Abstract
Objective:
We examined the relation between serum free testosterone and asthma, wheeze, asthma hospitalizations, and lung function in older adults.
Design:
Cross-sectional study
Setting:
United Kingdom
Participants:
256,419 adults aged 40 to 69 years, recruited from 2006 to 2010
Main outcome measures:
Multivariable logistic or linear regression was used for the analysis of free testosterone and physician-diagnosed asthma, current wheeze, asthma hospitalizations, and lung function measures, which was adjusted for serum estradiol, smoking status, and other covariates.
Results:
Free testosterone levels above the lowest quartile (Q1) were significantly associated with lower odds of asthma in both women (adjusted odds ratio [aOR] for Q4 [the highest quartile] vs. Q1=0.67, 95% confidence interval [CI]=0.64–0.71) and men (aOR for Q4 vs. Q1=0.87, 95% CI=0.82-0.91). Among subjects with asthma, free testosterone levels above Q1 were significantly associated with lower odds of current wheeze in women (aOR range=0.78 to 0.87), and free testosterone levels in Q4 were associated with lower odds of current wheeze in men (aOR for Q4 vs Q1=0.86, 95% CI=0.77-0.95). Among women with asthma, free testosterone levels in Q4 were also associated with lower odds of ≥1 asthma hospitalization. Among men, free testosterone was positively associated with FEV1 and FVC. Among women, free testosterone was negatively and weakly associated with FVC.
Conclusions:
In a large study of British adults, elevated free testosterone levels are associated with lower odds of asthma and current wheeze in women and men, lower odds of asthma hospitalizations in women, and higher FEV1 and FVC in men.
Keywords: testosterone, asthma, asthma hospitalization, lung function, UK Biobank
INTRODUCTION
Asthma affects approximately 339 million people worldwide.1 In the United Kingdom (U.K.), asthma leads to 933,000 hospitalizations per year, and 1.1 million children and 4.3 million adults are currently receiving treatment for asthma.2,3
The incidence of asthma varies widely by age and sex throughout the lifespan. Although asthma is more common in girls than in boys, adult women have higher prevalence and morbidity from asthma than adult men.4 Changes in sex hormone levels during the life course may partly explain this disparity.5,6
While estrogen and progesterone may enhance T-helper cell type 2 (Th2)-induced allergic airway inflammation, androgens such as testosterone and 5-alpha dihydrotestosterone (5α-DHT) may reduce such inflammation by suppressing innate and adaptive immune responses.7 Whereas fluctuation in estrogen or progesterone levels -related to the menstrual cycle, pregnancy, menopause, or intake of exogenous hormones- has been linked to increased asthma risk in women8, findings from experimental studies and a small observational study in children suggest that testosterone may be associated with reduced asthma risk in adult men.8,9 In a study of 7,615 adults aged 18 to 79 years in the U.S., we previously reported that a high serum testosterone level was associated with lower odds of current asthma in women (n=3,662) but not in men (n=3,953), a finding that could be explained by limited statistical power for the analysis in men (due to lower asthma prevalence in men than in women).10
Given a potential protective role of testosterone against asthma and our prior negative results for asthma in a relatively small sample of U.S. men10, we examined the relation between serum free testosterone and asthma, current wheeze, asthma hospitalizations, and lung function in an analysis of 256,419 British adults aged 40 to 69 years.
METHODS
Study design and study population
The UK Biobank (UKB) is a large prospective population-based study established to identify determinants of complex diseases of middle and old age. Approximately 9.2 million individuals aged 40–69 years who lived within twenty-two miles of assessment centers in England, Wales, and Scotland were invited to enter the cohort, and ~500,000 (5.5%) of those individuals participated in the baseline assessment11. Extensive data, including questionnaires, physical measures, and biological samples were collected at baseline, with longitudinal follow-up for a wide range of health-related outcomes.11,12 Details of the methods, protocols, and definitions used in the study can be found at the UKB website (www.ukbiobank.ac.uk/). The current study was conducted using the UKB Resource under application #43252 for a predictive model and constitutes an interim analysis. The algorithm used to select participants in this study is shown in Figure E1. Of 502,524 participants recruited at baseline, 256,419 (123,921 women [who did not report being pregnant] and 132,498 men) had complete information on asthma status, sex hormone levels, and relevant covariates, and were thus included in the current analysis of asthma. A subset of these 256,419 participants, including 87,137 women and 94,491 men, also had measures of forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], and FEV1/FVC, and were thus included in the current analysis of lung function.
