Abstract
The present study was designed to determine the effects of dexmedetomidine on immune function, renal function and inflammatory factors in patients undergoing percutaneous nephrolithotomy under general anesthesia. A total of 177 patients with kidney calculi who underwent percutaneous nephrolithotomy in The Second Affiliated Hospital of Fujian Medical University were enrolled, in which 91 patients were treated with dexmedetomidine during surgery (research group) and 86 patients were not sedated during surgery (control group). The vital signs, renal function, inflammatory factors and immune function during surgery between the two groups were compared. Patients in the research group showed improved vital signs, renal function, inflammatory factors and immune function compared with the control group (P<0.05), and also experienced a significantly shorter hospitalization time (P<0.001). Therefore, the present results suggested that with a relatively high safety profile, use of dexmedetomidine for sedation can effectively protect renal and immune functions, and reduce the inflammatory response of patients during percutaneous nephrolithotomy. Thus, dexmedetomidine may have be potentially applied in clinical practice.
Keywords: dexmedetomidine, percutaneous nephrolithotomy, kidney calculi, diastolic blood pressure, CD4+
Introduction
Calculi in the urinary system are an extremely common form of calculus in human body, which seriously affects the health of the patient (1). The incidence rate of calculus in the urinary system is 1-5% (2), and the most common forms are kidney calculi (3). Calculi are caused by abnormal aggregation of crystalline substances such as calcium, oxalic acid and uric acid (4). Furthermore, kidney calculi are common amongst young and middle-aged men (5), and the incidence of kidney calculi is rapidly increasing (6). Kidney calculi, a very common lithiasis, are usually accompanied by varying degrees of flank soreness and pain, which seriously affect everyday life and mobility (7). Kidney calculi are caused by numerous factors, including age, sex, heredity, environment, diet and occupation, and internal body influencing factors including abnormal metabolism, denutrition and urinary tract infection (8,9). Currently, kidney calculi are primarily treated by surgery and with further development of medical technology, percutaneous nephrolithotomy has become the first choice for the treatment of kidney calculi (10).
Percutaneous nephrolithotomy is a minimally invasive surgery associated with small trauma, a fast recovery effect and few postoperative complications, and its application value has been proven clinically. However, there are some limitations to the use of percutaneous nephrolithotomy in clinical practice, such as damage of the renal function of patients (10,11). Percutaneous nephrolithotomy may increase the intrapelvic pressure of patients, resulting in urine reflux in the renal pelvis, thereby damaging the patients' renal function (11,12). Therefore, it is important to choose a narcotic analgesic drug that has the ability to protect renal function. Moreover, in order to effectively lower the degree of renal injury caused to patients during surgery, anesthetics with protective effects on renal function are required in clinical practice. Dexmedetomidine is a α2 adrenoceptor agonist that not only has a protective effect on kidney tissues, cells and functions, but also has significant anti-inflammatory effects (13). Currently, there are limited studies on the application of dexmedetomidine in percutaneous nephrolithotomy. In order to determine the application value of dexmedetomidine, the present study enrolled patients undergoing kidney calculi surgery in The Second Affiliated Hospital of Fujian Medical University. The present study investigated the effects of dexmedetomidine on the renal function, inflammatory response and immune function of patients undergoing percutaneous nephrolithotomy. Thus, the present results may provide an accurate and reliable reference for future clinical selection of anesthetics for percutaneous nephrolithotomy.
Materials and methods
Patient data
A total of 177 patients with renal calculi who underwent percutaneous nephrolithotomy in the second affiliated Hospital of Fujian Medical University (Quanzhou, China) from October 2016 to October 2019 were enrolled. Patient characteristics are shown in Table I. Overall, 91 patients were treated with dexmedetomidine during surgery (research group) and 86 patients were not sedated during surgery (control group). The present study has been approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University and all patients or their direct families signed an informed consent form.
Table I.
Comparison of patient data.
| Research group (n=91) | Control group (n=86) | t or c2 | P-value | |
|---|---|---|---|---|
| Age, year | 48.21±5.12 | 49.13±5.29 | 1.176 | 0.241 |
| BMI, kg/cm2 | 25.62±2.26 | 25.73±2.44 | 0.311 | 0.756 |
| Operation time, min | 124.24±8.62 | 125.36±9.08 | 0.842 | 0.401 |
| Intraoperative blood loss, ml | 50.62±8.62 | 51.12±8.76 | 0.383 | 0.702 |
| Sex | 0.337 | 0.561 | ||
| Male | 79 (86.81) | 72 (83.72) | ||
| Female | 12 (13.19) | 14 (16.28) | ||
| Residential environment | 0.724 | 0.395 | ||
| Urban area | 58 (63.74) | 60 (69.77) | ||
| Rural area | 33 (36.26) | 26 (30.23) | ||
| Smoking | 1.187 | 0.276 | ||
| Yes | 72 (79.12) | 62 (72.09) | ||
| No | 19 (20.88) | 24 (27.91) | ||
| Exercise habit | 0.619 | 0.431 | ||
| Yes | 12 (13.19) | 15 (17.44) | ||
| None | 79 (86.81) | 71 (82.56) | ||
| Nationality | 1.683 | 0.195 | ||
| Han nationality | 87 (95.60) | 85 (98.84) | ||
| Minority nationality | 4 (4.40) | 1 (1.16) | ||
| ASA classification | 0.455 | 0.500 | ||
| Grade I | 43 (47.25) | 45 (52.33) | ||
| Grade II | 48 (52.75) | 41 (47.67) | ||
| Calculi site | 0.060 | 0.806 | ||
| Left kidney | 47 (51.65) | 46 (53.49) | ||
| Right kidney | 44 (48.35) | 40 (46.51) |
Data are presented as the mean ± SD or n (%). ASA, American Society of Anesthesiologists; BMI, body mass index.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) Patients with clinical manifestations in line with those of kidney calculi (14); ii) patients diagnosed with kidney calculi based on a series of examinations at The Second Affiliated Hospital of Fujian Medical University; iii) patients in line with indications of percutaneous nephrolithotomy and who underwent surgery in this hospital; iv) patients in grade I-II of the American Society of Anesthesiologists (ASA) classification (15); v) patients with complete case information; and vi) patients who were willing to cooperate with the experiment. Exclusion criteria were as follows: i) Patients with severe urinary tract obstructive diseases during surgery; ii) patients treated with a replaced surgery plan; iii) patients with tumors, other cardio-cerebrovascular diseases, other autoimmune diseases or neurological disorders; iv) patients allergic to some drugs; v) patients with organ failure, or hepatic and kidney function obstacle; vi) patients with a long-term history of taking sedative drugs; and vii) patients transferred to another hospital.
