Abstract
Objective.
To determine the frequency of chronic joint pain and stiffness 3 years after infection with chikungunya virus (CHIKV) in a Latin American cohort.
Methods.
A cross-sectional followup of 120 patients from an initial cohort of 500 patients who reported joint pain 2 years after infection from the Atlántico Department, Colombia. Patients were clinically diagnosed as having CHIKV during the 2014–2015 epidemic, and baseline and followup symptoms at 40 months were evaluated in serologically confirmed cases.
Results.
Of the initial 500 patients enrolled in the study, 482 had serologically confirmed chikungunya infection. From this group, 123 patients reported joint pain 20 months after infection, and 54% of those patients reported continued joint pain 40 months after infection. Therefore, 1 out of every 8 people who tested serologically positive for CHIKV infection had persistent joint pain 3 years after infection. Participants who followed up in person were predominantly adult (mean ± SD age 51 ± 14 yrs) and female (86%). The most common type of pain reported in these patients at 40 months post-infection was pain with periods of relief and subsequent reoccurrence, and over 75% reported stiffness after immobility, with 39% experiencing morning stiffness.
Conclusion.
To our knowledge, this is the first report to describe persistent joint pain and stiffness 40 months after viral infection. The high frequency of chronic disease highlights the need to develop prevention and treatment methods. Further studies should be conducted to understand the similarities between post-chikungunya joint pain and rheumatoid arthritis.
Keywords: INFECTION, ARTHRITIS, CLINICAL TRIALS, MORNING STIFFNESS, INFLAMMATION
Chikungunya virus (CHIKV) is an arthropod-borne illness that belongs to the Alphavirus genus within the Togaviridae family1. The virus is transmitted mainly by the Aedes aegypti mosquito vector. Acute chikungunya infection causes a wide range of clinical manifestations, most prominently fever, rash, headache, myalgia, and severe, disabling polyarthralgia2. While the acute symptoms associated with CHIKV typically disappear within 7–10 days, various studies report persistent joint pain in 30–70% of patients months or years after initial CHIKV infection3-9. There has consistently been a positive correlation between age and duration of CHIKV-associated persistent joint pain, as well as severe initial joint pain and high titers of CHIKV-specific IgG4,10,11. Currently, the Centers for Disease Control and Prevention recommends acetaminophen and nonsteroidal antiinflammatory drugs (NSAID) for treatment of acute CHIKV symptoms; however, there are currently no effective therapeutics or vaccines available3. While some success has been seen using disease-modifying antirheumatic drugs (DMARD), especially in combination with hydroxychloroquine, in relieving symptoms of chronic CHIKV, the majority of patients are put on a prolonged regimen of NSAID12,13.
Since its identification in Tanzania in the early 1950s, CHIKV has primarily caused small, sporadic outbreaks in Africa and Asia, with a brief quiet period from the mid- 1980s through 2004. An epidemic originating in Kenya in June 2004 spread first to Réunion Island and other Indian Ocean islands, followed by an epidemic in India that eventually spread through Southeast Asia, and lastly to the islands of the Pacific Ocean. Cases in the Western hemisphere were travel-related until October 2013, when the first autochthonous chikungunya transmission occurred on the Caribbean island of Saint Martin. By mid-February 2014, the virus had landed on the South American mainland14. Unlike the outbreak in Réunion Island that was believed to be caused by the East-Central-South African (ECSA) strain, sequence data have demonstrated that the CHIKV strain spreading throughout the Caribbean and Central/South America is within the Asian strain genotype, making it closely related to viruses isolated in China and the Philippines, with minimal evolution15,16,17. However, there have been recent reports of ECSA genotype chikungunya in circulation in Brazil, signifying that both lineages are circulating in the Americas18.
In a 36-month longitudinal study of patients infected during the 2006 outbreak in Réunion Island, the majority of patients (60–80%) had relapsing arthralgia, while only 20–40% had unremitting arthralgia19. High reported percentages of patients with persistent arthralgia is common in studies of Italian and French cohorts4,10,11, whereas that statistic is much lower in cohorts from India and Senegal9,20,21. Numerous studies have so far failed to elucidate the mechanisms behind the progression of CHIKV to the chronic stage, although many hypotheses do exist, including preexisting joint disease or metabolic syndrome features, and the persistent replication of virus or the presence of viral debris in joint tissues13. Previously, we investigated the virologic and immune factors associated with chronic CHIKV-associated joint pain. Synovial fluid samples were taken from patients who had persistent knee joint pain 22 months after laboratory-confirmed infection22. However, synovial fluid samples from all patients were negative for CHIKV on quantitative real-time PCR, no viral proteins were detected by mass spectrometry, and viral cultures were negative. Further, cytokine and chemokine data showed that interleukin (IL)-6, IL-12p70, monocyte chemoattractant protein 1, macrophage inflammatory protein 1β, and IL-8 were elevated in CHIKV patients with arthritis compared to controls, although it did not reach statistical significance. Further analysis of predictors of chronic joint pain are needed. In another study, we investigated serum cytokine concentrations during acute chikungunya infection, comparing values in patients who reported the presence of chronic joint pain at 20 months post-infection versus age- and sex-matched patients who reported recovery in the same amount of time23. Those results showed a correlation between a robust cytokine response during acute infection and decreased incidence of chronic joint pain, as well as the increased incidence of chronic joint pain in patients with low tumor necrosis factor–α, IL-13, IL-2, and IL-4 during acute infection.
