Why were the cohorts set up?
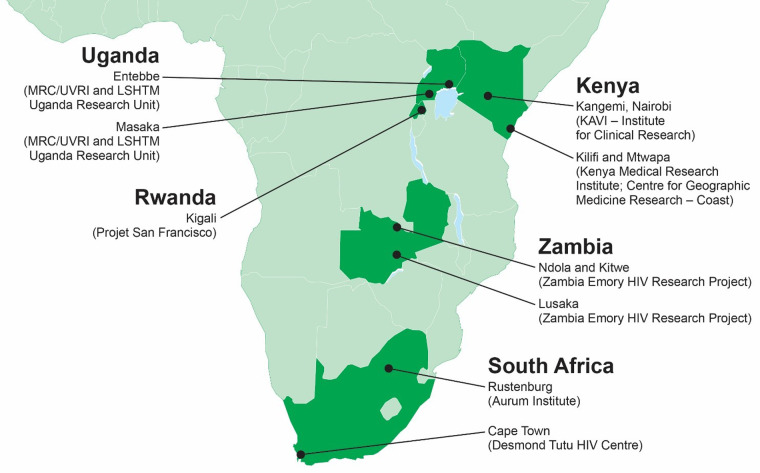
In 2003, IAVI (formerly the International AIDS Vaccine Initiative) identified several gaps in HIV epidemiology and vaccine research. IAVI launched cohort studies to better understand at-risk populations, their suitability for clinical trial participation and their unmet needs for preventive services and products. Our goals included (i) improving our understanding of HIV incidence and volunteer retention among ‘key populations’ of at-risk persons suitable for participation in large-scale HIV prevention trials; (ii) identifying and addressing unmet needs for health care, counselling and prevention among these key populations; (iii) understanding host–virus interactions both shortly after virus acquisition and longer term; (iv) generating data and reagents from recently transmitted HIV to support new vaccine product discovery; and (v) understanding clinical outcomes of HIV disease in the African context to define clinical trial endpoints where antiretroviral therapy (ART) was not (yet) widely available, while building the clinical, laboratory and quality systems to support future trials. To this end, starting in 2004, IAVI established partnerships with nine experienced clinical research centres in east and southern Africa to enrol suitable volunteers (Figure 1). This manuscript includes data from two broad protocols that followed persons at risk of HIV acquisition (1) ‘A prospective, open cohort, observational feasibility study to determine HIV incidence in preparation for future preventive HIV vaccine clinical trials’ (IAVI Protocol B) and (2) ‘Heterosexual transmission of HIV in Africa’ [Emory Heterosexual Transmission (HT) study]. Between 2005 and 2009, varying by research centre, through to December 2011, they served as the source populations for a third cohort study on the natural history of HIV infection, ‘A prospective, observational, multi-center study to evaluate laboratory, clinical, immunologic and viral markers of disease progression in recently HIV-infected volunteers’ (IAVI Protocol C). Table 1 summarizes the start and stop dates for each site and each cohort. As part of participating in each respective cohort study, clinical and laboratory teams were trained in good clinical practices and good clinical laboratory practices, laboratories were accredited, and all assays were standardized and conducted under an external quality control programme.1 Study teams met annually, typically in Africa, to share experiences and results and for additional training.
Figure 1.
Participating clinical research centres.
Table 1.
