Abstract
Objective
Antibodies against leucine-rich glioma-inactivated 1 (LGI1-Abs) characterize a limbic encephalitis (LE) strongly associated with HLA-DRB1*07:01, although some patients lack LGI1-Abs in CSF or do not carry this allele. Whether they represent a different subtype of disease or have different prognoses is unclear.
Methods
Retrospective analysis of clinical features, IgG isotypes, and outcome according to LGI1-Ab CSF positivity and DRB1*07:01 in a cohort of anti-LGI1 LE patients.
Results
Patients with LGI1-Abs detected in both CSF and serum (105/134, 78%) were compared with those who were CSF negative (29/134, 22%). Both groups had similar clinical features and serum levels, but CSF-positive patients had shorter diagnostic delay, more frequently hyponatremia, inflammatory CSF, and abnormal MRI (p < 0.05). Human leukocyte antigen (HLA) genotyping was performed in 72/134 (54%) patients and 63/72 (88%) carried DRB1*07:01. Noncarriers (9/72, 12%) were younger, more commonly women, and had less frequently psychiatric and frontal symptoms (p < 0.05). No difference in IgG isotypes according to CSF positivity or HLA was found (p > 0.05). HLA and IgG isotypes were not associated with poor outcome (mRS >2 at last follow-up) in univariate analyses; CSF positivity was only identified as a poor outcome predictor in the multivariate analysis including the complete follow-up, whereas age and female sex also remained when just the first year was considered.
Conclusions
LE without CSF LGI1-Abs is clinically indistinguishable and likely reflects just a lesser LGI1-Ab production. HLA association is sex and age biased and presents clinical particularities, suggesting subtle differences in the immune response. Long-term outcome depends mostly on demographic characteristics and the intensity of the intrathecal synthesis.
Patients with limbic encephalitis (LE) and antibodies against leucine-rich glioma-inactivated 1 (LGI1-Abs) are usually elderly men who develop severe anterograde amnesia, psychiatric symptoms, and seizures, along with medial temporal lobe abnormalities in brain MRI but, intriguingly, often without inflammatory signs in CSF routine analysis.1–5 Furthermore, contrary to other types of autoimmune encephalitis such as anti-NMDA receptor encephalitis,6 serum testing is more sensitive than CSF for the detection of LGI1-Abs in our experience, which has also been reported by others.3,7 The disease is also remarkably associated with the allele HLA-DRB1*07:01, which is found in nearly 90% of the patients.8–11 However, to date, whether patients without detectable LGI1-Abs in the CSF and those not carrying DRB1*07:01 show distinct particularities is still unclear. In addition, despite the response to immunotherapy in anti-LGI1 LE being satisfactory in most patients,1–3,12 the cognitive recovery is usually incomplete, and diverse prognostic factors have been reported.3,11,13,14 Nevertheless, the role of CSF positivity and human leukocyte antigen (HLA) on outcome has not been widely investigated.11,15
We therefore aimed to investigate whether clinical or immunologic differences exist among patients with anti-LGI1 LE according to CSF positivity for LGI1-Abs and HLA-DRB1*07:01 carrier status. In addition, we studied the evolution of disability and the prognostic factors, including the aforementioned biomarkers.
Methods
Patients and Clinical Data
All consecutive patients diagnosed in our center from January 2010 to November 2018 with LE and serum positive for LGI1-Abs were identified. Serum LGI1-Abs were detected with a cell-based assay (CBA), as previously described.5 Immunohistofluorescence (IHF) on rat brain sections was used as screening technique in CSF and further confirmed by CBA; only CSF samples positive for both techniques were therefore considered as positive. Demographic and clinical data were retrospectively collected from hospital charts, including first clinical feature (table e-1, links.lww.com/NXI/A430), disability at onset using the modified Rankin Scale (mRS), diagnostic delay, CSF positivity for LGI1-Abs, clinical features that developed during the course of the disease (table e-1), presence of cancer, intensive care unit (ICU) admission, presence of hyponatremia, inflammatory CSF (defined as levels exceeding the upper limit of normal of our laboratory reference values of protein content [>0.5 mg/dL] and/or white cell count [>2 cells/mL] and/or oligoclonal bands), brain MRI (normal or unilateral/bilateral medial temporal hypersignal), first-line treatment (corticosteroids, IV immunoglobulin [IVIG], and plasma exchange), second-line treatment (rituximab and cyclophosphamide), chronic immunotherapy (azathioprine and mycophenolate), dominant sequela at last follow-up, and mRS at diagnosis, and after 3, 6, 9, 12, 18, 24, and 36 months. Patients for whom only serum was tested and those without clinical data were excluded from the study.
Serum Levels of LGI1-Abs
Levels of serum LGI1-Abs were measured using an ELISA on a CBA. Culture medium was eliminated, and cells were washed once with phosphate-buffered saline (PBS; 200 μL/well). Sera were next diluted (1/10) using a dilution medium composed of Dulbecco's Modified Eagle Medium (DMEM), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), normal goat serum (NGS) 5%, and bovine serum albumin (BSA) 1% and then incubated for 30 minutes at 37°C (50 μL/well). Cells were washed once with PBS (200 μL/well) and fixed using 4% paraformaldehyde for 15 minutes at room temperature (100 μL/well). Cells were washed once again with PBS (200 μL/well) and twice with 0.05%PBS-Tween (200 μL/well), to be later incubated for 1 hour with a secondary anti-human antibody (1/10,000 dilution; Peroxidase AffiniPure Goat Anti-Human IgG Fcγ fragment specific, Jackson ImmunoResearch, Cambridge, UK) coupled to peroxidase for 30 minutes at 37°C (50 μL/well). Cells were washed using 0.05%PBS-Tween twice, and once with PBS (200 μL/well), wells aspirated, 3,3′, 5,5′-tetramethylbenzidine substrate solution (50 μL/well) added, and incubated for 30 minutes. The reaction was stopped using sulfuric acid (2 M; 50 μL/well). The plate was immediately read at 450 nm of wavelength using the SpectraMax Plus platform (Molecular Devices, San Jose, CA). All assays were performed in triplicate. Within each test, the mean of the 2 optic density (OD) values obtained was calculated for both transfected and nontransfected cells. The difference between these, labeled as Delta OD, was then obtained. For each sample, the mean of the Delta OD from the 3 experiments was used for statistical analysis. Twenty-five sera from patients negative for LGI1-Abs were used as controls for technical validation.