There have been many reports of relapsing-remitting arthralgia associated with CHIKV, including 1 study on Réunion Island hospital staff and their households that found that almost 50% of CHIKV-infected patients experienced at least 1 relapse of symptoms, and an increased occurrence with age24. Although there are no definitive causes for relapse, there are several suggested hypotheses, including the idea that virus particles or viral debris could reside within synovial tissue. In fact, Hoarau, et al found CHIKV RNA in synovial biopsy tissue, as well as virus antigen in macrophages, 18 months after infection in a single patient6. This was also demonstrated for the alphaviruses Sindbis virus and Ross River virus25,26,27. Further, longterm viral persistence was observed in macrophages in nonhuman primates up to 3 months after infection28. However, an analysis of 33 patients (many of whom had relapsing-remitting symptoms) did not find viral RNA or proteins in the synovial fluid of patients with persistent joint pain 22 months after infection22. The relative intensities of joint pain vary among patients, from less pain than that experienced during the acute phase, to more pain22,24.
Between 2015 and 2016, there were 376,436 cases of chikungunya reported in Colombia29. Colombia’s low elevation (< 1000 m above sea level, with the exception of the Andes Mountains) is favorable for A. aegypti proliferation and therefore, transmission to much of the population30. To date, longterm reports on the frequency of chronic arthritis in the Americas after chikungunya infection and detailed characterization of the clinical presentation of persistent arthritis years after infection have been limited.
The primary objective of this study was to describe the frequency of persistent joint pain in a Colombian cohort of CHIKV patients a median of 40 months post-infection and to better characterize the clinical symptoms, to better understand the longterm effect of the American outbreak. Our hypothesis was that one-third of the patients who reported persistent pain a median of 20 months post-infection would still be experiencing pain at 40 months.
MATERIALS AND METHODS
Ethics statement.
This study (IRB no. 121611, Trans no. 28283) was approved by the ethics committee of the Clinica de La Costa Ltda. and the George Washington University Committee on Human Research. A nonhuman subjects determination was made by the George Washington University Institutional Review Board for analysis of deidentified data. Written informed consent was obtained from all participants, and all samples were collected by qualified medical personnel5.
Study design.
In January 2015, 500 patients with clinically confirmed CHIKV infection were enrolled as part of a prospective cohort, and diagnosis was serologically confirmed by IgM and IgG antibody capture ELISA5. A baseline 33-item survey was conducted to established demographic characteristics, exposure history, and symptoms; subsequently, at a median of 20 months post-infection, a 56-item telephone survey included an assessment of the character and duration of persistent chikungunya arthritis symptoms, including swollen joint count, tender joint count, comorbidities, missed work or school, a global pain score during the last week (based on the 28-joint count Disease Activity Score)31, and therapies received5. At a median of 40 months after infection, a 30-item telephone survey was conducted with 120 patients who reported persistent arthritis during the 20-month followup. Of the 120 patients who participated in the phone survey, 82 also participated in an in-person assessment involving an 80-item survey that included the items mentioned above, as well as types of pain, what provokes relapse, and questions relating to stiffness, as devised and tested in patients with rheumatoid arthritis (RA)32.
Participants.
Cases were referred for enrollment after diagnosis with clinical chikungunya infection, defined by the Colombian Institute of Health as a fever of > 38°C, severe joint pain or arthritis, and acute onset of erythema multiform, with symptoms that could not be explained by other medical conditions among patients residing in or having visited a municipality with evidence of CHIKV transmission, or having traveled within 30 km of confirmed viral transmission33. Patients were from the Sabanalarga, Barranquilla, Juan de Acosta, Manati, Luruaco, and Baranoa municipalities in the Atlántico Department, Colombia, located on the Caribbean coastal plane. Clinically suspected cases of CHIKV were serologically confirmed for the purposes of our study5.
