Abstract
Background
Thromboprophylaxis for ambulatory patients with cancer is effective, although uncertainties remain on who should be targeted. Using D‐dimer values from individuals enrolled to the AVERT trial, we sought to identify and validate a more efficient venous thromboembolism (VTE) risk threshold for thromboprophylaxis.
Materials and Methods
The AVERT trial compared thromboprophylaxis with apixaban with placebo among patients with cancer with a Khorana Risk Score ≥2. The D‐dimer measured at randomization was used to calculate an individualized 6‐month VTE risk using the validated CATScore. A modified intention‐to‐treat analysis was used to assess efficacy (VTE) and safety (major and overall bleeding) in the (a) complete cohort and (b) ≥8% and < 8% 6‐month VTE risk thresholds.
Results
Five hundred seventy‐four patients were randomized in the AVERT trial; 466 (81%) with baseline D‐dimer were included in the study. Two hundred thirty‐seven subjects received apixaban; 229 received placebo. In the complete cohort, there were 13 (5.5%) VTE events in the apixaban arm compared with 26 (11.4%) events in the placebo arm (adjusted hazard ratio [aHR] 0.49 [0.25–0.95], p < .05). Number needed to treat (NNT) to prevent one VTE = 17. Eighty‐two (35%) and 72 (31%) patients in the apixaban and placebo arms, respectively, had a 6‐month VTE risk ≥8%. In this subgroup, 7 (8.4%) VTE events occurred with apixaban and 19 (26.3%) events with placebo (aHR 0.33 [0.14‐0.81], p < .05), NNT = 6. Individuals with a VTE risk <8% derived no benefit from apixaban thromboprophylaxis (aHR 0.89 [0.30–2.65), p = .84). Increased rates of overall bleeding were observed with apixaban in both the complete (aHR 2.11 [1.09–4.09], p < .05) and ≥ 8% predicted risk cohorts (aHR 2.87 [0.91–9.13], p = .07).
Conclusion
A 6‐month VTE risk threshold of ≥8% increases the efficiency of risk‐targeted thromboprophylaxis in ambulatory patients with cancer.
Implications for Practice
Ambulatory patients with cancer receiving chemotherapy have an increased risk of venous thromboembolism (VTE). A Khorana Risk Score (KRS) ≥2 is currently the suggested threshold for thromboprophylaxis. Using baseline D‐dimer values from individuals enrolled to the AVERT trial, this retrospective validation study identifies a 6‐month VTE risk of ≥8% as a more efficient threshold for thromboprophylaxis. At this threshold, the number needed to treat to prevent one VTE is 6, compared with 17 when using a KRS ≥2. Conversely, individuals with a predicted risk of <8% derive no clinical benefit from thromboprophylaxis. Future prospective studies should validate this threshold for outpatient thromboprophylaxis.
Keywords: Venous thromboembolism, Cancer‐associated thrombosis, Thromboprophylaxis, Apixaban, D‐dimer
Short abstract
Using individual patient data from the AVERT study, this study aimed to retrospectively identify a more efficient VTE risk threshold for thromboprophylaxis and to address the safety and efficacy of risk‐targeted thromboprophylaxis using the CATScore.
Introduction
The increased incidence of venous thromboembolism (VTE) among patients with cancer is well established [1], and the development of VTE portends a worse prognosis in this patient population [2, 3]. High‐quality randomized controlled trial (RCT) evidence suggests that thromboprophylaxis with either oral or parenteral anticoagulation is effective in reducing the incidence of VTE in ambulatory patients with cancer [4, 5, 6, 7]. Notably, the absolute risk reduction (ARR) in VTE achieved by thromboprophylaxis varies significantly by the treated cohort's baseline predicted risk of VTE.
The Khorana Risk Score (KRS) is the most widely used and externally validated risk prediction tool to categorize patients into low, intermediate, and high risk of VTE based on tumor type, as well as clinical and hematologic parameters [8]. A KRS ≥2 was an inclusion criterion in both the AVERT and CASSINI placebo‐controlled RCTs that demonstrated the superiority of the direct factor Xa inhibitors, apixaban and rivaroxaban (respectively), in reducing rates of VTE in ambulatory patients with cancer [6, 7]. Several guidelines now encourage consideration of thromboprophylaxis among ambulatory patients with cancer due to start chemotherapy who have a KRS ≥2 [9, 10, 11]. Prospective comparisons of several VTE risk prediction tools in patients with cancer have shown that models using thrombosis biomarkers, such D‐dimer and soluble p‐selectin, are able to better discriminate between low‐ and high‐VTE‐risk patients [12, 13, 14].
In the most recent iteration of the Vienna VTE risk prediction score, Pabinger et al. combined the clinical tumor site with the baseline plasma D‐dimer to develop and externally validate the 2018 Vienna “CATScore” in two large prospective cohorts [15]. This score provides an individualized 6‐month risk of VTE and demonstrated superior model performance compared with existing prediction models. In this retrospective validation study, we sought to use individual patient data from the AVERT study to (a) identify a more efficient VTE risk threshold for thromboprophylaxis using the CATScore and (b) perform a post hoc analysis of the AVERT study to address the safety and efficacy of risk‐targeted thromboprophylaxis using the CATScore.
