Abstract
Background
American Society of Clinical Oncology guidelines recommend that patients ≥65 years of age starting chemotherapy undergo a geriatric assessment (GA) to inform and guide management; however, little is known about resources available in community oncology practices to implement these guidelines and to facilitate geriatric oncology research.
Materials and Methods
Oncology practices within the National Cancer Institute Community Oncology Research Program (NCORP) were electronically surveyed in 2017 regarding the availability of specialty providers, supportive services, and practice characteristics, as part of a larger survey of cancer care delivery research capacity.
Results
Of the 943 NCORP practices, 504 (54%) responded to the survey, representing 210 practice groups. The median new cancer cases per year ≥65 years of age was 457 (interquartile range 227–939). Of respondents, only 2.0% of practices had a fellowship‐trained geriatric oncologist on staff. Geriatricians were available for consultation or comanagement at 37% of sites, and of those, only 13% had availability within the oncology clinic (5% of overall). Practice size of ≥1,000 new adult cancer cases (ages ≥18) per year was associated with higher odds (1.81, confidence interval 1.02–3.23) of geriatrician availability. Other multidisciplinary care professionals that could support GA were variably available onsite: social worker (84%), nurse navigator (81%), pharmacist (77%), dietician (71%), rehabilitative medicine (57%), psychologist (42%), and psychiatrist (37%).
Conclusion
Only a third of community oncology practices have access to a geriatrician within their group and only 5% of community sites have access within the oncology clinic. Use of primarily self‐administered GA tools that direct referrals to available services may be an effective implementation strategy for guideline‐based care.
Implications for Practice
Only a minority of community oncology practices in the U.S. have access to geriatric specialty care. Developing models of care that use patient‐reported measures and/or other geriatric screening tools to assess and guide interventions in older adults, rather than geriatric consultations, are likely the most practical methods to improve the care of this vulnerable population.
Keywords: Cancer care delivery, Aging, Geriatric oncology, Community oncology, Geriatric assessment
Short abstract
Treatment of cancer in older adults is complicated by the aging process. This article assesses the availability of geriatric specialty care to support the management of older adults with cancer in community oncology settings.
Introduction
Cancer is principally a disease of aging. Given changing demographics in the U.S., nearly 70% of all new cancer diagnoses will be among older adults over the age of 65 by 2030 [1]. The oncologic care of the older adult with cancer is often complicated by the heterogeneous aging process. Many older adults diagnosed with cancer have other comorbid conditions and functional impairments that often complicate treatment decision‐making [2, 3]. Furthermore, variable social support poses further challenges to the delivery of recommended treatments [4, 5]. Age and performance status alone are not adequate to fully characterize the health status of older patients with cancer to inform treatment decision‐making [6, 7]. A geriatric assessment (GA) that systematically and comprehensively evaluates the various domains of health and social support provides oncology providers with critical information to inform the development and tailoring of cancer treatment plans in older adults with cancer [8, 9].
American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines recommend that patients ≥65 years of age starting chemotherapy undergo a GA to inform and guide management [10, 11]. GA can help refine estimates of the risk/benefit ratio of treatment decisions and identify vulnerabilities that may be amenable to supportive care interventions [12, 13, 14, 15, 16]. Many older adults that undergo a GA are found to have impairments that may be amendable to interventions, such as those provided by physical/occupational therapists, social workers, dieticians, and/or pharmacists [6, 13, 17]. Finally, GA has recently been shown to improve patient‐centered communication in the care of older adults with cancer [18]. However, little is known about the resources available in community oncology practices to perform GAs and implement these guidelines. Furthermore, the accessibility of geriatric specialty care to aid in the management of older adults in community oncology clinics is unknown, and thus, the best model of care to facilitate these guidelines is uncertain. Additionally, understanding the available resources and capacity to conduct cancer and aging research within the National Cancer Institute (NCI) network of community oncology practices is critical to developing future implementation and care delivery studies in this population.