RESULTS
There were 500 participants with clinically confirmed CHIKV infection enrolled in our study, of whom 6 participants were excluded because of negative tests for IgM and IgG antibodies. Nine participants were excluded because joint pain status was not reported (Figure 1). Four hundred eighty-five participants were interviewed at a median of 20 months after chikungunya infection, 123 of whom reported persistent joint pain. Sixty-five of the 482 patients with serologically confirmed CHIKV infection reported persistent symptoms a median of 40 months after infection. Moreover, 54% of patients who reported persistent joint pain at a median 20 months post-infection reported still having pain at a median 40 months post-infection. This is clinically relevant because it appears that about 1 out of every 4 patients who tested serologically positive for CHIKV infection still have persistent joint pain associated with the virus 2 years after infection, and around 1 out of every 8 patients still have persistent joint pain 3 years after infection (Figure 2A). In response to the EQ-5D questionnaire (EuroQol Group; www.euroqol.org) that evaluates 5 dimensions of health status, over 30% of patients with persistent joint pain reported moderate or severe problems with pain and discomfort, while only around 10% reported moderate or severe problems with the other 4 areas, including mobility, self-care, daily activities, and depression or anxiety (Figure 2B).
Figure 1.
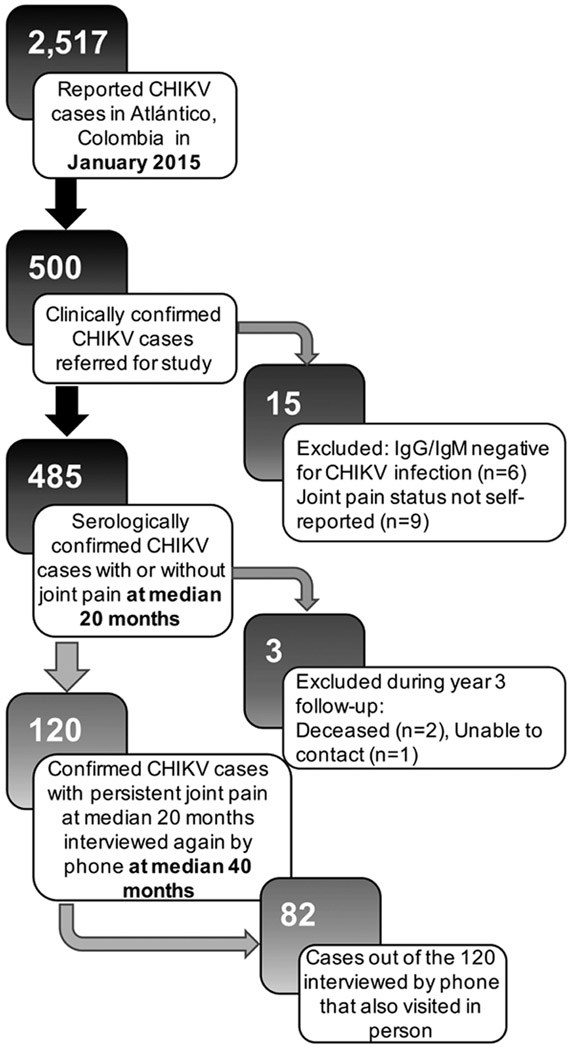
Study flow diagram. A chikungunya virus (CHIKV) epidemic occurred in 2014–2015 in the Atlántico Department of Colombia. Of the 2517 affected cases at study enrollment, 500 clinically confirmed cases were referred for the study, of which 485 participated in a followup telephone survey a median of 20 months after infection. Within this group, 123 patients reported having persistent joint pain, of whom 120 were subsequently interviewed by telephone a median of 40 months after infection, and 82 of whom also visited in person.
Figure 2.
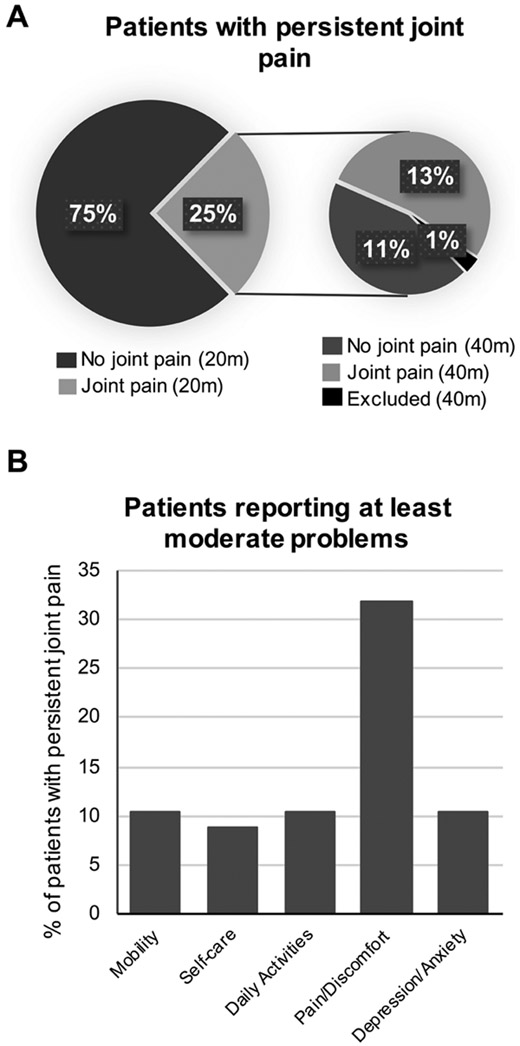
A. Pie chart depicting the percent of clinically confirmed patients enrolled in the study and followed up with 20 months post-CHIKV infection (n = 485) who reported persistent joint pain at 20 months (20 m) and 40 months (40 m) after infection. B. Graph showing percent of patients with persistent joint pain 40 months post-infection who reported at least moderate problems within each area of daily life. Patients were 3 times as likely to report problems with pain and discomfort than problems in any other area. CHIKV: chikungunya virus.