Materials and Methods
Study Participants
We used available data from patients enrolled in the AVERT study, a randomized, double‐blind, placebo‐controlled clinical trial that assessed thromboprophylaxis with low‐dose apixaban, 2.5 mg twice daily, in ambulatory patients with cancer due to start a minimum expected course of 3 months of cytotoxic chemotherapy. Patients with a KRS ≥2 were eligible for inclusion in the study. Supplemental online Table 1 describes the modified KRS used for patient selection; the full trial protocol and inclusion criteria have been previously described [7]. In this analysis, we excluded 108 patients for whom baseline D‐dimer measurements were not available prior to the first dose of study drug. Patients excluded from this analysis are described in supplemental online Table 2. The total treatment duration for the AVERT study was 180 days and patients were followed up to 210 days or death. This study was approved by all institutional review boards at participating organizations.
D‐Dimer Measurements
Blood samples for biomarker analyses were collected on the day of study enrollment (range day −28 to day 0 (i.e., day of chemotherapy initiation) and prior to administration of the first dose of either apixaban or placebo. Blood was drawn into 0.109‐M sodium citrate tubes. Within 1 hour of sample collection, platelet‐poor plasma was prepared by centrifugation for 15 minutes at 2,000g. Plasma samples for D‐dimer measurement were stored at −80°C after snap freezing. All D‐dimer assays were performed at the Ottawa Hospital Research Laboratory using an immunoturbidimetric assay (STA‐Liatest D‐Di 20; Diagnostica Stago, Asnières, France). When the initial assay reading was >4 μg/mL, the sample was diluted according to manufacturer specifications to yield a corrected assay range of 0.27–20 μg/mL.
CATScore and 6‐Month VTE Risk Prediction
The CATScore was developed and validated using data from two independent prospective cohorts designed to assess risk factors for VTE in patients with cancer. Participants in both studies had thrombosis biomarkers measured at the point of enrollment into the study and individuals receiving anticoagulation either therapeutic or prophylactic were excluded. In their model development, Pabinger et al. had maintained tumor risk site categorization as per the original KRS (supplemental online Table 1), adding colorectal cancer to the “high risk” category. Using prespecified variable selection process, the authors identified D‐dimer and tumor risk categorization for inclusion into the CATScore. Using tumor type and D‐dimer from the patients in the AVERT study, we calculated the 6‐month predicted risk of VTE for each individual using the published online calculator [15, 16]. The individual 6‐month predicted risk of VTE was calculated at “baseline,” that is, prior to receipt of placebo or apixaban.
Outcomes
The primary efficacy outcome of the AVERT trial was the first episode of objectively documented proximal deep vein thrombosis (DVT) or pulmonary embolism (PE). VTE was defined as any symptomatic or incidentally discovered proximal DVT of the lower or upper limbs, nonfatal symptomatic or incidentally discovered, or PE‐related death. The AVERT study did not perform routine ultrasonographic testing in asymptomatic patients.
The main safety outcome was major bleeding as defined by the International Society on Thrombosis and Hemostasis, that is, (a) fatal bleeding, (b) bleeding occurring in a critical site, or (c) a decrease in hemoglobin level of 2 g/dL or requiring transfusion of two or more units of packed red cells [17]. Clinically relevant nonmajor bleeding was defined as bleeding that did not meet the criteria for major bleeding but was associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of the assigned treatment, or impairment in daily activities. In this analysis, safety outcomes were reported separately for (a) major bleeding events and (b) overall bleeding (a combination of major and clinically relevant nonmajor bleeding events).
Statistical Analysis
All statistical analyses were performed with R Studio Version 1.2.5001. Model discrimination was assessed using the receiver operator characteristic (ROC) curve and quantified using the area under the ROC curve (AUC), with the 95% confidence interval (CI) calculated using the DeLong method [18]. A decision curve analysis was conducted to assess the net benefit at a range of threshold probability generated by the CATScore among patients randomized to the placebo arm [19, 20]. Among patients randomized to the placebo arm, sensitivity, specificity, and positive and negative predictive values were assessed at a range of 6‐month VTE risk thresholds as calculated by the CATScore and KRS ≥3. No statistical comparisons were made between the baseline characteristics of the complete cohort and the cohorts stratified by the ≥8% VTE Risk threshold cutoff. Categorical variables were described numerically and as percentages; continuous variables were described using means, standard deviations, and interquartile ranges. We estimated thrombosis‐free survival, major bleeding–free survival, and overall bleeding–free survival (combined outcome of major and clinically relevant nonmajor bleeding) between individuals randomized to apixaban versus placebo using the Kaplan‐Meier method and compared results between groups using the log‐rank test. We report the 180‐day estimate with 95% confidence intervals for both safety and efficacy event‐free survival outcomes. A multivariable Cox proportional hazards model adjusting for age and sex was used to provide the adjusted hazard ratios (aHRs) for VTE, major bleeding, and all clinically relevant bleeding over the time course of the AVERT study. All safety and efficacy outcomes were calculated using the modified intention‐to‐treat analysis. The ARR for VTE prevention was calculated by subtracting the event rate in the placebo arm from the event rate in the apixaban arm for the complete cohort and risk‐stratified cohort. The number needed to treat (NNT) is the inverse of the ARR. This analysis meets the recently established consensus guidelines on the analysis and reporting of risk‐based variation of benefit across trial populations [21].
