 2.67E-06 | -0.089 (0.017) | 2.80E-07 | -0.01 (0.033) | 0.765 |
| rs74933518 (DAPK2)
15:64303295 |
A/G | PEF | 0.025 | -0.072 (0.014) | 1.23E-07 | 0.025 | 0.007 (0.009) | 0.421 | 0.082 (0.016) | 3.05E-07 | 0.025 (0.043) | 0.568 |
| rs11247571 (ABR)
17:908502 |
G/A | PEF | 0.343 | -0.025 (0.005) | 3.65E-08 | 0.344 | 0.002 (0.003) | 0.569 | 0.027 (0.005) | 3.22E-07 | 0.010 (0.014) | 0.473 |
| rs707588
(RP11-154H17.1) 1:5711430 |
G/A | FEV 1 | 0.482 | -0.020 (0.004) | 3.23E-06 | 0.482 | 0.006 (0.003) | 0.029 | 0.025 (0.005) | 3.27E-07 | 0.014 (0.01) | 0.183 |
| rs138473298 (AUTS2)
7:69644989 |
T/C | PEF | 0.012 | -0.077 (0.020) | 0.0002 | 0.011 | 0.043 (0.014) | 0.002 | 0.122 (0.024) | 3.52E-07 | 0.037 (0.060) | 0.540 |
| rs139069254
(RP11-648K4.2) 15:88113916 |
A/G | FEV 1 | 0.018 | 0.071 (0.016) | 1.83E-05 | 0.018 | -0.027 (0.011) | 0.017 | -0.098 (0.019) | 4.66E-07 | -0.051 (0.041) | 0.216 |
| rs138163836 (PVRL3)
3:110952902 |
C/T | FVC | 0.021 | 0.064 (0.015) | 1.94E-05 | 0.020 | -0.025 (0.011) | 0.019 | -0.091 (0.018) | 5.07E-07 | -0.025 (0.038) | 0.5 |
| rs28493055 (XDH)
2:31573390 |
T/G | FEV 1 | 0.012 | 0.065 (0.020) | 0.002 | 0.013 | -0.055 (0.014) | 6.40E-05 | -0.119 (0.024) | 5.60E-07 | 0.035 (0.054) | 0.519 |
| rs117380804
18:76145905 |
T/C | FVC | 0.035 | 0.035 (0.012) | 0.003 | 0.036 | -0.035 (0.008) | 1.93E-05 | -0.070 (0.014) | 6.25E-07 | -0.034 (0.03) | 0.255 |
| rs602622 (RASGRP3)
2:33658226 |
C/G | PEF | 0.444 | -0.022 (0.004) | 2.11E-07 | 0.445 | 0.002 (0.003) | 0.444 | 0.025 (0.005) | 6.45E-07 | -0.013 (0.013) | 0.323 |
| rs2253718
(RF00019, SFTA2) 6:30900427 |
T/G | PEF | 0.409 | -0.049 (0.004) | 5.69E-30 | 0.405 | -0.027 (0.003) | 1.78E-20 | 0.025 (0.005) | 7.05E-07 | 0.002 (0.016) | 0.925 |
| rs2353939 (HHIP)
4:145729724 |
G/A | FVC | 0.437 | 0.016 (0.004) | 0.0002 | 0.435 | -0.009 (0.003) | 0.002 | -0.025 (0.005) | 7.55E-07 | -0.016 (0.01) | 0.124 |
| rs7691139 (ZNF280A)
22:22876151 |
G/C | FEV 1/FVC | 0.116 | -0.025 (0.007) | 0.0003 | 0.115 | 0.017 (0.005) | 0.002 | 0.043 (0.009) | 7.62E-07 | Not tested | |
| rs13020954
2:17296984 |
C/T | FEV 1/FVC | 0.014 | 0.050 (0.017) | 0.004 | 0.014 | -0.057 (0.014) | 3.83E-05 | -0.109 (0.022) | 7.88E-07 | -0.062 (0.043) | 0.148 |
| rs2731120 (MLF1)
3:158297633 |
A/C | FVC | 0.346 | 0.029 (0.004) | 3.72E-11 | 0.346 | 0.003 (0.003) | 0.310 | -0.026 (0.005) | 8.14E-07 | -0.008 (0.011) | 0.433 |
| rs355079
(LMCD1-AS1) 3:8643371 |
T/C | FVC | 0.337 | 0.015 (0.004) | 0.0007 | 0.339 | -0.011 (0.003) | 0.0004 | -0.026 (0.005) | 8.84E-07 | 0.001 (0.011) | 0.935 |
| rs7338055 (SPRYD7)
13:50504226 |
C/A | FVC | 0.259 | 0.018 (0.005) | 0.0001 | 0.259 | -0.009 (0.003) | 0.008 | -0.028 (0.006) | 9.81E-07 | -0.008 (0.012) | 0.478 |
| rs34490170 (NEUROD1/
CERKL) 2:182576419 |
C/T | FVC | 0.110 | -0.035 (0.007) | 6.41E-07 | 0.110 | 0.007 (0.005) | 0.186 | 0.041 (0.008) | 9.95E-07 | 0.009 (0.018) | 0.622 |
The SNPs are those that demonstrate a sex-interaction effect on lung function in UK Biobank (P<1x10 -6) (N = 303,612). Lung function traits were pre-adjusted for age, age 2, standing height and smoking status and the residuals rank-transformed to normality. The regression models also included genotyping array and the first ten ancestry-based principal components. For each SNP, columns 4-9 provide minor allele frequency (MAF), and beta-coefficients, standard errors and the P value for their association with lung function in males and females separately. Columns 10-11 show the results of the SNP-by-sex interaction in UK Biobank, where the effect is given in females relative to males. For example, the top SNP (rs7697189) shows a less positive effect in females compared to males and its beta coefficient is therefore negative. Columns 12-13 show the results of the SNP-by-sex interaction in 20 cohorts of the SpiroMeta consortium (N = 75,696). Bold text in final column indicates that the effect in SpiroMeta was in the same direction to the effect in UK Biobank.
