Abstract
Many interventions focus on preventing stunting in the first 1,000 days of life. We take a broader perspective on childhood growth to assess the proportions of children who suffer persistent stunting, recover, and falter and become newly stunted between birth and adolescence. We use longitudinal data collected on 7,128 children in Ethiopia, India, Peru, and Vietnam. Data were collected in five survey waves between the ages of 1 to 15 years. We use descriptive and graphical approaches to compare the trajectories of children first stunted by age 1, first stunted by age 5, and those remained not stunted until age 5. On average, 29.6% of children were first stunted by age 1, 12.9% of children were first stunted by the age 5, and 68.7% of children were not stunted at either age 1 or age 5. A larger percentage of children stunted by age 1 remained stunted at age 15 (40.7%) compared with those who were first stunted by age 5 (32.3%); 33.7% of children first stunted by age 1 and 31.1% of children first stunted by age 5 go on to recover, but then falter during later childhood. 13.1% of children who were not stunted at age 1 or age 5 become newly stunted between the ages of 8 and 15. Our results show that children both become stunted and recover from stunting into adolescence. More attention should be paid to interventions to support healthy growth throughout childhood.
Keywords: anthropometry, child health, longitudinal, low‐ and middle‐income countries, nutrition, stunting
Key messages.
A substantial proportion of stunting occurs after the first 1,000 days of life.
The data show that children become newly stunted, recover, and falter throughout childhood and into early adolescence.
There is significant variation across countries in the trajectories of children that recover from stunting and subsequently falter.
There is a need for child‐focused interventions to address the needs of children who are persistently stunted or at risk of being stunted past the first 1,000 days of life.
Future research is needed to explore the determinants of stunting and recovery throughout childhood in order to better support healthy growth into adolescence.
1. INTRODUCTION
The first 1,000 days of life, from conception until about 2 years of age, represent a critical period during which nutritional interventions can have a profound effect on setting a child up for a lifetime of healthy growth (Victora, de Onis, Hallal, Blössner, & Shrimpton, 2010). Concentrating on the first 1,000 days has been an effective advocacy tool in order to focus and scale up nutritional interventions to improve childhood anthropometric outcomes (Bhutta, 2013). Although ensuring proper nutrition during the first 1,000 days is critical for long‐term health, the focus on interventions during this period have caused many to shift their focus from interventions occurring outside this window under the belief that interventions outside this period are likely to be ineffective (Prentice et al., 2013).
Previous research suggests that stunting during early childhood is largely irreversible (Shrimpton et al., 2001) and associated with both shorter adult stature (Black et al., 2013) and decreased development of both cognitive and motor skills (Sudfeld et al., 2015). An excessive focus on the first 1,000 days assumes that children who are stunted by age 2 do not recover, and children who experience normal growth are not at risk of being stunted later in life (Black et al., 2013). Much of this research is based on repeated cross‐sectional data. Relatively few studies have examined these associations from a longitudinal perspective (Adair, 1999; Adair & Guilkey, 1997).
The findings from a few longitudinal studies that examine childhood growth trajectories over time challenge the notion that limited catch‐up growth occurs after the age of 2. Using data obtained from a cohort of 2,000 children in the Cebu Longitudinal Health and Nutrition Survey that followed children until the age 12 years, the study suggests that among children stunted at age 2, 30% were no longer stunted at 8.5 years, and 32.5% were no longer stunted at 12 years (Adair, 1999). Another analysis using data from the same longitudinal study in the Philippines that followed participants from birth until age 22 found a positive association between improved dietary intake and reduced morbidity during childhood on height during adolescence and at age 22 (Bhargava, 2015). Two longitudinal studies in Senegal also suggest the possibility of catch‐up growth during childhood (Simondon et al., 1998); one of these studies, conducted among 2,374 children with height measurements occurring between the ages of 1–5 years and then between the ages of 18–23 years, found that 80% of stunted participants achieved catch‐up by adulthood (Coly et al., 2006).
A few multi‐country studies also provide some evidence to point towards the potential for growth recovery after the first 1,000 days, as well as the risk for stunting during later childhood. These studies suggest that context‐specific variables may lead to differences in stunting and recovery during childhood. Analysis of compiled longitudinal data from Brazil, Guatemala, India, Philippines, and South Africa (n = 4,695) that followed children from birth to 48 or 102 months, depending on the country (Stein et al., 2010), found that substantial height catch‐up occurred between 24 months and mid‐childhood in all countries except for India (Prentice et al., 2013). A study using data from Young Lives obtained on 7,171 children followed between the ages of 1 and 8 in Ethiopia, India, Peru, and Vietnam found that between the ages of 5 to 8 years, stunting prevalence decreased by 5.0 percentage points in Vietnam to 12.7 percentage points in Peru, whereas incident stunting (stunting occurring among children not stunted at any previous round) also occurred between the ages of 8 and 5 years and ranged from 3% in India to 6% in Ethiopia (Lundeen et al., 2014). Using a different sample of 3,327 children aged between 8 and 15 years from Young Lives, another study found that 36% of children stunted at age 8 were no longer stunted by age 15, whereas growth faltering was considerable during late childhood and early adolescence (Rockers & Fink, 2014).
The majority of the existing longitudinal studies that focus on describing child growth trajectories over time examine changes in growth between rounds of data collection. As such, there remains limited exploration of the overall growth trajectories that children experience in order to understand the fluidity with which children become newly stunted, recover, and/or falter over the life course. Furthermore, there are few longitudinal studies that track children from birth into adolescence with data on height collected at regular intervals.
