Abstract
High‐salt (HS) intake is closely associated with the ignition and progression of hypertension. The mechanisms might be involved in endothelial dysfunction, nitric oxide deficiency, oxidative stress, and proinflammatory cytokines. Propolis is widely used as a natural antioxidant and is a well‐known functional food for its biological activities, which includes anti‐inflammation, antimicrobial, and liver detoxification. In this study, we successfully replicated a HS diet‐induced hypertensive rat model. We found that in the long‐term HS diet group, the myocardial function of the rats was altered and led to a significant decrease (around 49%) in heart function. However, doses of Chinese water‐soluble propolis (WSP) were found directly proportional (11%, 60%, 91%, respectively) to the myocardial function improvement in hypertensive rats. The results from the blood circulation test and hematoxylin‐eosin stains showed that propolis had protective effects on myocardial functions and blood vessels in hypertensive rats. Also, based on the results of western blot and polymerase chain reaction, WSP effectively regulated Nox2 and Nox4 levels and was responsible for a decrease in reactive oxygen species synthesis. Our findings demonstrate that Chinese WSP has a significant effect on the blood pressure of hypertensive rats and their cardiovascular functions that improved significantly. The improvement in the cardiovascular functions might be related to the process of anti‐oxidation, anti‐inflammation, and the improvements of the endothelial function in hypertensive rats.
Salt consumption has increased dramatically among the Chinese. A diet consisting of low salt concentrations could promote good cardiovascular health while a diet high in salt could be detrimental to health. 1 Therefore, it is well recognized that high‐salt (HS) intake is the main risk factor in the ignition and progression of hypertension. Many studies suggest that endothelial dysfunction, nitric oxide deficiency, oxidative stress, and proinflammatory cytokines contribute to the development of hypertension. 2 The HS diet has been associated with a dysregulation of the intrarenal renin‐angiotensin system (RAS), oxidative stress, and inflammatory cytokines that lead to excessive retention of Na+ increased vascular resistance, and high blood pressure. 3 HS intake affects cardiovascular functions through a mechanism that involves the transforming growth factor (TGF‐β1) and nitric oxide (NO). 4 Also, it has been reported that an HS diet promotes an increased generation of superoxide anion (O2 −) from nitric oxide synthase (NOS), which could, in turn, impair the endothelium‐dependent dilation through reduced NO bioavailability. Therefore, it is crucial to understand and prevent tissue injuries associated with oxidative stress and inflammatory cytokines.
Propolis is the generic name of a complex resinous mixture that is collected from plant buds and exudates by honey bees. Propolis is enriched with bee's saliva and enzyme‐containing secretions and used in the construction, adaptation, and protection of hives after pollen collection. 5 Nowadays, numerous studies show that honey and propolis have a beneficial effect on human health. For this reason, honey and propolis are widely used in cosmetics and are also popular alternatives for self‐treatment of various diseases. Propolis samples from Asia, South America, and Europe have different compositions and therefore varying biological activities. However, propolis generally shows great similarity in composition regardless of their botanical source. Propolis compounds have cardioprotective, antioxidant, antiangiogenic actions, antiatherosclerosis, vasoprotective, and anti‐inflammatory properties, which could be used in the modulation of cardiovascular disease.
Recent studies on Malaysian Propolis (MP) demonstrated antioxidant properties and cardioprotective activity against isoproterenol‐induced oxidative stress through direct cytotoxic radical‐scavenging. 6 Other studies on Brazilian red propolis have shown attenuated hypertension and renal damage in the 5/6 renal ablation model. 7 Studies on the Chinese poplar propolis have shown that it decreases oxidized low‐density lipoprotein‐induced endothelial cells injury. 8 The flavonoids extracted from propolis have the potential to inhibit the pathological cardiac hypertrophy progression and heart failure. 9
Chinese propolis could be considered a healthy food option; however, its beneficial effects on the protection of healthy cardiovascular function remain elusive. It has been reported that the protective effects of Chinese propolis on the damaged myocardial cells are induced by oxidative stress, platelet aggregation through inhibitory effects, and attenuate endothelial dysfunction. 10 This study aimed to investigate the protective effects of Chinese water‐soluble propolis (WSP) on hypertension induced by a HS diet and the discussion of the mechanisms involved.
Materials and Methods
Chemicals and drugs
Total RNA extraction reagent TRIzol, RNA reverse transcription kit, polymerase chain reaction (PCR) amplification kit, and primers were purchased from Invitrogen (USA). The Nox4 antibody was purchased from Abcam and Nox2 antibody was purchased from Boster Bioengineering Co., Ltd. The GAPDH antibody and horseradish peroxidase (HRP) was purchased from Shanghai Bioengineering Co., Ltd. The MSF (100 mM), SDS‐PAGE protein loading buffer (5×), SDS‐PAGE gel rapid preparation kit, pre‐stained protein standard molecular weight, SDS‐PAGE electrophoresis buffer, western semi‐dry transfer solution, hypersensitive ECL chemiluminescence kit, BeyoRed DNA loading buffer (6×), and Diethyl pyrocarbonate (DEPC) treated water were purchased from Biyuntian Biotechnology Research Institute. Ethidium bromide (EB) was purchased from Amresco (USA). Acetylcholine (ACh) and phenylephrine (Phe) were purchased from Hefei Bomei Biotechnology Co., Ltd.