To fill these knowledge gaps and better understand the capacity to implement these guidelines within community oncology practices, our goal was to assess the availability of geriatric specialty care and other multidisciplinary professionals that support the management of older adults with cancer in community oncology settings.
Materials and Methods
Overview
Data for the current study were drawn from the 2017 NCI Community Oncology Research Program (NCORP) Cancer Care Delivery Research (CCDR) Landscape Assessment. The NCORP is an NCI‐funded oncology research network of approximately 1,000 community oncology practices within the U.S. that conduct multisite trials in cancer prevention, screening, supportive care and symptom management, and cancer care delivery (ncorp.cancer.gov). The practices are clustered within 46 NCORP Community Sites, which are consortia of researchers, public hospitals, physician practices, academic medical centers, and other groups that provide health care services in communities across the U.S. NCORP sites are chosen through a competitive grant process with the goal of expanding access to clinical cancer research to a larger and more geographically and sociodemographically diverse patient population across the U.S. The Landscape Survey asked administrators and research staff employed at NCORP clinics about issues relevant to health care delivery and capacity to perform CCDR research. Community oncology practices were electronically surveyed in 2017 regarding practice characteristics and the availability of various providers and supportive services.
Survey Development and Distribution
A description of the development of the CCDR Landscape Assessment has been previously published [19, 20]. In brief, the survey was developed as an iterative process of question solicitation and review by the NCORP Research Bases and community oncology practices, with the goal of collecting data to inform future NCORP CCDR studies. Designated CCDR leads (research nurses or senior clinical research associates employed by the NCORP sites) were identified by the principal investigators at each of the 46 NCORP sites. These leads recruited practices and identified practice groups (multiple clinics that shared providers, patients, and infrastructure and generally had a common electronic health record). Staff at the practices were trained to collect the web‐based survey data via a series of webinars and frequently consulted a number of resources, including the local cancer registry and nurse managers, to obtain the information. Only one practice‐level response was submitted per practice group. The large majority (71.4%) of practices did represent a single physical location/practice.
Measures
For our specific research purposes, we were interested in the availability of geriatric specialized care and ancillary resources relevant to the management of older adults in community oncology clinics. The survey specifically asked for (a) the number of dually trained geriatric oncology providers at the practice, (b) whether geriatricians are available for consultation or comanagement, and (c) if yes, whether geriatricians were available in the clinic, hospital, or external outpatient consultation setting. In addition, we were interested in the availability of other multidisciplinary care professionals often integral to the care of older adults with cancer, including social workers, nurse navigators, pharmacists, dieticians, supportive caregiver services, rehabilitative medicine, psychologists, psychiatrists, integrative health specialists, and neuropsychologists. Lastly, we assessed the use of electronic health record systems, patient portals, and the use of patient‐reported outcomes to inform clinical care as these are resources that can be used to facilitate care management and communication. We also collected information about practices characteristics, including practice size (number of new cancer cases per year and number of oncology practitioners), self‐designation as a “safety net hospital,” designation by the by the Centers for Medicare and Medicaid Services (CMS) as a critical access hospital (located in a rural or underserved area), characteristics of new patients with cancer (proportion of patients covered by Medicaid), practice ownership, and participation in the CMS Innovation Center Oncology Care Model of payment and delivery (initiative to provide higher‐quality and more coordinated oncology care at the same or lower cost).