Overview of HIV epidemiology cohorts (Protocol B and Emory HT Study) and enrolment into the Early HIV Infection Cohort (Protocol C)
| Volunteers identified with incident HIV infection |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recruitment city | Country | Protocol | Study population/ descriptiona | Cohort size, nb | Earliest EDI | C Enrol stop date | Total, n | Enrolled into C, n | % | Others enrolled into Cc | Total enrolled into C, n |
| Masaka | Uganda | B | Rural community based | 1029 | 5 Sep 2005 | 30 Apr 2007 | 12 | 10 | 83.3 | 0 | 10 |
| Masaka | Uganda | B | DC | 1119 | 29 Jul 2006 | 31 Dec 2011 | 83 | 77 | 92.8 | 10 | 87 |
| Entebbe | Uganda | B | DC | 598 | 22 Feb 2006 | 17 Dec 2009 | 16 | 15 | 93.8 | 31 | 46 |
| Kigali | Rwanda | HT | DC | 1667 | 18 Feb 2005 | 15 Dec 2011 | 94 | 94 | 100.0 | 0 | 94 |
| Lusaka | Zambia | HT | DC | 1541 | 28 Jun 2005 | 15 Dec 2011 | 184 | 151 | 82.1 | 0 | 151 |
| Ndola | Zambia | HT | DC | 673 | 13 Oct 2005 | 15 Dec 2011 | 71 | 61 | 85.9 | 0 | 61 |
| Kitwe | Zambia | HT | DC | 243 | 14 Nov 2005 | 1 Dec 2008 | 25 | 22 | 88.0 | 0 | 22 |
| Rustenburg | South Africa | B | At-risk men and women | 599 | 2 May 2009 | 31 Dec 2011 | 22 | 21 | 95.5 | 1 | 22 |
| Cape Town | South Africa | B | At-risk younger men and women | 498 | 27 Oct 2006 | 27 Mar 2007 | 8 | 7 | 87.5 | 0 | 7 |
| Kilifi | Kenya | B | MSM | 508 | 15 Nov 2005 | 31 Dec 2011 | 77 | 67 | 87.0 | 1 | 68 |
| Kilifi | Kenya | B | Heterosexual men | 231 | 18 Sep 2006 | 31 Dec 2011 | 7 | 5 | 71.4 | 0 | 5 |
| Kilifi | Kenya | B | At-risk women (FSW, other) | 335 | 21 Mar 2006 | 31 Dec 2011 | 16 | 13 | 81.3 | 2 | 15 |
| Kangemi | Kenya | B | MSM | 301 | 9 Feb 2006 | 15 Sep 2010 | 19 | 17 | 89.5 | 4 | 21 |
| Kangemi | Kenya | B | Heterosexual men | 416 | 22 Jun 2009 | 15 Sep 2010 | 2 | 2 | 100.0 | 1 | 3 |
| Kangemi | Kenya | B | At-risk women (FSW, other) | 617 | 18 Sep 2006 | 15 Sep 2010 | 4 | 1 | 25.0 | 0 | 1 |
| Total: | 10 375 | 640 | 563 | 88.0 | 50 | 613 | |||||
DC, HIV discordant couples cohort; MSM, men who report sex with men; heterosexual men, men who report sex with women only; FSW, female sex workers.
C source population: volunteers who contributed at least one follow-up visit while recruitment for Protocol C was active.
Includes volunteers identified during serial voluntary counselling and testing visits (n = 31), prenatal visits (n = 1), discordant antibody test results (n = 7), participant in another epidemiology study with follow-up (n = 2) and p24-positive at screening for cohort study participation (n = 8).
Who is in the cohorts?
To prepare for HIV vaccine efficacy trials, each team began outreach to and recruitment of populations suitable for large-scale prevention trials. Enrolment criteria varied by research centre and cohort, but generally included screening adults (typically 18–49 years old) for risk behaviour associated with an increased risk of HIV acquisition. Protocol B maintained a diverse range of higher-risk study volunteers (see below) whereas the HT study focused primarily on heterosexual transmission risk in stable, HIV-discordant couples (Table 1).