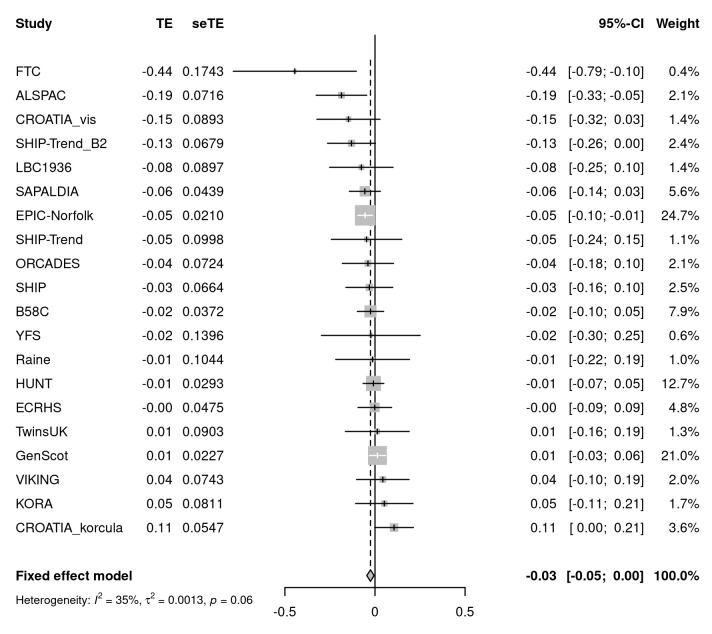
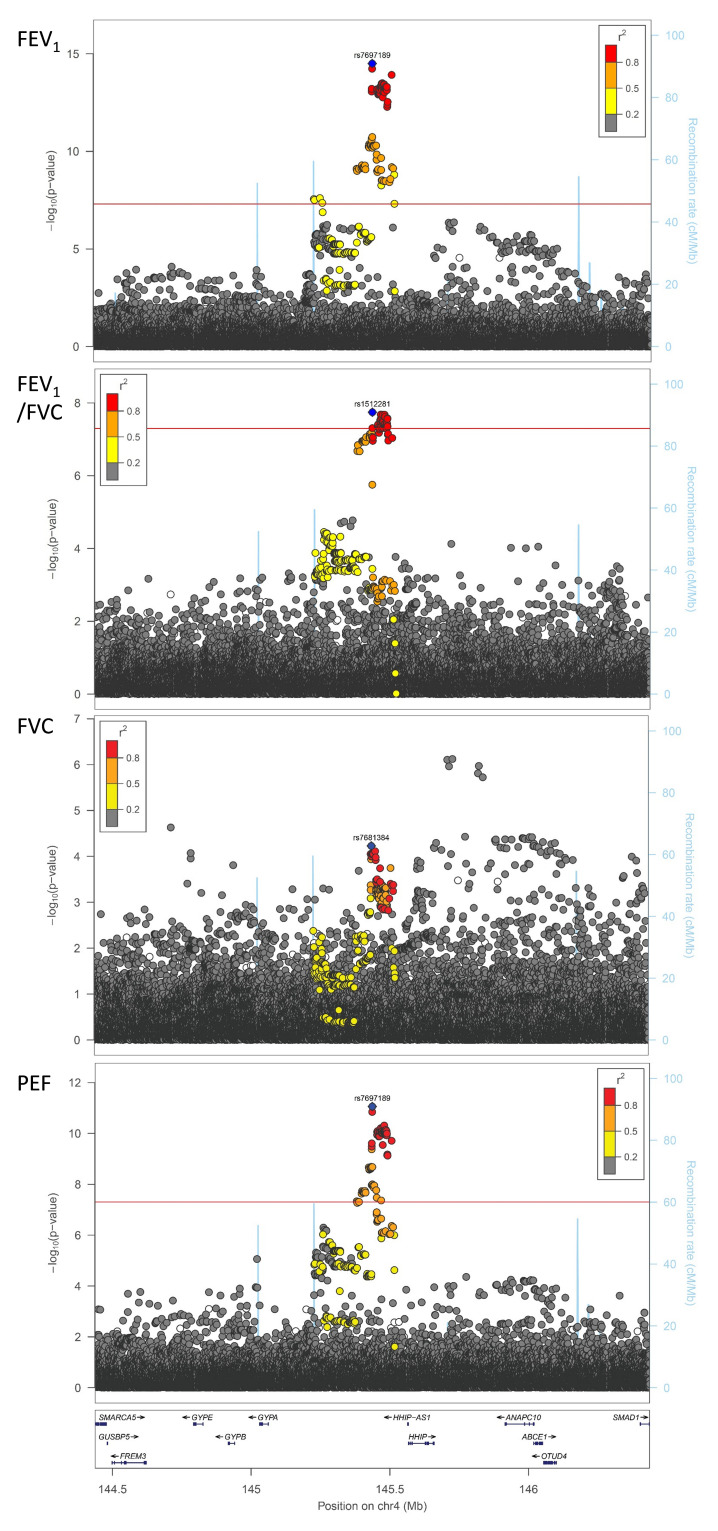
We sought evidence for replication of all 26 signals in up to 75,696 individuals from 20 cohorts of the SpiroMeta consortium. One variant, rs76911399, was excluded because it was poorly imputed in SpiroMeta cohorts and had no directly genotyped or well-imputed proxies (at r 2 threshold 0.8). Of the remaining 25 signals, 19 exhibited the same direction of interaction effect as in UK Biobank. Furthermore, the effect sizes (beta coefficients) from the regression analyses of all 25 SNPs in UK Biobank and SpiroMeta showed a correlation of 0.51 (Figure S2, Extended data 10). The SNP with the strongest evidence for interaction with sex on lung function in SpiroMeta cohorts was rs7697189 (near HHIP) (replication interaction P = 0.016) ( Table 1, Figure 1). The minor (C) allele of rs7697189 had a larger effect on lung function in males (β = 0.052 [SE 0.004], P = 2.13 x 10 -33) compared to females (β = 0.013 [SE 0.003], P = 1.16 x 10 -5) ( Table 1). This SNP resides upstream of the HHIP gene and is in linkage disequilibrium with two previously reported lung function-associated sentinel SNPs, rs13141641 16, 17 (r 2 = 0.91) and rs13116999 17 (r 2 = 0.56). SNP rs7697189 also showed some evidence of interaction with sex on PEF (β = -0.035 (0.005), P = 8.78 x 10 -12), FEV 1/FVC (β = -0.028 (0.005), P = 8.98 x 10 -8), and FVC (β = -0.020 (0.005), P = 8.71 x 10 -5) (Table S1, Extended data 10; Figure 2).