The UKB was approved by the UK National Health Service National Research Ethics Service (Ref 11/NW/0382) and informed consent was obtained from all participants.
Outcomes
Asthma was defined by inclusion of “asthma” as an answer to the following question: “Has a doctor ever told you that you have had any of the following conditions: blood clot in the leg, blood clot in the lung, emphysema/chronic bronchitis, asthma, hay fever, allergic rhinitis or eczema?” Control subjects were participants who did not include “asthma” as an answer to the question above. Current wheeze was defined as a “Yes” answer to the following question: “In the last year have you ever had wheeze or whistling in the chest?”. A hospitalization for asthma was defined as ever having had a hospitalization with an International Classification of Diseases Clinical Modification (ICD) code of main diagnosis compatible with asthma (ICD-9: 493.x or ICD-10: J45.x and J46.x), excluding hospitalizations with an ICD code for a main diagnosis consistent with chronic obstructive pulmonary disease (COPD ICD-9: J43, J44, J47 or ICD-10: 490, 491, 492, 494, 496).
Spirometry was performed using a Vitalograph Pneumotrac 6800 spirometer (Vitalograph Ltd., Buckingham, England), following European Respiratory Society/American Thoracic Society criteria for acceptability and reproducibility.13 Participants were excluded from spirometry testing if they were being treated for tuberculosis, or if they had a chest infection in the last month, history of a detached retina, a heart attack, eye surgery, surgery in the chest or abdomen in the previous 3 months, or history of a collapsed lung or a pneumothorax.
Serum sex hormone levels
The UKB collected blood samples during the baseline assessment visit.14 Serum total testosterone and estradiol were measured at the UKB central laboratory by competitive binding immunoassay analysis, and sex hormone binding globin (SHBG) was measured using a two-step sandwich immunoassay analysis on a Beckman Coulter Unicel Dxl 800 (Beckman Coulter [UK], Ltd). Internal quality control and external quality assurance schemes were used to verify assay performance. Samples with results exceeding the reportable range of the assay were diluted and re-analyzed (automatic dilution).15 Values below the analytical range for estradiol (175 pmol/L) were assigned a constant (123.7 pmol/L), calculated by dividing the lower limit of detection (LLOD) by the square root of 2 (LLOD / ).
Because testosterone circulates highly bound to SHBG, free testosterone was estimated using the empirical free testosterone (EFT) formula, as follows16:
Statistical analysis
Two-sided Wald chi-square and Wilcoxon rank sum tests were used for the bivariate analyses, as appropriate. Logistic regression was used for the multivariable analyses of serum free testosterone (as quartiles) and asthma, current wheeze, and at least one asthma hospitalization, which were conducted separately in men and women. Known or potential confounders of the relation between serum free testosterone and asthma were included in the multivariable models. All models were adjusted for age, race/ethnicity (Caucasian vs. other), annual household income (< vs. ≥ £31,000 per year, near the median household income for the UK in 201917), body mass index (BMI), smoking status (never, former, or current), pack-years of cigarette smoking, season and time of the day when the samples were collected (to account for daily and seasonal variation), serum estradiol level (see below), and (in women only) current use of oral contraceptives (OC) or hormone replacement therapy (HRT) and menopause (see below). Because most participants in the UKB were older adults, estradiol levels were below the LLOD in approximately 74% of women and 91% of men. Thus, serum estradiol was categorized as at or above vs. below the LLOD for data analysis. In turn, menopause was defined by a positive answer to the following question “have you had your menopause (periods stopped)” or (in the 15% of participants who answered “not sure”) by an age ≥51 years (the average age for menopause in the UK).18 On the basis of our prior work, we tested for an interaction between serum free testosterone level and two variables (obesity and menopause) after the final models were built.
Linear regression was used for the multivariable analysis of serum free testosterone and lung function measures (FEV1, FVC, and FEV1/FVC). All models were adjusted for age, race/ethnicity, annual household income, BMI, smoking status, pack-years of cigarette smoking, season and time of the day when the samples were collected, serum estradiol level, asthma status (for all participants), and (in women only) current use of OC or HRT, and menopause. Models for FEV1 and FVC were additionally adjusted for height and height squared. Participants with extreme values for FEV1 or FVC (< 1st or > 99th percentile) were excluded from the analysis.
Because 101,876 (28%) of the eligible participants were excluded from the analysis due to missing data for covariates (Figure E1), a multiple imputation procedure was used to include these participants in a sensitivity analysis. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results.