Experimental animals
We used male Sprague Dawley (SD) rats from the Hefei Laoshan Experimental Animal Breeding Center (animal certificate number: SCXK (Su) 2009‐0001). A total of 36 animals, 5–6 weeks old, weighing 160 ± 15 g were given a free diet, housed at a room temperature of 24℃, and were subjected to the adaptive feeding for 1 week.
In the first protocol, we randomly selected two different types of diets for 10 weeks. The normal control group (NC group) received distilled water and was fed with standard rat chow, and the HS group received distilled water and was fed with HS rat chow (4% NaCl). Blood pressure (BP) was measured before modeling and then weekly at a fixed time.
After 10 weeks, rats with a BP higher than 140 mmHg were selected for the second protocol. At this stage, the animals were randomly divided into five groups, and five drug treatments were administered for each group over 4 weeks. These groups included: (i) HS group, gavage 0.9% saline (50 mg/kg); (ii) HS + WSP‐L group, gavage WSP (50 mg/kg); (iii) HS + WSP‐M group, gavage WSP (100 mg/kg); (iv) HS + WSP‐H group, gavage WSP (200 mg/kg); (v) HS + WSP‐captopril (CAP) group, gavage CAP (50 mg/kg).
Specimen collection and detection
Rat tail artery blood pressure test
The rats were placed in a quiet environment for 5–10 minutes; tails were heated at 37℃ for 15 minutes, then put into a tail sleeve and connected to a transducer. BP was recorded with the RM6240BD signal acquisition and processing system. The data were measured trice to obtain average values.
Hemodynamic index detection
The rats were anesthetized and immobilized. A catheter (connected to a pressure transducer and filled with anticoagulant) was inserted into the ventricle through the right common carotid artery. After stabilization, the blood flow force was recorded using the Medlab biosignal acquisition system. The index was found to be 30 minutes.
Vascular ring function test
At the end of the experiment, the rats were euthanized and dissected. Segments of the thoracic and abdominal aorta were taken to create an anastomosis ring (3–4 mm long). The anastomosed artery was placed in a perfusion bath and given a preload of 1.5 g. After balance and stability were achieved the Medlab‐U/8C biosignal acquisition system was used to record the vascular tone. Later 10−6 mol/L Phe was used to shrink and stabilize the vascular ring; vascular endothelial integrity was examined with 10−5 mol/L of ACh. If ACh is added to make the Phe pre‐contracted vasodilatation 60–90%, the endothelium is considered intact otherwise, the endothelium could be considered destroyed. The maximum contraction amplitude was 100%, induced by 10−6 mol/L Phe. The changes in vascular tone were reflected by the ratio between the vascular tension amplitude after addition of the drug to the maximum contraction amplitude induced by Phe:
Biochemical indicator testing
Blood samples were collected from the carotid artery. The plasma was prepared by centrifugation at 3,000 rpm/minutes for 15 minutes and stored at −20℃. The aortic homogenate (10%) was prepared after the vascular ring function test and centrifuged at 3,500 rpm/minutes for 10 minutes at 4℃ to collect its supernatant and was stored at −20℃. The NO content in the vascular homogenate was detected with the nitrate reduction method. The content and activity of H2O2 and catalase (CAT) in the vascular homogenate were detected using spectrophotometry. We added each reagent following the kit instructions in a 37℃ water bath. The measuring wavelengths are NO 550 nm, H2O2 405 nm and CAT 405nm. TNF‐α, IL‐6, and eNOS content were detected using enzyme‐linked immunosorbent assay (ELISA) following the specific steps of the kit instructions.
Detection of ROS activity in blood vessels
Single‐cell suspensions were prepared with enzymatic digestion, and DCFH‐DA fluorescent probes were added. We then collected cell pellets after centrifugation for fluorescence detection. Blood vessel ROS activity was measured using flow cytometry.
Hematoxylin‐eosin stains of myocardial, vascular, and renal tissues
The rat aorta, heart, and kidney were suspended in a 10% formaldehyde solution. These organs were embedded in a wax block and later subjected to hydration and dewaxing. After dewaxing and hydration, the organs were sliced with a paraffin slicer and placed on a glass slide with a thickness of about 2 μm and allowed to dry. After drying, hematoxylin‐eosin (HE) stains were performed to observe the pathological changes of myocardium, blood vessels, and kidneys.