Figure 1. Meta-analysis of rs7697189-by-sex interaction effects on lung function in SpiroMeta cohorts.
The forest plot shows the beta-coefficients (test effects, TE) and standard errors for the interaction between rs7697189 and sex on forced expiratory volume in 1 second (FEV 1) in 20 cohorts of the SpiroMeta consortium (total N = 75,696). The overall effect size from fixed effects meta-analysis is represented by the diamond.
Figure 2. Genotype-by-sex interaction results within the HHIP region for lung function traits in UK Biobank.
The SNP with the strongest association in the rs7697189-proximal region is represented by a blue diamond. The FEV 1 and PEF sentinels are rs7697189, the FEV 1/FVC sentinel is rs1512281 (R 2 = 0.95 with rs7697189), and the FVC sentinel is rs7681384 (R 2 = 0.57 with rs7697189). Note that there is an independent suggestively significant signal from rs2353939 and surrounding SNPs for FVC, but this did not replicate in SpiroMeta cohorts. All other SNVs are colour coded according to their linkage disequilibrium (R 2) with the sentinel SNP (as shown in the key). All imputed SNVs are plotted irrespective of MAF, demonstrating that rarer variants are not exhibiting significant interactions with sex on lung function. The locations of genes in the region are shown in the lower panel of each plot. Recombination rate is represented by the blue lines. These plots were generated using LocusZoom software.
rs7697189 interacts with sex on lung function independently of height, smoking and pubertal timing
As SNPs in HHIP are also reported to be associated with height 18 and increased height is associated with increased lung function, it is possible that rs7697189 has differential effects on lung function in males and females through differential effects on height. However, the association of rs7697189 with standing height was not modified by sex in a combined analysis of UK Biobank males and females with a genotype-by-sex interaction term (interaction P = 0.806). We also conducted a sensitivity analysis showing that the effect of the rs7697189-by-sex interaction on FEV 1 was consistent with the original estimate after adjustment for sitting height (β = -0.04 [SE = 0.005], P = 1.97 × 10 -15).
Amongst the 303,612 UK Biobank participants in this study, the proportion of ever-smokers was higher in males (52.8%) than females (40.3%) (Table S2). A larger effect of rs7697189 on lung function in males compared to females could arise if there was an interaction effect with smoking. However, there was no interaction between rs7697189 and ever-smoking status on FEV 1 in this study (interaction P = 0.63). Pack years data was available for 94,750 UK Biobank participants. In sensitivity analyses we found a similar rs7697189-by-sex effect size on FEV 1 when adjusted for pack years (β = -0.033 [SE = 0.009], P = 3.50 × 10 -4) and no interaction between genotype and pack years on FEV 1 (interaction P = 0.80).
SNP rs7697189, and correlated SNPs in the region, have been shown to be associated with expression levels of HHIP in lung tissue 19. HHIP is a critical protein during early development and HHIP variants have been associated with lung function in infancy 20. We tested whether HHIP SNPs also have differential effects on lung function in females compared to males in childhood using data from children with an average age of eight years in the ALSPAC and Raine studies (N = 5645). In the meta-analysis of ALSPAC and Raine (Figure S3, Extended data 10), whilst we observed a point estimate for the rs7697189-by-sex interaction effect on FEV 1 that was consistent with the confidence intervals for the discovery effect observed in UK Biobank, the confidence intervals overlapped the null (which likely reflects in part the smaller numbers studied in these cohorts). Finally, as pubertal timing has been associated with adult lung function 21, we tested for an effect of relative age at puberty on the association between rs7697189 and lung function in a sex-stratified analysis. The association between HHIP SNPs and lung function was adjusted for relative age at voice breaking in males and for age at menarche in females, but adjusted effect estimates were highly consistent with the unadjusted estimates of the SNPs on lung function (Table S3, Extended data 10).
rs7697189 is associated with HHIP expression, but no interaction with sex
It is possible that rs7697189 interacts with sex on lung function through differential effects on HHIP expression. We confirmed that rs7697189 is associated with HHIP expression in lung tissue but we did not detect an interaction with sex on HHIP expression (Table S4, Extended data 10). However, HHIP (in all samples irrespective of genotype at rs7697189) did show differential expression between males and females, with females showing higher expression (Table S5; Extended data 10). This agrees with GTEx data on HHIP lung expression in males and females (Figure S4, Extended data 10).
rs7697189 is in linkage disequilibrium with a SNP predicted to disrupt SREBP and SRF motifs
HaploReg v4.1 22 was used to identify whether rs7697189, or SNPs in linkage disequilibrium, affected transcription factor binding motifs. This demonstrated that rs7697189 itself was predicted to change FAC1 and FOXO motifs and was within a chromatin mark indicative of enhancer activity in embryonic stem cell lines differentiated to CD56+ mesoderm and CD184+ endoderm cultured cells. A SNP (rs12504628) in complete linkage disequilibrium with rs7697189 changes SREBP and SRF motifs. These transcription factors have been reported to be involved in sex hormone signalling 23, 24.