Results
Of 574 randomized patients in the AVERT study, 466 were included in this analysis, as 108 had no available baseline D‐dimer assay. The baseline clinical characteristics of patients excluded from our study is described in supplemental online Table 2. Among individuals randomized to the placebo arm (n = 229), the CATScore demonstrated improved discrimination for VTE (AUC 0.75 [95% CI 0.65–0.86]) compared with the KRS (AUC 0.56 [95% CI 0.46–0.66]; supplemental online Fig. 1). The decision curve analysis demonstrates that the application of the CATScore has increased net benefit at a range of 6‐month predicted VTE risk thresholds compared with a KRS ≥2 (Fig. 1). A 6‐month VTE risk of 8% was the optimal threshold for risk stratification, and at this threshold the CATScore had a sensitivity of 73%, specificity of 74%, positive predictive value of 26%, and negative predictive value of 96% (supplemental online Table 3). In comparison, a similar proportion of patients in the placebo arm had a KRS ≥3 (n = 73 [32%]). However, the sensitivity was only 42% with a positive predictive value of 15% (supplemental online Table 3).
Figure 1.
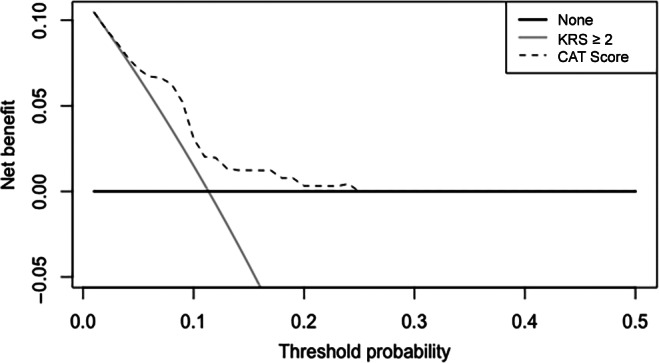
Decision curve analysis. A decision curve analysis among participants randomized to the placebo arm of the AVERT study. The net benefit (y‐axis) is calculated as the true positive rate minus the weighted false‐positive rate for venous thromboembolism and is demonstrated at a range of risk threshold probabilities (x‐axis—right truncated at 0.5). The dashed line demonstrates the net benefit for the use of the CATScore‐based selection for thromboprophylaxis, whereas the gray and black lines represent the net benefit of alternative strategies of the KRS of ≥2 (gray) or treating no one (black). Abbreviation: KRS, Khorana Risk Score.
The baseline clinical characteristics of individuals included in this analysis are summarized in Table 1. The mean 6‐month predicted VTE risk was 10.9% (95% CI 10.5%–11.3%) in the ≥8% risk cohort compared with 5.4% (95% CI 5.3%–5.5%) in the <8% risk cohort, with a mean D‐dimer of 4.0 μg/mL (95% CI 3.2–4.9 μg/mL) in the ≥8% risk cohort versus 1.2 μg/mL (95% CI 1.1–1.3 μg/mL) in the <8% risk cohort. Individuals in the ≥8% cohort were more likely to be male (59% vs. 33%) and have a lower body mass index (27.9 kg/m2 [95% CI 26.0–27.9] vs. 30.7 kg/m2 [95% CI 29.8–31.6]). All patients with a very high‐risk tumor type for VTE (i.e. pancreatic, gastric, and primary brain) had a predicted 6‐month VTE risk ≥8%.
Table 1.
Baseline clinical characteristics
| Characteristics | Complete cohort | CATScore ≥8% a | CATScore <8% a | |||
|---|---|---|---|---|---|---|
| Apixaban | Placebo | Apixaban | Placebo | Apixaban | Placebo | |
| n = 237 | n = 229 | n = 83 | n = 72 | n = 154 | n = 157 | |
| Age, years | ||||||
| Mean ± SD | 60.6 ± 12.6 | 61.0 ± 11.8 | 59.5 ± 11.7 | 61.8 ± 9.9 | 61.2 ± 13.0 | 60.6 ± 12.6 |
| IQR | 54–70 | 55–69 | 54–67 | 55 – 67 | 54–71 | 54–70 |
| Sex, n (%) | ||||||
| Male | 98 (41) | 98 (43) | 48 (58) | 44 (61) | 50 (32) | 54 (34) |
| CrCl, mL/min | ||||||
| Mean ± SD | 109 ± 42 | 107 ± 44 | 107 ± 40 | 104 ± 35 | 110 ± 43 | 109 ± 48 |
| IQR | 80–133 | 76–129 | 81–117 | 83–119 | 78–139 | 73–133 |
| Weight, kg | ||||||
| Mean ± SD | 81 ± 23 | 83 ± 22 | 76 ± 19 | 78 ± 18 | 83 ± 24 | 85 ± 23 |
| IQR | 62–96 | 67–95 | 61–87 | 63–87 | 64–98 | 68–97 |
| BMI, kg/m2 | ||||||