Western blot analysis of Nox2 and Nox4 protein expression in kidney, heart muscle, and vascular tissues
Total tissue protein was extracted and transferred into the PVDF membrane with SDS‐PAGE and then transferred into a blocking solution for 2 hours. The set‐up was incubated at room temperature for 1 to 2 hours or overnight at 4℃. The solution was eluted with TBST and incubated at room temperature for 2 hours. The solution was then eluted for 30 minutes. The immunocomplex assay was performed using a western blotting ECL kit.
PCR detection of Nox2 and Nox4 protein expression in the kidney, heart muscle, and vascular tissues
Total RNA was extracted from the kidney, myocardium, and vascular tissue using Trizol reagents. The purity and integrity of RNA were evaluated with Nanodrop2000 and agarose gel electrophoresis. RNA was reverse transcribed using the RNA reverse transcription kit. PCR amplification was performed using PCR amplification kit, primers, and S1000 ThermalCyclerPCR. Gene primer sequences are shown in Table S1 , and the targets had similar PCR efficiencies compared with the endogenous control.
Finally, the gel was placed into the electrophoresis tank, draw the DNA loading buffer to a ratio of 6:1, add the sample and DNA Ladder to the middle or both sides of the well, and run it at constant voltage of 100V until the loading buffer indicated the band Stop electrophoresis at 1–2 cm from the lower edge of the positive electrode of the gel. After the electrophoresis was completed, we removed the agarose gel and placed it into a camera to take a picture.
Statistical analysis
Data analysis and processing were performed using SPSS version 18.0. The data were expressed using the mean plus or minus standard deviation ( ). The mean comparison between groups was analyzed using one‐way ANOVA. The comparison between the two groups was performed using the SNK q test; P < 0.05 was considered statistically significant.
Results
Changes of BP in rat tail arteries
Compared with the NC group, BP of the HS group increased significantly after the third week. Compared with the HS group, the BP of HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group decreased significantly after the first week. BP on the HS + WSP ‐L group, decreased from the second week. Compared with the NC group, BP of the HS group kept rising. BP in the HS + WSP‐L group decreased. The BP decreased significantly in the HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group (Figure 1a ).
Figure 1.
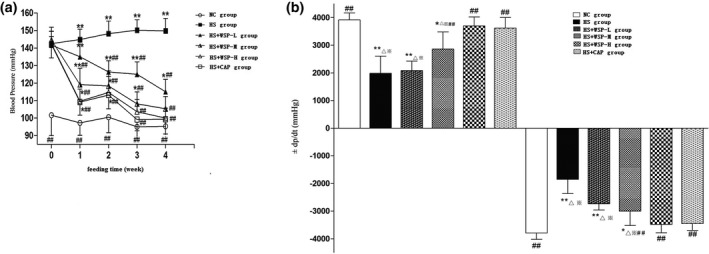
Detection of cardiac function and hemodynamics. (a) Changes of BP in each group. ( , n = 6, **P < 0.01 vs. NC group; ## P < 0.01 vs. HS group. (b) Changes of ±LV dp/dt max index in each group ( , n = 10). *P < 0.05, **P < 0.01 vs. NC group # P < 0.05, ## P < 0.01 vs. HS group; △ P < 0.01 vs. HS + WSP‐H group; ※ P < 0.01 vs. HS + CAP group. BP, blood pressure; HS, high salt; NC, normal control; WSP, Chinese water‐soluble propolis.
Changes in hemodynamic data
After 14 weeks (10 weeks of model establishment and 4 weeks of drug intervention), we detected hemodynamic data that reflect cardiac function (LVSP*HR and ± LV dp/dt max).
Figure 1b shows the changes in the ±LV dp/dt max index in each group. When compared with the NC group, +LV dp/dt max was decreased or the −LV dp/dt max was elevated significantly in the HS group, HS + WSP‐L group, and HS + WSP‐M group. Compared with the HS group, +LV dp/dt max was elevated or −LV dp/dt max was decreased significantly in the HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group.
Direct measurement of endothelial function
Effect of Phe on the thoracic aorta ring contraction
Phe (10−8 ~ 10−5 mol/L) was added to the bath after the arterial ring was stabilized. Figure 2a shows the effects of different concentrations of Phe on the arterial ring contraction rate in each group.
Figure 2.

Direct measurement of endothelial function. (a) Effects of different concentrations of Phe on the arterial ring contraction rate in each group ( , n = 6). *P < 0.05, **P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group. (b) Differences in ACh relaxation effect on pre‐contracted vascular rings induced by Phe ( , n = 6). *P < 0.05, **P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group. (c) Effect of L‐NAME on the relaxation rate of the rat thoracic aorta ring ( , n = 6). ACh, Acetylcholine; CAP, captopril; HS, high salt; NC, normal control; Phe, phenylephrine; WSP, Chinese water‐soluable propolis.
Relaxation effect of ACh on the pre‐contracted thoracic aorta ring induced by Phe
After the arterial ring was stabilized, we added Phe 10−6 mol/L to the bath and then ACh (10−8–10−5 mol/L). Figure 2b shows the differences in the relaxation effect of ACh on pre‐contracted vascular rings induced by Phe.