Methods
All patients were treated by percutaneous nephrolithotomy by senior surgeons from The Second Affiliated Hospital of Fujian Medical University. The patients were injected intramuscularly with 0.1 g of phenobarbital and 0.5 mg of atropine before anesthesia. Patients were closely monitored in terms of vital signs after entering the operating room and peripheral venous access was established. After local anesthesia (intramuscular injection of 0.1 g of phenobarbital and 0.5 mg of atropine), radial artery cannulation was performed and invasive arterial blood pressure was monitored. Patients in the research group were injected intravenously with 1 µg/kg dexmedetomidine (Jiangsu Heng Rui Pharmaceutical Co., Ltd.; State Food and Drug Administration approval no. H20090248) for 10 min prior to anesthesia induction and were then injected intravenously with dexmedetomidine at 0.5 µg/kg/h (16) until the end of the surgery. The control group was given an equal amount of normal saline. Patients in the two groups were injected intravenously with etomidate (0.3 mg-kg), fentanyl (0.3 µg/kg), midazolam (0.1 mg/kg) and atracurium (0.15 mg-kg) for anesthesia induction, and oral intubation was used for mechanical ventilation. The tidal volume was set to 8-10 ml/kg and respiratory rate to 11-13 times/min. The patients in the two groups were injected intravenously with propofol at 3-5 mg/kg/min and remifentanil at 0.1-0.2 µg-kg-min for maintenance of anesthesia.
Observation indexes
Changes in vital signs, including diastolic blood pressure (DBP), systolic blood pressure (SBP), heart rate (HR) and blood oxygen saturation (SpO2), were recorded before surgery (T0), 5 min after surgery (T1), 30 min after surgery (T2) and at the end of surgery (T3). The anesthesia wake-up time of the patient, which is the time from the end time point of surgery to the time point when the patient fully restored mental activity and could make sample movements (17), was recorded. The assessment of state of the patient at 24 h after surgery was based on the visual analogue scale (VAS) and Ramsay sedation scale (18,19). Furthermore, the incidence of adverse reactions including nausea, vomiting, arrhythmia and hypotension were calculated as follows: The incidence of adverse reactions = the number of patients with adverse reaction/the total number of patients x 100%. Fasting venous blood (4 ml) was sampled from the patients before surgery, 1 day after surgery and 3 days after surgery, and divided into two parts. One part was centrifuged for 10 min (1,505 x g, 4˚C) to obtain serum. Blood samples were analyzed using an automatic biochemistry analyzer for renal function determination, including blood urea nitrogen (BUN), serum creatinine (Scr), retinol binding protein (RBP) and cystatin C (Cys-C). Then, ELISA kits were used to determine tumor necrosis factor-α (TNF-α; Xiamen Huijia Biotechnology Co., Ltd.; cat. no. orb-EHJ130636), interleukin-6 (IL-6; Shanghai Xin Yu Biotech Co., Ltd.; cat. no. Bsk00040) and IL-8 (Shanghai Xiyuan Biotechnology Co., Ltd.; cat. no. XY-70R-35386) in the blood samples. Flow cytometry (BD canto II; BD Biosciences; Becton, Dickinson and Company) was used to detect the T lymphocyte subsets of patients, including the ratios of CD3+, CD4+, CD8+, CD4+/CD8+ and natural killer (NK) cells in another part of the blood samples, and the supporting CytExpert analysis software of the instrument (CytExpert v2.0; Beckman Coulter, Inc.) was adopted for analysis (20). The hospitalization time and hospitalization expense were compared between the two experimental groups.
Statistical analysis
All assays in the present study were repeated three times. Data were analyzed using SPSS 24.0 statistical software (Beijing Strong-Vinda Information Technology Co., Ltd.). All graphs were drawn using GraphPad 8 (Shenzhen SOFTHEAD Software Technology Co., Ltd.). Enumeration data, such as sex and incidence of adverse reactions, were presented as the rate, and comparison between these groups was analyzed using a χ2 test. Measurement data, such as DBP and HR, were expressed as the mean ± SD, and compared between groups using unpaired Student's t-test. Comparison of data before and after treatment was carried out using paired Student's t-test. In addition, comparison in measurement data at different time points was analyzed using a repeated measures ANOVA and Bonferroni post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of patient data
There was no significant difference between the two groups in age, body mass index, operation time, intraoperative blood loss, sex, residential environment, smoking, exercise habits, nationality, ASA classification and calculi sites (Table I).
Comparison of vital signs
The two study groups showed no significant differences in SBP, DBP, HR and SpO2 at T0. It was demonstrated that the research group had lower SBP, DBP and HR compared with the control group at T1, T2 and T3 (P<0.05; Fig. 1). Furthermore, the two groups showed lower SBP, DBP and HR at T1 compared with T0 (P<0.05), and had lower SBP, DBP and HR at T2 compared with both T0 and T1 (P<0.05). In addition, at T3, the two groups had the lowest level of SBP (P<0.05), showed no difference in DBP compared with T2, had a higher HR compared with T1 and T2 and had a lower HR compared with T0 (P<0.001; Fig. 1).
Figure 1.