At baseline (Table 1), confirmed cases that were followed up in person were predominately adult (mean age 51 ± SD 14 yrs) and female (86%), and had at least a high school level education (68%). All patients who reported race were of Mestizo ethnicity (n = 80; i.e., mixed European, often Iberian, with indigenous Latin American ancestry). Persistent symptoms forced 38% of patients to miss school or work, and 47% of patients said their capacity to continue normal activities was affected (Table 2). Among the patients who had persistent joint pain after 20 months, the survey on global pain score (range 0–100) produced a mean ± SD of 47 ± 20 (n = 123)5. However, at 40 months, the participants reporting persistent joint pain had a global pain score of 65 ± 20 (n = 67). Interestingly, 2 patients with persistent joint pain said they had developed RA after CHIKV infection, and another 17 said they had developed osteoarthritis.
Table 1.
Baseline demographic characteristics of serologically confirmed CHIKV study participants classified by joint pain status.
| Characteristics | All CHIKV Cases, n = 82 |
Persistent Joint Pain, n = 67 |
No Persistent Joint Pain, n = 15 |
|---|---|---|---|
| Age at baseline, yrs, mean ± SD, yrs | 51 ± 14 | 52 ± 14 | 46 ± 17 |
| Female | 70 (86) | 57 (85) | 13 (87) |
| Education level | |||
| High school or more | 56 (68) | 44 (66) | 12 (80) |
| Time since onset, mos, mean ± SD | 40 ± 4 | 40 ± 4 | 40 ± 2 |
| Prior arthritis | 2 (2.5) | 2 (3) | 0 (0) |
Values are n (%) unless otherwise indicated. CHIKV: chikungunya virus.
Table 2.
Pain symptoms reported by serologically confirmed CHIKV patients at 40 months post-infection classified by joint pain status.
| Variables | All CHIKV Cases, n = 82 |
Persistent Joint Pain, n = 67 |
No Persistent Joint Pain, n = 15 |
p |
|---|---|---|---|---|
| Symptoms caused patient to miss work/school | 31 (38) | 27 (40) | 4 (27) | 0.33 |
| Symptoms affecting capacity to continue normal activities† | 37 (47) | 35 (54) | 2 (14) | 0.0071 |
| Swollen joint count, mean ± SD | 0.4 ± 0.9 | 0.4 ± 0.9 | 0.4 ± 0.9 | 0.90‡ |
| Tender joint count, mean ± SD | 5.2 ± 6.7 | 6.2 ± 7.1 | 0.7 ± 1.0 | < 0.0001‡ |
| Global pain score in the last week, mean ± SD (range 0–100) | 56.8 ± 27.3 | 64.9 ± 20.3 | 20.7 ± 25.2 | < 0.0001‡ |
| Developed rheumatoid arthritis after CHIKV infection | 2 (2) | 2 (3) | 0 (0) | 0.50 |
| Developed osteoarthritis after CHIKV infection | 19 (23) | 17 (25) | 2 (13) | 0.32 |
| Developed depression after CHIKV infection† | 9 (11) | 6 (9) | 3 (20) | 0.22 |
Values are n (%) unless otherwise indicated.
Data were not available for all patients.
Nonparametric Wilcoxon rank-sum test. Values in bold face are statistically significant. CHIKV: chikungunya virus.