Effect of L‐NAME on the relaxation rate of the thoracic aorta ring
After the incubation of the arterial ring with the NO synthase inhibitor L‐NAME for 20 minutes, Phe 10−6 mol/L was added to the bath, and later ACh (10−8–10−5 mol/L) was added. Compared with the NC group, there were no significant differences in the change of relaxation rates among the HS group, HS + WSP‐L group, HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group (Figure 2c ).
Detection of oxidative stress‐related indicators
Serum NO content of the arterial homogenate in each group
The determination of NO in serum was detected using the nitrate reductase method. When compared with the NC group, the NO content in the HS group, HS + WSP‐L group and HS + WSP‐M group were significantly decreased. There was no significant difference between the HS + WSP‐H and HS + CAP groups. Compared with the HS group, the NO content in HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group were significantly increased. There was no significant difference in the HS + WSP‐L group (Figure 3a ).
Figure 3.
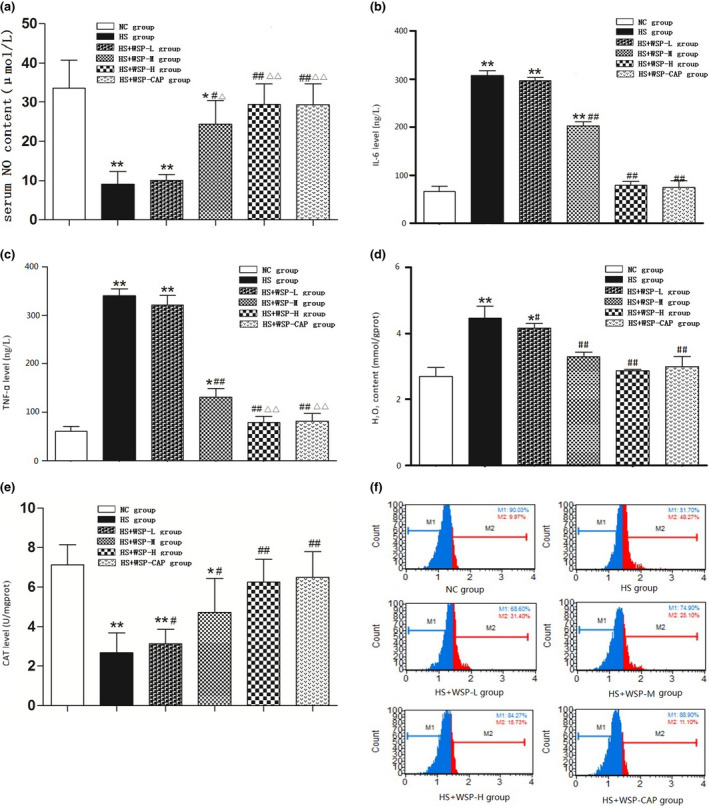
Detection of oxidative stress‐related indicators. (a) Serum NO content in each group ( , n = 6). (b) Comparison of IL‐6 levels in each group ( , n = 6). (c) Comparison of TNF‐α levels in each group ( , n = 6). (d) Comparison of H2O2 levels of arterial homogenate in each group ( , n = 6). (e) Comparison of CAT Levels in Vascular Tissues of Rats ( , n = 6). *P < 0.05, **P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group. △ P < 0.05, △△ P < 0.01 vs. WSP‐L group. (f) Detection of ROS content in vascular tissue using flow cytometry ( , n = 6). CAP, captopril; CAT, catalase; HS, high salt; NC, normal control; ROS, reactive oxygen species; WSP, Chinese water‐soluble propolis.
IL‐6, TNF‐α, and eNOS levels in the arterial homogenate of each group
The detection of IL‐6, TNF‐α, and eNOS levels in the arterial homogenate was carried with the ELISA method. When compared with the NC group, IL‐6 and TNF‐α levels in the HS group, HS + WSP‐L group, and HS + WSP‐M group were significantly elevated. Compared with the HS group, IL‐6 and TNF‐α levels in the HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group were significantly decreased (Figure 3b and c).
Table 1 shows that compared with the NC group, eNOS levels were significantly low in the HS group and HS + WSP‐L group. However, when compared with the HS group, eNOS levels were significantly elevated in the HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group.
Table 1.
eNOS levels in the arterial homogenate of each group ( , n = 6)
| Group | eNOS (ng/mL) | F value | P value |
|---|---|---|---|
| NC group | 49.76 ± 8.55 | 11.57 | 0.0000 |
| HS group | 29.07 ± 6.38** | ||
| HS + WSP‐L group | 32.7 ± 3.62** | ||
| HS + WSP‐M group | 42.44 ± 7.94# | ||
| HS + WSP‐H group | 53.75 ± 9.98## | ||
| HS + CAP group | 51.78 ± 8.97## |
Compared with the HS group, eNOS levels were significantly elevated in the HS+WSP‐M group, HS+WSP‐H group, and HS+CAP group.