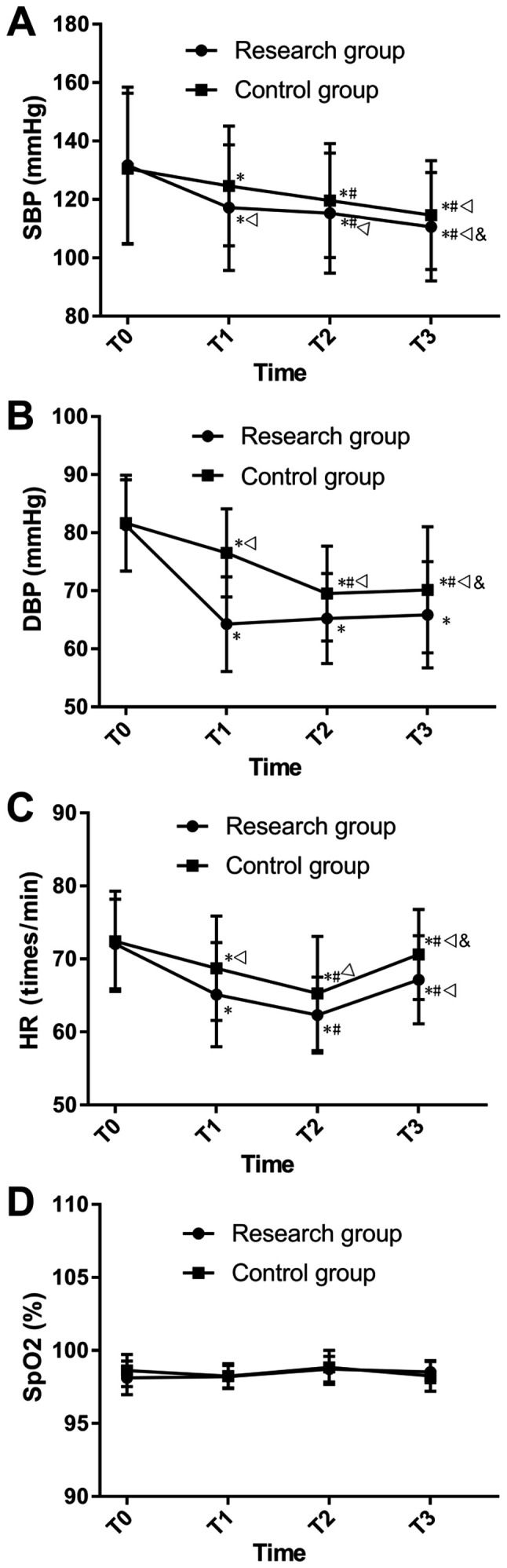
Comparison of vital signs. (A) Comparison of SBP. (B) Comparison of DBP. (C) Comparison of HR. (D) Comparison of SpO2. *P<0.05 vs. the same group at T0; #P<0.001 vs. the same group at T1; vP<0.001 vs. the same group at T2; &P<0.001 vs. the research group at the same time point. T0, before the surgery; T1, 5 min after the start of surgery; T2, 30 min after the start of surgery; T3, at the end of the surgery; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO2, blood oxygen saturation.
Comparisons of VAS score and Ramsay sedation scale score
The VAS score, Ramsay sedation scale score and anesthesia wake-up time of the research group were 0.57±0.26 points, 4.35±0.25 points and 216.22±16.27 min, respectively (Fig. 2). Moreover, those of the control group were 1.09±0.19 points, 2.42±0.32 points and 168.67±16.08 min, respectively. Thus, the present results suggested that the research group had a significantly lower VAS score and Ramsay sedation scale score compared with the control group (P<0.001; Fig. 2), and experienced a significantly longer anesthesia wake-up time compared with the control group (P<0.001).
Figure 2.
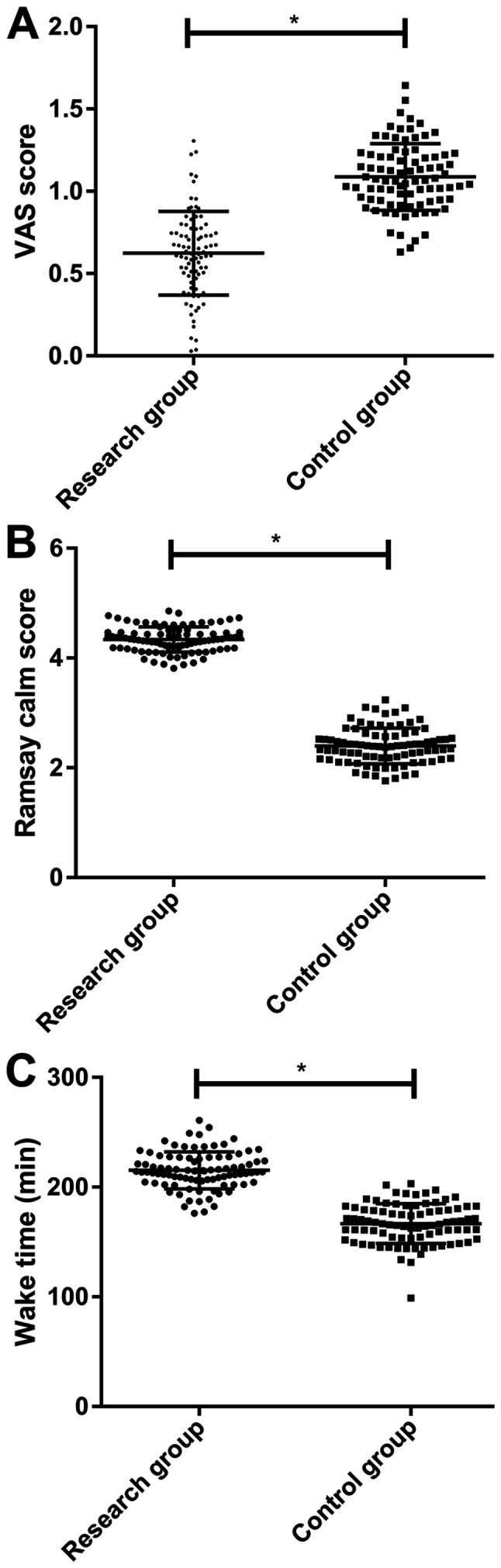
Comparison between the two groups in VAS score and Ramsay sedation scale score. (A) Comparison between the two groups in VAS score. (B) Comparison between the two groups in Ramsay sedation scale score. (C) Comparison between the two groups in anesthesia wake-up time. *P<0.001. VAS, visual analogue scale.
Comparison of adverse reactions
The research group showed an incidence of adverse reactions of 7.69%, with nausea in one patient (1.10%), vomiting in two patients (2.20%), hypotension in two patients (2.20%) and diarrhea in two patients (2.20%; Table II). The control group had an incidence of adverse reactions of 13.95%, with nausea in one patient (1.16%), vomiting in two patients (2.33%), arrhythmia in one patient (1.16%), hypotension in three patients (3.49%), diarrhea in three patients (3.49%) and coma in two patients (2.33%). The two groups showed no significant difference in incidence of adverse reactions (Table II).