The most common type of pain experienced by patients with persistent joint pain 40 months after CHIKV infection was pain with periods of relief and subsequent reoccurrence (Figure 3A). More than half of the patients reported this type of pain, while another 22% reported constant pain with changes in intensity. The activity that patients reported provoked pain the most was exercise, followed by infection (Figure 3B). Eighteen percent of patients reported other activities provoked pain relapse, including immobility, bending over, kneeling, work, cold temperatures, alcohol, and household chores. Stiffness occurred in the morning in 39% of patients with chronic joint pain and 30% experienced stiffness at other times of the day (Figure 3C). After periods of immobility, over three-quarters of patients with persistent joint pain reported stiffness, with 46% reporting mild stiffness in 1–3 joints and 32% reporting moderate stiffness in 3–5 joints or severe stiffness in over 5 joints (Figure 3D). Further, patients with chronic CHIKV scored a mean of 28.9% of the maximum possible impact (SD of 21.8) for determining the impact of stiffness on physical activity, which was higher than the 13.9% in the patients without arthritis (SD not calculated). This was not the case for mean score for the psychosocial impact of stiffness, which amounted to 30.1% of the maximum impact (SD 14.1) in patients with arthritis and 33.3% in patients without arthritis.
Figure 3.
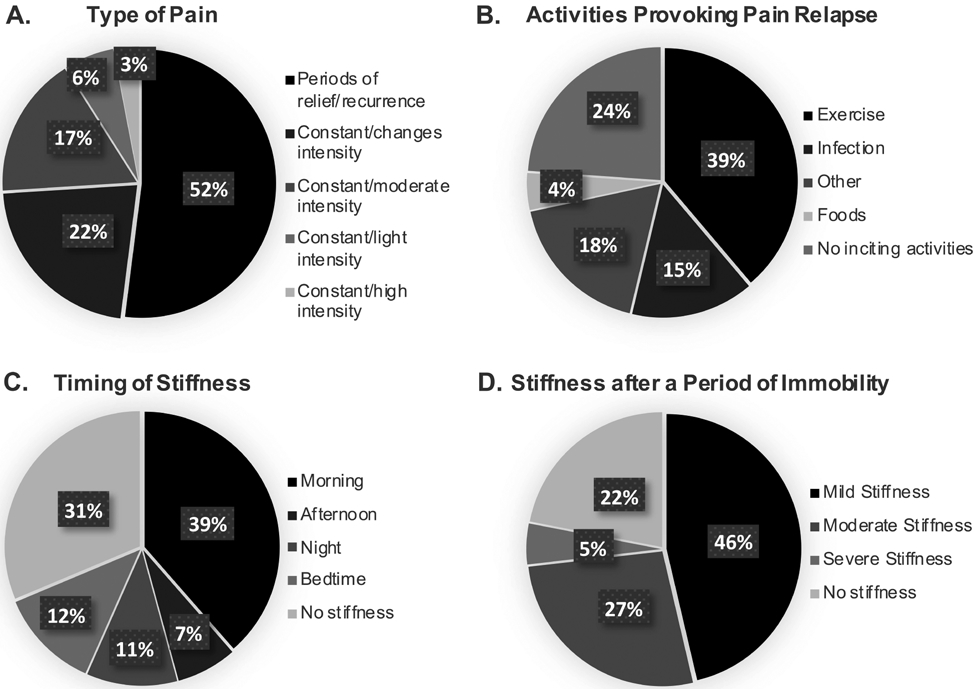
Graphs show percent of patients with persistent joint pain at 40 months post-infection who reported different types of pain (A; n = 65), activities that provoke the relapse of pain (B; n = 67), the timing of any reported stiffness (C; n = 67), and the level of stiffness they have after a period of immobility (D; n = 41).
DISCUSSION
Our primary findings include a high prevalence of continued self-reported joint pain in patients 3 years post-CHIKV infection characterized in over half of the cases by relapsing-remitting symptoms; three-fourths of patients experienced mild to moderate stiffness after a period of immobility and over one-third experienced stiffness in the morning. There have been few reports regarding the relapsing-remitting features of chronic arthralgia among chikungunya patients to date; however, it is a known symptom of post-infection rheumatism4,24. Similar to our findings that over half of chronic CHIKV patients experienced relapsing-remitting symptoms, Schilte, et al found that 24% of the patients involved in a 36-month longitudinal study experienced partial recovery from CHIKV-associated arthralgia at months 4, 6, or 16, then relapsed19. Further, in the same study those that experienced symptoms at 36 months post-infection reported arthralgia was provoked by a change in ambient temperature (43.5%) or physical effort (8%), whereas in our study, exercise was named as the most frequent arthritis trigger (39%), and infection as the second most frequent cause (15%).
There are many resemblances between CHIKV-associated arthritis and RA. Both are more common in women than in men, and a higher prevalence is seen in older patients34. While inflammation in RA is due to an autoimmune process, similarities have been seen in the cytokine secretion and induced genes in mouse models of both CHIKV infection and RA35,36. In CHIKV-associated arthritis, proinflammatory cytokines and chemokines are produced by infected skeletal muscle cells, macrophages, synoviocytes, and osteoblasts, whereas in RA, proliferating synovial lining cells and infiltrating inflammatory cells produce the cytokines and chemokines that cause inflammation. Periarticular and systemic bone loss are also common in both RA and CHIKV-associated arthritis, resulting from an inflamed synovial membrane; however, the focal bone erosion and articular cartilage thinning found in patients with RA is not seen in post-CHIKV arthritis37.