Statistical Analyses
Descriptive statistics were used to report prevalence of resources available at each practice. Logistic regression models were used to evaluate associations between practice factors and access to geriatricians for consultation or comanagement at site, along with potential confounding variables including number of new adult cancer cases (ages ≥18) per year (<1,000 vs. ≥1,000 [median number of cases]), number of practitioners, practice group region, practice ownership type, proportion of Medicaid ≤10% (approximate mean), and participation in oncology care model. Minimal missing data were present in the respondents (<10% for all observations) and managed as missing at random without any imputation. Dichotomization of new adult cancer cases per year and proportion of Medicaid cases were selected at approximate median levels seen in the Landscape survey. All tests of significance were two sided, and analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Practice Group Characteristics
Of the 943 NCORP discrete practice locations at the time of survey, 504 (54%) responded to the survey alone or as part of a practice groups, representing 227 distinct practice groups; 17 were excluded because they serve pediatric patients exclusively. Of these 210 practice groups, 58% included a free‐standing clinic or private/group practice, 81.4% included a hospital‐based outpatient clinic, and 82% included inpatient services (practice groupings of the nonrespondents is unknown). The median number of new cancer cases per year for patients ≥65 years of age per site was 457 (interquartile range [IQR] 227–939; Table 1). The median number of medical oncology providers was 5 (IQR 3–11). About half of practices were located in the Midwest (53%), and 23% and 21% were self‐designated as safety net hospital (provides significant level of care to low‐income or uninsured populations) and critical access (provides care in a rural or underserved area), respectively. Most sites used electronic health record systems (95%) and patient portals (91%), and one third (33%) reported using patient‐reported outcomes to inform clinical care or telemedicine services (31%) for delivery of care.
Table 1.
NCORP oncology practice group characteristics (n = 210)
| Characteristics | n (%) |
|---|---|
| Number of oncology providers, median (IQR) | 5 (3–11) |
| Number of new cancer cases per year ≥65 years of age, median (IQR) | 457 (227–939) |
| Practice group region | |
| Midwestern | 111 (53) |
| Western | 44 (21) |
| Northeastern | 13 (6) |
| Southern | 42 (20) |
| Practice ownership type | |
| Independently owned | 75 (36) |
| Owned by large regional/multistate health system | 113 (54) |
| Other (HMO/payer, publicly or university owned) | 20 (10) |
| Missing | 2 (1) |
| Proportion of Medicaid‐only or dual Medicare–Medicaid cases | |
| ≤10% | 141 (67) |
| >10% | 57 (27) |
| Missing | 12 (6) |
| Safety‐net hospital | 48 (23) |
| Designated as critical access a | 44 (21) |
| Minority or underserved site under NCORP b | 36 (17) |
| Participate in Oncology Care Model c | 60 (29) |
| Use of outpatient electronic medical records | 198 (95) |
| Use of patient portals | 189 (91) |
| Use of patient‐reported outcomes to inform clinical care | 67 (33) |
| Use of telemedicine services for delivery of care | 64 (31) |
Located in a rural or underserved area.
NCORP designation indicating that the site has a patient population comprising at least 30% racial/ethnic minorities or rural residents.
Oncology Care Model is a Medicare–Medicaid innovation initiative to provide higher‐quality and more coordinated oncology care at the same or lower cost.
Abbreviations: HMO, health maintenance organization; IQR, interquartile range; NCORP, National Cancer Institute Community Oncology Research Program.
Geriatric and Ancillary Care Resources
Of respondents, only 2.0% of practices had a dual fellowship–trained geriatric oncologist on staff (Table 2). Geriatricians were available for consultation or comanagement for 37% of sites. However, few practice sites (13%) had geriatricians that could see patients within the oncology clinic (Fig. 1). Among those with access to geriatricians, 54% had access to inpatient consultation and 90% to outpatient consultation external to the practice.
Table 2.
Availability of geriatric and ancillary care services in NCORP oncology practice groups (n = 210)
| n | Denominator | % | |
|---|---|---|---|
| Geriatric Service care | |||
| Availability of geriatric oncology (fellowship‐trained) providers at site | 3 | 202 | 2 |
| Availability of geriatricians for consultation or comanagement at site | 77 | 207 | 37 |
| If geriatricians available, available in oncology clinic | 10 | 78 | 13 |
| If geriatricians available, available for inpatient consultation | 42 | 78 | 54 |
| If geriatricians available, available for outpatient consultation external to the oncology practice | 70 | 78 | 90 |
| Other multidisciplinary care professionals | |||
| Availability of social workers at site | 174 | 207 | 84 |
| Availability of nurse navigators at site | 167 | 207 | 81 |
| Availability of pharmacists at site | 159 | 207 | 77 |
| Availability of dieticians at site | 147 | 206 | 71 |
| Availability of supportive caregiver services providers at site | 130 | 204 | 64 |
| Availability of rehabilitative medicine at site | 117 | 207 | 57 |
| Availability of psychologists at site | 80 | 207 | 42 |
| Availability of psychiatrists at site | 77 | 207 | 37 |
| Availability of integrative health specialists at site | 52 | 207 | 25 |
| Availability of neuropsychologists at site | 40 | 207 | 19 |
Abbreviation: NCORP, National Cancer Institute Community Oncology Research Program.