Table II.
Comparison between the two groups in adverse reactions.
| Research group (n=91) | Control group (n=86) | c2 | P-value | |
|---|---|---|---|---|
| Nausea | 1 (1.10) | 1 (1.16) | 0.002 | 0.968 |
| Vomiting | 2 (2.20) | 2 (2.33) | 0.003 | 0.954 |
| Arrhythmia | 0 (0.00) | 1 (1.16) | 1.064 | 0.302 |
| Hypotension | 2 (2.20) | 3 (3.49) | 0.268 | 0.605 |
| Diarrhea | 2 (2.20) | 3 (3.49) | 0.268 | 0.605 |
| Coma | 0 (0.00) | 2 (2.33) | 2.140 | 0.144 |
| Incidence, % | 7.69 | 13.95 | 1.809 | 0.179 |
Data are presented as the n (%).
Comparison of renal function
The two groups showed no significant difference in BUN, RBP and Cys-C before surgery, and also showed no significant difference in Scr at the different time points. The research group showed a significantly lower level of BUN, RBP and Cys-C compared with the control group at days 1 and 3 after surgery (P<0.05; Fig. 3). Moreover, at days 1 and 3 after surgery, both groups showed no significant difference in Scr compare with levels before surgery, but showed significantly increased levels of BUN, RBP and Cys-C (P<0.05; Fig. 3). Furthermore, it was found that 3 days after surgery, both groups showed significantly lower levels BUN, RBP and Cys-C compared with levels 1 day after surgery (P<0.05; Fig. 3).
Figure 3.
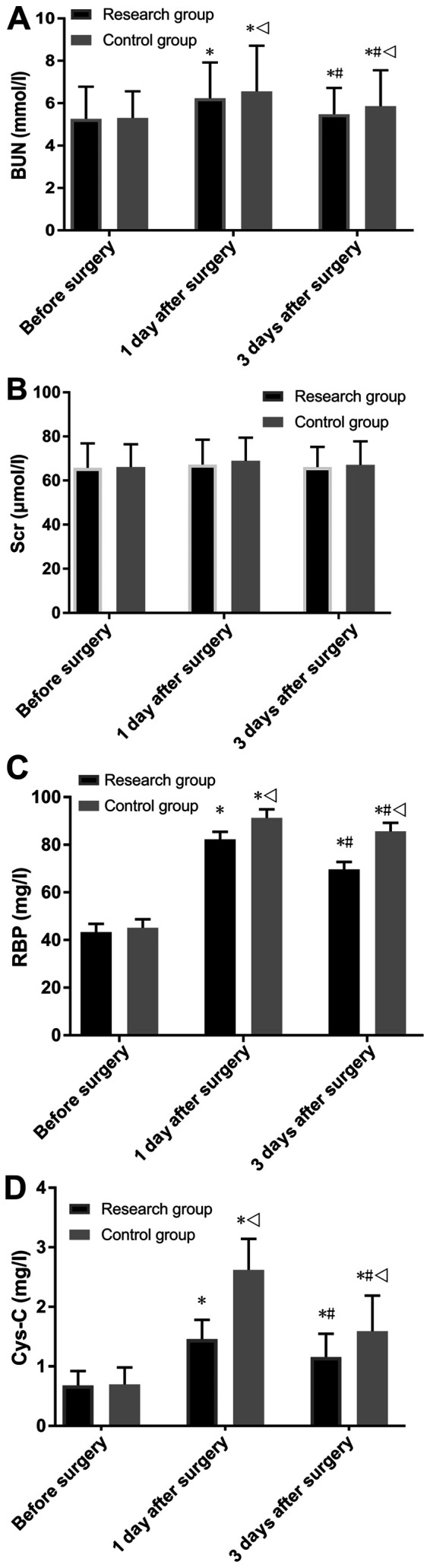
Comparison of renal function. (A) Comparison of BUN. (B) Comparison of Scr. (C) Comparison of RBP. (D) Comparison in Cys-C. *P<0.001 vs. the same group before surgery; #P<0.001 vs. the same group 1 day after surgery; vP<0.001 vs. the research group at the same time point. BUN, blood urea nitrogen; Scr, serum creatinine; RBP, retinol binding protein; Cys-C, cystatin C.