| Mean ± SD | 29.3 ± 7.8 | 29.6 ± 7.4 | 27.0 ± 6.5 | 27.0 ± 5.6 | 30.6 ± 8.1 | 30.9 ± 7.8 |
| IQR | 23–36 | 24–35 | 23–30 | 24–29 | 24–37 | 25–36 |
| Tumor type, n (%) | ||||||
| Brain | 13 (5.4) | 8 (3.5) | 13 (15.7) | 8 (11.1) | 0 (0) | 0 (0) |
| Lung | 24 (10.1) | 23 (10.0) | 2 (2.4) | 2 (2.8) | 22 (14.2) | 21 (13.4) |
| Stomach | 23 (9.7) | 16 (7.0) | 23 (27.7) | 16 (22.2) | 0 (0) | 0 (0) |
| Pancreatic | 27 (11.4) | 30 (13.1) | 27 (32.5) | 30 (41.7) | 0 (0) | 0 (0) |
| Lymphoma | 62 (26.1) | 58 (25.3) | 9 (10.8) | 4 (5.6) | 53 (34.4) | 54 (34.3) |
| Gynecologic | 65 (27) | 61 (26.6) | 8 (9.6) | 10 (13.9) | 57 (37.0) | 51 (32.5) |
| Other | 23 (9.8) | 33 (14.4) | 1 (1.2) | 2 (2.8) | 22 (14.2) | 31 (19.7) |
| Khorana Risk Score, n (%) | ||||||
| 2 | 151 (63.7) | 156 (68.1) | 45 (54.2) | 44 (61.1) | 106 (68.8) | 112 (71.3) |
| 3 | 65 (27.4) | 57 (24.9) | 26 (31.3) | 20 (27.8) | 39 (25.3) | 37 (23.5) |
| 4 | 21 (8.9) | 16 (7.0) | 12 (14.4) | 8 (11.1) | 9 (5.8) | 8 (5.1) |
| D‐dimer, μg/mL | ||||||
| Mean ± SD | 2.2 ± 3.5 | 2.1 ± 3.5 | 3.9 ± 5.3 | 4.1 ± 5.5 | 1.3 ± 1.0 | 1.2 ± 1.0 |
| IQR | 0.6–2.4 | 0.4–2.0 | 0.6–4.9 | 0.5–6.7 | 0.5–1.7 | 0.4–1.6 |
| 6‐month predicted risk, % | ||||||
| Mean ± SD | 7.4 ± 3.1 | 7.1 ± 3.3 | 10.9 ± 2.5 | 11.0 ± 3.0 | 5.5 ± 1.0 | 5.3 ± 1.1 |
| IQR | 5.1–9.6 | 4.9–8.9 | 9.3–11.6 | 9.1–11.8 | 4.9–6.1 | 4.7–6.0 |
Each patient was stratified by their individualized 6‐month predicted risk of VTE [15].
Abbreviations: BMI, body mass index; CATScore, 2018 Vienna CATScore; CrCl, creatinine clearance; IQR, interquartile range.
VTE Outcomes by Risk Cohorts
The median duration of follow‐up was 196 days (interquartile range 188–204 days) in the complete cohort and was identical in the ≥8% and < 8% risk cohorts. In the complete cohort, there were 13 (5.5%) VTE events in the apixaban arm and 26 (11.4%) events in the placebo arm (aHR 0.49 [95% CI 0.25–0.95], p < .05; Table 2). The 180‐day thrombosis‐free survival in the apixaban arm was 95% (95% CI 92%–98%) compared with 89% (95% CI 85%–93%) in the placebo arm (Fig. 2). When selecting patients for thromboprophylaxis based on a KRS ≥2 (i.e., the complete cohort), the ARR of 5.9% equates to a corresponding NNT with apixaban of 17 to prevent one VTE.
Table 2.
Efficacy and safety data of complete and risk‐stratified populations
| Complete analytic AVERT cohort | CATScore ≥8% a | CATScore <8% a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Apixaban | Placebo | Hazard ratio | Apixaban | Placebo | Hazard ratio | Apixaban | Placebo | Hazard ratio |
| n = 237 | n = 229 | (95% CI) | n = 83 | n = 72 | (95% CI) | n = 154 | n = 157 | (95% CI) | |
| VTE, n (%) | 13 (5.5) | 26 (11.4) | 0.49 (0.25–0.95) b | 7 (8.4) | 19 (26.3) | 0.33 (0.14–0.81) b | 6 (3.9) | 7 (4.5) | 0.89 (0.30–2.65) |
| Major bleeding, n (%) | 9 (3.8) | 5 (2.2) | 1.83 (0.61–5.45) | 5 (6.0) | 3 (4.2) | 1.91 (0.44–8.19) | 4 (2.6) | 2 (1.3) | 2.07 (0.38–11.3) |
| Overall bleeding, n (%) | 27 (11.4) | 13 (5.7) | 2.11 (1.09–4.09) b | 11 (13) | 4 (5.6) | 2.87 (0.91–9.13) | 16 (10.3) | 9 (5.7) | 1.89 (0.83–4.27) |
The complete analytic cohort comprises all patients enrolled into the AVERT study for whom baseline D‐dimer was available.
Each patient was stratified by their individualized 6‐month predicted risk of VTE [15].
Adjusted hazard ratios p < .05 (adjusted for age and sex).
Abbreviations: CATScore, 2018 Vienna CATScore; CI, confidence interval; VTE, venous thromboembolism.
Figure 2.