Figure 1.
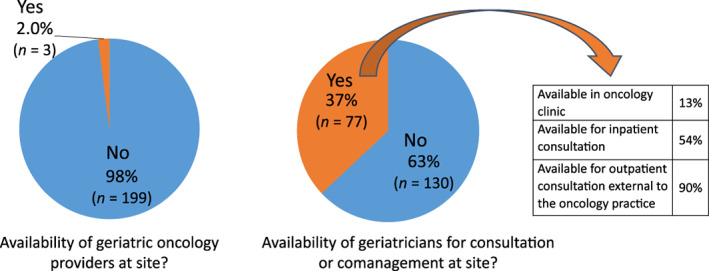
Availability of geriatric‐trained providers in community oncology practices in the U.S.
Other multidisciplinary care professionals that could support oncology providers in caring for older adults with cancer were variably available onsite: social worker (84%), nurse navigator (81%), pharmacist (77%), dietician (71%), supportive caregiver services (64%), rehabilitative medicine (57%), psychologist (42%), psychiatrist (37%), integrative health specialist (25%), and neuropsychologist (19%). No significant differences in the proportion of available social worker, nurse navigator, pharmacist, rehabilitative medicine, psychiatrist, or integrative health specialist existed between sites with and without geriatric specialty access, but we found lower proportions of availability for dieticians, supportive caregiver services, psychologist, and neuropsychologist (supplemental online Table 1).
When using logistic models to examine practice factors (including number of practitioners, practice group region, practice ownership type, proportion of dual Medicare–Medicaid, and participation in oncology care model) associated with access to geriatricians for consultation or comanagement, only practice size, defined as at least 1,000 new adult cancer cases (ages ≥18) per year, was associated with a greater odds (1.81, confidence interval 1.02–3.22) of having access to a geriatrician (Table 3).
Table 3.
Logistic models to examine practice factors associated with access to geriatricians for consultation or comanagement among NCORP adult practice groups (n = 210)
| Variable | Odds ratio | Confidence intervals | p value |
|---|---|---|---|
| Number of new adult cancer cases (ages ≥18) per year ≥1,000 (REF <1,000) | 1.81 | 1.02–3.22 | .04 |
| Number of practitioners (medical oncologist, radiation oncologist, and surgical oncologist) | 1.0 | 0.99–1.02 | .36 |
| Practice group region | .55 | ||
| Midwestern | REF | REF | |
| Western | 0.97 | 0.47–1.99 | |
| Northeastern | 0.66 | 0.19–2.27 | |
| Southern | 0.59 | 0.27–1.28 | |
| Practice ownership type | .42 | ||
| Independently owned | REF | REF | |
| Owned by large regional/multistate health system | 1.41 | 0.76–2.62 | |
| Other | 1.78 | 0.65–4.88 | |
| Proportion of Medicaid cases ≤10% (REF >10%) | 1.23 | 0.65–2.34 | .52 |
| Participate in Oncology Care Model | 0.74 | 0.40–1.37 | .33 |
Bold indicates statistical significance.
Abbreviation: NCORP, National Cancer Institute Community Oncology Research Program; REF, reference.
Discussion
Using the NCORP Landscape Assessment, we were able to characterize the availability of geriatric specialty care at community oncology clinics across the U.S. Availability of geriatric‐trained providers is limited in community oncology practices. Only one third of responding practices have access to a geriatrician for consultation, with only about 5% of community sites having access within the oncology clinic. Practice size, as identified by the number of new adult cancer cases (ages ≥18) per year, was the only factor associated with increased access to geriatric specialty care. Access is particularly limited in the outpatient setting and most often only available external to the oncology practice. This represents a critical gap in the ability of community cancer practices to care for older adults with cancer.