**P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group.
Hydrogen peroxide content and CAT activity of the arterial homogenate in each group
The detection of the hydrogen peroxide (H2O2) content and CAT activity was performed by using spectrophotometry. The results suggest that compared with the NC group, the H2O2 content in the HS group and HS + WSP‐L group were elevated significantly. Compared with the HS group, the H2O2 content was low in the HS + WSP‐L group, HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group (Figure 3d ). Also, when compared with the HS group, CAT activity in the HS + WSP‐L group, HS + WSP‐M group, HS + WSP‐H group, and HS + CAP group was significantly high (P < 0.05 or P < 0.01) which had a concentration‐dependent upward trend (Figure 3e ).
Vascular tissue ROS content in each group
The ROS content in vascular tissue was detected using flow cytometry. The results showed that compared with the NC group, ROS content in the HS, HS + WSP‐L, and HS + WSP‐M group was significantly high. Compared with the HS group, ROS content in the HS + WSP‐L, HS + WSP‐M, HS + WSP‐H, and HS + CAP group were significantly low and tended to decrease when increasing the concentration (Figure 3f and Table 2 ).
Table 2.
Statistics of ROS content in vascular tissues of each group
| Group | ROS (GATE%) | F value | P value |
|---|---|---|---|
| NC group | 10.46 ± 0.43 | 2119.38 | 0.0000 |
| HS group | 48.56 ± 0.7** | ||
| HS + WSP‐L group | 31.23 ± 0.36**# | ||
| HS + WSP‐M group | 26.12 ± 1.4**## | ||
| HS + WSP‐H group | 16.6 ± 0.91## | ||
| HS + CAP group | 10.82 ± 0.18## |
Compared with the HS group, ROS content in the HS+WSP‐L, HS+WSP‐M, HS+WSP‐H, and HS+CAP group were significantly low.
CAP, captopril; HS, high salt; NC, normal control; ROS, reactive oxygen species; WSP, Chinese water‐soluble propolis.
**P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group. ( , n = 6).
HE stains in rat myocardium, vascular, and kidney tissues
The results after observing NC group tissues under a light microscope were as follows: (1) The cardiomyocytes in the NC group were arranged neatly and in normal shape, (2) The aortic endothelial cells are flattened and arranged evenly and in close alignment. The cells in the middle layer were arranged neatly, and the thickness of the vessel wall was uniform and (3) The renal morphological structure was normal, and no lesions in the small blood vessels of the kidney were observed (Figure 4a – c ).
Figure 4.
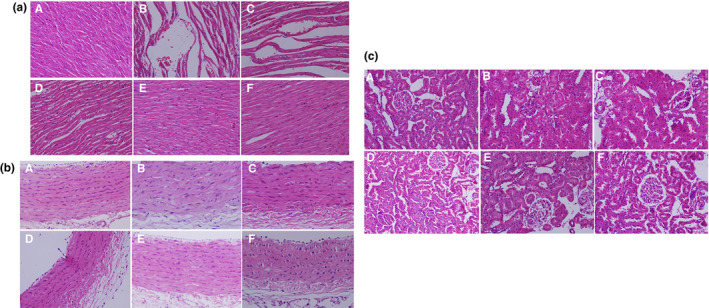
Morphological changes of rat myocardium, vascular, and kidney tissues in each group (HE stains × 400). (a) In myocardial tissues. (b) In vascular tissue. (c) In kidney tissue. (A: NC group; B: HS group; C: HS + WSP‐L group; D: HS + WSP‐M group; E: HS + WSP‐H group; F: HS + CAP group). CAP, captopril; HE, hematoxylin‐eosin; HS, high salt; NC, normal control; WSP, Chinese water‐soluble propolis.
The histological findings in the HS group were as follows: (i) Myocardial fibers showed gap widening and fiber breakage; (ii) Aortic intima showed significant damage which included wall thickening, irregular arrangement of endothelial cells with morphological changes; and (iii) Renal body atrophy and aortic glass degeneration in renal tissue were observed (Figure 4a – c ).
The histological findings of the HS + WSP‐L and HS + WSP‐M groups were: (i) Myocardial fibers were distorted and the gap widened; (ii) Aortic intima was damaged,vascular wall cells were not arranged neatly,and had morphological changes; and (iii) Mild vitreous degeneration of renal arterioles and slight atrophy of the renal corpuscle was observed in the HS + WSP‐L group, while the above phenomenon was not present in the HS + WSP‐M group (Figure 4a – c ).
The histological findings of the HS + WSP‐H and HS + WSP‐CAP groups were: (i) Normal cardiomyocyte arrangement and morphology; (ii) Aortic intima was intact and the middle layer cells were normal; and (iii) No small vessel disease was found in the kidney, and the renal corpuscle structure was normal (Figure 4a – c ).