Comparison of inflammatory factors
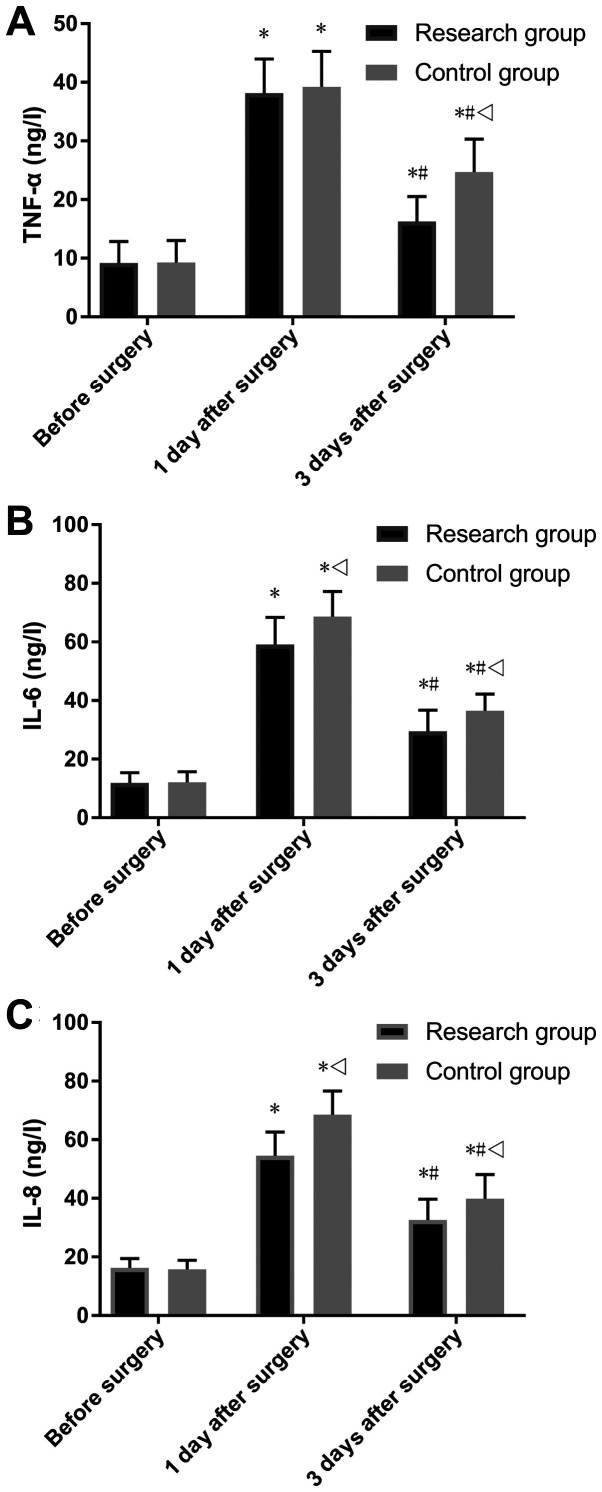
Both groups showed no significant difference in the levels of TNF-α, IL-6 and IL-8 before surgery (Fig. 4). It was found that the level of TNF-α was not significantly different in the research group compared with the control group. However, the research group was demonstrated to have significantly lower levels of IL-6 and IL-8 compared with the control group 1 day after surgery (P<0.05), and lower levels of TNF-α, IL-6 and IL-8 3 days after surgery (P<0.05). Moreover, both groups were found to have significantly increased levels of TNF-α, IL-6 and IL-8 1 day after surgery compared with T0 (P<0.05), and showed lower levels 3 days after surgery compared with levels 1 day after surgery (P<0.05; Fig. 4).
Figure 4.
Comparison of inflammatory factors. (A) Comparison of TNF-α. (B) Comparison of IL-6. (C) Comparison of IL-8. *P<0.05 vs. the group before surgery; #P<0.05 vs. the group 1 day after surgery; vP<0.05 vs. the research group at the same time point. TNF-α, tumor necrosis factor α; IL, interleukin.
Comparison of immune function
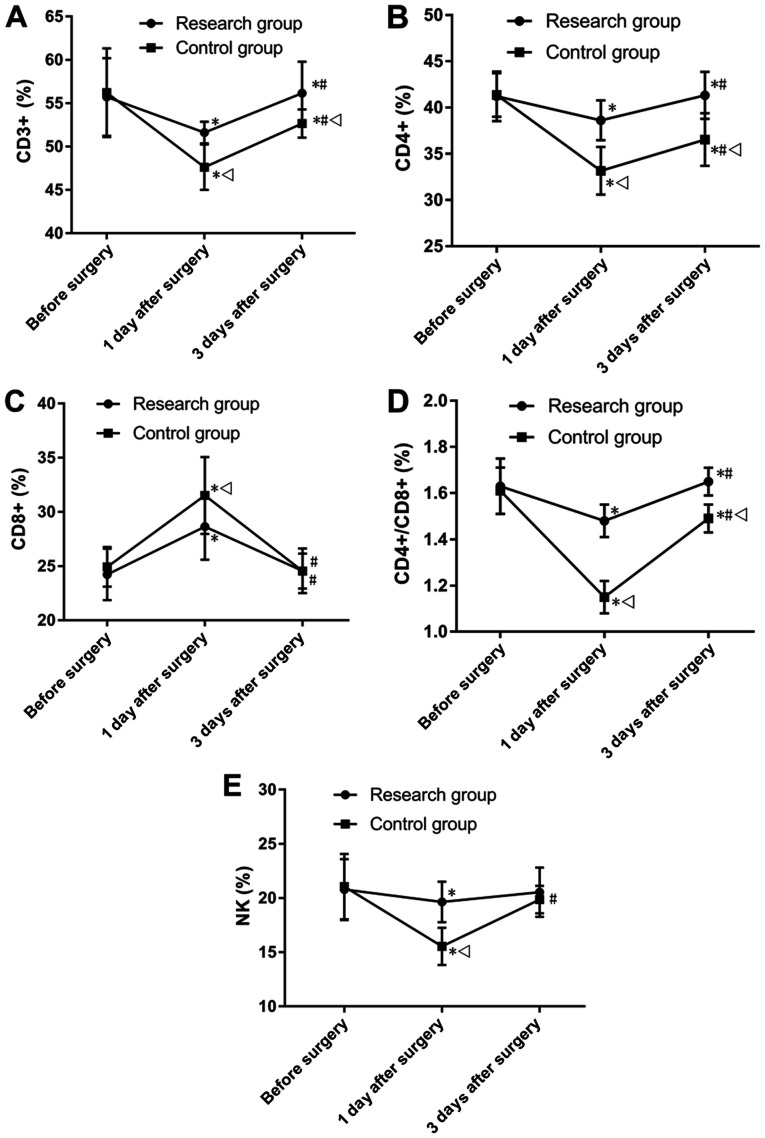
The present results suggested that the two groups showed no significant differences in the expression levels of CD3+, CD4+, CD8+, CD4+/CD8+ and NK. However, 1 day after surgery, the research group had significantly higher expression levels of CD3+, CD4+, CD4+-CD8+ and NK cells, and significantly lower levels of CD8+ compared with the control group (P<0.001; Fig. 5). Moreover, 3 days after surgery, no significant difference was found between the two groups for the expression levels of CD8+ and NK cells, however the research group had significantly higher levels of CD3+, CD4+ and CD4+/CD8+ compared with the control group (P<0.001; Fig. 5).
Figure 5.
Comparison of immune function. (A) Comparison of CD3+. (B) Comparison in CD4+. (C) Comparison in CD8+. (D) Comparison in CD4+/CD8+. (E) Comparison between the two groups in NK cells. *P<0.001 vs. the group before surgery; #P<0.001 vs. the group 1 day after surgery; vP<0.001 vs. the research group at the same time point. NK, natural killer.