In this paper, we question the exclusive focus on the first 1,000 days of life and take a broader perspective on childhood experience to assess stunting trajectories of children from 1 to 15 years of age using longitudinal data from Young Lives collected in Ethiopia, India, Peru, and Vietnam. The purpose of this paper is to track children over time in each of the four study countries to explore the extent to which children first stunted in early childhood (first stunted by age 1 and by age 5) and those not stunted in early childhood suffer from persistent stunting, recover and falter, and become newly stunted between birth and adolescence.
2. METHODS
2.1. Data source
The data used in this study are from Young Lives (YL). YL is a longitudinal study of 8,062 children from Ethiopia, India, Peru, and Vietnam. Data collection began when the participants were aged approximately 1 year. Data collection began in the year 2002 (Round 1), with subsequent rounds of data collection occurring every 3 to 4 years: 2006 when the children were aged 5 years (Round 2), 2009 when the children were aged 8 years (Round 3), 2013 when the children were aged 12 years (Round 4), and 2016 when the children were aged 15 years (Round 5).
YL employed a multistage sampling design. In each country, 20 clusters were identified through a non‐random selection process in order to ensure geographic diversity and overrepresentation of the poorest communities (Flores & Escobal, 2008; Kumra, 2008; Nguyen, 2008; Outes‐Leon & Sanchez, 2008). Cluster selection was nationwide in all counties but India, where cluster selection only included Andhra Pradesh. Approximately 100 households were randomly selected from each cluster (Wilson, Huttly, & Fenn, 2006). Ethical approval for this study was granted by the London School of Hygiene and Tropic Medicine Ethics Committee along with local institutional review boards in each country (Young Lives).
2.2. Height measurement
Supine length at 1 year of age and height at ages 5, 8, 12, and 15 years was measured using standardized length boards and stadiometers to the nearest millimetre. For children aged less than 60 months, WHO Growth Standards were used (Organization, 2006), and for children aged over 60 months, WHO Growth References were used (Onis et al., 2007) to calculate height‐for‐age z‐scores (HAZ). Stunting is defined as −2 z‐scores of the WHO child/adolescent reference.
2.3. Study sample
Children were included in the analysis if they had HAZs available for each of the five rounds of data collection. The initial sample at Round 1 included 1,999 children in Ethiopia, 2,011 children in India, 2,052 children in Peru, and 1,999 children in Vietnam. By Round 5, 282 children (14.11%) in Ethiopia, 184 children (9.15%) in India, 301 children (14.67%) in Peru, and 167 children (8.35%) in Vietnam were missing data on height in at least one round of data collection and were therefore excluded from the study. The final study sample included 7,128 children.
2.4. Analysis
We use descriptive statistics and graphical analysis to compare the populations of stunted children across the four countries included in the study at five different time points. Specifically, we divide children into three cohorts that correspond to stunting status at survey rounds 1 and 2:
First stunted by age 1: children who first became stunted between birth and age 1
First stunted by age 5: children who first became stunted between the age of 1 and age 5
Not stunted at age 1 or 5: children who remained unstunted until age 5
To track stunting status across each cohort over time at the ages of 1, 5, 8, 12, and 15 years, we developed alluvial plots for each country. Alluvial plots illustrate how key variables in a sample change over time by showing the composition of a population with regard to a categorical variable of interest, as do bar plots; however, they also show how the composition of the population changes over time, by showing how different cohorts of the population move between categories over time. The width of the bands that flow between each time point represent the percentage of the population within that cohort. The alluvial plots presented in this paper follow the trajectories of children at each age, tracking cohorts of children who were persistently stunted, recovered, faltered (became newly stunted), or never stunted across each survey round.
3. RESULTS
Demographic characteristics of the children in the final sample and those who were excluded from the sample (either lost to follow‐up or who were missing data on height) are presented in Table 1 by country. The only significant differences found between the final sample of children and children who were excluded were that excluded children tended to be wealthier in Vietnam (p < .01), and a larger percentage came from urban areas in India and Vietnam (p < .001). The mean age at the time of enrolment (Round 1) was 11.5 months, ranging between 5 and 22 months (Figure S1).
Table 1.
Demographic characteristics by country comparing children included and excluded from the study sample
| Characteristic by country | Included in study sample | Excluded from sample[Link] | p value |
|---|---|---|---|
| Ethiopia | |||
| Number of children | 1,717 | 282 | |
| Child's sex (% male) | 53.1 | 48.6 | |
| Age at enrolment in months (mean) | 11.7 | 11.6 | |
| Birthweight in grams (mean) | 3,147.3 | 3,234.1 | |
| Wealth tertile (% poorest) | 33.6 | 32.7 | |
| Residence location (% rural) | 65.8 | 59.9 | |
| India | |||
| Number of children | 1,827 | 184 | |
| Child's sex (% male) | 54.0 | 51.9 | |
| Age at enrolment in months (mean) | 11.8 | 12.2 | |
| Birthweight in grams (mean) | 2,771.4 | 2,679.7 | |
| Wealth tertile (% poorest) | 33.1 | 36.4 | |
| Residence location (% rural) | 75.6 | 66.3 | ** |
| Peru | |||
| Number of children | 1,751 | 301 | |
| Child's sex (% male) | 50.5 | 47.5 | |
| Age at enrolment in months (mean) | 11.5 | 11.5 | |
| Birthweight in grams (mean) | 3,201.4 | 3,197.1 | |
| Wealth tertile (% poorest) | 32.6 | 38.0 | |
| Residence location (% rural) | 31.2 | 33.2 | |
| Vietnam | |||
| Number of children | 1,833 | 167 | |
| Child's sex (% male) | 50.9 | 56.3 | |
| Age at enrolment in months (mean) | 11.7 | 11.1 | |
| Birthweight in grams (mean) | 3,100.0 | 3,102.1 | |
| Wealth tertile (% poorest) | 34.3 | 31.9 | *** |
| Residence location (% rural) | 81.8 | 60.5 | *** |
Table 2 presents the stunting status for children by survey round across countries. Ethiopia has the largest percentage of children stunted at 1 year (41.1%), and Vietnam has the lowest percentage (20.8%). By age 5, both India (35.3%) and Peru (32.6%) have a larger percentage of children who are stunted than Ethiopia (30.5%). India continues to have the highest proportion of children stunted between 8 and 15 years compared with the other countries in the study. The percentage of children stunted decreases as age increases in all survey rounds, except in Ethiopia. The percentage of children stunted in Ethiopia increases between age 8 (20.6%) and age 12 (28.4%). By age 15, India has the largest percentage of stunted children (27.0%), and Vietnam has the least (12.3%).