Determination of NADPH oxidase activity
Measuring Nox2 and Nox4 protein expression by western blot
The detection of Nox2 and Nox4 protein expression in the myocardium, kidney, and vascular tissues in each group was made with western blot. We observed that Nox2 protein expression in groups HS, HS + WSP‐L and HS + WSP‐M was found to be more than the NC group. There were no differences in groups HS + WSP‐H and HS + WSP‐CAP. Compared with the HS group, the expression of Nox2 in groups HS + WSP‐L and HS + WSP‐M was not different. While the expression in groups HS + WSP‐H and HS + WSP‐CAP were significantly low (Figure 5a ).
Figure 5.
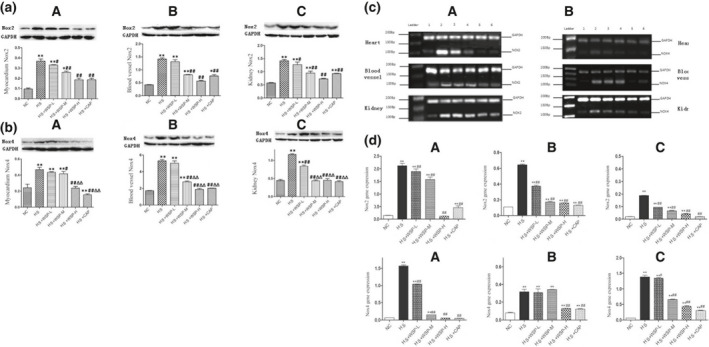
Determination of NADPH oxidase activity. (a) and (b) represent Nox2/Nox4 protein expression in myocardial, vascular and kidney tissues of each group, respectively. ( , n = 6). *P < 0.05, **P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group (A: Myocardial tissue; B: Vascular tissue; C: Kidney tissue). (c) The expression level of the Nox2 and Nox4 gene in each group by real‐time fluorescent PCR (A: Nox2 gene expression; B: Nox4 gene expression). (d) PCR statistical results of Nox2 and Nox4 gene expression ( , n = 6). (ABC represents different gene expression sites; A: Heart, B: Vessel, C: Kidney). *P < 0.05, **P < 0.01 vs. NC group; # P < 0.05, ## P < 0.01 vs. HS group. CAP, captopril; HS, high salt; NC, normal control; PCR, polymerase chain reaction; WSP, water‐soluable Chinese propolis.
Nox4 protein expression in groups HS, HS + WSP‐L, and HS + WSP‐M is more than the NC group. There were no differences in the groups HS + WSP‐H and HS + WSP‐CAP. Compared with the HS group, the expression of Nox4 was low, especially in the groups HS + WSP‐M, HS + WSP‐H, and HS + WSP‐CAP (Figure 5b ).
Monitoring the expression level of Nox2 and Nox4 gene by real‐time fluorescent PCR
Figure 5c shows that real‐time fluorescent PCR specifically identified the expression of Nox2 and Nox4 genes in each group.
Figure 5d shows the statistical results of Nox2 and Nox4 genes expression in each group for the myocardial tissue, vascular tissue, and kidney tissue. Compared with the NC group, the expression of Nox2 and Nox4 genes in the HS group was significantly high. The expression levels of Nox2 and Nox4 genes showed a gradual reduction as the concentration of propolis increased. There were no significant differences in Nox2 and Nox4 genes expression between HS + WSP‐H group and HS + CAP group.
Discussion
Salt restriction in the range of 2–2.6 g/day promotes good cardiovascular health. However, many people consume salt in ranges of 4–10 g/day. It has been observed that salt intake using a dose‐dependent method consisting of a standard diet with increasing amounts of salt showed a decrease in rat survival. 11 Previous studies have reported that the hypertensive rat model induced with HS diet is consistent with clinical hypertension models. 12
It has been reported that the HS diet increased mortality by accelerating arteriosclerosis and renal parenchymal damage. 13 Proper treatment of hypertensive patients will significantly improve the quality of life and prolong it. Propolis is widely used as a natural antioxidant and is a well‐known functional food for its biological activities, which includes anti‐ inflammation, antimicrobial, and liver detoxification. 14 Recently, the cardioprotective effect of propolis extract has been investigated both in vitro and in vivo experiments.
Effect of WSP on lowering BP and improving heart function
We have shown that HS intake elevated BP and weakened the heart contractile function. HE stains also confirm that the HS diet causes significant changes in the morphological structure of rat myocardium, intima, and kidney. While WSP can improve heart contractile function and induce morphological changes to decrease BP levels in a concentration‐dependent manner. Our data indicate that WSP plays a vital role in protecting heart function like CAP, angiotensin II receptor blocker, and could be used as an antihypertensive drugs.