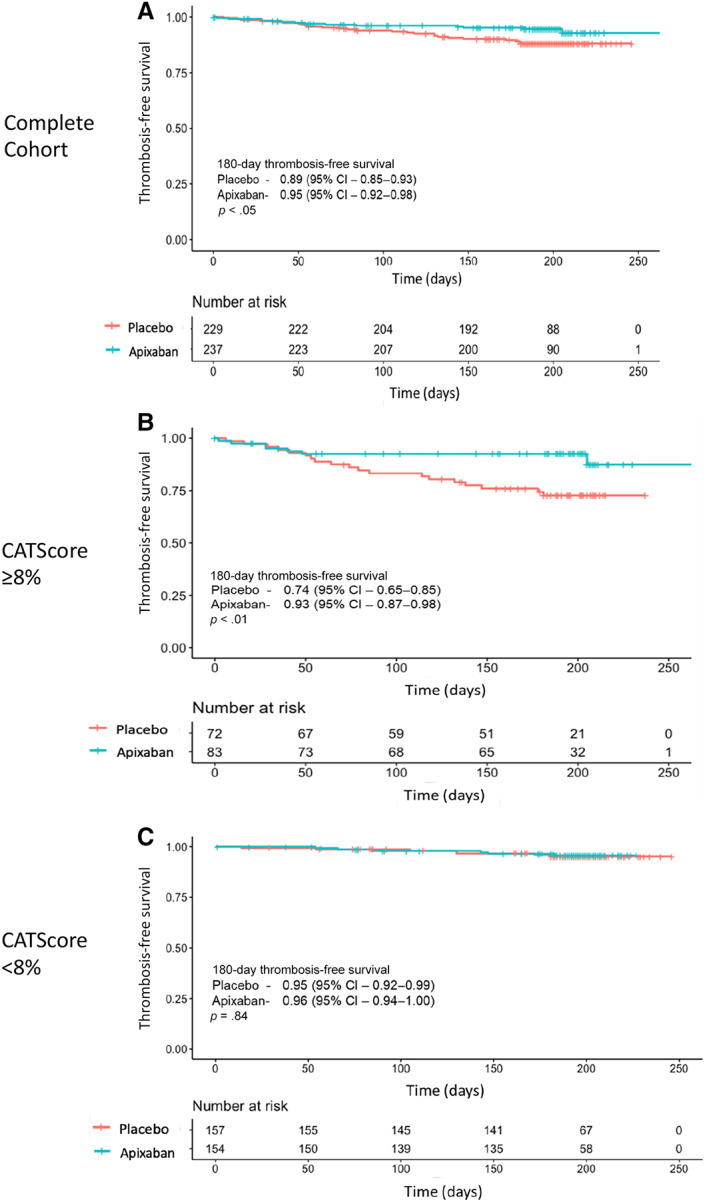
Kaplan‐Meier analysis of thrombosis‐free survival. A Kaplan‐Meier analysis with log‐rank test was used to compare the thrombosis‐free survival between twice‐daily apixaban 2.5 mg versus placebo for (A) all patients enrolled into the AVERT study for whom baseline D‐dimer values were available and (B) individuals in the AVERT study with a 6‐month predicted risk of venous thromboembolism ≥8% or (C) 6‐month predicted risk of <8%. (A): Thromboprophylaxis with apixaban led to an improved thrombosis‐free survival compared with placebo with an absolute risk reduction (ARR) of 5.9% at 180 days (number needed to treat [NNT] = 17; p < .05). (B): Among patients with a 6‐month predicted risk ≥8%, apixaban had a 180‐day ARR of 17.9% (NNT = 6; p < .01). (C): Individuals with a 6‐month predicted risk <8% derived no benefit in thrombosis‐free survival when receiving prophylaxis with apixaban compared with placebo (p = .84). Abbreviation: CI, confidence interval.
In the ≥8% 6‐month VTE risk cohort, there were 7 (8.4%) VTE events in the apixaban arm and 19 (26.3%) in the placebo arm (aHR 0.33 [0.14–0.81], p < .05) and the corresponding 180‐day thrombosis‐free survival was 93% (95% CI 87%–98%) in the apixaban arm versus 74% (95% CI 65%–85%, p < .01) in the placebo arm. When selecting patients for thromboprophylaxis using a CATScore 6‐month VTE risk threshold of ≥8%, the ARR is 17.9% with a corresponding NNT with apixaban of 6.
In the <8% risk cohort, there was no significant difference in the VTE events between patients treated with apixaban (n = 6 [3.9%]) versus placebo (n = 7 [4.5%]; aHR 0.89 [0.30–2.65], p = .84; Table 2). In this cohort, the 180‐day thrombosis free survival was 96% (95% CI 94%–100%) in the apixaban arm compared with 95% (95% CI 92%–99%) in the placebo arm (Fig. 2).
Bleeding Outcomes by Risk Cohort
In the complete cohort, there were no significant differences in major bleeding events in the apixaban arm (n = 9 [3.8%]) compared with the placebo arm (n = 5 [2.2%]; aHR 1.83 [95% CI 0.61–5.45, p = .28). Patients receiving apixaban had increased overall bleeding rates compared with placebo (n = 27 [11.4%] vs. 13 [5.7%]; aHR 2.11 [95% CI 1.09–4.09], p < .05; Table 2). Patients receiving apixaban in the complete cohort had a 180‐day major bleeding–free survival of 96% (95% CI 94%–99%) and 180‐day overall bleeding–free survival of 88% (95% CI 84%–92%; Fig. 3).
Figure 3.