IgG Isotypes
Isotyping was performed using mouse anti-human antibodies that specifically recognize IgG1 (mouse anti-human IgG1 CH2 domain; Bio-Rad, Hercules, CA), IgG2 (purified mouse anti-human IgG2 clone G18-21 [RUO]; BD Biosciences, San Jose, CA), IgG3 (mouse anti-human IgG3; Bio-RAD), or IgG4 (purified mouse anti-human IgG4 clone G17-4 [RUO]; BD Biosciences). Either 100 or 200 μL (according to whether 96 or 24-well plate were used) of the IgG solution of interest (diluted 1/1,000 for IgG1 and IgG3 and 1/500 for IgG2 and IgG4) in DMEM-HEPES-NGS5%-BSA1% was used and incubated for 1 hour at room temperature in the dark. After aspiration, cells were washed with DMEM-HEPES (100/200 μL) and once again with PBS (100/200 μL). A secondary goat anti-mouse antibody (100/200 μL) coupled to a fluorochrome (Alexa Fluor 555; ThermoFisher Scientific, Waltham, MA), diluted 1/1,000 in DMEM-HEPES-NGS5%-BSA1%, was added and then incubated for 1 hour at room temperature in the dark. After aspiration, cells were washed with DMEM-HEPES (100/200 μL) and with PBS (100/200 μL). In addition, titration of IgG1 and IgG4 subclasses in serum and CSF was performed only in the double-positive samples and determined as the lowest dilution with positive signal by CBA. All the CBAs were read using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany).
To validate the specificity of the secondary antibodies used for IgG isotyping, a Western blot was performed with 1 μg of every purified IgG subclass (IgG1, HCA192; IgG2, HCA193; IgG3, HCA194; IgG4, HCA195; Bio-Rad) load on each well. After migration, transfer, and saturation, membranes were separately incubated with each anti-human IgG subtype (1/1,000 dilution for anti-IgG1 and 3, 1/500 for anti-IgG2 and 4 antibodies). Revelation was performed with goat anti-mouse IgG (1/20,000; 115-036-003, Jackson ImmunoResearch, Suffolk, United Kingdom).
HLA Analysis
HLA genotyping was performed in patients with available DNA using next-generation sequencing on a MiSeq sequencer system (Illumina, San Diego, CA) and is reported at a 4-digit level resolution. Controls (courtesy of EFS Auvergne-Rhône-Alpes) were 300 healthy subjects of Caucasian ethnic origin, genotyped for HLA A, B, C, DR, and DQ, previously obtained using next-generation sequencing technology (Omixon, Budapest, Hungary).
Statistical Analysis
An exploratory approach,16 using χ2 and Fisher exact tests for categorical variables and the Wilcoxon signed-rank test for quantitative data, was used to compare patients according to CSF positivity for LGI1-Abs, DRB1*07:01 carrier status, or IgG isotypes.
To search for factors associated with good (defined as last mRs ≤2) or poor prognosis (mRs >2), a 2-step approach was used: first, a univariate binary logistic regression (with continuous variables categorized), and second, a multivariate analysis using a generalized linear mixed model for binary distribution (SAS Glimmix procedure). This mixed model assessed repeated measures of mRS for each patient; we declared the patient as random effect and the remaining factors as fixed effect. The dependent variable was good/poor prognosis as defined above, and the choice of independent variables included was based on clinical criteria: the demographic (age, sex) and main clinical (amnesia, faciobrachial dystonic seizures [FBDSs], other seizures, psychiatric symptoms, and frontal syndrome) characteristics that are present at disease onset; CSF positivity for LGI1-Abs as a biomarker (HLA was excluded due to the small sample size compared with the overall cohort); and diagnostic delay, length of follow-up, and treatment as possible confounder factors. The probability to switch from good to poor outcome was modeled. Statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). All p values were 2 tailed, and p values <0.05 were considered statistically significant.
Differences in HLA carrier frequencies between patients and controls were analyzed using the 2-tailed Fisher's exact tests (SPSS software package version 25.0; IBM Corp., Armonk, NY). Allele frequency comparisons were Bonferroni corrected using the number of alleles for each locus; corrected p < 0.05 values were considered significant.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all patients for the storage and use of biological samples and clinical information for research purposes. The Institutional Review Board of the Université Claude Bernard Lyon 1 and Hospices Civils de Lyon approved the study (ICARE NCT-04106596).
Data Availability
Any data not published within the article are available and will be shared by request from any qualified investigator.