Discussion
We identified a genome-wide significant genotype-by-sex interaction signal at a locus previously reported for association with lung function upstream of the HHIP gene (rs7697189, FEV 1 interaction P = 3.15 × 10 -15). The SNP showed some evidence of replication in 75,696 individuals from 20 independent studies of the SpiroMeta consortium (β = -0.025 (0.01), P = 0.016), although it did not pass a Bonferroni correction for multiple testing. We demonstrated that the differential effects of this SNP in males and females (FEV 1 β = 0.052 (0.004) in males and 0.013 (0.003) in females, corresponding to an untransformed FEV 1 β = 0.028 [SE 0.0022] litres in males vs β = 0.009 [SE 0.0014] litres in females) did not appear to be mediated by effects on height, smoking behaviour or pubertal age.
There was evidence that SNPs at the HHIP locus demonstrated interactions with sex on two additional lung function traits in UK Biobank: FEV 1/FVC and PEF (β = -0.028 (0.005), P = 8.78 × 10 -12 and β = -0.035 (0.005), P = 8.78 × 10 -12, respectively). Stratified analyses in males and females demonstrated that these SNPs appeared to have a stronger effect on lung function in males compared to females. There was no interaction between these SNPs and ever-smoking status on lung function in UK Biobank, suggesting that the stronger effect in males is not due to differences in smoking behaviour. We also demonstrate that an association between these SNPs and standing height is not modified by sex, suggesting that differential effects on height in males and females do not explain the genotype-by-sex interaction on lung function. It should be noted, however, that lung function is more closely related to sitting and thoracic height than standing height. We conducted sensitivity analyses showing that the rs7697189-by-sex interaction remained after adjustment for sitting height, but thoracic height was not available.
In contrast to these results, a recent study found comparatively weak evidence of an interaction effect between a SNP (rs13140176) in high LD with rs7697189 (r 2 = 0.93) and sex on risk of COPD in UK Biobank 25. This is likely in part to be due to reduced power to detect interaction effects on a binary trait. Indeed, in our study, the rs13140176-by-sex interaction effect on FEV 1/FVC passes the conventional threshold for genome-wide significance (P<5×10 -8) but when COPD was defined as FEV 1/FVC<0.7 this threshold was not met (P=0.023). Nevertheless, rs13140176 shows a consistent direction of effect between the studies: the lung function-lowering allele increases risk of COPD to a greater extent in males than females 25.
The genome-wide significant sex interaction locus is located upstream of the HHIP gene, a region previously reported to be associated with lung function 12, 15 and HHIP gene expression 19. The HHIP gene encodes hedgehog-interacting protein, a negative regulator of hedgehog signalling. The hedgehog signalling pathway regulates numerous physiological processes such as growth, self-renewal, cell survival, differentiation, migration, and tissue polarity and plays a vital role in the morphogenesis of lung and other organs 26. Hedgehog signalling has also been shown to participate in regulation of stem and progenitor cell populations in adult tissues, impacting tissue homeostasis and repair 27. SNP rs7697189, showing the strongest sex interaction on lung function in our study, is in strong linkage disequilibrium (R 2 = 0.93) with SNPs residing in an HHIP enhancer region 19. These enhancer-region SNPs were reported to be associated with enhancer activity and HHIP expression in lung tissues. They also exhibit genome-wide significant genotype-by-sex interactions on lung function in our data. We therefore tested the effect of rs7697189 on HHIP expression in lung tissue from 472 males and 566 females to look for sex differential effects. In contrast to the previous study 19, we found that the lung-function lowering G allele was associated with enhanced expression of HHIP in both males and females, and that expression was lower in males than females. However, the association between rs7697189 and HHIP expression was not modified by sex. This may be because there is no sex differential effect on expression, or the study might have been underpowered to detect an interaction effect. It is also possible that sex-differential effects of HHIP SNPs are only detectable in particular cell types. We therefore propose that HHIP eQTLs could be tested in larger numbers of males and females and in different cell types. Our in silico analyses predict that rs7697189 and a SNP in linkage disequilibrium (rs12504628) change transcription factor motifs that may be relevant to the effect of sex hormones on lung development, but experimental analyses will be required to test these hypotheses.
Investigating the effects of HHIP at different stages of development by sex may help to shed light on its mechanism of action. In our study we had access to genetic and lung function data from 5645 children with an average age of eight years. Though underpowered to detect the association between rs7697189 and FEV 1 seen in UK Biobank adults, the lack of a similar trend in children suggests that HHIP variants may have differential effects at different developmental stages (though the genotype-by-sex interaction is in the same direction as in adults). We also looked for an effect of timing of puberty on the association between rs7697189 and lung function in adults, but adjustment for relative age of voice breaking in males and relative age at menarche in females made no difference to the relationship between rs7697189 and lung function. As UK Biobank participants were aged between 40 and 69 years at recruitment, we did not have the longitudinal data to investigate the effect of HHIP SNPs on trajectories of lung function decline throughout life 28, but this could be an interesting area for future studies.
We identified four additional genome-wide significant (interaction P<5x10 -8) sex-by-genotype interactions on lung function in our discovery analysis in UK Biobank, with a further 21 that met a less stringent threshold of interaction (P<1x10 -6). As far as we are aware, this is the first genome-wide sex-by-genotype interaction study for lung function traits. We did not find a significant genotype-by-sex interaction on lung function or COPD at the CELSR1 locus (interaction P = 0.525 and P = 0.503, respectively) previously reported to have sex-specific effects on risk of COPD 8.