Starting in late 2004, the team in Masaka began recruiting volunteers in rural villages where previous research studies had found a higher HIV prevalence than the Ugandan national average.2 Annual HIV incidence was found to be low (1%), and the study team began to recruit the HIV-uninfected partner of HIV-discordant couples in 2006; this remained the source population for study volunteers at this recruitment centre for the duration of this study.3 Discordant-couple recruitment expanded to Entebbe in 2006.4 Also in 2004, IAVI began the support and expansion of discordant couple cohorts in Rwanda and Zambia, recruited through voluntary counselling and testing (VCT) sessions for couples.5–7 In Kilifi, recruitment began in 2005 with walk-in VCT attendees, then expanded to female sex workers and their clients. As the research centre became recognized as a site for non-judgmental care of sex workers, male sex workers began to attend outreach sessions and asked to be considered for enrolment; thus started the first prospective HIV epidemiology study among men who have sex with men (MSM) in Africa.8,9 In Nairobi, enrolment began in 2005 and volunteers included female sex workers, their clients, and MSM.8 In Cape Town, enrolment began in 2006, and because of high regional HIV prevalence and the generalized nature of the epidemic, recruitment included all persons reporting for VCT who reported any sexual activity; the Cape Town team was the only one to include adolescents, with an enrolment age range of 16–40 years old.8 Rustenburg initiated Protocol B in 2009, also focusing on men and women with more relaxed ‘risk’ criteria (any sexual activity). Many of these cohorts were active before recruitment for the Early HIV Infection Cohort began and continued after enrolment for that study ended. Additional details and a wider perspective on this work have been published elsewhere.4
Enrolment for Protocol C, the Early HIV Infection Cohort, began in February 2006, but included some volunteers whose HIV infection was diagnosed in 2005 and who were enrolled once the study started. These latter volunteers, diagnosed prior to the start of the Early HIV Infection Cohort, were invited to remain in their respective HIV incidence study where we provided CD4 T cell counts, counselling and appropriate care. We did not collect peripheral blood mononuclear cells (PBMCs) from those volunteers during these visits, as this was not permitted in the HIV epidemiology studies at that time. We did allow (with permission from the volunteers via the consent process) data and samples (in this case, frozen blood plasma only) from the incidence studies to ‘roll over’ into the Early HIV Infection Cohort Study once it began. Over 90% of Protocol C volunteers were identified via Protocol B and the HT Study, with the remainder either identified at the time of screening for Protocol B by detectable p24 antigen in the absence of HIV antibodies (suggesting incident HIV infection) or through other sources (Table 1, see also10). Suspected transmitting partners were also invited to enrol for a single study visit and 406 partners were enrolled, primarily from the HIV-discordant couple cohorts. In 2006, ART programmes in east and southern Africa were in their infancy, and ART was typically initiated when CD4 T cell counts were ≤200 cells/mm3. These programmes evolved considerably over the course of the Early HIV Infection Cohort, with 17 major guideline changes across five countries from 2005 to 2011. Volunteers’ health was monitored closely and ART was initiated per guidelines that existed at the time.
How often have they been followed up?
Volunteers in Protocol B and the HT Study were typically followed quarterly, with a subset deemed at higher risk for HIV acquisition followed monthly for more prevention counselling and HIV testing to detect incident HIV soon after transmission (see ‘What has been measured?’ below). As volunteers were diagnosed with HIV infection they were invited to enrol in the Early HIV Infection Cohort and their follow-up schedule was determined based on their estimated date of HIV infection (EDI). The EDI was defined as the midpoint between the date of the last negative and first positive test in the case of detection by HIV antibody assay, 14 days prior to the test date in the case of detection by p24 assay only, or 10 days prior to the test date for those volunteers with a polymerase chain reaction (PCR)-positive result prior to antibody or p24 detection. If a volunteer could identify an obvious exposure event, the date of this event could be adopted as the EDI at the discretion of the research team. Once enrolled into the Early HIV Infection Cohort, volunteers were followed monthly for the first 3 months after EDI, quarterly through 2 years post-EDI, and every 6 months thereafter. Of the Early HIV Infection Cohort volunteers, 112 (18%) volunteers were identified very soon after acquiring HIV infection (typically prior to full HIV antibody seroconversion) and were invited into a different visit schedule for the first 3 months: weekly follow-up visits with PBMC collection in the first month following enrolment, then every 2 weeks for the second and third month. Subsequently, their follow-up schedule matched the other Early HIV Infection Cohort volunteers.
What has been measured?
Each HIV epidemiology cohort study measured basic demographics at enrolment, with risk behaviour assessed at enrolment and at each quarterly visit thereafter. Medical history and physical examination were performed at baseline, genital examination was performed either at baseline or as indicated if sexually transmitted infection (STI) was suspected. HIV testing was done quarterly and followed the national guidelines; typically, they recommended two rapid tests followed by a tiebreaker if needed. A p24 antigen test was also performed to detect HIV infection prior to seroconversion. These data are summarized in Table 2. Plasma was stored from each visit. If incident HIV infection was detected, additional sampling and tests were done, including PCR testing of the preceding study visit sample to detect HIV infection prior to seroconversion (Table 2). Newly transmitted HIV was subtyped by sequencing the pol region of the genome and transmitted antiretroviral mutations were assessed, as described.11 Risk was evaluated at month 12 in Protocol B only, and volunteers who were no longer eligible in terms of behaviour risk were taken off study. Volunteers in the HT study continued follow-up while in a sexual relationship with an HIV-positive person. Once enrolled into the Early HIV Infection Cohort, larger volumes of blood were collected to allow processing and storage of plasma and PBMCs, HIV viral load testing, and CD4 and CD8 T cell counts. Human leukocyte antigen system (HLA) characterization was done for all Early HIV Infection Cohort volunteers, as described elsewhere.12 Data on medication history, including antiretroviral drugs, was also collected. As these volunteers went on antiretroviral therapy, they were followed to assure they were medically stable and enrolled in a treatment programme, then taken off study.