Results
Characteristics of Patients According to LGI1-Ab CSF Positivity
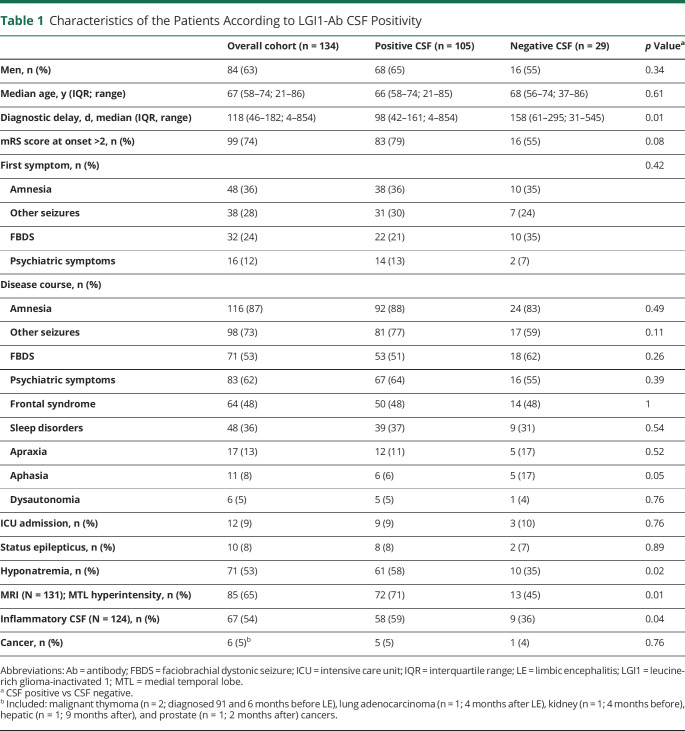
A total of 142 patients with LE and serum LGI1-Abs were identified. Eight (8/142, 6%) patients were excluded either because clinical data were lacking or because LGI1-Abs were not assayed in CSF. Among the remaining 134 patients positive for LGI1-Abs in serum, 105 (78%) were also CSF positive. Twenty-nine (22%) patients were considered to be CSF negative: 23 (79%) were negative for both IHF and CBA, 5 (17%) were positive for IHF but negative for CBA, and 1 subject (3%) was negative for only CBA. No significant difference in sex, age, and clinical presentation was observed between the 2 groups. Diagnostic delay was longer in the CSF-negative (median 158 days, interquartile range [IQR] 61–295) vs the CSF-positive (median 98 days, IQR 42–161; p = 0.01) group. Patients with positive CSF had more frequently hyponatremia (61/105, 58%, vs 10/29, 35%; p = 0.02), inflammatory CSF findings (58/99, 59%; vs 9/25, 36%; p = 0.04), and abnormal MRIs (72/102, 71%; vs 13/29, 45%; p = 0.01; table 1).
Table 1.
Characteristics of the Patients According to LGI1-Ab CSF Positivity
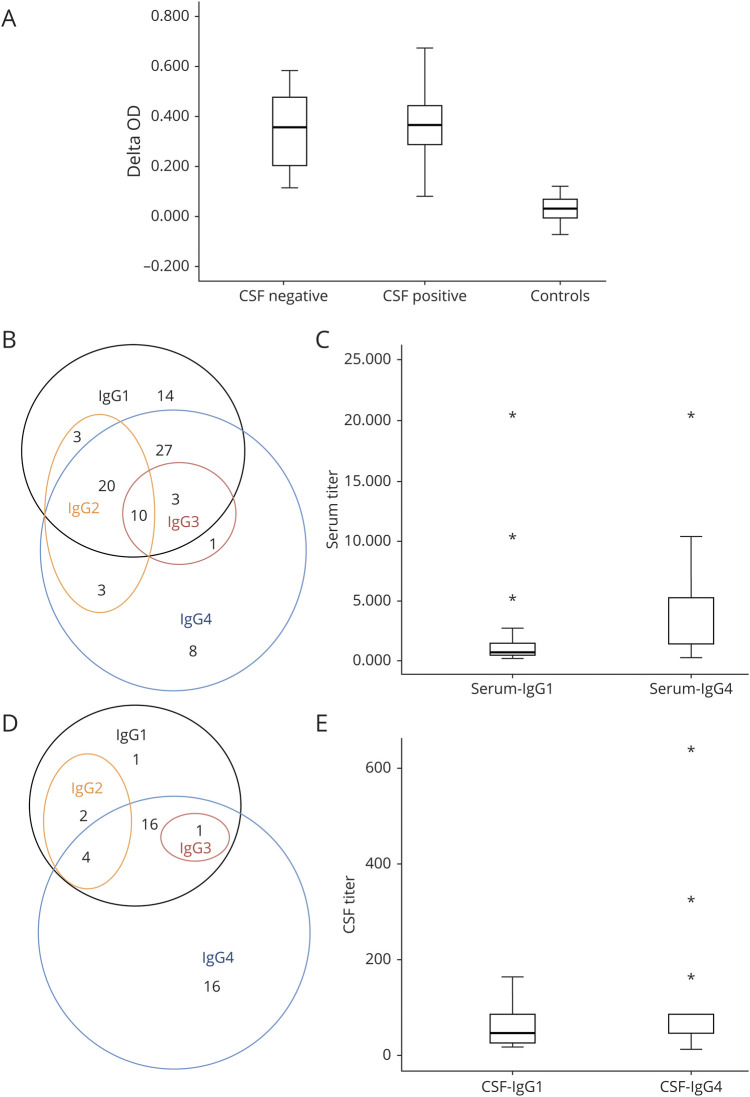
Serum levels were determined by ELISA-CBA in 60 patients with available samples; 48 of these patients (80%) had LGI1-Abs in both serum and CSF, while 12 (20%) had LGI1-Abs only in the serum. No significant difference in serum levels was found between CSF-positive and CSF-negative patients (p = 0.91; figure 1A).