Table 2.
Percentage of children stunted at ages 1, 5, 8, 12, and 15 years in Ethiopia, India, Peru, and Vietnam
| Stunting status by child's age in years | |||||
|---|---|---|---|---|---|
| Ethiopia (n = 1,717) % (n) | India (n = 1,827) % (n) | Peru (n = 1,751) % (n) | Vietnam (n = 1,833) % (n) | Total (n = 7,123) % (n) | |
| Age 1 | |||||
| Stunted | 41.1 (706) | 30.3 (554) | 27.1 (474) | 20.8 (382) | 29.7 (2,116) |
| Not stunted | 58.9 (1,011) | 69.7 (1,273) | 72.9 (1,277) | 79.2 (1,451) | 70.3 (5,012) |
| Age 5 | |||||
| Stunted | 30.5 (524) | 35.3 (645) | 32.6 (571) | 25.4 (466) | 31.0 (2,206) |
| Not stunted | 69.5 (1,193) | 64.7 (1,182) | 67.4 (1,180) | 74.6 (1,367) | 69.0 (4,922) |
| Age 8 | |||||
| Stunted | 20.6 (353) | 28.6 (523) | 19.7 (344) | 20.0 (367) | 22.3 (1,587) |
| Not stunted | 79.4 (1,364) | 71.4 (1,304) | 80.3 (1,407) | 80.0 (1,466) | 77.7 (5,541) |
| Age 12 | |||||
| Stunted | 28.4 (488) | 28.5 (520) | 18.2 (318) | 19.4 (356) | 23.6 (1,682) |
| Not stunted | 71.6 (1,229) | 71.5 (1,307) | 81.8 (1,433) | 80.6 (1,477) | 76.4 (5,446) |
| Age 15 | |||||
| Stunted | 25.4 (436) | 27.0 (494) | 15.4 (270) | 12.3 (226) | 20.0 (1,426) |
| Not stunted | 74.6 (1,281) | 73.0 (1,233) | 84.6 (1,481) | 87.7 (1,607) | 80.0 (5,702) |
Table 3 shows the relationship between stunting status in early childhood and at age 15.
Table 3.
Percentage of children first stunted by age 1, first stunted by age 5, and not stunted at age 1 or 5 in relation to their stunting status at age 15 years in Ethiopia, India, Peru, and Vietnam
| First stunted by age 1 | |||||
|---|---|---|---|---|---|
| Ethiopia (n = 706) | India (n = 554) | Peru (n = 474) | Vietnam (n = 382) | Total (n = 2,116) | |
| Status at age 15 | % (n) | % (n) | % (n) | % (n) | % (n) |
| Stunted | 40.4 (285) | 46.9 (260) | 36.7 (174) | 37.4 (143) | 40.7 (862) |
| Recovered | 59.6 (421) | 53.1 (294) | 63.3 (300) | 62.6 (239) | 59.3 (1,254) |
| First stunted by age 5 | |||||
|---|---|---|---|---|---|
| Ethiopia (n = 185) | India (n = 289) | Peru (n = 254) | Vietnam (n = 192) | Total (n = 920) | |
| Status at age 15 | % (n) | % (n) | % (n) | % (n) | % (n) |
| Stunted | 34.1 (63) | 44.3 (128) | 23.2 (59) | 24.5 (47) | 32.3 (297) |
| Recovered | 66.0 (122) | 55.7 (161) | 76.8 (195) | 75.5 (145) | 67.7 (623) |
| Not stunted at age 1 or 5 | |||||
|---|---|---|---|---|---|
| Ethiopia (n = 826) | India (n = 984) | Peru (n = 1023) | Vietnam (n = 1259) | Total (n = 4092) | |
| Status at age 15 | % (n) | % (n) | % (n) | % (n) | % (n) |
| Faltered | 10.7 (88) | 10.7 (106) | 3.6 (37) | 2.9 (36) | 6.5 (257) |
| Never stunted | 89.4 (738) | 89.2 (161) | 96.4 (986) | 97.1 (1,223) | 93.5 (3,825) |
| Total number of children | 1,717 | 1,827 | 1,751 | 1,833 | 7,128 |
Note. Stunted = stunted at age 1 or 5 and at age 15. Recovered = stunted at age 1 or 5 and not stunted at age 15. Faltered = not stunted at age 1 or 5 and stunted at age 15. Never stunted = not stunted at either age 1 or 5 or age 15.