Comparisons of hospitalization time and hospitalization expense
The hospitalization time of the research group was significantly shorter compared with the control group (3.62±1.20 and 5.24±2.26 days, respectively; P<0.001; Fig. 6). Furthermore, the present results suggested that there was no significant difference between the research group and control group in hospitalization expense (4563.62±315.64 and 4602.56±275.64 CNY, respectively).
Figure 6.
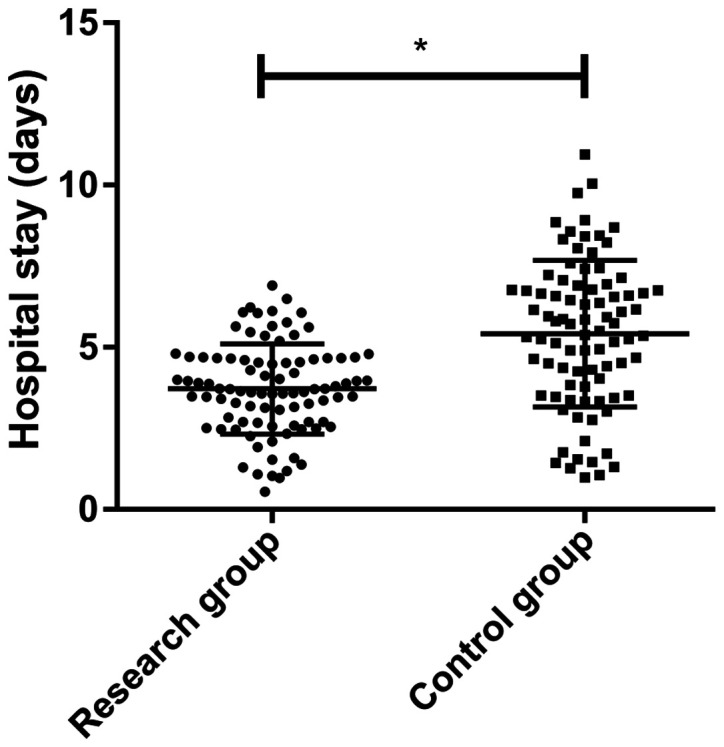
Comparison of the hospitalization time between the two groups. *P<0.001.
Discussion
Percutaneous nephrolithotomy is a minimally invasive surgery associated with a small amount of intraoperative blood loss and fast recovery. However, during percutaneous nephrolithotomy, high pressure pouring is required, which can increase renal pelvic pressure in patients and lead to urine countercurrent in renal pelvis (21). Therefore, the correct anesthetic drug for percutaneous nephrolithotomy is needed to avoid renal damage. In order reduce the damage on renal function of patients with kidney calculi during operation, several studies have investigated and confirmed the role of different anesthetics in protecting renal function (22,23).
Dexmedetomidine, an imidazole derivative, has a very high selective binding with α2 adrenergic receptor and acts as a α2 adrenergic agonist, with high efficiency and high selectivity. The binding products of dexmedetomidine not only inhibit the sympathetic nervous system, but also can reduce the release of norepinephrine, which helps provide protection to the kidneys (24). Ammar et al (25) demonstrated that dexmedetomidine has a protective effect on kidney function in cardiac surgery.
The present results suggested that the vital sign parameters of the research group were lower compared with the control group after anesthesia, suggesting that dexmedetomidine may effectively reduce the fluctuation of blood flow parameters during the perioperative period of percutaneous nephrolithotomy. Furthermore, it was found that SpO2 was not significantly different between the two groups at different time points, suggesting that dexmedetomidine did not inhibit the respiratory function and blood circulation of patients. The present results were consistent with previous results from Liu et al (26), which demonstrated the effects of dexmedetomidine on the microcirculation of patients during cardiac surgery. Moreover, it was found that the VAS and Ramsay sedation scale scores of the research group were different from those of the control group after surgery, suggesting that dexmedetomidine may be an effective sedative and analgesic agent for patients during the perioperative period. The research group experienced longer anesthesia wake-up time compared with the control group, which may be due to the fact that dexmedetomidine plays a synergistic role in enhancing the intensity of local anesthetic drugs and prolongs the action time of drugs (27). In addition, patients in the control group relied on intravenous injection for maintenance of intraoperative anesthesia, as this group did not receive dexmedetomidine anesthesia and intravenous anesthesia was stopped when the surgery ended.
A previous study has confirmed that conventional anesthesia is not as effective as dexmedetomidine in surgical operations (28). The present results suggested that there were no significant differences between the two groups for the incidence of adverse reactions, suggesting that dexmedetomidine had a relatively high safety profile in percutaneous nephrolithotomy. However, Jessen Lundorf et al (29) found that patients treated with dexmedetomidine for analgesia were more likely to have hypotension, while the incidence of hypotension in the present study was only 2.20% (two patients). This inconsistency may be because the anesthetics used by Jessen Lundorf et al (29) and the present study were not identical, so the difference may be related to a specific reaction from dexmedetomidine combined with other anesthetics, which increases the likelihood of hypotension in patients. Furthermore, the difference in results may be because the surgery performed by Jessen Lundorf et al (29) was an abdominal surgery with relatively large trauma, while percutaneous nephrolithotomy is a minimally invasive surgery with a small amount of intraoperative blood loss. The hemodynamics of patients undergoing percutaneous nephrolithotomy can be maintained at a relatively stable state, so the possibility of decreased blood pressure was low. However, further studies are required to investigate the safety of dexmedetomidine for percutaneous nephrolithotomy.
The renal function, inflammatory factors, and immune function were compared between the two groups, and the results indicated that that the renal function and immune function of the research group were both improved compared with the control group, while the levels of inflammatory factors in the research group were lower compared with the control group. As routine conventional indexes of renal injury, BUN and Scr levels were not significantly different in the present study, which may be due to the fact that the renal injury caused by percutaneous nephrolithotomy was small. It has been previously reported that BUN and Scr show a significant increase when the renal injury reaches a relatively severe state (30). Thus, further studies are required to investigate this effect. As a good index of early renal injury, Cys-C can be completely reabsorbed by the proximal convoluted tubule after being filtered by the renal tubular (31), and RBP is a sensitive index for the degree of injury of the proximal convoluted tubule (32). In the present study, Cys-C and RBP levels were increased after surgery in the two groups, while those of the research group were lower compared with the control group, which suggested the protective effect of dexmedetomidine on renal function.