RESULTS
Table 1 shows the main characteristics of participants by asthma status, separately in men and women. Estimates of the prevalence of physician-diagnosed asthma (heretofore called “asthma”) were 11.3% in women and 9.3% in men. Compared to women without asthma (n=109,865), those with asthma (n=14,056) were younger and more likely to be Caucasian and non-current smokers, to have a higher BMI and a detectable serum estradiol level, and to have a lower serum free testosterone level and lower lung function measures. Women with asthma were also more likely to report current use of OC or HRT and to be pre-menopausal than those without asthma. Compared with men without asthma (n=118,251), those with asthma (n=14,247) were more likely to be younger and non-current smokers, and to have lower household income, lower serum free testosterone levels, and lower lung function measures.
Table 1.
Main characteristics of study participants, by sex and asthma status
| Women (n=123,921) | Men (n=132,498) | |||
|---|---|---|---|---|
| Characteristics | Controls (n=109,865) |
Asthma (n=14,056) |
Controls (n=118,251) |
Asthma (n=14,247) |
| Age at recruitment (years) | 55.4 ± 8.0 | 54.3 ± 8.1* | 56.4 ± 8.1 | 55.3 ± 8.4* |
| Caucasian ethnicity | 104,642 (95.3) | 13,301 (94.6)* | 112,602 (95.2) | 13,598 (95.4) |
| Annual household income < £31,000 | 54,863 (49.9) | 7,077 (50.4) | 52,906 (44.7) | 6,172 (43.2)* |
| Body mass index (BMI, kg/m2) | 27.0 ± 5.1 | 28.4 ± 6.0* | 27.8 ± 4.2 | 28.0 ± 4.4* |
| Obesity (BMI ≥ 30 kg/m2) | 25,807 (23.5) | 4,545 (32.3)* | 29,624 (25.1) | 3,919 (27.5)* |
| Smoking status | ||||
| Never | 75,812 (69.0) | 9,506 (67.6)* | 69,392 (58.7) | 8,558 (60.1)* |
| Former | 24,056 (21.9) | 3,258 (23.2) | 35,436 (30.0) | 4,330 (30.4) |
| Current | 9,997 (9.1) | 1,292 (9.2) | 13,423 (11.4) | 1,359 (9.5) |
| Pack-years of smoking | 6.1 ± 12.3 | 6.7 ± 13.4* | 10.6 ± 18.2 | 10.3 ± 18.6 |
| Current use of OC or HRT | 8023 (7.3) | 1212 (8.6)* | - | - |
| Had menopause | 75,474 (68.7) | 8,966 (63.8)* | - | - |
| Free testosterone (pmol/L) | 11.9 ± 8.8 | 11.4 ± 8.6* | 164.9 ± 60.1 | 163.2 ± 61.7* |
| Estradiol (pmol/L) | 241.4 ± 302.7 | 259.1 ± 314.6* | 132.9 ± 0.1 | 133.2 ± 0.4 |
| FEV1 (mL)† | 2,500 ± 489 | 2,359 ± 506* | 3,368 ± 660 | 3,099 ± 726* |
| FVC (mL)† | 3,266 ± 593 | 3,179 ± 606* | 4,457 ± 794 | 4,331 ± 842* |
| FEV1/FVC (%)† | 76.7 ± 5.4 | 74.1 ± 6.6* | 75.5 ± 6.3 | 70.9 ± 8.2* |
| Wheeze in the last year | - | 8,826 (63.7) | - | 9,341 (66.5) |
| At least one asthma hospitalization | - | 484 (3.6) | - | 354 (2.6) |
OC= oral contraceptives and HRT= hormone replacement therapy
Results are shown as mean ± standard deviation (SD) for continuous variables, and as N (%) for binary variables.
P<0.05 for comparison of participants with current asthma vs. controls.
Numbers (%) may vary due to missingness.