On average across all countries, 12.9% (920/7,128) of children were first stunted by the age 5 (10.8% [185/1,717] in Ethiopia, 15.8% [289/1,827] in India, 14.5% [254/1,751] in Peru, and 10.5% [254/1,833] in Vietnam), and 68.7% (4,092/7,128) of children were not stunted at either age 1 or age 5 (57.4% [826/1,717] in Ethiopia, 48.1% [984/1,827] in India, 53.9% [1,023/1,751] in Peru, and 58.4% [1,259/1,833] in Vietnam). In all countries, a larger percentage of children stunted by age 1 remained stunted at age 15 compared with those who were first stunted by age 5. India had the largest percentage of children stunted at age 1 who remained stunted at age 15 (46.9%) and also the largest percentage of children first stunted at age 5 who remained stunted at age 15 (44.3%). Peru had the largest percentage of children who were stunted at age 1 who recovered by age 15 (63.3%) as well as the largest percentage of children first stunted by age 5 who recovered by age 15 (76.8%). Among children who were never stunted at age 1 or age 5, the majority remained unstunted at age 15 (93.5%). Vietnam has the lowest percentage of children not stunted at age 1 or age 5 who falter at age 15 (2.9%), and Ethiopia and India have the highest percentage (10.7%).
Figure 1 shows the stunting trajectories of all children in the sample at all survey rounds from age 1 through age 15 in each of the four countries included in the study. In each chart, the specific trajectories of different cohorts of children are displayed, based on their stunting status at each survey round. The charts illustrate that in all countries, a substantial proportion of children move between stunting categories between the ages 1 and 15 years. Figures S2–S5 provide flow charts showing the raw populations of children who move between stunting categories at each survey round.
Figure 1.
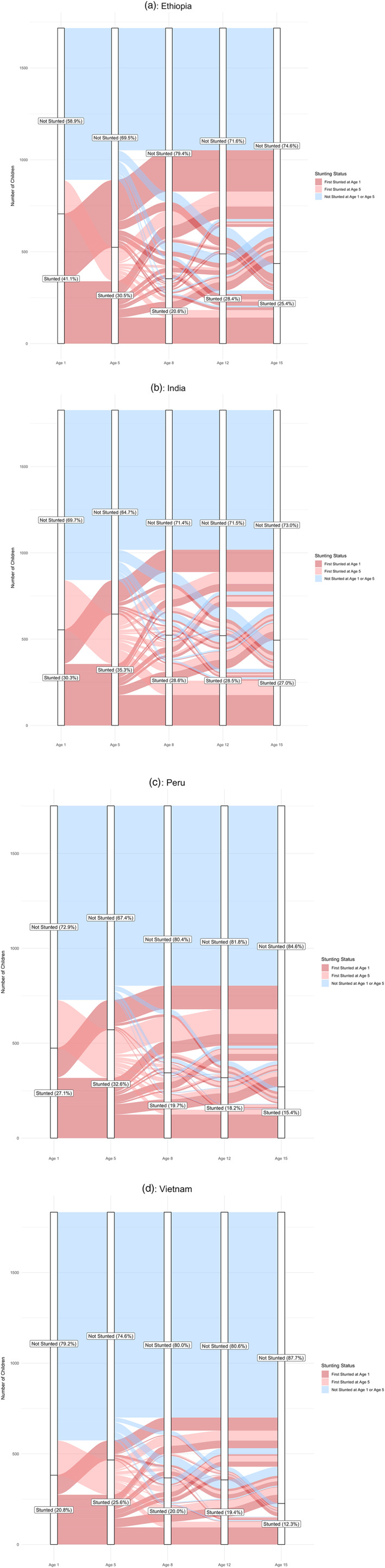
Alluvial plots illustrating percentages of children aged between 1 and 15 years stunted at each survey round and the percentage of children in different cohorts who move between stunting categories in each survey round in (a) Ethiopia, (b) India, (c) Peru, and (d) Vietnam
Figure 2.
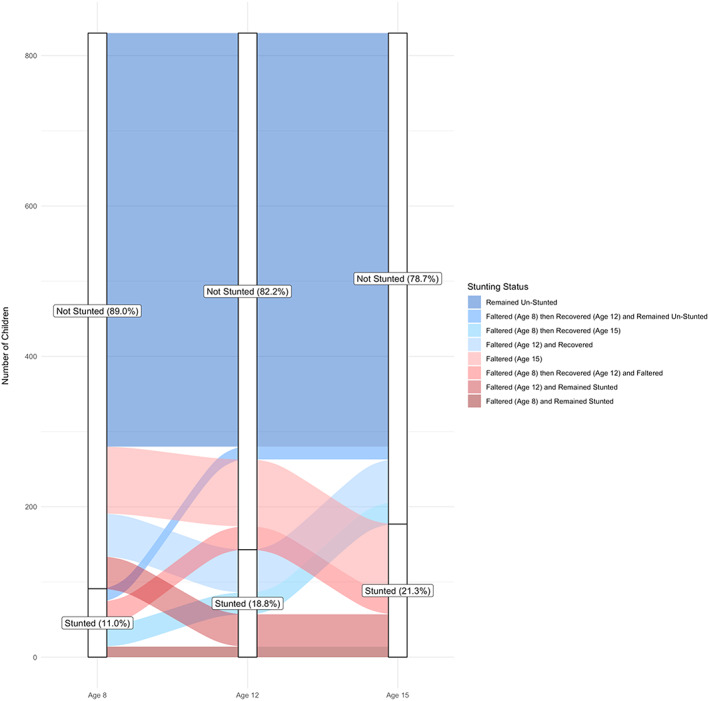
Alluvial plot illustrating the proportion of children stunted and not stunted at each survey round and the proportion of children who moved between stunting categories at each time point, among children who recovered from stunting at age 1 by age 5 years in all study countries combined (Ethiopia, India, Peru, and Vietnam)
The trajectories of children who were first stunted by age 1 are shown on each of the four charts in Figure 1 in dark red. A considerable proportion of children (25.3%) who are first stunted by age 1 remain persistently stunted at all time points until age 15: 20.0% in Ethiopia, 37.2% in India, 26.2% in Peru, and 24.5% in Vietnam. Overall, 60.8% of children first stunted at age 1 remain stunted at age 5, ranging from 48.0% in Ethiopia to 71.7% in Vietnam. Persistent stunting until age 15 is even more pronounced among children stunted at both ages 1 and 5, with 41.6% in Ethiopia, 49.4% in India, 39.1% in Peru, and 34.3% in Vietnam remaining stunted until age 15.