Effect of WSP on improving endothelial function
The vascular endothelium plays a vital role in the maintenance of vascular homeostasis. Endothelial dysfunction (ED) is one of the major pathologic changes that lie between exposure to cardiovascular risk factors and the development of cardiovascular diseases. ED could be defined as a reduction in the arterial endothelium‐dependent vasodilation. 15 An increase in salt intake directly impacts endothelial cells functions like the regulation of nitric oxide (NO) production. The hallmark of ED is the impaired bioavailability of the NO, which is a critical endothelium‐derived relaxing factor. Muscarinic agonists, such as ACh, could be used to assess the NO‐dependent endothelial function. 16 It also should be noted that the action of eNOS forms NO.
Moreover there is a loss of NO influence on the resting arteriolar tone. 17 A plausible candidate for this loss is O2 ‐ generation. NOS could act as an additional O2 − source through a process called “NOS uncoupling” in the HS diet of rats. 18 NO scavenging by O2 − plays an essential role in hypertension induced by HS diet. In our study, it was observed that WSP increased the arterial ring relaxation rate and affected the NO level and eNOS content in a concentration‐dependent manner.
Several studies have demonstrated that oxidative stress plays a vital role in the development of various vascular diseases. 19 It is believed that maintaining vascular homeostasis is beneficial for the regulation of blood pressure. 20 However, oxidative stress injury reduces the synthesis of eNOS and decreases the bioavailability of NO in vessel walls, which leads to vascular dysfunction and the destruction of vascular homeostasis. Our study concludes that WSP could be employed as a potential treatment in hypertension induced by HS diet due to its capacity of restoring normal endothelial function.
WSP reduces inflammation and involvement of IL‐6 and TNF‐α
Recently, inflammation has been reported as having a causative relationship with the development of hypertension. 21 Moreover, it should also be noted that inflammation is another common underlying mechanism of ED. Low‐grade inflammation in multiple organs occurs during hypertension. These inflammatory tissues release a variety of inflammatory factors that causes changes in blood pressure and those changes in blood pressure can stimulate the release of inflammatory factors, thus becoming a vicious circle. Several studies have reported that an influx of neutrophils characterizes inflammation into the inflammatory area in which the cytokines play a crucial role. 22 our results indicate that WPS reduces the HS‐induced inflammatory response. It could be hypothesized that WPS can protect vascular endothelial function by reducing the production of proinflammatory cytokines like IL‐6 and TNF‐α and decreasing the oxidative stress of inflammatory factors.
Effect of WSP on Nox protein
A growing body of evidence supports the hypothesis that overproduction of O2‐ through Nox and mitochondria, a reduced O2 − metabolism by SOD, and other antioxidant enzymes can initiate the development of hypertension. 23 Considering several previous studies, there is more evidence suggesting that the intake of a HS diet has become a significant risk factor for the cardiovascular system and an independent disease factor for the promotion of hypertension. In some individuals, previous studies revealed that risk factors for HS dietary intake are associated with a critical feature that limits the nitric oxide bioavailability of endothelium‐dependent dilated blood vessels, the reduction of NO, and the NADPH oxidase of the vessel wall. The increase in the level of reactive oxygen species produced by xanthine oxidase or unconjugated endothelial nitric oxide synthase is closely related.
Nox is the primary source of active oxygen. The activity of Nox is up‐regulated in the body along with long‐term HS intake, and NADPH is used as a common reaction substrate. 24 The regulation of oxygen free radicals in the body is closely related to Nox and its many subtypes of protein. Among them, Nox2 and Nox4 are distributed in myocardial and vascular tissues whereas oxygen‐free radicals regulate NO. Our results show that the expression of Nox2 and Nox4 was gradually decreased in groups administered with WSP. Furthermore, it should be noted that the expressions of Nox2 and Nox4 genes in the HS + WSP‐H group and HS + CAP group showed a high level of similarity.
Conclusion
The study concludes that WPS has a significant effect on the blood pressure of hypertensive rats and their cardiovascular functions that improved significantly. The improvement in the cardiovascular functions might be related to the process of anti‐oxidation, anti‐inflammation, and the improvements of the endothelial function in hypertensive rats.
Funding
This study was funded by a grant from General Project of Quality Engineering Teaching Research in Anhui Province (No: 2018jyxm0759).
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
H.Z. wrote the manuscript. H.H.W. designed the research. H.Z., H.H.W., N.S., and F.W. performed the research. H.Z. and N.S. analyzed the data.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Chinese water‐soluble propolis (WSP), a healthy food option, has a significant effect on the blood pressure of hypertensive rats and their cardiovascular functions that improved significantly.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ It presented a way to study Chinese WSP had a protective of cardiovascular function in rats against a high‐salt diet.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ WSP could improve the cardiovascular functions that might be related to the process of anti‐oxidation, anti‐inflammation, and the improvements of the endothelial function in hypertensive rats.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ This study implies the potential of Chinese WSP as a potential drug for high‐salt diet‐induced hypertension treatment in the clinic.
Supporting information
Table S1.
Footnotes
CAP, captopril; HS, high salt; NC, normal control; WSP, Chinese water‐soluble propolis.