Figure 1. Immunologic Characteristics of Anti-LGI1 Encephalitis.
(A) Box plot showing LGI1-Abs serum levels according to CSF positivity. No significant difference in serum levels, represented as Delta OD measured by ELISA-CBA, was found between CSF-positive (median Delta OD = 0.37, IQR 0.29–0.44) and CSF-negative (median Delta OD = 0.37, IQR 0.19–0.48) patients (p = 0.91). Control sera from patients without LGI1-Abs were used for technical validation. (B) Venn diagram showing serum IgG isotypes: most sera (60/89, 67%) were positive for both IgG1 and IgG4. (C) Box plot representing IgG1/4 serum titers: IgG4 titers were higher than IgG1 titers in 59 double-positive samples analyzed. (D) Venn diagram showing CSF IgG isotypes: IgG4 was the most detected isotype (37/40, 93%), but 21 samples were also positive for IgG1. (E) Box plot representing IgG1/4 CSF titers: IgG4 titers were higher than IgG1 titers in the 29 double-positive samples analyzed. CBA = cell-based assay; IQR = interquartile range; LGI1-Abs = antibodies against leucine-rich glioma-inactivated 1 protein; OD = optic density.
HLA Association in Anti-LGI1 Encephalitis
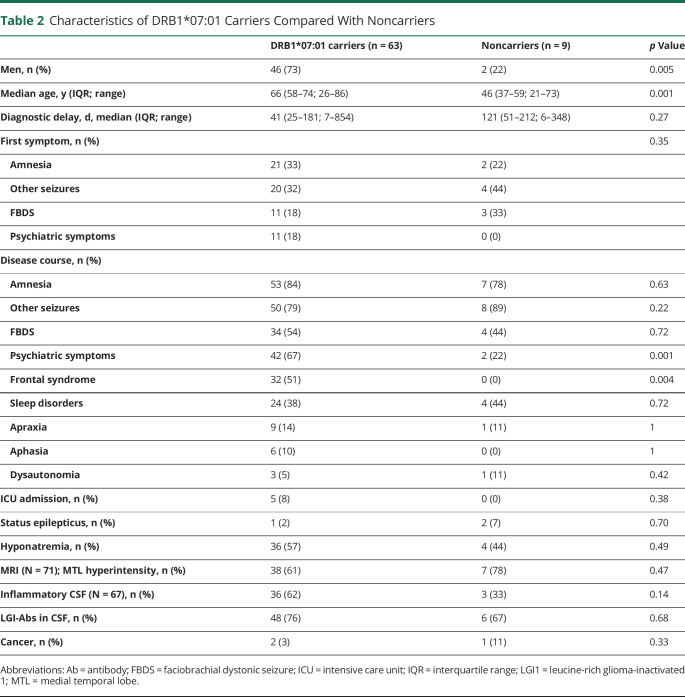
DNA was available in 72/134 (54%) patients; 68/72 (94%) of whom were of Caucasian origin. DRB1*07:01 was carried by 63/72 (88%) patients; this was significantly more frequent than in the control group (86/300, 29%; corrected p < 0.0001; OR = 17.42, 95% CI 8.29–36.58). Several alleles in linkage disequilibrium with DRB1*07:01 were also found more commonly in patients: A*30:01 (10/72, 14%, vs 7/300, 2% in the control group; corrected p = 0.009; OR = 6.75, 95% CI 2.47–18.42), C*06:02 (25/72, 35%, vs 36/300, 12% in the control group; corrected p < 0.001; OR = 3.90, 95% CI 2.15–7.09), and DQB1*02:02 (53/72, 74%, vs 78/300, 26% in the control group; corrected p < 0.0001; OR = 7.94, 95% CI 4.43–14.24). Another DRB1 allele, DRB1*04:02 was also significantly more frequent among patients (9/72, 13%), compared with controls (7/300, 2%; corrected p = 0.03; OR = 5.9, 95% CI 2.2–16.7); however, 7/9 (78%) DRB1*04:02 carriers were also DRB1*07:01 carriers. Four alleles were found to be significantly more common in controls than in patients, but this finding was likely a consequence of an overrepresentation of DRB1*07:01 and its linked alleles in the patients group (table e-2, links.lww.com/NXI/A430).
Clinical Characteristics Per DRB1*07:01 Status
The non-DRB1*07:01 carriers were more frequently women (7/9, 78%; vs 17/63, 27% of DRB1*07:01 carriers; p = 0.005) and younger (median age 46 years, IQR 37–59; vs 66, IQR 58–74 for DRB1*07:01 carriers; p = 0.001). Only 3 patients with an associated cancer were analyzed for HLA genotyping, 2 of them (with a kidney and a hepatic cancer) were DRB1*07:01 carriers, whereas 1 with a malignant thymoma was not. There was no significant difference according to HLA status for most of the variables analyzed; however, noncarriers presented less frequently with psychiatric symptoms (2/9, 22%, vs 42/63, 67%; p = 0.001) and frontal syndrome (0/9, vs 32/63, 51%; p = 0.004; table 2).
Table 2.
Characteristics of DRB1*07:01 Carriers Compared With Noncarriers
IgG Isotyping in Serum and CSF
IgG isotypes were determined in 89 sera and 40 CSF; 38 samples were pairs (91 patients in total). Twenty sera (20/89, 23%) with negative CSF for LGI1-Abs could not be isotyped in CSF due to nondetectable Abs. HLA status was known for 68/91 (75%) patients, and 60/68 (88%) were DRB1*07:01 carriers.