The care model for delivery of specialized care to the older adult with cancer varies worldwide [21]. In select high‐resource areas with ready availability of geriatric specialty care, a geriatrician is often a member of the multidisciplinary team and performs a GA within the oncology clinic [22, 23, 24, 25, 26]. This offers the distinct advantage of incorporating a provider trained specifically in the management of medically complex older adults within the clinic to potentially aid in treatment decision‐making. Furthermore, the geriatrician then has the ability to directly perform and/or provide referrals for GA‐based interventions that could improve outcomes in vulnerable older adults [22]. In contrast, in other clinical settings without such access to a geriatric specialist, a GA can only be performed by the oncology team [27]. In the settings without access to a geriatrician, a patient‐reported or nurse‐led GA can be performed, but it is up to the oncology team to incorporate the findings of the GA results into treatment decision‐making, identify appropriate GA‐based interventions, and then provide appropriate referrals [28, 29]. The results of our study suggest that this is the clinical scenario for most community practice settings (particularly smaller clinical practices) and ultimately should be the model of the focus of ongoing research given its broad applicability to clinical sites in the U.S. Developing GA tools for use by the oncology care team and providing education on how to use these results to personalize treatment and guide interventions are warranted.
Given the lack of geriatric specialty care in most community oncology clinics in the U.S., there should be an increased focus on developing and testing GA tools that are feasible for use by the oncology team. Furthermore, GA‐based interventions that can be implemented by the oncology team or primary care providers within community settings should also be developed that leverage available ancillary services, including social workers, nurse navigators, pharmacists, and dietitians, that are readily available in community settings. Care pathways that rely on referrals to geriatricians to perform GA and/or GA‐guided interventions will not be optimal for the majority of community cancer clinics in the U.S. given lack of access to subspecialty geriatrics care [23, 30]. To effectively implement ASCO and NCCN guidelines, research and education should focus on testing primarily self‐administered GA tools or toolkits to be used by the clinical team that can effectively direct referrals to needed available ancillary services [13].
Importantly, although access to geriatricians is limited in community practices, many important multidisciplinary professionals that are needed to provide comprehensive care to older patients with cancer are widely available. In particular, social workers, nurse navigators, pharmacists, and nutritionists are readily accessible. These results also have research implications for the design and testing of both implementation and new care delivery models. Understanding the availability of and any potential barriers to ancillary services is critically important in study design, as poor adherence to interventions is a common problem facing many studies in the older adult population [17, 31, 32]. Notably, most sites do not have access to psychological support and only a little over half have rehabilitative medicine at their site. Given that anxiety/depression and functional impairments are common in this population, the low availability of these services are areas of concern [33, 34, 35]. Conversely, there is widespread use of electronic medical systems, patient portals, social work, and nurse navigators at NCORP community practice sites. In particular, nurse navigators could be used to perform GAs and help ensure that intervention recommendations are performed, as nurse‐led and navigation interventions have shown particular promise in similar cancer settings [36, 37]. Moreover, the use of patient‐reported outcomes and telemedicine at community sites is higher than anticipated, and the incorporation of these new technologies in the development of future studies in community oncology clinics appears potentially feasible.
Given the availability of electronic health systems and patient portals in most practices, this provides an opportunity to administer many patient‐reported assessments efficiently [38]. Additionally, patient‐reported outcome measures are becoming increasingly used (33% of our sample) as part of routine care and used to inform clinical care. Based on the report of several recent seminal publications demonstrating improved symptom management, reduced hospitalizations, improved health‐related quality of life, and decreased mortality with the incorporation of patient‐reported measures [39, 40, 41, 42], there appears to be growing uptake in the use of patient‐reported measures in community oncology clinics. These increasingly available tools could be readily adapted to assist in identifying needs relevant to the older adult with cancer. Incorporating geriatric screening tools and/or patient‐reported GA tools into these platforms may not only improve the feasibility of using the GA in clinical practice but also streamline incorporation of the GA into oncologic decision‐making and improve adherence to GA‐based interventions [43]. However, older adults may have increased barriers to the use of new technologies and unique issues related to digital literacy, warranting further examination of the digital divide and tailoring intervention to the older adult population. A recent secondary analysis by Nipp et al. found that age moderated the beneficial effects of an electronic symptom monitoring intervention on the risk of emergency room visits and survival, thus highlighting important age‐related differences in electronic health interventions [44].