In conclusion, we have identified a novel genotype-by-sex interaction at SNPs at a putative enhancer region upstream of the hedgehog-interacting protein ( HHIP) gene. Establishing the mechanism by which HHIP has sex differential effects on lung function will be important for our understanding of the biological underpinnings of COPD in males and females. This knowledge, in turn, will be crucial to optimising treatment in males and females.
Materials and Methods
Ethics and consent
This study used anonymised data from UK Biobank (RRID: SCR_012815), which comprises over 500,000 volunteer participants aged 40–69 years recruited across Great Britain between 2006 and 2010. The protocol and consent were approved by the UK Biobank’s Research Ethics Committee. Our analysis was conducted under approved UK Biobank data application number 648. For SpiroMeta consortium cohorts, all participants provided written informed consent and studies were approved by local Research Ethics Committees and/or Institutional Review boards. Full ethics statements for each SpiroMeta consortium cohort is included in the S1 Appendix ( Extended data, 10).
UK Biobank
The UK Biobank is described here: http://www.ukbiobank.ac.uk. Individuals were included in this study if (i) they had no missing data for sex, age, height, and smoking status, (ii) their spirometry data passed quality control, as described previously 7, (iii) their genetically inferred sex matched their reported sex, (iv) they had genome-wide imputed genetic data, (v) they were of genetically determined European ancestry, and (vi) they were not first- or second-degree relatives of any other individual included in the study. In total, 303,612 individuals met these criteria (Table S2, Extended data 10).
Participants’ DNA was genotyped using either the Affymetrix Axiom ® UK BiLEVE array or the Affymetrix Axiom ® UK Biobank array 29. Genotypes were imputed based on the Human Reference Consortium (HRC) panel, as described elsewhere 29. Variants with minor allele frequency (MAF)<0.01 were excluded, as were variants with imputation quality scores <0.3.
SpiroMeta consortium
The SpiroMeta consortium meta-analysis comprised 75,696 individuals from 20 studies (see S1 Appendix for details, Extended data 10). Ten studies (N=17,280) were imputed using 1000 Genomes Phase 1 reference panel 30, 31, nine (N=37,919) were imputed using the Haplotype Reference Consortium (HRC) panel 29, and one (N=2077) was imputed using the HapMap CEU Build 36 Release 22. The ALSPAC (RRID: SCR_007260) and Raine studies also provided data on children with an average age of eight years (N=4426 and N=1219, respectively). Tables S6 and S7 show definitions of all abbreviations, study characteristics, details of genotyping platforms and imputation panels and methods ( Extended data 10). Measurements of spirometry for each study are as previously described 7, 21. Fourteen SpiroMeta studies had data on PEF (N=51,555).
Statistical analysis
Spirometry-based lung function traits FEV 1, FEV 1/FVC, FVC, and PEF were pre-adjusted for age, age 2, standing height (or sitting height in the sensitivity analysis) and smoking status and the residuals rank-transformed to normality using the rntransform function of the GenABEL package (RRID: SCR_001842) in R (RRID: SCR_001905). To test each imputed autosomal variant for an interaction effect, a linear regression model with genotype (additive effect), sex, genotype-by-sex interaction, genotyping array and the first ten principal components included as covariates was implemented using Plink 2.0 software (RRID: SCR_001757). Step-wise conditional analyses to identify independently associated variants were undertaken using GCTA software 32, 33.
Regression analysis to test genotype-by-sex interactions on height were conducted using a model including genotype (additive effect), age, age 2, sex, genotyping array and the first ten principal components as covariates. Interactions between smoking status and genotype on lung function were tested using lung function traits transformed as described above (with sex included in the model instead of ever-smoking status). The linear regression model included genotype (additive effect), ever-smoking status, a genotype-by-smoking interaction term, genotyping array and the first ten principal components.
To test whether pubertal timing has differential effects on the association between SNPs and lung function in males and females, the regression model was adjusted for relative age at menarche in females and relative age at voice breaking in males. Relative age at voice breaking is categorised as earlier than average (1), around average (2) and later than average (3) in UK Biobank. Age at menarche is given as the participant’s age at menarche in years. To make these variables comparable, age at menarche was categorised as early (<12 years old), average (12–14 years old) and late (>14 years old) as in a previous study 34. As in the lung function analyses, ancestry-based principal components and genotyping array were included in all the regression models.
For the SpiroMeta consortium, summary statistics were generated by each contributing cohort separately according to the same analysis plan as the UK Biobank data. Meta-analysis of SpiroMeta cohorts was conducted using inverse-variance weighted fixed effects meta-analysis using the metagen function of the meta package in R.
The lung eQTL study
The lung expression quantitative trait loci (eQTL) study database has been described previously 35– 37 and in S1 Appendix ( Extended data 10). HHIP differential gene expression analysis between females and males was performed using linear regression. Association of rs7697189 and rs7697189-by-sex interaction with gene expression was tested in 1,038 subjects with genotypes using MatrixEQTL package in R. All analyses were done separately in Laval, UBC and Groningen, and then combined using a meta-analysis with fixed-effects model and inverse-variance weights.
Data availability
Underlying data
UK Biobank data is an open access resource available to bona fide researchers undertaking health-related research. Researchers must apply for access (see https://www.ukbiobank.ac.uk/researchers/ for more details). Genome-wide interaction study summary statistics are available on Figshare (see below).