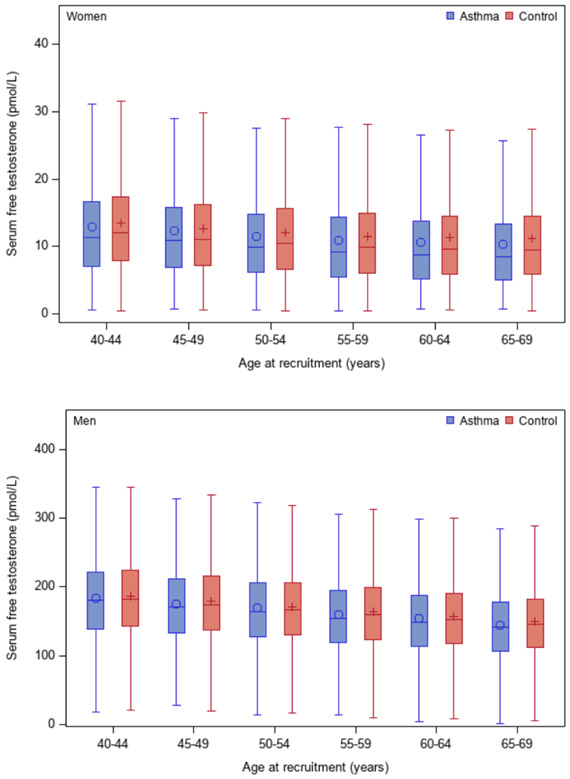
Figure 1 shows serum free testosterone levels by age and asthma status, separately in men and women. Serum free testosterone levels decreased slightly with age in both men and women, with slightly higher levels among control subjects. In a multivariable analysis (Figure 2 and Model 1 in Table E1), women whose serum free testosterone level was above the first or lowest quartile (Q1) had 16% to 33% significantly lower odds of physician-diagnosed asthma than those with levels in Q1 (e.g., adjusted odds ratio [aOR] for Q4 (the fourth or highest quartile) vs. Q1=0.67, 95% confidence interval [CI]=0.64-0.71). In this analysis, women who had a serum estradiol at or above the LLOD (detectable) had 8% significantly higher odds of asthma than those with a serum estradiol below the LLOD (undetectable). Moreover, the observed association between serum free testosterone or serum estradiol and asthma was essentially unchanged after the analysis was additionally adjusted for number of livebirths and menstruation on the day of examination (Model 2 in Table E1) or after excluding women with missing data for the question about menopause (Model 3 in Table E1). In the multivariable analysis in men (Model 1 in Table E1 and Figure 2), subjects with serum free testosterone levels in Q4 had 13% lower odds of asthma than those with serum free testosterone levels in Q1 (95% CI for aOR=0.82-0.91). There was no significant association between serum estradiol and asthma in men.
Figure 1-.
Serum levels of free testosterone, by age and asthma status, in women (upper panel) and men (lower panel)
Figure 2-.
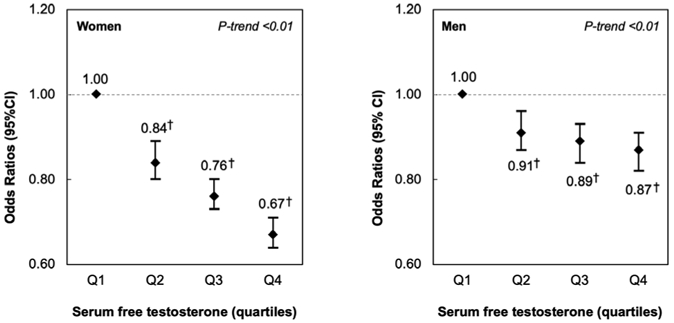
Multivariable analysis of serum free testosterone levels and asthma, in women (left panel) and men (right panel)
Footnote: All models were adjusted for age, race/ethnicity, annual household income, body mass index, smoking status, pack-years of smoking, current use of birth control pills or hormone replacement therapy and menopause status (in women), the season and the time of the day when the examination was performed, and serum estradiol level. †P<0.01
To reduce the impact of potential misclassification of COPD as asthma, we repeated the multivariable analysis of asthma after stratification by smoking status (current smokers vs. never smokers and former smokers with <10 pack-years of smoking). In this analysis (Table E2), a serum free testosterone level above Q1 was significantly associated with lower odds of asthma in men and women, regardless of smoking status. A detectable serum estradiol was significantly associated with increased odds of asthma in women who were never smokers or former smokers with <10 pack-years of smoking, but not in current smokers. A detectable serum estradiol was not significantly associated with asthma in men, regardless of smoking status.
Table 2 shows the results of the multivariable analysis of serum free testosterone level and current wheeze and at least one asthma hospitalization. In this analysis, women with asthma whose testosterone levels were in Q4 had 22% significantly lower odds of current wheeze and 27% significantly lower odds of ever having had an asthma-related hospitalization than women with asthma and free testosterone levels in Q1. Among men with asthma, a serum free testosterone level in Q4 was significantly associated with 14% lower odds of current wheeze, but there was no significant association between serum free testosterone and at least one asthma hospitalization. A detectable serum estradiol level was not significantly associated with current wheeze or asthma hospitalizations, either in men or in women.
Table 2.