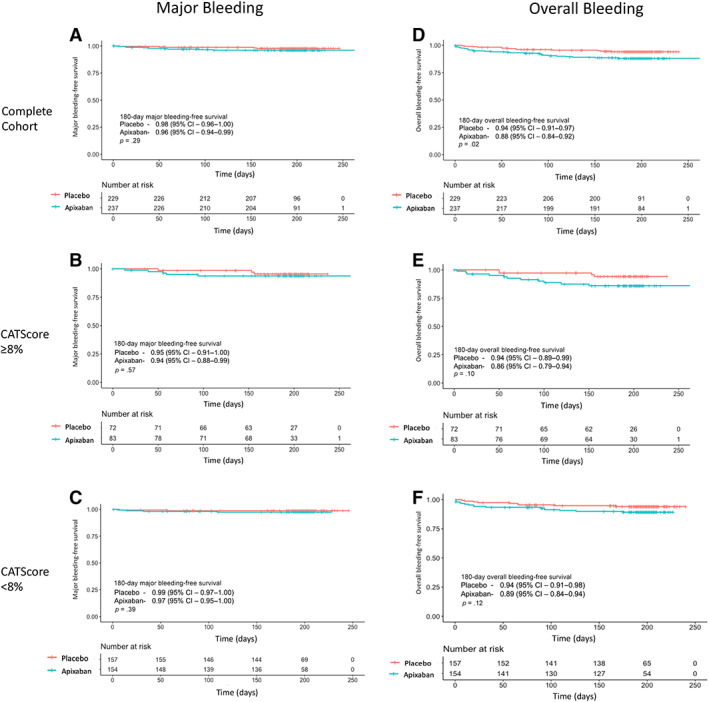
Kaplan‐Meier analysis of major and overall bleeding events. A Kaplan‐Meier analysis with log‐rank test demonstrated no difference in major bleeding events in patients randomized to thromboprophylaxis with apixaban 2.5 mg twice daily versus placebo in (A) all patients enrolled into the AVERT study for whom baseline D‐dimer was available (p = .29), (B) individuals with a 6‐month predicted venous thromboembolism (VTE) rate of ≥8% (p = .57), or (C) individuals with a 6‐month predicted VTE rate < 8% (p = .39). There was an increased rate of overall bleeding (composite of major and clinically relevant nonmajor bleeding) in all patients randomized to apixaban 2.5 mg b.i.d. versus placebo; this was significant in the complete analytic cohort (p = .02) with no significance in patients with a 6‐month predicted VTE risk ≥8% (E; p = .10) or those with a 6‐month predicted VTE risk <8% (F; p = .12). Abbreviation: CI, confidence interval.
In the ≥8% risk cohort, there was no significant difference in major bleeding between apixaban and placebo (n = 5 [6%] vs. 3 [4.2%]; aHR 1.91 [95% CI 0.44–8.19], p = .39). However, although the rates of overall bleeding were increased in the apixaban arm (n = 11 [13%] vs. 4 [5.6%] in the placebo arm), this was not statistically significant (aHR 2.87 [95% CI 0.91–9.13], p = .07; Table 2). In the ≥8% cohort, patients receiving apixaban had a 180‐day major bleeding–free survival of 94% (95% CI 88%–99%) and 180‐day overall bleeding–free survival of 86% (95% CI 79%–94%; Fig. 3).
In the <8% risk cohort, major and overall bleeding events were lower compared with the ≥8% risk cohorts. There was no significant difference in major bleeding events between apixaban and placebo (n = 4 [2.6%] vs. n = 2 [1.3%]; aHR 2.07 [95% CI 0.38–11.3], p = .40). Although there were increased rates of overall bleeding in the apixaban arm (n = 16 [10.3] vs. 9 [5.7%]), this was not statistically significant (aHR 1.89 [95% CI 0.83–4.27], p = .13). In the <8% cohort, patients receiving apixaban had a 180‐day major bleeding–free survival of 97% (95% CI 95%–100%) and 180‐day overall bleeding–free survival of 89% (95% CI 84%–94%; Fig. 3).
Discussion
Despite the longstanding and robust evidence on the utility of thromboprophylaxis in reducing the VTE burden among ambulatory patients with cancer [4, 5, 6, 7], methods for identifying the appropriate “high‐risk” population for the most efficient use of thromboprophylaxis continues to generate much debate [22]. In this study, we retrospectively validated the 2018 Vienna CATScore [15] in a cohort of ambulatory patients with cancer with a Khorana Risk Score ≥ 2 who were enrolled into the placebo‐controlled AVERT thromboprophylaxis trial [7]. We confirm the excellent discrimination of the CATScore for VTE prediction; furthermore, we propose a 6‐month VTE risk cutoff of ≥8% as a risk threshold for consideration of thromboprophylaxis among ambulatory patients with cancer. At this threshold, the NNT to prevent one VTE with apixaban thromboprophylaxis is only 6, compared with an NNT of 17 when using a KRS ≥2. Patients with a 6‐month predicted risk of VTE <8% appear to derive no benefit in terms of VTE prevention from thromboprophylaxis with apixaban. Patients in the ≥8% and < 8% cohorts did not experience increased rates of major bleeding. Similar rates of overall clinically relevant bleeding with apixaban were seen in the complete and risk‐stratified cohorts.
The Khorana Risk Score was published in 2008 and was developed from a prospective cohort of patients enrolled in the Awareness of Neutropenia Study Group Registry [8]. A significant advantage and key to the initial popularity of the KRS is the readily available clinical and hematologic parameters required at the time of risk stratification, without the need for additional measurements of thrombotic biomarkers. However, subsequent prospective validation studies and systematic reviews have demonstrated the limitation of the KRS in terms of positive predictive value for VTE and that the key component for risk prediction of the KRS is the categorization and weighting of the primary tumor type [12, 22, 23]. When developing and validating the 2018 Vienna CATScore, Pabinger et al. maintained the tumor type categorization as per the KRS and added D‐dimer on a continuous scale for improved risk prediction [15]. In their external validation cohort, the CATScore had an AUC of 0.68 (95% CI 0.62–0.74) versus 0.56 (95% CI 0.50–0.63) for the KRS in the same cohort. Similarly, we demonstrate the excellent discrimination of the CATScore when applied to the placebo arm of the AVERT study. This is in contrast to the poor sensitivity of the KRS that has been previously highlighted [23]. Although the sensitivity of a KRS ≥3 in the placebo arm of the AVERT of 42% is an improvement from previously published figures [22, 23], it does not compare favorably with the 73% sensitivity seen with a CATScore at a threshold of ≥8%.