Figshare: Genome-wide sex interaction study summary statistics for lung function traits in UK Biobank. https://doi.org/10.6084/m9.figshare.12298736.v1 38
Extended data
Figshare: Variants associated with HHIP expression have sex-differential effects on lung function: supplementary material. https://doi.org/10.6084/m9.figshare.12129207 10
This project contains Fawcett_et_al_Extended_data_supplement.docx, which contains the following extended data:
Supplementary materials and methods
Figure S1. Genome-wide interaction SNP-by-sex interaction results on four measures of lung function in UK Biobank
Figure S2. Correlation between genotype-by-sex interaction effect sizes in UK Biobank and the SpiroMeta studies
Figure S3. Association between rs7697189 and FEV 1 in children from the ALSPAC and Raine cohorts
Figure S4. GTEx data on expression of HHIP by sex in different tissues
Table S1. Association between rs7697189 and lung function traits in males and females, and genotype-by-sex interaction results
Table S2. UK Biobank demographics
Table S3. Sex-stratified association between rs7697189 and lung function before and after adjustment for pubertal timing
Table S4. Association between rs7697189 and HHIP expression and rs7697189-by-sex interaction on HHIP expression
Table S5. Differential expression of HHIP in males compared to females
Table S6. SpiroMeta studies
Table S7. SpiroMeta analysis methods
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
We gratefully acknowledge the contributions of co-authors Professor John M. Starr and Professor John Henderson, both of whom died prior to the publication of this manuscript. We thank UK Biobank and all the participants for generating this important health research resource. This study used the ALICE and SPECTRE High Performance Computing Facilities at the University of Leicester. The ALSPAC study team are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The ECRHS study would like to thank the participants, field workers and researchers who have participated in the ECRHS study for their time and cooperation. The EPIC-Norfolk study team are grateful to all the participants who have been part of the EPIC-Norfolk project and to the many members of the study teams at the University of Cambridge who have enabled this research. Generation Scotland is grateful to all the families who took part, the general practitioners and the Scottish School of Primary Care for their help in recruiting them, and the whole Generation Scotland team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, healthcare assistants and nurses. The HUNT study team are grateful for the contributions from He Zhang and Hyun Min Kang and would also like to acknowledge the support given to them by the Genotyping core and Jin Chen. We thank the LBC1936 participants and team members who contributed to this study. The ORCADES study would like to acknowledge the invaluable contributions of the research nurses in Shetland, the administrative team in Edinburgh and the people of Shetland. The VIKING study would like to acknowledge the invaluable contributions of the research nurses in Orkney, the administrative team in Edinburgh and the people of Orkney. The Viking Health Study – Shetland (VIKING) DNA extractions and genotyping were performed at the Edinburgh Clinical Research Facility, University of Edinburgh. The Orkney Complex Disease Study (ORCADES) DNA extractions were performed at the Wellcome Trust Clinical Research Facility in Edinburgh. The Raine study would like to acknowledge the continued contribution of Raine Study participants and their families, Raine Study team for cohort coordination and data collection, NHMRC for long term funding over last 30 years, The University of Western Australia, Curtin University, Women and Infants Research Foundation, Telethon Kids Institute, Edith Cowan University, Murdoch University, The University of Notre Dame Australia, and The Raine Medical Research Foundation for providing funding for Core Management of the Raine Study. The Raine study would also like to acknowledge The University of Western Australia (Division of Obstetrics and Gynaecology, King Edward Memorial Hospital and Medical School, Royal Perth Hospital), and Telethon Kids Institute for providing in-kind support for the storage and curation of biological samples, and Pawsey Supercomputing Centre with funding from Australian Government and the Government of Western Australia for providing computation resource to carry out analyses required.
Funding Statement
This work was supported by the Wellcome Trust through a Wellcome Trust/BHF Fellowship awarded to A.L.G.; an Investigator Award awarded to M.D.T (202849); core support for ALSPAC (102215); a Strategic Award as support for Generation Scotland (104036); and GABRIEL project funding as support for SAPALDIA (084703). Sources of support: K.A.F. holds an Asthma UK fellowship. A.L.G. is supported by a Wellcome Trust/BHF fellowship. C.J. holds a Medical Research Council (MRC) Clinical Research Training Fellowship (MR/P00167X/1). L.V.W. holds a GSK/British Lung Foundation Chair in Respiratory Research. M.D.T. is supported by a Wellcome Trust Investigator Award (202849). M.D.T. and L.V. Wain have been supported by the MRC (MR/N011317/1). The research was partially supported by the NIHR Leicester Biomedical Research Centre; the views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. I.P.H.: The research was partially supported by the NIHR Nottingham Biomedical Research Centre; the views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Study-specific sources of support: ALSPAC: The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); Lung function measurements at 24 years were specifically funded by MRC MR/M022501/1. This publication is the work of the authors and will serve as guarantors for the contents of this paper. CROATIA-Korcula/Split/Vis: MRC, University Unit Programme Grant (MC_PC_U127592696), European Union, EUROSPAN project (contract no. LSHG-CT-2006-018947), Croatian Ministry of Science (216-1080315-0302) (I.R.) ECRHS: This work was supported by a contract from the European Commission (018996), Fondo de Investigación Sanitaria (91/0016-060-05/E, 92/0319, 93/0393, 97/0035-01, 99/0034-01 and 99/0034-02), Hospital General de Albacete, Hospital General Ramón