A substantial percentage of children who are stunted by age 1 recover between the ages of 5 and 15 years. Among children first stunted by age 1, 26.0% remain unstunted at all subsequent survey rounds, ranging from 18.9% in Vietnam to 31.2% in Ethiopia. Overall, 39.2% of stunted children recover from stunting between the ages 1 and 5, ranging from 28.3% in Vietnam to 52.0% in Ethiopia. Among the children who recover by age 5, 66.3% remain unstunted at all time points between the ages of 5 and 12, ranging from 68.1% in Ethiopia to 81.2% in Vietnam. Children first stunted at age 1 continue to recover at points later in childhood: 21.1% of those stunted at age 8 move from stunted to unstunted by age 12 (ranging from 14.9% in Ethiopia to 28.7% in Vietnam), whereas 34.5% of those stunted at age 12 move from stunted to unstunted by age 15 (ranging from 28.07% in India to 45.0% in Vietnam).
Despite high rates of recovery among children first stunted by age 1, children continue to be at risk of growth faltering and recurrent stunting at subsequent ages. We find that 33.7% of children recover by age 5 but then falter during later childhood. Among these children, 11.0% do so by age 8 (ranging from 7.1% in Peru to 14.8% in Vietnam), 12.1% do so by age 12 (ranging from 5.1% in Peru to 15.3% in Ethiopia), and 10.7% do so by age 15 (ranging from 4.6% in Vietnam to 13.1% in Ethiopia).
The trajectories of children who were first stunted by age 5 are shown on each of the four figures in light red. Among the children first stunted at age 5, 19.6% remain persistently stunted at all time points until age 15: 22.2% in Ethiopia, 28.7% in India, 9.8% in Peru, and 16.25% in Vietnam. Of children first stunted by age 5, 36.4% recover and remain unstunted at all subsequent survey rounds. Overall, 52.8% of children who are first stunted by age 5 recover by age 8, ranging from 40.5% in India to 67.3% in Peru, of whom 68.9% remain unstunted at all subsequent time points, ranging from 59.0% in India to 76.0% in Peru. Additionally, 28.1% of children first stunted by age 5 move from stunted to unstunted between the ages of 8 and 12 (ranging from 12.9% in Ethiopia to 39.8% in Peru), whereas 47.8% of children first stunted by age 5 move from stunted to unstunted between the ages of 12 and 15 (ranging from 39.1% in Ethiopia to 63.0% in Vietnam).
As observed among the children first stunted by age 1, a substantial percentage of children first stunted by age 5 who then recover also go on to falter later in childhood. Among the 31.1% of children who were first stunted by age 5, recovered by age 8, and then faltered during later childhood, 20.6% do so by age 8 (ranging from 16.4% in Peru to 24.1% in Vietnam), and the remaining 10.5% do so by age 15 (ranging from 7.0% in Ethiopia to 18.8% in India).
The blue cohort in each of the four figures represents the trajectories of the children who were not stunted at either age 1 or age 5. Among these children, 13.1% become stunted between the ages of 8 and 15: 19.5% in Ethiopia, 17.8% in India, 7.3% in Peru, and 10.0% in Vietnam. Overall, 3.9% of children who were not stunted at age 1 or 5 falter between the ages of 5 and 8 (ranging from 2.5% in Peru to 4.5% in India), 5.0% between the ages of 8 and 12 (ranging from 2.3% in Peru to 8.3% in Ethiopia), and 4.2% between the ages of 12 and 15 (ranging from 1.9% in Vietnam to 6.9% in Ethiopia and India).
4. DISCUSSION
Our results show that a substantial proportion of stunting occurs beyond the first 1,000 days. Specifically, our findings highlight three key points: (a) catch‐up growth and recurrent stunting occur throughout childhood, (b) there is wide variation across countries with regard to children's growth and recovery trajectories, and (c) there may be critical windows later into childhood and adolescence to improve child growth. Although focusing on the first 1,000 days of a child's life is critical for ensuring healthy growth and development into early adolescence, stunting and recovery trajectories between the ages of 5 and 15 years illustrate a more complex picture. A perspective that takes into account growth trajectories beyond the first 1,000 days is needed to examine longer term outcomes.