In a report describing the features of 10 American travelers who were simultaneously infected with CHIKV while visiting Haiti in 2014, Miner, et al noted the similarities in peripheral T cell phenotypes between CHIKV-infected patients and patients with RA38. Eight patients reported persistent arthritis lasting 6 weeks, along with severe joint pain and morning stiffness lasting at least 8 weeks. The authors pointed out that had they not known about the patients’ travel history, these patients would have met the 2010 American College of Rheumatology/European League Against Rheumatism definition of RA.
Morning stiffness is a hallmark of RA and is often an even more predominant symptom than pain. A 10-month study of CHIKV cases from the Karnataka State in India reported morning stiffness lasting > 1 h in 78.7% of patients with chronic CHIKV39. Further, at Month 36 of the Schilte, et al study in Réunion Island, 75.5% of patients with chronic symptoms related to CHIKV experienced arthralgia caused by stiffness, with 67.7% reporting a need to stretch in the morning19. Similarly, a 32-month followup study at Réunion Island showed that 79.1% of patients experienced morning stiffness, with 71% experiencing it for more than 30 min40. Further, 4% of the 328 patients in that study initially examined at the time of infection were subsequently diagnosed with RA. In contrast, our study found that only 39% of patients with chronic CHIKV experienced morning stiffness at 40 months after infection; however, nearly three-fourths of patients with chronic CHIKV experienced mild or moderate stiffness after immobility. Based on the results from questions aimed at determining the effect of stiffness, patients with chronic CHIKV reported an effect on physical activity score more than double that reported by patients without chronic arthritis. On the other hand, patients with and without chronic arthritis scored the same when it came to the psychosocial effect of stiffness. While it is important to note that only 3 patients without arthritis supplied answers to these questions, the results emphasize the importance of including stiffness impact scores in future followup studies to compare between arthritis disease states. It would also be interesting to compare the scores for chronic CHIKV arthritis with those from patients with RA.
The current recommendations for therapeutic management of post-CHIKV chronic arthritis were developed based on its similarities to RA and are patient-specific. The recognition and management of patients with chronic CHIKV-associated arthritis, and subsequently relieving the pain and inflammation associated with the condition, is critical in limiting joint stiffness, loss of muscle tone, and loss of physical fitness in the short term, and joint degradation and bone loss in the long term. Analgesics or NSAID for pain relief, combined with physical therapy on any areas that are persistently painful to relieve pain and preserve muscle tone and range of motion, are the primary therapeutic approach. While there is no indication that DMARD are helpful prior to 8 weeks after the end of the acute stage, methotrexate is recommended for patients who meet the definition of post-CHIKV chronic inflammatory rheumatism. Systemic corticosteroids and local antiinflammatory therapies are also recommended for patients displaying certain symptoms, such as tenosynovitis or active synovitis41.
As with RA, post-chikungunya arthritis is a great personal burden on the individuals having longterm disease, as well as a significant societal economic burden. In our study, patients with chronic joint pain scored a mean clinical Health Assessment Questionnaire disability score that denoted mild functional losses42. Almost half of patients reported missing school or work because of symptoms, and over half reported that symptoms affected their ability to perform the normal activities of their daily life. Although our study found that the largest problem for patients was pain and discomfort, and that only 10% of patients with joint pain reported feeling anxious or depressed, other studies reported a continued effect on the mental health of patients as well as their physical health11.
There were various limitations to our study. Because of the lack of a control group, we cannot rule out the possibility that a few of the study participants may have developed joint pain and symptoms over the course of 40 months from another etiology but attributed it to CHIKV infection. Similarly, patients were not tested for other arboviral infections, such as Zika, dengue, and Mayaro viruses, which can cause joint pain. Although Mayaro virus was not known to be circulating in the Atlántico Department during the chikungunya epidemic, it is known to sporadically affect the Colombia Amazon region43. Another limitation includes the lack of serological markers of inflammation with a primary outcome of self-reported joint pain. Similar to all other previous longterm studies, the absence of systematic clinical and biological examinations represents a significant limitation.
To our knowledge, our study is the first to report persistent joint pain 40 months after CHIKV infection in the Americas. In light of our findings, recommendations can be made about medical followup of CHIKV-infected patients until they are fully recovered. Upon diagnosis with acute CHIKV infection, patients should be made aware of the slow, yet progressive, return to normal, the relapsing-remitting characteristics of the symptoms, and of the potential for chronic symptoms lasting years. Owing to the effect chronic disease has on quality of life, patients may benefit from mental health support.