Jiménez, Consejería de Sanidad del Principado de Asturias, CIRIT (1997SGR 00079, 1999SGR 00241), and Servicio Andaluz de Salud, SEPAR, Public Health Service (R01 HL62633-01), RCESP (C03/09), Red RESPIRA (C03/011), Basque Health Department, Swiss National Science Foundation, Swiss Federal Office for Education and Science, Swiss National Accident Insurance Fund (SUVA), GSF-National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (FR 1526/1-1, MA 711/4-1), Programme Hospitalier de Recherche Clinique-DRC de Grenoble 2000 no. 2610, Ministry of Health, Direction de la Recherche Clinique, Ministere de l’Emploi et de la Solidarite, Direction Generale de la Sante, CHU de Grenoble, Comite des Maladies Respiratoires de l’Isere. UCB-Pharma (France), Aventis (France), Glaxo France. Estonian Science Foundation, and Asthma UK (formerly known as National Asthma Campaign UK). EPIC-Norfolk: Medical Research Council (MR/N003284/1, MC-UU_12015/1, MC_PC_13048) Cancer Research UK (C864/A14136). FTC: Academy of Finland (308248, 312073) (J.K.), Sigrid Juselius Foundation (J.K.), Academy of Finland (213506 ) (T.R.), Academy of Finland (272376, 314383, 266286) (K.P.), Finnish Medical Foundation (K.P.), Novo Nordisk Foundation (K.P.), Finnish Diabetes Research Foundation (K.P.), State Research Funds (K.P.), University of Helsinki (K.P.) Generation Scotland: MRC, University Unit Programme Grant (MC_PC_U127592696), Wellcome Trust Strategic Award (104036), Chief Scientist Office (CZD/16/6), Scottish Funding Council (HR03006). HUNT: Stiftelsen Kristian Gerhard Jebsen (K.H.), The Liaison Committee for education, research and innovation in Central Norway (K.H., B.M.B.), NIH (HL135824, HL109946, HL127564) (C.W.): The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Center (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Nord-Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. B.M.B. received a research grant from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology. KORA: MC-Health, LMUinnovativ: The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Furthermore, KORA research was supported within the Munich Center of Health Sciences (MC-Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. LBC1936: Biotechnology and Biological Sciences Research Council (BBSRC) (BB/F019394/1), Age UK (The Disconnected Mind Project) (DCM and DCM PHASE 2), Cross Council Lifelong Health and Wellbeing Initiative (MR/K026992/1). ORCADES/VIKING: Chief Scientist Office (CZB/4/276, CZB/4/710) (J.F.W.), MRC (MC_UU_00007/10) (J.F.W.), MRC (MR/N013166/1) (S.M.), EU FP6 (LSHG-CT-2006-018947) (J.F.W.), Royal Society (URF to J.F.W.). The work of LK was supported by an RCUK Innovation Fellowship from the National Productivity Investment Fund (MR/R026408/1). The Raine Study: National Health and Medical Research Council of Australia (NHMRC) (572613, 403981, 003209), Canadian Institutes of Health Research (CIHR) (MOP-82893), Raine Medical Research Foundation. SAPALDIA: Swiss National Science Foundation (33CS30-148470/1&2, 33CSCO-134276/1, 33CSCO-108796, 324730_135673, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247-065896, 3100-059302, 3200-052720, 3200-042532, 4026-028099, PMPDP3_129021/1, PMPDP3_141671/1) (SAPALDIA1 to SAPALIDA5), Canton's government of Aargau, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais, and Zürich, Federal Offices of Environment, of Public Health, and of Roads and Transport, Cantonal lung leages of Basel Stadt/ Basel Landschaft, Geneva, Ticino, Valais, Graubünden and Zurich, Horizon 2020 (633212) (ALEC Project), European Commission (308610, 018996) (GABRIEL Project), Freie Akademische Gesellschaft (N.P.), UBS Wealth Foundation (N.P.), Wellcome Trust (084703) (GABRIEL Project), Talecris Biotherapeutics GmbH (N.P.), Abbott Diagnostics (N.P.) SHIP/SHIP_Trend/SHIP_Trend_B2: German Federal Ministry of Education and Research (01ZZ9603, 01ZZ0103, and 01ZZ0403), German Research Foundation (GR 1912/5-1) YFS: Academy of Finland (286284, 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi)), Social Insurance Institution of Finland, Competitive State Research Financing of the Expert Responsibility area of Kuopio, Tampere and Turku University Hospitals, Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation for Cardiovascular Research, Finnish Cultural Foundation, The Sigrid Juselius Foundation, Tampere Tuberculosis Foundation, Emil Aaltonen Foundation, Yrjö Jahnsson Foundation, Signe and Ane Gyllenberg Foundation, Diabetes Research Foundation of Finnish Diabetes Association, EU Horizon 2020 (755320 for TAXINOMISIS), European Research Council (742927 for MULTIEPIGEN), Tampere University Hospital Supporting Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. GBD 2016 Causes of Death Collaborators: Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. LoMauro A, Aliverti A: Sex differences in respiratory function. Breathe (Sheff). 2018;14(2):131–40. 10.1183/20734735.000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kocurek EG, Hemnes AR: Women's Health and Lung Development and Disease. Obstet Gynecol Clin North Am. 2016;43(2):307–23. 10.1016/j.ogc.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 4. Townsend EA, Miller VM, Prakash YS: Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33(1):1–47. 10.1210/er.2010-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aryal S, Diaz-Guzman E, Mannino DM: COPD and gender differences: an update. Transl Res. 2013;162(4):208–18. 10.1016/j.trsl.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 6. Sorheim IC, Johannessen A, Gulsvik A, et al. : Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–5. 10.1136/thx.2009.122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shrine N, Guyatt AL, Erzurumluoglu AM, et al. : New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51(3):481–93. 10.1038/s41588-018-0321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hardin M, Cho MH, Sharma S, et al. : Sex-Based Genetic Association Study Identifies CELSR1 as a Possible Chronic Obstructive Pulmonary Disease Risk Locus among Women. Am J Respir Cell Mol Biol. 2017;56(3):332–41. 10.1165/rcmb.2016-0172OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khramtsova EA, Davis LK, Stranger BE: The role of sex in the genomics of human complex traits. Nat Rev Genet. 2019;20(3):173–190. 10.1038/s41576-018-0083-1 [DOI] [PubMed] [Google Scholar]