Multivariable analysis of serum free testosterone level and current wheeze and at least one asthma hospitalization
| Serum levels of sex hormones | Current wheeze | At least one asthma hospitalization |
|---|---|---|
| Odds ratio (95% confidence interval) | ||
| Women with asthma (n=14,056) | n=8,826 | n=484 |
| Quartiles (Q) of free testosterone, pmol/L | ||
| Q1 (< 5.9) | 1.0 | 1.0 |
| Q2 (5.9- < 9.8) | 0.87 (0.79, 0.96)† | 0.91 (0.71, 1.17) |
| Q3 (9.8- < 14.8) | 0.78 (0.70, 0.86)† | 0.86 (0.66, 1.11) |
| Q4 (≥ 14.8) | 0.78 (0.71, 0.87)†‡ | 0.73 (0.56, 0.95)*‡ |
| Estradiol, pmol/L | ||
| < 123.7 | 1.0 | 1.0 |
| ≥ 123.7 | 1.10 (0.99-1.22) | 1.12 (0.86, 1.45) |
| Men with asthma (n=14,247) | n=9,341 | n=354 |
| Quartiles (Q) of free testosterone, pmol/L | ||
| Q1 (< 121.0) | 1.0 | 1.0 |
| Q2 (121.0- < 158.5) | 1.01 (0.91, 1.12) | 0.91 (0.67, 1.22) |
| Q3 (158.5- < 199.2) | 0.93 (0.84, 1.03) | 0.86 (0.64, 1.17) |
| Q4 (≥ 199.2) | 0.86 (0.77, 0.95)†‡ | 0.84 (0.61, 1.15) |
| Estradiol, pmol/L | ||
| < 123.7 | 1.0 | 1.0 |
| ≥ 123.7 | 0.96 (0.84, 1.08) | 1.20 (0.85, 1.70) |
All models included both serum free testosterone and serum estradiol levels, and were adjusted for age, race/ethnicity, annual household income, body mass index, smoking status, pack-years of smoking, current use of birth control pills or hormone replacement therapy and menopause status (in women), and the season and the time of the day when the examination was performed.
P<0.05
P<0.01
P for trend < 0.05
Table 3 shows the results of the multivariable analysis of serum free testosterone and lung function measures. Among men, each quartile increment in free testosterone was significantly associated with 26 ml higher FEV1 and 36 ml higher FVC, while having a detectable serum estradiol level was significantly associated with lower FEV1 (by 44.7 ml), lower FVC (by 50.1 ml), and lower FEV1/FVC (by 0.21%). Among women, each quartile increment in free testosterone was significantly associated with 4.3 ml lower FVC, while a detectable serum estradiol level was significantly associated with higher FVC (by 11.1 ml) and lower FEV1/FVC (by 0.12%). We obtained similar results in a secondary analysis of lung function in which z-scores were calculated for lung function measures, based on the Global Lung Function Initiative 201219 (Table E3).
Table 3 –
Multivariable analysis of serum free testosterone and lung function measures
| Women (n = 87,137) |
Men (n = 94,491) |
|
|---|---|---|
| Serum levels of sex hormones | β (95% confidence interval) | |
| Free testosterone (pmol/L), per quartile increment | ||
| FEV1 (mL) | −2.3 (−4.6, 0.3) | 26.0 (22.8, 29.2)* |
| FVC (mL) | −4.3 (−7.1, −1.5)* | 36.0 (32.3, 39.8)* |
| FEV1/FVC (%) | 0.03 (−0.01, 0.06) | −0.004 (−0.04, 0.04) |
| Estradiol ≥123.7 pmol/L | ||
| FEV1 (mL) | 5.40 (−2.15, 12.94) | −44.70 (−56.52, −38.88)† |
| FVC (mL) | 11.13 (1.99, 20.28)* | −50.11 (−63.94, −36.28)† |
| FEV1/FVC (%) | −0.12 (−0.23, −0.02)* | −0.21 (−0.34, −0.07)† |
All models included both serum free testosterone and serum estradiol, and were adjusted for age, race/ethnicity, annual household income, body mass index, asthma status, smoking status, pack-years of cigarette smoking, current use of birth control pills or hormone replacement therapy and menopause status (in women), and the season and the time of the day when the examination was performed. Models for FEV1 and FVC were additionally adjusted for height and height squared.