We find that a considerable percentage of children move between stunting categories throughout childhood into adolescence, suggesting that not only does catch‐up growth occur throughout childhood but also children continue to remain at risk of new and recurrent stunting into early adolescence. For example, in our study, only 26% of children first stunted by age 1 recovered and remained unstunted at all subsequent survey rounds. At age 15, however, nearly 60% of these children were found to be not stunted. Among children first stunted by age 5, a similarly low percentage (36%) of children recovered and remained unstunted at all subsequent survey rounds. However, we found that by age 15, 68% of the children first stunted at age 5 had recovered. First, it is worth noting that in our study, nearly 2/3 (62%) of children stunted by either age 1 or age 5 recovered by the age of 15 years. Furthermore, these discrepancies suggest that 34% of children first stunted by age 1 and 32% of children first stunted by age 5 experience a trajectory that includes a combination of recurrent stunting and recovery throughout late childhood into adolescence. Furthermore, even though only a relatively small percentage of children who were not stunted at age 1 or age 5 became stunted between the ages of 8 and 15, our results show that these children amount to 7.5% of all of children in the study, and as such, they represent a sizeable population of children at risk of stunting later in childhood that are not considered in interventions that focus on the first 1,000 days.
Childhood stunting is often linked to long‐term adverse physical and cognitive outcomes and is often used as an indicator of childhood undernutrition and overall well‐being (De Onis & Branca, 2016). The utility of stunting as an individual‐based measure, however, has been called into question given the absence of a marked inflection point at −2 HAZ with regard to physical and cognitive outcomes (Perumal, Bassani, & Roth, 2018). In addition, a focus on individual stunting may shift attention from the underlying contextual determinants of childhood growth in favour of short‐term nutritional interventions with limited impact on linear height (Beaton, 1989; Subramanian, Mejía‐Guevara, & Krishna, 2016). Given that a large percentage of children in this study follow complex growth trajectories that consist of both growth recovery and faltering through early adolescence, our research raises the question about whether there is an overreliance on stunting as a key indicator reflecting early childhood nutrition and whether its use as a predictor of longer term health and growth potential represents a false dichotomy.
Our results also indicate that there is considerable variation across the four study countries in terms of child growth trajectories, which may suggest the importance of environmental context. Ethiopia and India typically had higher proportions of persistent stunting, lower proportions of recovery, and higher percentages of growth faltering across survey rounds in comparison with Peru and Vietnam. All of the countries included in the study have undertaken large‐scale, national‐level interventions focused on improving child nutrition over the last decades. Peru achieved dramatic success in reducing stunting and improving childhood nutrition between 2005 and 2011, which has been attributed to a combination of factors, including a shift in national nutrition policy, engagement of civil society, and poverty reduction (Mejía Acosta & Haddad, 2014). In India, although large national programs have been implemented to improve child nutrition, challenges related to the complexity of the interventions identified often leads to gaps in implementation (Avula, Raykar, Menon, & Laxminarayan, 2016). Large‐scale childhood nutrition programs have also been implemented in Ethiopia and Vietnam (Lemma & Matji, 2013; Piwoz, Baker, & Frongillo, 2013), with Ethiopia experiencing an overall decline in childhood stunting from 58% to 44% between the years of 2000 and 2011 (Central Statistical Agency [Ethiopia] & ICF International, 2012). Future research could explore child growth trajectories across the four countries included in this study with more depth to better understand what factors may contribute to these different patterns of growth faltering and recovery at different time points throughout childhood and into adolescence. Better understanding of these determinants may support the identification of both critical windows and specific opportunities for intervention beyond the first 1,000 days.
Although previous studies indicate that the first 1,000 days is critical for healthy long‐term development through later childhood, stunting in later childhood may also carry with it long‐term health and development challenges (Rockers & Fink, 2014); however, research remains limited in this area. Other research has found that recovery from early growth faltering after the first 1,000 days may provide significant cognitive benefit; thus, intervention designed to improve growth after the first 1,000 days may offer important developmental benefits (Crookston et al., 2013). Although the importance of rapid brain development is well established in early childhood, such results may also suggest that a relationship exists between growth and brain development in later childhood. A research question that remains is whether linear growth is a proxy measure for brain development (Perez‐Escamilla, 2013). We find that in our study, stunting by age 1 and stunting by age 5 both have important ramifications on future childhood growth. Our results show that approximately 25% of children first stunted by age 1 and 20% of children first stunted by age 5 suffer from persistent stunting at all subsequent survey rounds. As such, children who become stunted after the first 1,000 days may also have poor rates of recovery in childhood, and further research should explore whether such children are also at risk of similarly adverse health outcomes as those stunted earlier.
A narrow focus on the first 1,000 days may also ignore the potential for critical periods of catch‐up growth later in childhood. A more inclusive approach would enable to identification of opportunities to mitigate the long‐term health and developmental consequences of stunting that occur after the first 1,000 days. Other research has identified possible periods later in childhood to promote catch‐up growth, such as prior to and during puberty (Prentice et al., 2013). Our study may provide support to these findings in that the large percentage of recovery observed in our study between the ages of 12 and 15 may indicate that puberty could be another important window to promote growth.