References
- 1. Kendig, M.D. & Morris, M.J. Reviewing the effects of dietary salt on cognition: mechanisms and future directions. Asia Pac. J. Clin. Nutr. 28, 6–14 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Hamlyn, J.M. & Blaustein, M.P. Salt sensitivity, endogenous ouabain and hypertension. Curr. Opin. Nephrol. Hypertens. 22, 51–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majid, D.S. , Prieto, M.C. & Navar, L.G. Salt‐sensitive hypertension: perpectives on intrarenal mechanisms. Curr. Hypertens Rev. 11, 38–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franco, M. , Pérez‐Méndez, O. , Kulthinee, S. & Navar, L.G. Integration of purinergic and angiotensin II receptor function in renal vascular responses and renal injury in angiotensin II‐dependen hypertension. Purinergic Signal. 15, 277–285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takashima, M. , Ichihara, K. & Hirata, Y. Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food Chem. Toxicol. 132, 110669 (2019). [DOI] [PubMed] [Google Scholar]
- 6. Ahmed, R. et al. Antioxidant properties and cardioprotective mechanism of Malaysian propolis in rats. Evid. Based Compl. Alternat. Med. 2017, 5370545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teles, F. et al. Brazilian red propolis attenuates hypertension and renal damage in 5/6 renal ablation model. PLoS ONE 10, e0116535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang, H. , Yuan, W. , Wu, H. , Yin, X. & Xuan, H. Bioactive components and mechanisms of Chinese poplar propolis alleviates oxidized low‐density lipoprotein‐induced endothelial cells injury. BMC Complement. Altern. Med. 18, 142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vazhappilly, C.G. et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacology 27, 863–869 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Wang, K. et al. Effects of Chinese propolis in protecting bovine mammary epithelial cells against mastitis pathogens‐induced cell damage. Mediators Inflamm. 2016, 8028291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng, W. , Dell'Italia, L.J. & Sanders, P.W. Novel paradigms of salt and hypertension. J. Am. Soc. Nephrol. 28, 1362–1369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vedanthan, R. et al. Hypertension management in rural western Kenya: a needs‐based health workforce estimation model. Hum. Resour. Health 17, 57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duś‐Żuchowska, M. et al. The Central European diet as an alternative to the Mediterranean diet in atherosclerosis prevention in postmenopausal obese women with a high risk of metabolic syndrome ‐ a randomized nutrition‐al trial. Acta Sci. Pol. Technol. Aliment. 17, 399–407 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Shi, Y.Z. et al. Ethanol extract of Chinese propolis attenuates early diabetic retinopathy by protecting the blood‐retinal barrier in Streptozotocin‐induced diabetic rats. J. Food Sci. 84, 358–369 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Zhang, G. , Lin, X. , Shao, Y. , Su, C. , Tao, J. & Liu, X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin. Exp. Hypertens. 20, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 16. Li, Q.Y. , Zhu, M.J. & Chen, L. Effects of Aging on Endothelium‐dependent Vasodilation of Human Artery. Sichuan Da Xue Xue Bao Yi Xue Ban 50, 210–214 (2019). [PubMed] [Google Scholar]
- 17. Huang, G. et al. Protective effect of Xin‐Ji‐Er‐Kang on cardiovascular remodeling in high salt‐induced hypertensive mice. Exp. Ther. Med. 17, 1551–1562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebhart, V. , Reiß, K. , Kollau, A. , Mayer, B. & Gorren, A.C.F. Site and mechanism of uncoupling of nitric‐oxide synthase: Uncoupling by monomerization and other misconceptions. Nitric Oxide 89, 14–21 (2019). [DOI] [PubMed] [Google Scholar]
- 19. Mi, C. , Qin, X. , Hou, Z. & Gao, F. Moderate‐intensity exercise allows enhanced protection against oxidative stress‐induced cardiac dysfunction in spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 52, e8009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshino, D. & Sato, M. Early‐stage dynamics in vascular endothelial cells exposed to hydrodynamic pressure. J. Biomech. Eng. 141 (2019). [DOI] [PubMed] [Google Scholar]
- 21. Barrows, I.R. , Ramezani, A. & Raj, D.S. Inflammation, immunity, and oxidative stress in hypertension‐partners in crime? Adv. Chronic. Kidney. Dis. 26, 122–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arokiasamy, S. , Zakian, C. , Dilliway, J. , Wang, W. , Nourshargh, S. & Voisin, M.B. Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation. Sci. Rep. 7, 44189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frazziano, G. , Al Ghouleh, I. , Baust, J. , Shiva, S. , Champion, H.C. & Pagano, P.J. Nox‐derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am. J. Physiol. Heart Circ. Physiol. 306, H197–H205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bryk, D. , Olejarz, W. & Zapolska‐Downar, D. The role of oxidative stress and NADPH oxidase in the pathogenesis of atherosclerosis. Postepy Hig. Med. Dosw. 71, 57–68 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