- 10. Fawcett K, Obeidat M, Melbourne C, et al. : Fawcett_et_al_Extended_data_supplement.docx. figshare.Journal contribution.2020. 10.6084/m9.figshare.12129207.v1 [DOI]
- 11. Kichaev G, Bhatia G, Loh PR, et al. : Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am J Hum Genet. 2019;104(1):65–75. 10.1016/j.ajhg.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pillai SG, Ge D, Zhu G, et al. : A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5(3):e1000421. 10.1371/journal.pgen.1000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soler Artigas M, Wain LV, Miller S, et al. : Sixteen new lung function signals identified through 1000 Genomes Project reference panel imputation. Nat Commun. 2015;6:8658. 10.1038/ncomms9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terzikhan N, Sun F, Verhamme FM, et al. : Heritability and genome-wide association study of diffusing capacity of the lung. Eur Respir J. 2018;52(3):1800647. 10.1183/13993003.00647-2018 [DOI] [PubMed] [Google Scholar]
- 15. Van Durme YM, Eijgelsheim M, Joos GF, et al. : Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam Study. Eur Respir J. 2010;36(1):89–95. 10.1183/09031936.00129509 [DOI] [PubMed] [Google Scholar]
- 16. Wilk JB, Chen TH, Gottlieb DJ, et al. : A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5(3):e1000429. two studies of COPD genetics (2004-2008), and consulting fees (2006-2008) from GlaxoSmithKline. EKS received an honorarium from Wyeth for a talk on COPD genetics in 2004. EKS received an honorarium from Bayer for a symposium at the ERS Meeting in 2005. EKS received honoraria for talks in 2007 and 2008 and consulting fees in 2008 from AstraZeneca. 10.1371/journal.pgen.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shrine N, Guyatt AL, Erzurumluoglu AM, et al. : New genetic signals for lung function highlight pathways and pleiotropy, and chronic obstructive pulmonary disease associations across multiple ancestries. bioRxiv. 2018. 10.1101/343293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weedon MN, Lango H, Lindgren CM, et al. : Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40(5):575–83. 10.1038/ng.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou X, Baron RM, Hardin M, et al. : Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet. 2012;21(6):1325–35. 10.1093/hmg/ddr569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins SA, Lucas JS, Inskip HM, et al. : HHIP, HDAC4, NCR3 and RARB polymorphisms affect fetal, childhood and adult lung function. Eur Respir J. 2013;41(3):756–7. 10.1183/09031936.00171712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahmoud O, Granell R, Tilling K, et al. : Association of Height Growth in Puberty with Lung Function. A Longitudinal Study. Am J Respir Crit Care Med. 2018;198(12):1539–1548. 10.1164/rccm.201802-0274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ward LD, Kellis M: HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–4. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heemers HV, Verhoeven G, Swinnen JV: Androgen activation of the sterol regulatory element-binding protein pathway: Current insights. Mol Endocrinol. 2006;20(10):2265–77. 10.1210/me.2005-0479 [DOI] [PubMed] [Google Scholar]
- 24. Leimgruber C, Quintar AA, Peinetti N, et al. : Testosterone Rescues the De-Differentiation of Smooth Muscle Cells Through Serum Response Factor/Myocardin. J Cell Physiol. 2017;232(10):2806–17. 10.1002/jcp.25679 [DOI] [PubMed] [Google Scholar]
- 25. Sakornsakolpat P, Prokopenko D, Lamontagne M, et al. : Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019;51(3):494–505. 10.1038/s41588-018-0342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kugler MC, Joyner AL, Loomis CA, et al. : Sonic hedgehog signaling in the lung. From development to disease. Am J Respir Cell Mol Biol. 2015;52(1):1–13. 10.1165/rcmb.2014-0132TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrova R, Joyner AL: Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141(18):3445–57. 10.1242/dev.083691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lange P, Celli B, Agustí A, et al. : Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–22. 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 29. Bycroft C, Freeman C, Petkova D, et al. : Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv. 2017. 10.1101/166298 [DOI] [Google Scholar]
- 30. Battram T, Hoskins L, Hughes DA, et al. : Coronary artery disease, genetic risk and the metabolome in young individuals [version 2; peer review: 2 approved]. Wellcome Open Res. 2018;3:114. 10.12688/wellcomeopenres.14788.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. 1000 Genomes Project Consortium, . Abecasis GR, Altshuler D, et al. : A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. 10.1038/nature09534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang J, Ferreira T, Morris AP, et al. : Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369–75, S1-3. 10.1038/ng.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang J, Lee SH, Goddard ME, et al. : GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minelli C, van der Plaat DA, Leynaert B, et al. : Age at puberty and risk of asthma: A Mendelian randomisation study. PLoS Med. 2018;15(8):e1002634. following competing interests: CM and GDS are members of the Editorial Board of PLOS Medicine. 10.1371/journal.pmed.1002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hao K, Bossé Y, Nickle DC, et al. : Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8(11):e1003029. 10.1371/journal.pgen.1003029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamontagne M, Couture C, Postma DS, et al. : Refining susceptibility loci of chronic obstructive pulmonary disease with lung eqtls. PLoS One. 2013;8(7):e70220. 10.1371/journal.pone.0070220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Obeidat M, Miller S, Probert K, et al. : GSTCD and INTS12 regulation and expression in the human lung. PLoS One. 2013;8(9):e74630. 10.1371/journal.pone.0074630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fawcett K, Obeidat M, Melbourne C, et al. : Genome-wide sex interaction study summary statistics for lung function traits in UK Biobank. figshare.Journal contribution.2020. 10.6084/m9.figshare.12298736.v1 [DOI]