Our study is not without limitations. Although we were able to survey a large number of community oncology practices across the U.S. from within the NCORP research network, only 227 practice groups (representing 54% of total NCORP sites) responded to the questionnaire, which may have resulted in some sample bias. Unfortunately, we are unable to examine potential differences in responding and nonresponding practices for this survey, as equivalent data are not available for nonresponding practices. Based on our data collection experience, one of the biggest factors related to response was interest among practice leadership in research, specifically in cancer care delivery research. Practices that had participated in past cancer care delivery research studies or intended to in the future were more likely to respond. Furthermore, community practices within the NCORP research network may not be representative of all practices in the U.S., as these sites undergo a competitive selection process and at the consortium level generally have a successful history of accrual to cancer clinical trials. Our participating sites are quite heterogeneous and thus represent an addition to samples consisting primarily of academia centers. Furthermore, we were unable to measure whether access existed to geriatric services nearby but outside of a particular practice or health system, such as proximity to larger tertiary centers. Lastly, although we measured availability of geriatric specialty services, these results do not reflect the actual use of such services among older patients with cancer.
Conclusion
Access to geriatrics specialty care in community oncology practices in the U.S. appears limited, but many ancillary services are widely available. Providing additional geriatrics training to the oncology care team, using patient‐reported measures relevant to older adults, and using GA screening tools that assist in identifying care needs are likely the most practical methods to improve the care of the growing number of older adults with cancer in the U.S.
Next steps for research to foster implementation of guideline‐based GA into community practices should emphasize testing of strategies to integrate direct data capture of patient‐reported GA into electronic medical record as part of routine clinical workflow and navigation care delivery strategies to match GA‐guided supportive care recommendation to local resources. Education should include a focus on aging‐related training for nurses and advance practice providers as well as patients and caregivers. Advocacy to support integration of geriatric measures into the oncology quality rubric would provide additional incentive to develop practice‐level solutions to incorporate GA into care pathways.
Author Contributions
Conception/design: Grant R. Williams, Kathryn E. Weaver, Glenn J. Lesser, Heidi D. Klepin
Provision of study material or patients: Kathryn E. Weaver, Glenn J. Lesser, Heidi D. Klepin
Collection and/or assembly of data: Kathryn E. Weaver, Emily Dressler, Heidi D. Klepin
Data analysis and interpretation: Grant R. Williams, Kathryn E. Weaver, Glenn J. Lesser, Emily Dressler, Karen M. Winkfield, Heather B. Neuman, Anne E. Kazak, Ruth Carlos, Lucy J. Gansauer, Charles S. Kamen, Joseph M. Unger, Supriya Mohile, Heidi D. Klepin
Manuscript writing: Grant R. Williams, Kathryn E. Weaver, Glenn J. Lesser, Emily Dressler, Karen M. Winkfield, Heather B. Neuman, Anne E. Kazak, Ruth Carlos, Lucy J. Gansauer, Charles S. Kamen, Joseph M. Unger, Supriya Mohile, Heidi D. Klepin
Final approval of manuscript: Grant R. Williams, Kathryn E. Weaver, Glenn J. Lesser, Emily Dressler, Karen M. Winkfield, Heather B. Neuman, Anne E. Kazak, Ruth Carlos, Lucy J. Gansauer, Charles S. Kamen, Joseph M. Unger, Supriya Mohile, Heidi D. Klepin
Disclosures