P<0.05
P<0.01
Because of our prior findings in a U.S. cohort of adults aged 18 to 79 years, we tested for an interaction between obesity (a BMI ≥30 kg/m2) and serum free testosterone on asthma. This interaction was not significant in women (P=0.53) or men (P=0.10). Among women, we also tested for an interaction between menopause and serum free testosterone on asthma, finding no significant interaction (P=0.37).
After imputing data for missing covariates, we repeated the multivariable analysis of serum free testosterone and asthma in all eligible participants (n=358,295). This sensitivity analysis yielded similar results to those from the analysis that excluded participants with missing covariates (Table E4).
DISCUSSION
In a large population-based study of British adults, elevated serum levels of free testosterone were significantly associated with lower odds of physician-diagnosed asthma and current wheeze in middle-aged and older women and men. Moreover, an elevated free testosterone level was associated with decreased odds of at least one asthma hospitalization in women, and with higher FEV1 and FVC in men.
Few epidemiological studies have examined free testosterone and asthma in adults. In our previous analysis of adults (ages 18 to 79 years) who participated in the U.S. National Health and Nutrition Examination Survey (NHANES), we reported that higher levels of serum free testosterone were associated with lower odds of current asthma in women but not in men.10 Our finding of a significant inverse association between a high serum free testosterone level and asthma among men in the current analysis is thus novel and likely due to greater statistical power, due to a much larger sample size in the UKB than in NHANES.10 Alternatively, these results and our finding of no interaction between obesity and free testosterone on asthma in women in the UKB cohort could be explained by differences in the age range of participants in NHANES vs. those in the UKB. Consistent with our current results, a human phase II clinical trial reported that nebulized dehydroepiandrosterone-3-sulfate (DHEAS) improved asthma control and symptoms in adults with poorly controlled moderate to severe asthma on inhaled corticosteroids and long-acting ß2-agonists.20 Moreover, a study of 450 U.S. men aged 40-63 years with COPD showed that subjects on testosterone replacement therapy had a 4.2%-9.1% reduction in hospitalizations compared with those on no such therapy.21
Testosterone may protect against asthma through systemic and airway-specific anti-inflammatory effects. In a murine model, testosterone was shown to decrease dust mite-induced eosinophilic and neutrophilic inflammation in the lungs, partially through androgen receptor (AR) signaling.22 In another murine model, testosterone was shown to decrease Alternaria-extract-induced IL-5, IL-13, and lung eosinophils by attenuating group 2 innate lymphoid cells (ILC2).23 Other studies have shown that androgens can induce airway smooth muscle relaxation. In guinea pig airways, testosterone at physiological concentrations reduced reactivity of smooth muscle by diminishing intracellular calcium [Ca2+]i increment through modifying IP3 receptor (ITPR).24 Androgens have also been shown to weaken TNFα or IL-13-induced enhancement of [Ca2+]i in human airway smooth muscle cells, which lessens airway responsiveness.25 Collectively, bronchodilating and anti-inflammatory effects of testosterone may explain our results for asthma, current wheeze, and asthma hospitalizations.
Little is known about the effects of testosterone on lung function in adult women. In a study of 1,768 community-dwelling adult men (of whom 14% reported asthma and 2.7% reported COPD), total testosterone and dihydrotestosterone (DHT) were positively associated with FEV1 and FVC.26 Among 2,197 men with an average age of 66 years (of whom 4.7% reported COPD), higher total and free testosterone levels were significantly associated with increased FEV1 and FVC.27 In a study of boys with current asthma (aged 6-18 years), DHEAS was positively associated with FEV1 and FVC and improved asthma control.28 Our results are thus consistent with those of prior studies in men, but this is (to our knowledge) the first study to examine free testosterone and lung function in women. The observed weak negative effect of free testosterone on FVC (but not on FEV1 or FEV1/FVC) in women could be explained by residual confounding due to unmeasured factors affecting hormonal variations (e.g., type/length/dose of hormonal contraception or replacement therapy, or follicular phase of the menstrual cycle).
The discrepant direction and magnitude of the estimated effects of testosterone on lung function between women and men are intriguing. In general, adult men have higher FEV1 and FVC than adult women (Figure E2), which may explain the larger effect size of free testosterone on lung function in men. Moreover, unmeasured androgens (e.g., DHT or DHNES) or androgen receptors may partly explain these findings. In mouse models, DHT downregulated Th2 inflammation in the lung, but androgen receptors enhanced such inflammation by upregulating M2 macrophage polarization that induces eosinophil recruitment.29
In our previous analysis in NHANES, in which there were both a lower limit of detection and a broader range of serum estradiol levels than those in the current study10, we found no significant association between serum estradiol and asthma in our main analysis. Although our current finding of an association between a detectable serum estradiol and asthma and reduced FEV1/FVC in women and reduced lung function measures (FEV1, FVC, and FEV1/FVC) in men may be due to true enhancement of allergic airway inflammation7, these results must be cautiously interpreted because of the high proportion of participants with undetectable estradiol levels and lack of a significant association between a detectable serum estradiol and current wheeze or asthma hospitalizations in participating men and women.