The main serum isotypes detected were IgG1 (77/89, 87%) and IgG4 (72/89, 81%; figure 1B). However, IgG4 titers were significantly higher (median 1/1,280, IQR 1/1280–1/5,120) than IgG1 titers (median 1/640, IQR 1/320–1/1,280; p = 0.0001) in 59 double-positive samples analyzed (figure 1C). In the CSF, IgG4 (37/40, 93%) was the most frequent isotype, followed by IgG1 (24/40, 60%; figure 1D). When 21 double-positive CSF samples were analyzed, IgG4 titers were significantly higher (median 1/80, IQR 1/40–1/120) than those of IgG1 (median 1/40, IQR 1/15–1/80; p = 0.008; figure 1E).
There was no significant difference in serum isotypes or IgG1/4 titers according to CSF positivity nor in serum or CSF isotypes and IgG1/4 titers according to DRB1*07:01 carrier status; in addition, there was no significant difference in clinical and paraclinical features according to the presence/absence of IgG1/4 in serum/CSF (data not shown).
The validating Western blot performed showed high specificity for the anti-IgG1, anti-IgG3, and IgG4 used, whereas anti-IgG2 presented slight cross-reactivity with all other IgG isotypes (data not shown).
Treatment and Prognosis
A total of 130/134 (97%) patients received immunotherapy; among them, first-line treatments were used in all patients (130/130, 100%), whereas second-line treatments were used in 64/130 (49%). First-line treatments included corticosteroids (109/130, 84%), IVIG (110/130, 85%), and plasma exchange (8/130, 6%). Second-line treatments were rituximab (45/64, 70%) and cyclophosphamide (50/64, 78%). Chronic immunotherapy was used in only 19 patients; it was either azathioprine (5/19, 26%) or mycophenolate (14/19, 74%).
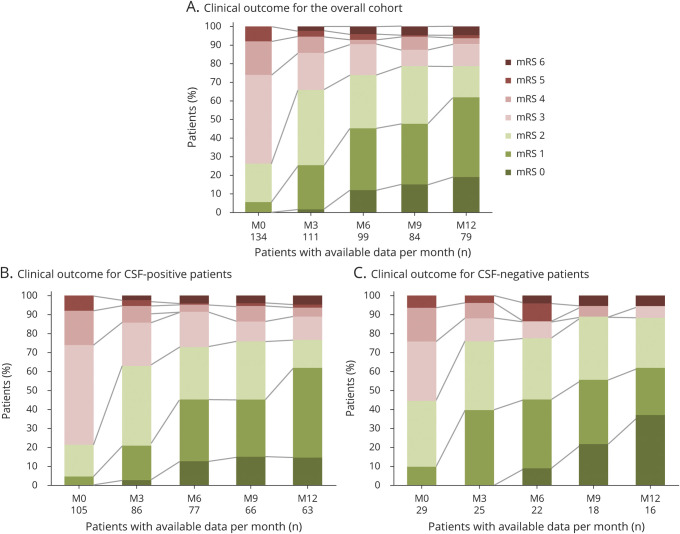
Median follow-up was 16.2 months (IQR 9–24 months) for the entire cohort, 18 months (IQR 9–36 months) for CSF-positive patients, and 12 months (IQR 9–18 months) for CSF-negative patients. At last follow-up, the median mRS score was 1 (IQR 1–2) for the total cohort or either of the 2 subgroups. How disability (mRS) evolved over the first 12 months in each subgroup and across the entire cohort is shown in figure 2. Among the 129 patients still alive at last follow-up, the most frequent dominant sequela was memory impairment (78/129, 61%), followed by psychiatric symptoms (9/129, 7%), other seizures (8/129, 6%), and FBDS (3/129, 2%); 31/129 (24%) patients did not report any sequela.
Figure 2. Evolution of Disability (mRS) With Long-term Follow-up.
Data shown only for the first 12 months of follow-up for the overall cohort (A; n = 134), and for patients with CSF-positive (B; n = 105), or CSF-negative (C; n = 29) for LGI1-Abs. Within every figure, abscissa axis indicates for each month the number of patients with available data, whereas ordinate axis represents the percentage of patients with a certain mRS score. LGI1-Abs = antibodies against leucine-rich glioma-inactivated 1 protein; M = month; mRS = modified Rankin Scale.
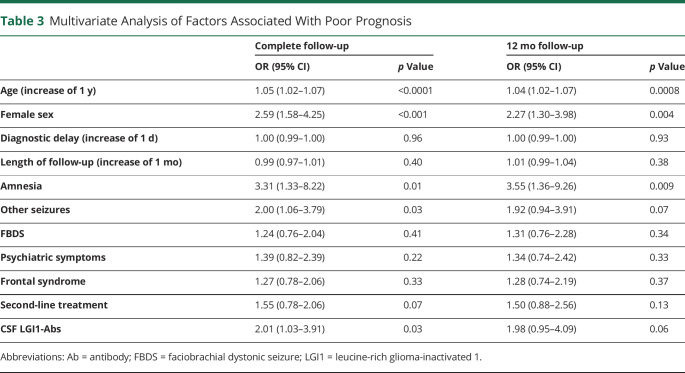
Univariate analysis only identified 3 factors associated with poor prognosis: increasing age (p = 0.03), higher mRS at onset (p < 0.001), and status epilepticus (p = 0.02; table e-3, links.lww.com/NXI/A430). Multivariate analysis was based on the variables described in the Methods section, although only second-line treatment was included as all treated patients received first-line medications. Multivariate analysis identified increasing age (for an increase of 1 additional year; OR = 1.05, 95% CI 1.02–1.07), female sex (OR = 2.59, 95% CI 1.58–4.25), amnesia (OR = 3.31, 95% CI 1.33–8.22), other seizures (OR = 2.00, 95% CI 1.06–3.79), and CSF-positivity (OR = 2.01, 95% CI 1.03–3.91) as factors associated with poor prognosis; when a 12-month cutoff was applied, only increasing age (for an increase of 1 additional year; OR = 1.04, 95% CI 1.02–1.07), female sex (OR = 2.27, 95% CI 1.30–3.98), and amnesia (OR = 3.55, 95% CI 1.36–9.26) remained (table 3).