Table 2.
Data collected in Protocol B, the Emory H) Study and Protocol C
| Baseline demographics | Medical history, physical exam | Risk assessment | Risk behaviour data | HIV VCT | HIV testing | Other testing | |
|---|---|---|---|---|---|---|---|
| Protocol B | Age, sex, education, race, tribe where available (e.g. recording data on tribe is not legal in Rwanda), country of birth, education, religion, marital status (including polygamous marriage), duration of time in current domicile | Baseline and quarterly. Includes external genital examination at baseline, symptoms-directed examinations at quarterly follow up | Quarterlya except at Masaka where it was done every 6 months | Alcohol and drug use, self-reported STI symptoms, number of sex partners, number who were new, condom frequency, knowledge of partners' HIV status, commercial sex, forced sex/rape, group sex, anal sex | Quarterlya | Two antibody rapid tests, with third tiebreaker (rapid or ELISAb), p24 antigen ELISA | Syphilis annually, symptoms-directed STI testing, urine pregnancy testing quarterly. Safety labsc, plasma and PBMC collection if incident HIV detected |
| HT Study | Age, sex, literacy (local language and English), occupation, income, length of stay in city, religion, tribe, alcoholic history, polygamy (men) and type of marriage (men) | Baseline and quarterly. Genital examination at baseline, symptom- directed and at annual follow up | Quarterlya | Frequency of sex with and without condom with HIV+ partner, frequency of sex with additional partners (if any) | Quarterlya | Two antibody rapid tests, with third tiebreaker (rapid or ELISA), p24 antigen ELISA | Hematocrit and syphilis quarterly. Symptoms-directed STI testing. Safety labs, plasma and PBMC collection if incident HIV detected |
| Protocol C | Age, sex, education, race, tribe where available, country of birth, education, marital status (including polygamous marriage) | Baseline and quarterly. Includes external genital examination at baseline, symptom-directed examinations and concomitant medications (including ARVsd) at quarterly follow up | Baseline only | High-risk activities in 3 months prior to enrolment, suspected route of exposure for those volunteers who could recall a specific exposure event | Not applicable | Confirmation of p24 antigen status and/or antibody seroconversion, viral load PCR at every visit | Syphilis annually, symptom-directed STI testing, urine pregnancy testing quarterly. Safety labs, plasma and PBMC collection. HLA characterization |
Monthly in a subset of volunteers deemed at higher risk.
ELISA, enzyme-linked immunosorbent assay.
Safety labs include hematology (Complete Blood Count (CBC), differential, platelets), serum chemistries (alanine transaminase (ALT), creatinine (Cr)), CD4 and CD8 T cell counts.
ARV, antiretroviral drugs.
What has been found? Key findings and publications
To date, data and samples from these cohorts have contributed to over 220 peer reviewed manuscripts. Highlights of this research include the following.