The study has both several strengths and limitations worth noting. A strength of this study is that the data used represent a large cohort of children from four diverse low‐ and middle‐income countries followed longitudinally, spanning from birth to the age of 15. Relatively few studies follow children in this manner from birth into adolescence. Furthermore, loss to follow up is relatively low in this study, especially considering the study's duration. That said, it is worth noting that the loss‐to‐follow‐up experienced in this study may limit the generalizability of our findings. Although we found few differences between children who continued in the study until the age of 15 and those who were excluded from the study due to missing data, according to their socio‐economic characteristics, children who were lost to follow up may have experienced higher rates of morbidity that could have ultimately affected their growth, thereby influencing our results. Another important limitation of this study is that our data are limited temporally to the survey rounds that are spaced between 3 and 4 years apart. Therefore, it is not possible to determine from the data precisely when children first became stunted between the ages of 1 and 5 years. As children in Round 1 were enrolled before 2 years of age, we can expect that some of children in the cohort defined as first stunted by age 5 may have been stunted before the first 1,000 days. Despite this concern, our study still shows complex growth trajectories in which children move between stunting categories throughout later childhood and adolescence, between the ages of 5 and 15, well beyond the first 1,000‐day mark. Furthermore, although loss to follow up is relatively low considering the length of this study, selection bias could influence our results if the children who are lost to follow up suffer from worse health than those who remain in the study.
Although the first 1,000 days represent a critical period during which interventions can have a substantial impact on setting children up for a lifetime of healthy growth, we believe that our study suggests that an exclusive focus on the first 1,000 days is limited from two perspectives. First, it is too narrow in focus and does not consider the factors that contribute to stunting and catch‐up growth later in childhood. Second, as is highlighted in this study, there is a growing body of evidence that children are able to recover from stunting at later ages and that children continue to be at risk of becoming stunted. Although the first 1,000 days argument places more emphasis on maternal health and is arguably focused more on primary prevention, we need to also think about child‐focused interventions for children who are already stunted or at risk of being stunted after the first 1,000 days of life.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
All authors conceptualized the study. JG performed the data analysis and wrote the first draft. RK and SVS provided critical input and revisions. All authors approved the final draft.
Supporting information
Figure S1: Age (in months) of children at the time of enrollment in Ethiopia, India, Peru, and Vietnam
Figure S2: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Ethiopia (n = 1,717 children)
Figure S3: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in India (n = 1,827 children)
Figure S4: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Peru (n = 1,751 children)
Figure S5: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Vietnam (n = 1,833 children)
ACKNOWLEDGMENTS
The data used in this publication come from Young Lives, a 15‐year study of the changing nature of childhood poverty in Ethiopia, India, Peru, and Vietnam (www.younglives.org.uk). Young Lives is funded by UK aid from the Department for International Development (DFID). The views expressed here are those of the author(s). They are not necessarily those of Young Lives, the University of Oxford, DFID, or other funders.
Gausman J, Kim R, Subramanian SV. Stunting trajectories from post‐infancy to adolescence in Ethiopia, India, Peru, and Vietnam. Matern Child Nutr. 2019;15:e12835. 10.1111/mcn.12835
Footnotes
p < .01.
p < .001
REFERENCES
- Adair, L. S. (1999). Filipino children exhibit catch‐up growth from age 2 to 12 years. The Journal of Nutrition, 129(6), 1140–1148. 10.1093/jn/129.6.1140 [DOI] [PubMed] [Google Scholar]
- Adair, L. S. , & Guilkey, D. K. (1997). Age‐specific determinants of stunting in Filipino children. The Journal of Nutrition, 127(2), 314–320. 10.1093/jn/127.2.314 [DOI] [PubMed] [Google Scholar]
- Avula, R. , Raykar, N. , Menon, P. , & Laxminarayan, R. (2016). Reducing stunting in India: What investments are needed? Maternal & Child Nutrition, 12, 249–252. 10.1111/mcn.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton, G. H. (1989). Small but healthy? Are we asking the right question? Human Organization, 48, 30–39. 10.17730/humo.48.1.04n1174r2712g727 [DOI] [Google Scholar]
- Bhargava, A. (2015). Protein and micronutrient intakes are associated with child growth and morbidity from infancy to adulthood in the Philippines, 2. The Journal of Nutrition, 146(1), 133–141. 10.3945/jn.115.222869 [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. (2013). Early nutrition and adult outcomes: Pieces of the puzzle. The Lancet, 382(9891), 486–487. 10.1016/S0140-6736(13)60716-3 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , De Onis, M. , … Martorell, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Central Statistical Agency [Ethiopia], & ICF International . (2012). Ethiopia demographic and health survey 2011. Retrieved from Addis Ababa, Ethiopia and Calverton, MD, USA:
- Coly, A. N. , Milet, J. , Diallo, A. , Ndiaye, T. , Bénéfice, E. , Simondon, F. , … Simondon, K. B. (2006). Preschool stunting, adolescent migration, catch‐up growth, and adult height in young Senegalese men and women of rural origin. The Journal of Nutrition, 136(9), 2412–2420. 10.1093/jn/136.9.2412 [DOI] [PubMed] [Google Scholar]
- Crookston, B. T. , Schott, W. , Cueto, S. , Dearden, K. A. , Engle, P. , Georgiadis, A. , … Behrman, J. R. (2013). Postinfancy growth, schooling, and cognitive achievement: Young Lives. The American Journal of Clinical Nutrition, 98(6), 1555–1563. 10.3945/ajcn.113.067561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Onis, M. , & Branca, F. (2016). Childhood stunting: A global perspective. Maternal & Child Nutrition, 12, 12–26. 10.1111/mcn.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores, E. , & Escobal, J. (2008). An assessment of the Young Lives sampling approach in Peru.
- Kumra, N. (2008). An assessment of the Young Lives sampling approach in Andhra Pradesh, India.