Table 3.
Multivariate Analysis of Factors Associated With Poor Prognosis
Discussion
The absence of detectable Abs or of any overt inflammatory abnormalities in the CSF of some patients with anti-LGI1 LE has raised the question of how these cases could have significant CNS pathology. In addition, it was unclear whether these patients were phenotypically or immunologically different. Herein, we found no significant difference in clinical presentation between LGI1-Abs CSF-positive and -negative patients. Nevertheless, patients with detectable LGI1-Abs in the CSF had more frequently an inflammatory CSF, hyponatremia, and MRI abnormalities, suggesting a more intense immune response.
Immunologic differences found in patients with LE with and without LGI1-Abs in the CSF seem to be more quantitative than qualitative. Recently, LGI1-Abs producing B cells were found in the CSF from patients without LGI1-Abs or other abnormalities in the CSF, demonstrating that even in these patients there is an intrathecal synthesis, although it may be not strong enough to be detected by current techniques.17 In addition, we demonstrated herein that serum levels were not significantly different between patients with and without LGI1-Abs in the CSF, supporting the intrathecal origin of CSF LGI1-Abs and arguing against a simple passive transfer from the serum. This is further reinforced by the recent report of B-cell maturation occurring at least partially inside the CNS.18
Previous studies have reported that LGI1-Abs were mainly of IgG4 isotype.13,19–21 In the current series, we confirmed this predominance in both serum and CSF, but we also found that co-occurrence of other isotypes including IgG1, though at lower titers, is very common. The detection of IgG1, which, in contrast to IgG4, can activate the complement and therefore could produce irreversible structural damages, has been previously associated with poor prognosis and cognitive sequelae,20 although we and others did not find any association between isotypes and outcome.15,21 We have also shown that the serum isotypes or IgG1/4 titers did not differ significantly between LGI1-Abs CSF-positive and -negative LE, reinforcing the hypothesis of a similar autoimmune response in these 2 subgroups that is only distinguishable by the amount of LGI1-Abs intrathecally synthetized.
Neurologic autoimmune diseases mediated by IgG4-Abs are strongly associated with particular HLA class II haplotypes.22 The present study confirms previous reports,8,9,11 indicating that DRB1*07:01 is carried by nearly 90% of patients with LGI1-Abs LE. Of interest, we also found that DRB1*07:01 noncarriers were younger and more frequently women. Recently, a Chinese study reported lack of DRB1*07:01 association; however, more than 60% of their patients were women, and the median age was nearly 40 years,23 which could therefore support our results. A higher frequency of the unusual sex and atypical age at onset among the noncarriers of the associated HLA haplotype has been described in other autoimmune diseases, such as MS or ankylosing spondylitis.24,25 Despite sex bias on immune response being well described, most of our knowledge about this phenomenon is based on the immune stimulating effects of estrogens, explaining the female predominance in the majority of the autoimmune diseases.26 Conversely, much less is known about the influence of sex on HLA, although some evidence exists that estrogens may alter HLA expression27,28 and that sex also affects how HLA modifies T-cell receptor repertoires.29 Intriguingly, anti-CASPR2 (contactin-associated protein-like 2) LE is, similarly to anti-LGI1 LE, more common in elderly men and strongly associated with a particular HLA (DRB1*11:01).30 Thus, the interaction between sex and HLA is far from elucidated, especially the underlying mechanisms of sex bias in some HLA class II–associated diseases.
We also found herein that anti-LGI1 LE patients not carrying DRB1*07:01 presented less frequently with psychiatric and frontal symptoms, which has not been reported in previous studies8,9,11,15; while there was no association between HLA status and outcome, as it has been already described.11,15 In IgLON5 encephalitis, another IgG4-mediated CNS disorder tightly linked to HLA, clinical presentation, and predominant IgG subclasses were different according to DRB1*10:01 carrier status, likely reflecting an heterogeneous pathogenesis.31 Conversely, we demonstrated herein that CSF positivity and IgG isotypes did not significantly differ according to DRB1*07:01 carrier status in LGI1-Abs LE. Nevertheless, other immune effectors such as cytokines may vary depending on the HLA involved,32 explaining the differential clinical presentation. However, larger cohorts are necessary to further investigate the differential characteristics of patients according to their HLA.
Anti-LGI1 LE is usually a nonparaneoplastic disorder, although tumors, mainly malignant thymoma, have been described in few patients.2,3,13,14 Initially, a lack of HLA association in paraneoplastic cases was suggested9; however, this finding was not further confirmed in a more recent study, although it did not include malignant thymomas but other prevalent tumors, notably basal carcinoma.11 Herein, 3 patients with cancer were genotyped, and the one with malignant thymoma did not carry DRB1*07:01. We thus hypothesize that HLA may be useful in differentiating the truly paraneoplastic associations from others in anti-LGI1 LE. Furthermore, the strong HLA association in nonparaneoplastic cases and its absence in paraneoplastic ones might suggest different pathogenic pathways, as previously reported in Lambert-Eaton myasthenic syndrome33 and recently described in anti-CASPR2 diseases.30 Because of the scarcity of tumors in anti-LGI1 LE, international collaborative studies are needed to collect large enough series and confirm these results.