Due to limited experimental conditions, the present study was not able to investigate the protective mechanism of dexmedetomidine on renal function, which is a limitation. The present study hypothesized that the protection mechanism of dexmedetomidine may be as follows: Dexmedetomidine mainly acted in the sympatho-adrenomedullary system and did not bind to cytokines in peripheral blood to increase the concentration of catecholamine (33), which caused dilatation of small arteries in the renal tubular and relieved the pressure in renal capsule to maintain a relatively stable state of renal function. The present results suggested that the research group had lower levels of TNF-α, IL-6 and IL-8 compared with the control group, suggesting that dexmedetomidine also had a beneficial anti-inflammatory effect. Previous results by Luo et al (34) and Li and Liu (35) are in line with results from the present study. Therefore, the protective effect of dexmedetomidine on renal function may also be related to its anti-inflammatory effect. Thus, dexmedetomidine may protect the kidney by reducing the inflammatory response in the body and lowering the possibility of oxidative stress in patients.
The present study also compared T lymphocyte subsets in the two groups and found that all patients showed decreased levels of CD3+, CD4+, CD4+/CD8+ and NK and increased CD8+, but that these cells gradually recovered 3 days after surgery. A previous study has shown that anesthetics cause changes in the immune function of patients at any duration of administration (36), which was also verified by the results of the present study. It was also demonstrated that the immune function of the patient was inhibited after surgery and anesthesia, but returned to the original level with the recovery of the patient. However, the research group had increased changes in immune function compared with the control, which suggested that dexmedetomidine also had a protective effect on the immune function. At present, there are few studies on the effects of dexmedetomidine on human immune function and the underlying mechanisms are not fully understood. Based on the present results, dexmedetomidine may reduce the immunosuppressive stress response on body by inhibiting sympathetic activation, or may reduce immunosuppression caused by the inflammatory response by downregulating inflammatory factors. Moreover, a previous study has shown that opiate drugs, such as propofol, have a more significant inhibitory effect on immune function compared with other opiate drugs (37). Furthermore, Chen et al (38) revealed that dexmedetomidine can inhibit the maturation and function of dendritic cells of human cord blood by impacting the synthesis of IL-12 and IL-23, and also has a regulatory effect on the normal operation of the immune function. Thus, this may be why the research group showed higher levels of CD3+ and CD4+, and lower levels of CD8+ compared with the control group in the present study. However, due to the absence of in vitro experiments, it is difficult to determine the underlying mechanisms, thus additional studies are required. The present results suggested that the research group had a significantly improved rehabilitation process compared with the control group and that there was no difference in the hospitalization expense between the two groups, which indicated that it may be feasible to use dexmedetomidine in clinical practice.
The present study had some limitations. Experiments were not performed to determine the mechanism of dexmedetomidine on inflammatory indexes. In addition, due to the limited number of patients, homogenous ethnicity and small age range of patients, it is not possible to determine if the difference in performance of dexmedetomidine may be caused by changes in body functions. Therefore, further experiments are required, with in-depth analysis and discussion of the application of dexmedetomidine in percutaneous nephrolithotomy.
In conclusion, with relatively high safety profile, dexmedetomidine for sedation can effectively protect renal and immune functions and reduce the inflammatory response of patients during percutaneous nephrolithotomy, and thus may be used for application in clinical practice.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YB conceived the study and wrote the manuscript. HH was responsible for the ELISA. PZ analyzed and interpreted the patient data. WL and LH helped with statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University. Patients who participated in this research, signed the informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Alatab S, Pourmand G, El Howairis Mel F, Buchholz N, Najafi I, Pourmand MR, Mashhadi R, Pourmand N. National profiles of urinary calculi: A comparison between developing and developed worlds. Iran J Kidney Dis. 2016;10:51–61. [PubMed] [Google Scholar]
- 2.Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, Pace KT, Pais VM Jr, Pearle MS, Preminger GM, et al. Surgical management of stones:american urological association/endourological society guideline, Part I. J Urol. 2016;196:1153–1160. doi: 10.1016/j.juro.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 4.Daudon M, Jungers P, Bazin D, Williams JC Jr. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis. 2018;46:459–470. doi: 10.1007/s00240-018-1043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, Traxer O, Tiselius HG. Kidney stones. Nat Rev Dis Primers. 2016;2(16008) doi: 10.1038/nrdp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero V, Akpinar H, Assimos DG. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 7.Heers H, Turney BW. Trends in urological stone disease: A 5-year update of hospital episode statistics. BJU Int. 2016;118:785–789. doi: 10.1111/bju.13520. [DOI] [PubMed] [Google Scholar]
- 8.Sofia NH, Walter TM. Prevalence and risk factors of kidney stone. GJRA. 2016;5:183–187. [Google Scholar]
- 9.Abeywickarama B, Ralapanawa U, Chandrajith R. Geoenvironmental factors related to high incidence of human urinary calculi (kidney stones) in Central Highlands of Sri Lanka. Environ Geochem Health. 2016;38:1203–1214. doi: 10.1007/s10653-015-9785-x. [DOI] [PubMed] [Google Scholar]
- 10.Ghani KR, Andonian S, Bultitude M, Desai M, Giusti G, Okhunov Z, Preminger GM, de la Rosette J. Preminger GMand de la Rosette J: Percutaneous nephrolithotomy:update, trends, and future directions. Eur Urol. 2016;70:382–396. doi: 10.1016/j.eururo.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Gorbachinsky I, Wood K, Colaco M, Hemal S, Mettu J, Mirzazadeh M, Assimos DG, Gutierrez-Aćeves J. Evaluation of renal function after percutaneous nephrolithotomy-does the number of percutaneous access tracts matter? J Urol. 2016;196:131–136. doi: 10.1016/j.juro.2016.01.121. [DOI] [PubMed] [Google Scholar]