Grant R. Williams: Carevive Systems (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Differences in availability of multidisciplinary care professionals between sites with and without access of geriatrics specialty care.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health through the NCI Community Oncology Research Program (NCORP), including Awards 1UG1CA189824 (Wake Forest Health Sciences NCORP Grant), UG1CA189961 (University of Rochester NCORP Grant), 5UG1CA189828 (Eastern Cooperative Oncology Group American College of Radiology Imaging Network), K24AG056589 (SGM), R21AG059206 (SGM), and K08CA234225 (G.R.W). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Portions of this work were previously presented as a poster presentation at American Society of Clinical Oncology Annual Meeting in Chicago, Illinois, on June 1, 2019.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 2. Williams GR, Mackenzie A, Magnuson A et al. Comorbidity in older adults with cancer. J Geriatr Oncol 2016;7:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Couderc AL, Boulahssass R, Nouguerede E et al. Functional status in a geriatric oncology setting: A review. J Geriatr Oncol 2019;10:884–894. [DOI] [PubMed] [Google Scholar]
- 4. Williams GR, Pisu M, Rocque GB et al. Unmet social support needs among older adults with cancer. Cancer 2019;125:473–481. [DOI] [PubMed] [Google Scholar]
- 5. Kadambi S, Soto‐Perez‐de‐Celis E, Garg T et al. Social support for older adults with cancer: Young international society of geriatric oncology review paper. J Geriatr Oncol 2020;11:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jolly TA, Deal AM, Nyrop KA et al. Geriatric assessment‐identified deficits in older cancer patients with normal performance status. The Oncologist 2015;20:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkhus L, Saltyte Benth J, Rostoft S et al. Geriatric assessment is superior to oncologists' clinical judgement in identifying frailty. Br J Cancer 2017;117:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamaker ME, Te Molder M, Thielen N et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients ‐ A systematic review. J Geriatr Oncol 2018;9:430–440. [DOI] [PubMed] [Google Scholar]
- 9. Bruijnen CP, van Harten‐Krouwel DG, Koldenhof JJ et al. Predictive value of each geriatric assessment domain for older patients with cancer: A systematic review. J Geriatr Oncol 2019;10:859–873. [DOI] [PubMed] [Google Scholar]
- 10. Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. VanderWalde N, Jagsi R, Dotan E et al. NCCN guidelines insights: Older adult oncology, version 2.2016. J Natl Compr Cancer Netw 2016;14:1357–1370. [DOI] [PubMed] [Google Scholar]
- 12. Hamaker ME, Schiphorst AH, ten Bokkel Huinink D et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients–A systematic review. Acta Oncol 2014;53:289–296. [DOI] [PubMed] [Google Scholar]
- 13. Magnuson A, Allore H, Cohen HJ et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol 2016;7:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Extermann M, Boler I, Reich RR et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 15. Hurria A, Mohile S, Gajra A et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 2016;34:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gajra A, Loh KP, Hurria A et al. Comprehensive geriatric assessment‐guided therapy does improve outcomes of older patients with advanced lung cancer. J Clin Oncol 2016;34:4047–4048. [DOI] [PubMed] [Google Scholar]
- 17. Kenis C, Decoster L, Flamaing J et al. Adherence to geriatric assessment‐based recommendations in older patients with cancer: A multicenter prospective cohort study in Belgium. Ann Oncol 2018;29:1987–1994. [DOI] [PubMed] [Google Scholar]
- 18. Mohile SG, Epstein RM, Hurria A et al. Communication with older patients with cancer using geriatric assessment: A cluster‐randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol 2019;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cathcart‐Rake EJ, Zemla T, Jatoi A et al. Acquisition of sexual orientation and gender identity data among NCI Community Oncology Research Program practice groups. Cancer 2019;125:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlos RC, Sicks JD, Chang GJ et al. Capacity for cancer care delivery research in National Cancer Institute Community Oncology research program community practices: Availability of radiology and primary care research partners. J Am Coll Radiol 2017;14:1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnuson A, Dale W, Mohile S. Models of care in geriatric oncology. Curr Geriatr Rep 2014;3:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magnuson A, Canin B, van Londen GJ et al. Incorporating geriatric medicine providers into the care of the older adult with cancer. Curr Oncol Rep 2016;18:65. [DOI] [PubMed] [Google Scholar]