At our recommended 6‐month VTE risk threshold of ≥8%, similar to a KRS ≥2, all patients categorized with “very high‐risk” tumor types would receive thromboprophylaxis. The measurement of D‐dimer and evaluation of CATScore would thus have limited utility in the decision to provide thromboprophylaxis in this patient population. However, as the CATScore has not been widely adopted into clinical practice and still needs further validation, we would advocate the ongoing calculation of the CATScore even among very high‐risk tumor types. Future studies, ideally including patients with KRS 0 or 1, may identify a higher 6‐month VTE risk threshold, which would thus necessitate measurement of D‐dimer and calculation of the CATScore in all tumor types.
D‐dimer is a global marker of fibrinolysis and is a key component of the CATScore. It is widely available in clinical laboratories and commonly used for its negative predictive value to exclude low‐risk VTE [24, 25]. D‐dimer is now also being used to assess the risk of VTE recurrence on cessation of oral anticoagulation [26]. Most prior risk prediction tools use D‐dimer as a dichotomous variable (i.e., either normal or elevated) [13, 24, 25, 26]; however, dichotomization is known to result in significant loss of vital clinical information [27]. By maintaining D‐dimer on a continuous scale, Pabinger et al. are better able to use this thrombotic biomarker for risk prediction [15]. Interestingly, the Vienna Prediction Model for VTE recurrence, similar to the CATScore, used D‐dimer on a continuous scale and demonstrated improved discrimination between high‐ and low‐risk patients [28].
Unlike the KRS, the requirement for a D‐dimer assay for the CATScore poses additional practical hurdles in real‐world implementation of risk‐targeted thromboprophylaxis in ambulatory patients with cancer. Additionally, increasing efforts will need to be placed on the operating characteristics of the large variety of commercially available D‐dimer assays, as the results from the nomogram generated by Pabinger et al. may not translate directly to all D‐dimer assays [29]. Despite these potential limitations, prior quality‐improvement strategies that incorporate electronic health records to provide personalized VTE prophylaxis to ambulatory patients with cancer have been shown to be an effective tool to increase thromboprophylaxis uptake rates [30].
Limitations
There are several important limitations in our study. First, the model performance of the CATScore was assessed in a cohort of patients with a KRS ≥2 enrolled in the AVERT trial. It remains uncertain if the risk discrimination will be as robust when applied to a wider cohort of ambulatory patients with cancer with lower baseline predicted risks of VTE. Notably, the low‐risk group (i.e., KRS of 0 or 1) accounted for greater than 50% of the individuals enrolled into the prospective cohorts used for the development and validation of the CATScore [15]. However, our study is not able to evaluate the proportion of patients with a KRS <2 who would be categorized as having a CATScore ≥8%. Second, in this post hoc analysis, we excluded 108 randomized patients owing to the omission of baseline D‐dimer measurement. Although there does not appear to be a systematic etiology for this omission and the excluded patients had similar baseline clinical characteristics, the possibility of an inadvertent selection bias and confounding remains. Third, given the exclusion of patients at high risk of bleeding from clinical trials evaluating thromboprophylaxis and the caveats of translating trial results into real‐world practice [6, 7, 31], most societal guidelines still recommend an individualized patient‐centered approach when deciding on thromboprophylaxis [9, 10]. Interestingly, similar to the evolution of primary and secondary prevention in cardiovascular disease, an individualized risk percentile as generated by the CATScore may aid in this shared decision‐making process [32]. Fourth, we are mindful to highlight that the inclusion criteria for the AVERT study required patients to have a minimum intent of 3 months of outpatient cytotoxic chemotherapy. The role of thromboprophylaxis among those receiving immunotherapy, targeted therapy, or hormonal therapy alone has not been fully outlined. Finally, we demonstrate that there are increased rates of overall bleeding with apixaban in the complete cohort analysis; however, the rates of overall bleeding were not statistically significant in the cohorts stratified by the CATScore. With the reduced sample size, we would be mindful for the possibility of a type II error in this instance.
Conclusion
Thromboprophylaxis in patients with cancer due to start outpatient chemotherapy has been shown to be effective. Using baseline D‐dimer from individuals enrolled into the AVERT thromboprophylaxis trial, we demonstrated the improved efficiency of risk‐targeted thromboprophylaxis using the 2018 Vienna CATScore. We propose a 6‐month VTE risk of ≥8% as a threshold for patient selection, where the number needed to treat to prevent one VTE is only 6. Future prospective studies should aim to further validate the CATScore and our recommended threshold.