The overall outcome was initially described as favorable in anti-LGI1 LE,1–3,12 but subsequent studies that performed deeper evaluations found that at least three-quarters of cases have cognitive sequelae,3,34 close to the current cohort. Conversely, long-term epilepsy was uncommon herein, which has been already reported and likely reflects a better response to immunotherapy compared with memory disturbances.35 In addition, because of the heterogeneity and retrospective nature of most studies, several variables have been inconsistently associated with a poor outcome, such as the extension of MRI abnormalities,34,36 the development of hippocampal atrophy,14 the detection of IgG1-LGI1-Abs and higher LGI1-Abs serum titers,20 elevated IgG index and higher LGI1-Abs CSF titers,21 and CSF positivity for LGI1-Abs in the present series, all of them likely reflecting the intensity of the immune response against the brain leading to structural damages and permanent deficits. Herein, the worse outcome associated with increasing age is likely the result of a decrease in cognitive reserve34; whether older patients also develop a greater neuronal loss, measured through neurodegenerative biomarkers in CSF,37,38 has not been investigated yet. In addition, immune responses in women are usually stronger than those in men,26 which could explain the association between female sex and poor outcome that we found in the current study.
The benefit of first-line immunotherapy, mainly corticosteroids, is proven for FBDS and has been shown to prevent the development of cognitive dysfunction when used promptly.3,4,13,34,36,39 In contrast, the role of second-line treatments is less clear, as regimens are not uniform, studies are retrospective, and they are generally used in more severely affected patients.14,21,34 Herein, we did not find a positive effect of second-line treatment on long-term prognosis, and we could not analyze the role of first-line treatment as they were used in all patients. Recently, the first randomized trial with IVIG including a small sample of anti-LGI1 LE patients reported a significant response in seizure control and a trend toward a better cognitive outcome.15 Thus, further prospective studies with a comprehensive evaluation of cognitive dysfunction are needed to establish the appropriate treatment for anti-LGI1 LE.
The main limitations of the present study are its retrospective nature, the heterogeneity of the duration of follow-up, the adopted classification of pleocytosis, and the small sample size of the LGI1-Abs CSF-negative and DRB1*07:01 noncarrier groups; our results should therefore be confirmed in larger cohorts. Another limitation is the use of mRS for disability assessment, which is not the most accurate scale for cognitive evaluation, although it has been widely used even in other types of autoimmune encephalitis.40 It is also worth mentioning that higher sensitivity of CSF compared with serum has been also reported13; whether this finding represents a technical discrepancy (such as samples dilution or fixation processes) or a difference in the intrathecal synthesis has not been yet clarified. Furthermore, given this fact and the longer diagnostic delay observed herein for CSF-negative patients, it is highly recommended to send both kinds of samples to avoid underdiagnosis.
In conclusion, LGI1-Abs CSF-positive and -negative patients are clinically similar; while DRB1*07:01 status is associated with particular demographic and clinical characteristics. Potential therapeutic interventions should be investigated in large prospective cohorts as long-term outcome depends on a complex interaction of demographic and immunologic factors.
Acknowledgment
The authors thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples. They thank Philip Robinson for help in manuscript preparation (Direction de la Recherche Clinique, Hospices Civils de Lyon).
Glossary
- BSA
bovine serum albumin
- CBA
cell-based assay
- DMEM
Dulbecco's Modified Eagle Medium
- FBDS
faciobrachial dystonic seizure
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HLA
human leukocyte antigen
- ICU
intensive care unit
- IHF
immunohistofluorescence
- IQR
interquartile range
- IVIG
IV immunoglobulin
- LE
limbic encephalitis
- LGI1-Abs
antibodies against leucine-rich glioma-inactivated 1
- mRS
modified Rankin Scale
- NGS
normal goat serum
- OD
optic density
- PBS
phosphate-buffered saline
Appendix. Authors
Contributor Information
Sergio Muñiz-Castrillo, Email: ext-sergio.muniz@chu-lyon.fr.
Julie Haesebaert, Email: julie.haesebaert01@chu-lyon.fr.
Laure Thomas, Email: laure.thomas@chu-lyon.fr.
Alberto Vogrig, Email: alberto.vogrig@gmail.com.
Anne-Laurie Pinto, Email: anne-laurie.pinto@chu-lyon.fr.
Géraldine Picard, Email: geraldine.picard@chu-lyon.fr.
Charlotte Blanc, Email: blanc.charlotte@outlook.com.
Le-Duy Do, Email: le-duy.do@chu-lyon.fr.
Bastien Joubert, Email: bastien.joubert@chu-lyon.fr.
Giulia Berzero, Email: giulia.berzero01@universitadipavia.it.
Dimitri Psimaras, Email: dimitri.psimaras@aphp.fr.
Agusti Alentorn, Email: agusti.alentorn@aphp.fr.
Véronique Rogemond, Email: veronique.rogemond@chu-lyon.fr.
Valérie Dubois, Email: valerie.dubois@efs.sante.fr.
Aditya Ambati, Email: ambati@stanford.edu.
Ryad Tamouza, Email: tamouza.ryad@gmail.com.
Emmanuel Mignot, Email: mignot@stanford.edu.
Study Funding
This study is supported by FRM (Fondation pour la recherche médicale) DQ20170336751. This work has been developed within the BETPSY project, which is supported by a public grant overseen by the French National Research Agency (ANR), as part of the second “Investissements d'Avenir” program (reference ANR-18-RHUS-0012). S. Muñiz-Castrillo is supported by a research grant from Fundación Alfonso Martín Escudero (Spain).