- 23. Puts MTE, Hsu T, Mariano C et al. Clinical and Cost‐effectiveness of a Comprehensive geriatric assessment and management for Canadian elders with Cancer‐The 5C study: A study protocol for a randomised controlled phase III trial. BMJ Open 2019;9:e024485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nadaraja S, Matzen LE, Jorgensen TL et al. The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: A randomized parallel‐group clinical trial. J Geriatr Oncol 2020;11:488–495. [DOI] [PubMed] [Google Scholar]
- 25. Molga A, Wall M, Chhetri R et al. Comprehensive geriatric assessment predicts azacitidine treatment duration and survival in older patients with myelodysplastic syndromes. J Geriatr Oncol 2020;11:114–120. [DOI] [PubMed] [Google Scholar]
- 26. Caillet P, Canoui‐Poitrine F, Vouriot J et al. Comprehensive geriatric assessment in the decision‐making process in elderly patients with cancer: ELCAPA study. J Clin Oncol 2011;29:3636–3642. [DOI] [PubMed] [Google Scholar]
- 27. Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol 2007;25:1936–1944. [DOI] [PubMed] [Google Scholar]
- 28. Williams GR, Kenzik KM, Parman M et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol 2020;11:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Festen S, Kok M, Hopstaken JS et al. How to incorporate geriatric assessment in clinical decision‐making for older patients with cancer. An implementation study. J Geriatr Oncol 2019;10:951–959. [DOI] [PubMed] [Google Scholar]
- 30. Klepin HD, Wildes TM. Fighting for the integration of geriatric principles into oncology. J Geriatr Oncol 2018;9:705–706. [DOI] [PubMed] [Google Scholar]
- 31. Pergolotti M, Deal AM, Williams GR et al. Older adults with cancer: A randomized controlled trial of occupational and physical therapy. J Am Geriatr Soc 2019;67:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magnuson A, Lemelman T, Pandya C et al. Geriatric assessment with management intervention in older adults with cancer: A randomized pilot study. Support Care Cancer 2018;26:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pergolotti M, Deal AM, Lavery J et al. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J Geriatr Oncol 2015;6:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pergolotti M, Langer MM, Deal AM et al. Mental status evaluation in older adults with cancer: Development of the Mental Health Index‐13. J Geriatr Oncol 2019;10:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiss Wiesel TR, Nelson CJ, Tew WP et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology 2015;24:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCorkle R, Strumpf NE, Nuamah IF et al. A specialized home care intervention improves survival among older post‐surgical cancer patients. J Am Geriatr Soc 2000;48:1707–1713. [DOI] [PubMed] [Google Scholar]
- 37. Rocque GB, Pisu M, Jackson BE et al. Resource use and medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol 2017;3:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basch E. Patient‐reported outcomes ‐ Harnessing patients' voices to improve clinical care. N Engl J Med 2017;376:105–108. [DOI] [PubMed] [Google Scholar]
- 39. Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strasser F, Blum D, von Moos R et al. The effect of real‐time electronic monitoring of patient‐reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E‐MOSAIC, a multicenter cluster‐randomized phase III study (SAKK 95/06). Ann Oncol 2016;27:324–332. [DOI] [PubMed] [Google Scholar]
- 42. Denis F, Basch E, Septans AL et al. Two‐year survival comparing web‐based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA 2019;321:306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loh KP, Ramsdale E, Culakova E et al. Novel mHealth app to deliver geriatric assessment‐driven interventions for older adults with cancer: Pilot feasibility and usability study. JMIR Cancer 2018;4:e10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nipp RD, Horick NK, Deal AM et al. Differential effects of an electronic symptom monitoring intervention based on the age of patients with advanced cancer. Ann Oncol 2020;31:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Differences in availability of multidisciplinary care professionals between sites with and without access of geriatrics specialty care.