Author Contributions
Conception/design: Vaibhav Kumar, Joseph Shaw, Nigel S. Key, Phil Wells, Marc Carrier
Provision of study material or patients: Phil Wells, Marc Carrier
Collection and/or assembly of data: Vaibhav Kumar, Joseph Shaw, Anton Ilich, Ranjeeta Mallick
Data analysis and interpretation: Vaibhav Kumar, Joseph Shaw, Nigel S. Key, Anton Ilich, Ranjeeta Mallick, Phil Wells, Marc Carrier
Manuscript writing: Vaibhav Kumar, Joseph Shaw
Final approval of manuscript: Vaibhav Kumar, Joseph Shaw, Nigel S. Key, Anton Ilich, Ranjeeta Mallick, Phil Wells, Marc Carrier
Disclosures
Vaibhav Kumar: Diagnostica Stago (H); Phil Wells: Bristol‐Myers Squibb/Pfizer, Bayer Healthcare (H [to institution]); Marc Carrier: Bristol‐Myers Squibb, Pfizer, Leo Pharma (RF), Bayer, Bristol‐Myers Squibb, Pfizer, Leo Pharma, Servier, Sanofi (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Tables
Acknowledgments
The AVERT trial was funded by the Canadian Institute for Health Research and the BMS‐Pfizer Alliance. M Carrier is the recipient of a Research Chair from the University of Ottawa and the Department of Medicine on Venous Thromboembolism and Cancer. V Kumar receives NIH funding through the NIH 5T32HL007149‐43.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Blom JW, Vanderschoot JPM, Oostindiër MJ et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J Thromb Haemost 2006;4:529–535. [DOI] [PubMed] [Google Scholar]
- 2. Sørensen HT, Mellemkjær L, Olsen JH et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–1850. [DOI] [PubMed] [Google Scholar]
- 3. Wun T, White RH. Epidemiology of cancer‐related venous thromboembolism. Best Pract Res Clin Haematol 2009;22:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agnelli G, Gussoni G, Bianchini C et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo‐controlled, double‐blind study. Lancet Oncol 2009;10:943–949. [DOI] [PubMed] [Google Scholar]
- 5. Agnelli G, George DJ, Kakkar AK et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601–609. [DOI] [PubMed] [Google Scholar]
- 6. Khorana AA, Soff GA, Kakkar AK et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 7. Carrier M, Abou‐Nassar K, Mallick R et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 8. Khorana AA, Kuderer NM, Culakova E et al. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2019;38:496–523. [DOI] [PubMed] [Google Scholar]
- 10. Wang TF, Zwicker JI, Ay C et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory patients with cancer: Guidance from the SSC of the ISTH. J Thromb Haemost 2019;17:1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farge D, Frere C, Connors JM et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–e581. [DOI] [PubMed] [Google Scholar]
- 12. van Es N, Di Nisio M, Cesarman G et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017;102:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ay C, Dunkler D, Marosi C et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–5383. [DOI] [PubMed] [Google Scholar]
- 14. Alexander M, Ball D, Solomon B et al. Dynamic thromboembolic risk modelling to target appropriate preventative strategies for patients with non‐small cell lung cancer. Cancers (Basel) 2019;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pabinger I, van Es N, Heinze G et al. A clinical prediction model for cancer‐associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.catscore: A clinical prediction model for cancer‐associated venous thromboembolism. Available at https://cemsiis.meduniwien.ac.at/en/kb/science‐research/software/clinical‐software/cancer‐vte/. Accessed January 13, 2020.
- 17. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 18. Robin X, Turck N, Hainard A et al. pROC: An open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Mak 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Memorial Sloan Kettering Cancer Center . Biostatistics: Decision Curve Analysis. Available at https://www.mskcc.org/departments/epidemiology‐biostatistics/biostatistics/decision‐curve‐analysis. Accessed February 14, 2020.
- 21. Kent DM, Paulus JK, Van Klaveren D et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med 2020;172:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulder FI, Bosch FTM, Van Es N. Primary thromboprophylaxis in ambulatory cancer patients: Where do we stand? Cancers (Basel) 2020;12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulder FI, Candeloro M, Kamphuisen PW et al. The khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta‐analysis. Haematologica 2019;104:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells PS, Anderson DR, Rodger M et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer. Ann Intern Med 2001;135:98–107. [DOI] [PubMed] [Google Scholar]
- 25. Wells PS, Anderson DR, Rodger M et al. Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis. N Engl J Med 2003;349:1227–1235. [DOI] [PubMed] [Google Scholar]
- 26. Palareti G, Legnani C, Cosmi B et al. Risk of venous thromboembolism recurrence: High negative predictive value of D‐dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002;87:7–12. [PubMed] [Google Scholar]
- 27. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eichinger S, Heinze G, Kyrle PA. D‐dimer levels over time and the risk of recurrent venous thromboembolism: An update of the Vienna prediction model. J Am Heart Assoc 2014;3:e000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linkins L‐A, Takach Lapner S. Review of D‐dimer testing: Good, bad, and ugly. Int J Lab Hematol 2017;39:98–103. [DOI] [PubMed] [Google Scholar]
- 30. Ades S, Gilchrist A, Holm A et al. Venous thromboembolism prevention in the ambulatory cancer clinic (VTE‐PACC): A systems‐based, personalized, multidisciplinary program to increase venous thromboembolism (VTE) risk assessment, education and anticoagulant prophylaxis in cancer outpatients. Blood 2017;130(suppl 1):217. [Google Scholar]
- 31. Najafzadeh M, Schneeweiss S. From trial to target populations ‐ Calibrating real‐world data. N Engl J Med 2017;376:1203–1205. [DOI] [PubMed] [Google Scholar]
- 32. Lloyd‐Jones DM, Braun LT, Ndumele CE et al. Use of risk assessment tools to guide decision‐making in the primary prevention of atherosclerotic cardiovascular disease: A special report from the American Heart Association and American College of Cardiology. Circulation 2019;139:e1162–e1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Tables