Disclosure
The authors report no conflict of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 2016;87:1449–1456. [DOI] [PubMed] [Google Scholar]
- 4.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. [DOI] [PubMed] [Google Scholar]
- 5.Navarro V, Kas A, Apartis E, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 2016;139:1079–1093. [DOI] [PubMed] [Google Scholar]
- 6.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients: LGI1/CASPR2-IgG + Patients. Ann Neurol 2017;82:79–92. [DOI] [PubMed] [Google Scholar]
- 8.Kim T-J, Lee S-T, Moon J, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes: HLA Subtypes in Anti-LGI1 Encephalitis. Ann Neurol 2017;81:183–192. [DOI] [PubMed] [Google Scholar]
- 9.van Sonderen A, Roelen DL, Stoop JA, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4: anti-LGI1 Encephalitis. Ann Neurol 2017;81:193–198. [DOI] [PubMed] [Google Scholar]
- 10.Mueller SH, Färber A, Prüss H, et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis: GWAS Autoimmune Encephalitis. Ann Neurol 2018;83:863–869. [DOI] [PubMed] [Google Scholar]
- 11.Binks S, Varley J, Lee W, et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 2018;141:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celicanin M, Blaabjerg M, Maersk-Moller C, et al. Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies: a national cohort study. Eur J Neurol 2017;24:999–1005. [DOI] [PubMed] [Google Scholar]
- 13.Ariño H, Armangué T, Petit-Pedrol M, et al. Anti-LGI1–associated cognitive impairment: presentation and long-term outcome. Neurology 2016;87:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol 2017;74:50. [DOI] [PubMed] [Google Scholar]
- 15.Dubey D, Britton J, McKeon A, et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann Neurol 2020;87:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 17.Kornau H-C, Kreye J, Stumpf A, et al. Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol 2020;87:405–418. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann-Horn K, Irani SR, Wang S, et al. Intrathecal B-cell activation in LGI1 antibody encephalitis. Neurol Neuroimmunol Neuroinflamm 2020;7:e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 2012;72:241–255. [DOI] [PubMed] [Google Scholar]
- 20.Thompson J, Bi M, Murchison AG, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain J Neurol 2018;141:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadoth A, Zekeridou A, Klein CJ, et al. Elevated LGI1-IgG CSF index predicts worse neurological outcome. Ann Clin Transl Neurol 2018;5:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñiz-Castrillo S, Vogrig A, Honnorat J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Autoimmun Highlights 2020;11:2. 10.1186/s13317-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu F, Liu X, Zhang L, et al. Novel findings of HLA association with anti-LGI1 encephalitis: HLA-DRB1*03:01 and HLA-DQB1*02:01. J Neuroimmunol 2020;344:577243. 10.1016/j.jneuroim.2020.577243. [DOI] [PubMed] [Google Scholar]
- 24.Hensiek AE, Sawcer SJ, Feakes R, et al. HLA-DR 15 is associated with female sex and younger age at diagnosis in multiple sclerosis. J Neurol Neurosurg Psychiatry 2002;72:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Xu M, Pan X, et al. Epidemiological comparison of clinical manifestations according to HLA-B*27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens 2013;82:338–343. [DOI] [PubMed] [Google Scholar]
- 26.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 27.Kaur M, Schmeier S, MacPherson CR, et al. Prioritizing genes of potential relevance to diseases affected by sex hormones: an example of myasthenia gravis. BMC Genomics 2008;9:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taneja V. Sex hormones determine immune response. Front Immunol 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider-Hohendorf T, Görlich D, Savola P, et al. Sex bias in MHC I-associated shaping of the adaptive immune system. Proc Natl Acad Sci USA 2018;115:2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muñiz-Castrillo S, Joubert B, Elsensohn MH, et al. Anti-CASPR2 clinical phenotypes correlate with HLA and immunological features. J Neurol Neurosurg Psychiatry 2020;91:1076–1084. [DOI] [PubMed] [Google Scholar]
- 31.Gaig C, Ercilla G, Daura X, et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm 2019;6:e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Çebi M, Durmuş H, Yılmaz V, et al. Relation of HLA‐DRB1 to IgG4 autoantibody and cytokine production in muscle‐specific tyrosine kinase myasthenia gravis (MuSK‐MG). Clin Exp Immunol 2019;197:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirtz PW, Willcox N, van der Slik AR, et al. HLA and smoking in prediction and prognosis of small cell lung cancer in autoimmune Lambert–Eaton myasthenic syndrome. J Neuroimmunol 2005;159:230–237. [DOI] [PubMed] [Google Scholar]
- 34.Sola-Valls N, Ariño H, Escudero D, et al. Telemedicine assessment of long-term cognitive and functional status in anti-leucine-rich, glioma-inactivated 1 encephalitis. Neurol Neuroimmunol Neuroinflamm 2020;7:e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology 2019;92:e2185–e2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013;265:75–81. [DOI] [PubMed] [Google Scholar]
- 37.Constantinescu R, Krýsl D, Andrén K, et al. Cerebrospinal fluid markers of neuronal and glial cell damage in patients with autoimmune neurologic syndromes with and without underlying malignancies. J Neuroimmunol 2017;306:25–30. [DOI] [PubMed] [Google Scholar]
- 38.Körtvelyessy P, Prüss H, Thurner L, et al. Biomarkers of neurodegeneration in autoimmune-mediated encephalitis. Front Neurol 2018;9:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136:3151–3162. [DOI] [PubMed] [Google Scholar]
- 40.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article are available and will be shared by request from any qualified investigator.