ACKNOWLEDGMENT
The authors thank Mariana Encinales, Lorena Encinales, Cuello Bendek, and Gerardo Javier for their help on this study, as well as the Allied Research Society, especially Ariel Gonzalez and Martha Utrera.
This study was supported by a pilot award from the Rheumatology Research Foundation and the US National Institutes of Health (NIH; National Center for Advancing Translational Sciences grants UL1-TR-001876 and KL2-TR-001877). The views expressed herein are solely the responsibility of the authors and do not necessarily represent the official views of the Rheumatology Research Foundation or the NIH.
Contributor Information
Sarah R. Tritsch, George Washington University, San Diego
Liliana Encinales, Allied Research Society LLC, San Diego.
Nelly Pacheco, Allied Research Society LLC, San Diego.
Andres Cadena, Clinica de La Costa Ltda, San Diego.
Carlos Cure, Biomelab, San Diego.
Elizabeth McMahon, George Washington University, San Diego.
Hugh Watson, Evotec ID, San Diego.
Alexandra Porras Ramirez, Universidad El Bosque, San Diego.
Alejandro Rico Mendoza, Universidad El Bosque, San Diego.
Guangzhao Li, George Washington University, San Diego.
Kunal Khurana, George Mason University, San Diego.
Juan Jose Jaller-Raad, Centro de Reumatología y Ortopedia, San Diego.
Stella Mejia Castillo, Universidad Simón Bolívar, San Diego.
Onaldo Barrios Taborda, Universidad Simón Bolívar, San Diego.
Juan Jose Jaller-Char, Centro de Reumatología y Ortopedia, San Diego.
Lil Avendaño Echavez, Universidad Simón Bolívar, San Diego.
Dennys Jiménez, Clinica de La Costa Ltda, San Diego.
Andres Gonzalez Coba, Clinica de La Costa Ltda, San Diego.
Magda Alarcon Gomez, Universidad Simón Bolívar, San Diego.
Dores Ariza Orozco, Allied Research Society LLC, San Diego.
Eyda Bravo, Allied Research Society LLC, San Diego.
Victor Martinez, Clinica de La Costa Ltda, San Diego.
Brenda Guerra, Clinica de La Costa Ltda, San Diego.
Gary Simon, George Washington University, San Diego.
Gary S. Firestein, University of California, San Diego
Aileen Y. Chang, George Washington University.
REFERENCES
- 1.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I. Clinical features. Trans R Soc Trop Med Hyg 1955;49:28–32. [DOI] [PubMed] [Google Scholar]
- 2.Paul BJ, Sadanand S. Chikungunya infection: A re-emerging epidemic. Rheumatol Ther 2018;5:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Chikungunya virus: resources for healthcare providers. [Internet. Accessed October 11, 2019.] Available from: www.cdc.gov/chikungunya/hc/resources.html
- 4.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on Réunion Island. Clin Infect Dis 2008;47:469–75. [DOI] [PubMed] [Google Scholar]
- 5.Chang AY, Encinales L, Porras A, Pacheco N, Reid SP, Martins KAO, et al. Frequency of chronic joint pain following chikungunya virus infection: a Colombian cohort study. Arthritis Rheumatol 2018;70:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 2010;184:5914–27. [DOI] [PubMed] [Google Scholar]
- 7.Soumahoro MK, Gérardin P, Boëlle PY, Perrau J, Fianu A, Pouchot J, et al. Impact of chikungunya virus infection on health status and quality of life: a retrospective cohort study. PLos One 2009;4:e7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahim AA, Thekkekara RJ, Bina T, Paul BJ. Disability with persistent pain following an epidemic of chikungunya in rural south India. J Rheumatol 2016;43:440–4. [DOI] [PubMed] [Google Scholar]
- 9.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J 1983;63:313–5. [PubMed] [Google Scholar]
- 10.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic chikungunya disease on Réunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis 2009;3:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couturier E, Guillemin F, Mura M, Léon L, Virion JM, Letort MJ, et al. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatology 2012;51:1315–22. [DOI] [PubMed] [Google Scholar]
- 12.Ravindran V, Alias G. Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: a 24-week randomized controlled open label study. Clin Rheumatol 2017;36:1335–40. [DOI] [PubMed] [Google Scholar]
- 13.Zaid A, Gérardin P, Taylor A, Mostafavi H, Malvy D, Mahalingam S. Review: Chikungunya arthritis: implications of acute and chronic inflammation mechanisms on disease management. Arthritis Rheumatol 2017;70:484–95. [DOI] [PubMed] [Google Scholar]
- 14.Nasci RS. Movement of chikungunya virus into the Western hemisphere. Emerg Infect Diseases 2014;20:1394–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet 2014;383:514. [DOI] [PubMed] [Google Scholar]