- 12.Daggülli M, Utangaç MM, Dede O, Bodakci MN, Hatipoglu NK, Penbegül N, Sancaktutar AA, Bozkurt Y, Söylemez H. Potential biomarkers for the early detection of acute kidney injury after percutaneous nephrolithotripsy. Ren Fail. 2016;38:151–156. doi: 10.3109/0886022X.2015.1073494. [DOI] [PubMed] [Google Scholar]
- 13.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, Palsson R. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol. 2013;28:1923–1942. doi: 10.1007/s00467-012-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle DJ, Goyal A, Bansal P, Garmon EH. American Society of Anesthesiologists Classification. 2020 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 16.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–652. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 17.Lai HC, Chan SM, Lu CH, Wong CS, Cherng CH, Wu ZF. Planning for operating room efficiency and faster anesthesia wake-up time in open major upper abdominal surgery. Medicine (Baltimore) 2017;96(e6148) doi: 10.1097/MD.0000000000006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline ME, Herman J, Shaw ER, Morton RD. Standardization of the visual analogue scale. Nurs Res. 1992;41:378–380. [PubMed] [Google Scholar]
- 19.Dawson R, von Fintel N, Nairn S. Sedation assessment using the Ramsay scale. Emerg Nurse. 2010;18:18–20. doi: 10.7748/en2010.06.18.3.18.c7825. [DOI] [PubMed] [Google Scholar]
- 20.Bhowmick D, Sheridan RTC, Bushnell TP, Spalding KL. Practical Guidelines for Optimization and Characterization of the Beckman Coulter CytoFLEX™ Platform. Cytometry A. 2020;97(8):800–810. doi: 10.1002/cyto.a.23998. [DOI] [PubMed] [Google Scholar]
- 21.Ozayar E, Gulec H, Bayraktaroglu M, Tutal ZB, Kurtay A, Babayigit M, Ozayar A, Horasanli E. Comparison of retrograde intrarenal surgery and percutaneous nephrolithotomy: From the view of an anesthesiologist. J Endourol. 2016;30:184–188. doi: 10.1089/end.2015.0517. [DOI] [PubMed] [Google Scholar]
- 22.Motayagheni N, Phan S, Eshraghi C, Nozari A, Atala A. A review of anesthetic effects on renal function: Potential organ protection. Am J Nephrol. 2017;46:380–389. doi: 10.1159/000482014. [DOI] [PubMed] [Google Scholar]
- 23.Kim N, Lee JG, Lee S, Nam KS, Shu JW, Paik HC, Yoo YC. A comparison of propofol based total intravenous anesthesia and sevoflurane based balanced anesthesia on renal protection during lung transplantation under extracorporeal membrane oxygenation-A prospective, randomized trial. J Heart Lung Transplant. 2017;36:S116–S117. [Google Scholar]
- 24.Palomino J, Echavarria R, Franco-Acevedo A, Moreno-Carranza B, Melo Z. Opioids preconditioning upon renal function and ischemia-reperfusion injury: A Narrative Review. Medicina (Kaunas) 2019;55(522) doi: 10.3390/medicina55090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammar AS, Mahmoud KM, Kasemy ZA, Helwa MA. Cardiac and renal protective effects of dexmedetomidine in cardiac surgeries: A randomized controlled trial. Saudi J Anaesth. 2016;10:395–401. doi: 10.4103/1658-354X.177340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Zhang K, Wang W, Xie G, Cheng B, Wang Y, Hu Y, Fang X. Dexmedetomidine versus propofol sedation improves sublingual microcirculation after cardiac surgery: A randomized controlled trial. J Cardiothorac Vasc Anesth. 2016;30:1509–1515. doi: 10.1053/j.jvca.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Xu W, Ning C, Li M, Zhao G, Jiang W, Ding J, Chen X. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials. 2018;181:378–391. doi: 10.1016/j.biomaterials.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Niu J, Li Z, Lv H, Cai H. The efficacy and safety of dexmedetomidine in cardiac surgery patients: A systematic review and meta-analysis. PLoS One. 2018;13(e0202620) doi: 10.1371/journal.pone.0202620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessen Lundorf L, Korvenius Nedergaard H, Møller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev. 2016;2(CD010358) doi: 10.1002/14651858.CD010358.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan WW, Waltman Johnson K, Friedman HS, Navaratnam P. Association between cardiac, renal, and hepatic biomarkers and outcomes in patients with acute heart failure. Hosp Pract 1995. 2016;44:138–145. doi: 10.1080/21548331.2016.1197760. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Li Y, Yang X, Shan H, Zhang Q, Ming Z, Xie Y, Chen H, Liu Y, Zhang J. Serum cystatin C as an inflammatory marker in exacerbated and convalescent COPD patients. Inflammation. 2016;39:625–631. doi: 10.1007/s10753-015-0287-x. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016;89:58–67. doi: 10.1016/j.kint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Sichrovsky TC, Mittal S, Steinberg JS. Dexmedetomidine sedation leading to refractory cardiogenic shock. Anesth Analg. 2008;106:1784–1786. doi: 10.1213/ane.0b013e318172fafc. [DOI] [PubMed] [Google Scholar]
- 34.Luo X, Zheng X, Huang H. Protective effects of dexmedetomidine on brain function of glioma patients undergoing craniotomy resection and its underlying mechanism. Clin Neurol Neurosurg. 2016;146:105–108. doi: 10.1016/j.clineuro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Liu S. The effect of dexmedetomidine on oxidative stress response following cerebral ischemia-reperfusion in rats and the expression of intracellular adhesion molecule-1 (ICAM-1) and S100B. Med Sci Monit. 2017;23:867–873. doi: 10.12659/msm.899855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q, Shi NJ, Zhang H, Zhu YM. Effects of combined general-epidural anesthesia and total intravenous anesthesia on cellular immunity and prognosis in patients with non-small cell lung cancer: A comparative study. Mol Med Rep. 2017;16:4445–4454. doi: 10.3892/mmr.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Wu L, Zhang M, Zhao L. Effects of general anesthesia with combined epidural anesthesia on inflammatory response in patients with early-stage gastric cancer undergoing tumor resection. Exp Ther Med. 2019;17:35–40. doi: 10.3892/etm.2018.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Le Y, Zhou L, Gong L, Li X, Li Y, Liao Q, Duan K, Tong J, Ouyang W. Dexmedetomidine inhibits maturation and function of human cord blood-derived dendritic cells by interfering with synthesis and secretion of IL-12 and IL-23. PLoS One. 2016;11(e0153288) doi: 10.1371/journal.pone.0153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.