We acknowledge additional study limitations. First, we cannot examine temporal relationships in this cross-sectional study. Second, serum progesterone was not measured in the UKB, and thus we could not examine whether progesterone affects asthma or lung function. Third, a “healthy volunteer” selection bias has been suggested for the UK Biobank study. Compared with the general population, participants in the UKB were less likely to be obese or to smoke and had fewer self-reported health conditions.30 Thus, our findings may not be generalizable to the British population at large. Fourth, recall bias and misclassification of asthma are possible, as this disease was defined using a combination of self-reported (ever) asthma (which the participant had to select from a list including other chronic conditions) and self-reported (ever) physician-diagnosed asthma. However, we obtained similar results for current wheeze, and our findings for asthma were unchanged in an analysis of data from never smokers or former smokers with <10 pack-years of smoking. Lastly, we lack data on several potential confounders, including allergic sensitization, insulin resistance, and environmental exposure to endocrine disruptors.
In summary, we found that elevated serum levels of free testosterone were significantly associated with reduced odds of asthma and current wheeze in middle-aged and older British men and women. In these adults, we also show that elevated free testosterone levels were associated with decreased odds of at least one asthma-related hospitalization in women, as well as higher FEV1 and FVC in men. Taken together with prior results from experimental and observational studies, our findings suggest that free testosterone contributes to the pathogenesis of asthma in adults.
Supplementary Material
What is the key question? Are elevated serum levels of free testosterone associated with lower risk of asthma and higher lung function in adults of both sexes?
What is the bottom line? In a large population-based study of British adults aged 40 to 69 years, an elevated serum level of free testosterone was associated not only with lower odds of physician-diagnosed asthma and current wheeze in women and men, but also with lower odds of at least one asthma hospitalization in women and higher FEV1 and FVC in men.
Why read on? The findings from this study suggest that serum free testosterone is linked to lower risk of asthma in both men and women.
Dissemination to participants and related patient and public communities: The results of the study will be linked to the UK Biobank website.
ACKNOWLEDGEMENTS
We thank the UK Biobank participants. This research was conducted using the UK Biobank Resource (application # 43252).
Funding
Dr. Han’s contribution was supported by grant MD011764 from the U.S. National Institutes of Health (NIH). Dr. Celedón’s contribution was supported by grants HL117191, HL119952, and MD011764 from the U.S. NIH. Dr. Forno’s contribution was supported by grants HL149693 from the U.S. NIH. Dr. Yan’s contribution was supported by grant HL138098 from the U.S. NIH. This study was conducted using the UK Biobank Resource under Application Number 43252.
Footnotes
Competing interests
Dr. Celedón has received research materials from Merck and GSK (inhaled steroids), and Pharmavite (vitamin D and placebo capsules), to provide medications free of cost to participants in NIH-funded studies, unrelated to the current work. The other authors report no conflicts of interest.
Data sharing
The UK Biobank data are available from the UK Biobank upon request (www.ukbiobank.ac.uk/).
REFERENCES
- 1.Global Asthma Network. The Global Asthma Report 2018. Auckland, New Zealand [Google Scholar]
- 2.Mukherjee M, Stoddart A, Gupta RP, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med 2016;14(1):113. doi: 10.1186/s12916-016-0657-8 [published Online First: 2016/08/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asthma UK. Asthma facts and statistics London, England: 2020. [Available from: https://www.asthma.org.uk/about/media/facts-and-statistics/ accessed Feb 5 2020. [Google Scholar]
- 4.Almqvist C, Worm M, Leynaert B, et al. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008;63(1):47–57. doi: 10.1111/j.1398-9995.2007.01524.x [published Online First: 2007/09/08] [DOI] [PubMed] [Google Scholar]
- 5.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007 [published Online First: 2015/01/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCleary N, Nwaru BI, Nurmatov UB, et al. Endogenous and exogenous sex steroid hormones in asthma and allergy in females: A systematic review and meta-analysis. J Allergy Clin Immunol 2018;141(4):1510–13 e8. doi: 10.1016/j.jaci.2017.11.034 [published Online First: 2018/01/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Curr Allergy Asthma Rep 2017;17(3):19. doi: 10.1007/s11882-017-0686-1 [published Online First: 2017/03/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah R, Newcomb DC. Sex Bias in Asthma Prevalence and Pathogenesis. Front Immunol 2018;9:2997. doi: 10.3389/fimmu.2018.02997 [published Online First: 2019/01/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol 2018;120(5):488–94. doi: 10.1016/j.anai.2018.01.016 [published Online First: 2018/02/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han YY, Forno E, Celedon JC. Sex Steroid Hormones and Asthma in a Nationwide Study of U.S. Adults. Am J Respir Crit Care Med 2020;201(2):158–66. doi: 10.1164/rccm.201905-0996OC [published Online First: 2019/09/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [published Online First: 2015/04/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Biobank. Key documents for a large-scale prospective epidemiological resource [Available from: http://www.ukbiobank.ac.uk/key-documents/ accessed Feb 4 2020.