- Lemma, F. , & Matji, J. (2013). Delivery platforms for sustained nutrition in Ethiopia. The Lancet, 382(9891), 488–489. 10.1016/S0140-6736(13)61054-5 [DOI] [PubMed] [Google Scholar]
- Lundeen, E. A. , Behrman, J. R. , Crookston, B. T. , Dearden, K. A. , Engle, P. , Georgiadis, A. , … Consequences of child growth project, T (2014). Growth faltering and recovery in children aged 1–8 years in four low‐ and middle‐income countries: Young Lives. Public Health Nutrition, 17(9), 2131–2137. 10.1017/S1368980013003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía Acosta, A. , & Haddad, L. (2014). The politics of success in the fight against malnutrition in Peru. Food Policy, 44, 26–35. 10.1016/j.foodpol.2013.10.009 [DOI] [Google Scholar]
- Nguyen, N. (2008). An assessment of the Young Lives sampling approach in Vietnam.
- Onis, M. d. , Onyango, A. W. , Borghi, E. , Siyam, A. , Nishida, C. , & Siekmann, J. (2007). Development of a WHO growth reference for school‐aged children and adolescents. Bulletin of the World Health Organization, 85, 660–667. 10.2471/BLT.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H . (2006). WHO child growth standards: Length/height for age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age, methods and development: World Health Organization.
- Outes‐Leon, I. , & Sanchez, A. (2008). An assessment of the Young Lives sampling approach in Ethiopia. Young Lives Technical Note, 1, 1–37. [Google Scholar]
- Perez‐Escamilla, R. (2013). Post‐1000 days growth trajectories and child cognitive development in low‐ and middle‐income countries. American Journal of Clinical Nutrition, 98(6), 1375–1376. 10.3945/ajcn.113.074757 [DOI] [PubMed] [Google Scholar]
- Perumal, N. , Bassani, D. G. , & Roth, D. E. (2018). Use and misuse of stunting as a measure of child health. The Journal of Nutrition, 148(3), 311–315. 10.1093/jn/nxx064 [DOI] [PubMed] [Google Scholar]
- Piwoz, E. , Baker, J. , & Frongillo, E. A. (2013). Documenting large‐scale programs to improve infant and young child feeding is key to facilitating progress in child nutrition. Food and Nutrition Bulletin, 34(3_suppl2), S143–S145. 10.1177/15648265130343S201 [DOI] [PubMed] [Google Scholar]
- Prentice, A. , Fulford, A. J. , Goldberg, G. R. , Ward, K. A. , Jarjou, L. M. , Moore, S. E. , & Prentice, A. M. (2013). Critical windows for nutritional interventions against stunting. The American Journal of Clinical Nutrition, 97(5), 911–918. 10.3945/ajcn.112.052332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockers, P. C. , & Fink, G. (2014). Childhood growth, schooling, and cognitive development: further evidence from the Young Lives study. The American Journal of Clinical Nutrition, 100(1), 182–188. 10.3945/ajcn.113.080960 [DOI] [PubMed] [Google Scholar]
- Shrimpton, R. , Victora, C. G. , de Onis, M. , Lima, R. C. , Blössner, M. , & Clugston, G. (2001). Worldwide timing of growth faltering: Implications for nutritional interventions. Pediatrics, 107(5), e75–e75. 10.1542/peds.107.5.e75 [DOI] [PubMed] [Google Scholar]
- Simondon, K. , Simondon, F. , Simon, I. , Diallo, A. , Bénéfice, E. , Traissac, P. , & Maire, B. (1998). Preschool stunting, age at menarche and adolescent height: A longitudinal study in rural Senegal. European Journal of Clinical Nutrition, 52(6), 412–418. 10.1038/sj.ejcn.1600577 [DOI] [PubMed] [Google Scholar]
- Stein, A. D. , Wang, M. , Martorell, R. , Norris, S. A. , Adair, L. S. , Bas, I. , … Gigante, D. P. (2010). Growth patterns in early childhood and final attained stature: Data from five birth cohorts from low‐and middle‐income countries. American Journal of Human Biology, 22(3), 353–359. 10.1002/ajhb.20998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S. , Mejía‐Guevara, I. , & Krishna, A. (2016). Rethinking policy perspectives on childhood stunting: Time to formulate a structural and multifactorial strategy. Maternal & Child Nutrition, 12, 219–236. 10.1111/mcn.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudfeld, C. R. , McCoy, D. C. , Danaei, G. , Fink, G. , Ezzati, M. , Andrews, K. G. , & Fawzi, W. W. (2015). Linear growth and child development in low‐and middle‐income countries: A meta‐analysis. Pediatrics, 135(5), e1266–e1275. 10.1542/peds.2014-3111 [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , de Onis, M. , Hallal, P. C. , Blössner, M. , & Shrimpton, R. (2010). Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics, Peds., 2009–1519. [DOI] [PubMed] [Google Scholar]
- Wilson, I. , Huttly, S. R. , & Fenn, B. (2006). A case study of sample design for longitudinal research: Young Lives. International Journal of Social Research Methodology, 9(5), 351–365. 10.1080/13645570600658716 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Age (in months) of children at the time of enrollment in Ethiopia, India, Peru, and Vietnam
Figure S2: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Ethiopia (n = 1,717 children)
Figure S3: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in India (n = 1,827 children)
Figure S4: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Peru (n = 1,751 children)
Figure S5: Flow Chart Showing Stunting Trajectories for Children Stunted and Not Stunted at Age 1 through Age 15 in Vietnam (n = 1,833 children)