- 16.Lanciotti RS, Valadere AM. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Diseases 2014; 20:1400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Lambert AJ. Phylogenetic analysis of chikungunya virus strains circulating in the Western hemisphere. Am J Trop Med Hyg 2016;94:800–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa-da-Silva AL, Ioshino RS, Petersen V, Lima AF, Cunha MDP, Wiley MR, et al. First report of naturally infected Aedes aegypti with chikungunya virus genotype ECSA in the Americas. PLoS Negl Trop Dis 2017;11:e0005630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis 2013;7:e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew AJ, Goyal V, George E, Thekkemuriyil DV, Jayakumar B, Chopra A; Trivandrum COPCORD Study Group. Rheumatic-musculoskeletal pain and disorders in a naive group of individuals 15 months following a chikungunya viral epidemic in south India: a population based observational study. Int J Clin Pract 2011;65:1306–12. [DOI] [PubMed] [Google Scholar]
- 21.Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect 2012;140:842–50. [DOI] [PubMed] [Google Scholar]
- 22.Chang AY, Martins KA, Encinales L, Reid SP, Acuna M, Encinales C, et al. Chikungunya arthritis mechanisms in the Americas: a cross-sectional analysis of chikungunya arthritis patients twenty-two months after infection demonstrating no detectable viral persistence in synovial fluid. Arthritis Rheumatol 2018;70:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang AY, Tritsch S, Reid SP, Martins K, Encinales L, Pacheco N, et al. The cytokine profile in acute chikungunya infection is predictive of chronic arthritis 20 months post infection. Diseases 2018;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staikowsky F, Le Roux K, Schuffenecker I, Laurent P, Grivard P, Develay A, et al. Retrospective survey of chikungunya disease in Réunion Island hospital staff. Epidemiol Infect 2008;136:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heise MT, Simpson DA, Johnston RE. Sindbis-group alphavirus replication in periosteum and endosteum of long bones in adult mice. J Virol 2000;74:9294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, et al. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum 2000;43:365–9. [DOI] [PubMed] [Google Scholar]
- 27.Rulli NE, Suhrbier A, Hueston L, Heise MT, Tupanceska D, Zaid A, et al. Ross river virus: molecular and cellular aspects of disease pathogenesis. Pharmacol Ther 2005;107:329–42. [DOI] [PubMed] [Google Scholar]
- 28.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010;120:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan American Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory: 2016, cumulative cases. [Internet. Accessed October 11, 2019.] Available from: www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=37867&lang=en
- 30.World Health Organization. Chikungunya. [Internet. Accessed October 11, 2019.] Available from: www.who.int/news-room/fact-sheets/detail/chikungunya
- 31.Prevoo ML, Van’T Hof MA, Kuper HH, Van Leeuwen MA, Van De Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 32.Halls S, Dures E, Kirwan J, Pollock J, Baker G, Edmunds A, et al. AB1128-HPR developing a new rheumatoid arthritis (RA) stiffness patient reported outcome measure (PROM). Ann Rheum Dis 2016;75:1317. [DOI] [PubMed] [Google Scholar]
- 33.Ospina ML, Martínez Duran ME, Pacheco García OE, Quijada Bonilla H. Protocolo de vigilancia en salud púlica: chikunguña. [Article in Spanish] [Internet. Accessed October 21, 2019.] Available from: https://cruevalle.org/files/PRO-Chikungunya.pdf
- 34.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. Gene profiling of chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum 2012;64:3553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assunção-Miranda I, Cruz-Oliveira C, Da Poian AT. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed Res Int 2013;2013:973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Foo SS, Sims NA, Herrero LJ, Walsh NC, Mahalingam S. Arthritogenic alphaviruses: new insights into arthritis and bone pathology. Trends Microbiol 2015;23:35–43. [DOI] [PubMed] [Google Scholar]
- 38.Miner JJ, Aw-Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol 2015;67:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg 2010;104:392–9. [DOI] [PubMed] [Google Scholar]
- 40.Bouquillard E, Fianu A, Bangil M, Charlette N, Ribera A, Michault A, et al. Rheumatic manifestations associated with chikungunya virus infection: a study of 307 patients with 32-month follow-up (RHUMATOCHIK study). Joint Bone Spine 2018;85:207–10. [DOI] [PubMed] [Google Scholar]
- 41.Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, et al. ; Societe de pathologie infectieuse de langue francaise. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect 2015;45:243–63. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe F, Michaud K, Pincus T. Development and validation of the Health Assessment Questionnaire II: a revised version of the Health Assessment Questionnaire. Arthritis Rheum 2004;50:3296–305. [DOI] [PubMed] [Google Scholar]
- 43.Groot H, Morales A, Vidales H. Virus isolations from forest mosquitoes in San Vicente de Chucuri, Colombia. Am J Trop Med Hyg 1961;10:397–402. [DOI] [PubMed] [Google Scholar]