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805 [published Online First: 2005/08/02] [DOI] [PubMed] [Google Scholar]
- 14.Elliott P, Peakman TC, Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol 2008;37(2):234–44. doi: 10.1093/ije/dym276 [published Online First: 2008/04/03] [DOI] [PubMed] [Google Scholar]
- 15.UK Biobank. UK Biobank Biomarker Project - Companion Document to Accompany Serum Biomarker Data 2019. [Available from: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf accessed Feb 4 2020. [Google Scholar]
- 16.Ly LP, Handelsman DJ. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur J Endocrinol 2005;152(3):471–8. doi: 10.1530/eje.1.01844 [published Online First: 2005/03/11] [DOI] [PubMed] [Google Scholar]
- 17.Office for National Statistics. Average household income, UK: financial year ending 2019. London, England, 2020. [Google Scholar]
- 18.National Health Service. Overview -Menopause United Kingdom 2018. [Available from: https://www.nhs.uk/conditions/menopause/ accessed March 10 2020.
- 19.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40(6):1324–43. doi: 10.1183/09031936.00080312 [published Online First: 2012/06/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzel SE, Robinson CB, Leonard JM, et al. Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy and asthma proceedings : the official journal of regional and state allergy societies 2010;31(6):461–71. doi: 10.2500/aap.2010.31.3384 [DOI] [PubMed] [Google Scholar]
- 21.Baillargeon J, Urban RJ, Zhang W, et al. Testosterone replacement therapy and hospitalization rates in men with COPD. Chron Respir Dis 2019;16:1479972318793004. doi: 10.1177/1479972318793004 [published Online First: 2018/09/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuseini H, Yung JA, Cephus JY, et al. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J Immunol 2018;201(7):1843–54. doi: 10.4049/jimmunol.1800293 [published Online First: 2018/08/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cephus JY, Stier MT, Fuseini H, et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep 2017;21(9):2487–99. doi: 10.1016/j.celrep.2017.10.110 [published Online First: 2017/12/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montano LM, Flores-Soto E, Reyes-Garcia J, et al. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol Cell Endocrinol 2018;473:17–30. doi: 10.1016/j.mce.2017.12.010 [published Online First: 2017/12/25] [DOI] [PubMed] [Google Scholar]
- 25.Kalidhindi RSR, Katragadda R, Beauchamp KL, et al. Androgen Receptor-Mediated Regulation of Intracellular Calcium in Human Airway Smooth Muscle Cells. Cell Physiol Biochem 2019;53(1):215–28. doi: 10.33594/000000131 [published Online First: 2019/07/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan SS, Knuiman MW, Divitini ML, et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin Endocrinol (Oxf) 2015;83(2):268–76. doi: 10.1111/cen.12738 [published Online First: 2015/02/11] [DOI] [PubMed] [Google Scholar]
- 27.Svartberg J, Schirmer H, Medbo A, et al. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromso study. Eur J Epidemiol 2007;22(2):107–12. doi: 10.1007/s10654-006-9095-9 [published Online First: 2007/01/30] [DOI] [PubMed] [Google Scholar]
- 28.DeBoer MD, Phillips BR, Mauger DT, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med 2018;18(1):58. doi: 10.1186/s12890-018-0612-x [published Online First: 2018/04/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becerra-Diaz M, Strickland AB, Keselman A, et al. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol 2018;201(10):2923–33. doi: 10.4049/jimmunol.1800352 [published Online First: 2018/10/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol 2017;186(9):1026–34. doi: 10.1093/aje/kwx246 [published Online First: 2017/06/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.