HIV epidemiology: Even in the context of regular counselling and testing (and well before the era of widespread ART availability, pre-exposure prophylaxis and test and treat programmes) HIV incidence remained high. In these cohorts, we observed annual HIV incidences ranging from ∼1% in rural Ugandans, 4% in Ugandan discordant couples, 3% in Rwandan discordant couples, 8% in Zambian discordant couples, 6–7% in Kenyan MSM, 9–10% in South African women and 2–3% in Kenyan female sex workers.2–4,8 HIV incidence tended to vary by sex, time on study, and sometimes by calendar year though there was considerable heterogeneity across cohorts.4 Our Kenyan cohort of MSM was the first of its kind in Africa and highlighted many similar risk factors for HIV acquisition to non-African MSM, including unprotected sex, group sex, other STIs (e.g. gonorrhea) and receptive anal intercourse.13 Working with these men, we have created training modules for health care workers to improve health care delivery and reduce prejudice.14,15 Additional work included some of the first published reports on transmitted drug resistance mutations in Africa,11 characterizing pregnancy outcomes in women with HIV infection,16 describing a novel HLA type associated with favourable clinical outcomes, B*44 (12), and confirmation of other HLA types and other factors in disease progression.17,18 Ground-breaking work with HIV discordant couples has reinforced the importance of couples’ VCT in lowering HIV incidence,19–21 leading the World Health Organization (WHO) to recommend couples’ counselling wherever VCT is available.22
Clinical course of HIV infection: When the Early HIV Infection Cohort began, national ART programmes in the study countries were just beginning and criteria for when to start varied. Volunteers were followed closely, their CD4 T cell counts and viral loads monitored at every visit and they were referred for treatment according to national guidelines current at the time. The cohorts included regions with very different epidemic dynamics and viral diversity, allowing comparison of clinical outcomes across infecting HIV-1 subtype. We observed that HIV disease progression measured by three endpoints varied by subtype, with C-infected volunteers tending to progress to (i) AIDS, (ii) viral load ≥100 000 copies/mL and (iii) CD4 T cell count ≤350 cells/µl faster than those infected with subtype A.23 Because we also enrolled volunteers with incident HIV infection, typically within 1–2 months of their EDI, we were able to see patterns in acute retroviral syndrome; those infected with subtype A appeared to have worse symptoms shortly after infection, with greater report of headache, lymphadenopathy, fever and other symptoms.24 T cell decline in very early infection was much more pronounced than expected, with a majority of volunteers falling below 500 cells/µL (the WHO-recommended threshold to start treatment in 2013) within 6 months of acquiring HIV infection.25 We also observed that ∼5% of volunteers appeared to control the virus, keeping viral load ≤2000 copies/mL. This too varied by infecting subtype; those with subtype A were more likely to control the virus compared with those with subtype C.10
HIV transmission: Enrolment of suspected transmitting partners allowed for in-depth analysis of events around the time of HIV transmission. We observed that 67–100% of suspected transmitting partners were truly the index case by comparing sequence between partners, and that this varied by study site.4 Infection is typically established by a single genetic variant from the HIV swarm in the index case; we observed selection bias towards more fit viral variants establishing new infection, and that this bias was increased in men compared with women, suggesting a more permissive transmission environment in the female genital tract.26 We also observed that these bottleneck events were not as pronounced when inflammation and/or STIs were present, which presumably compromised the mucosal barrier to viral entry allowing transmission of greater numbers and/or diversity of variants.26,27 Pre-adapted HIV, that is, transmitted HIV that came from someone with a similar HLA profile to the recipient’s, was also found to be associated with more negative clinical outcomes—this preadaptation likely grants recently transmitted HIV a level of invisibility to the new host’s immune system to which it may, in part, have already been adapted through prior immune escape.28,29
HIV virology: Pol sequence was determined at an early timepoint for all volunteers, to estimate the HIV subtype.12 Efforts to generate full-length sequence and infectious molecular clones from very early samples (typically within 2 months of the EDI) are underway to further define the extent of genetic recombination between regions and subtypes. Viral replicative capacity, as defined by cloning the virus’ gag sequence into a replication-competent viral backbone (MJ4 and NL-43) and quantifying subsequent viral replication via an in vitro cell culture assay,30 was found to correlate with immune decline, independent of viral load and HLA type; viruses with high replicative capacity were also found to more readily infect memory T cells, suggesting a more efficient seeding of the latent viral reservoir.31
Neutralizing antibodies to HIV infection: Understanding how the body produces neutralizing antibodies to HIV is currently an exciting topic for HIV vaccine design. Although individuals infected with HIV make neutralizing antibodies to the infecting virus, it rapidly escapes through mutations.32,33 A goal, therefore, of the Early HIV Infection Cohort Study was to characterize the frequency and development of broad and potent neutralizing antibodies to HIV. In this cohort, we observed that ∼15% of volunteers developed broadly neutralizing antibodies to HIV, typically between 2 and 4 years post-infection. Higher viral loads, lower CD4 T cell counts, HLA A*03 allele and infection with subtype C HIV were all independently associated with the development of neutralizing antibodies.34 In-depth characterization of the process over time by which these antibodies are developed provides guidance for immunogen design for an HIV vaccine to elicit neutralizing antibodies against a wide array of epitopes.35–37
Contributing to larger-scale analyses: Samples and data from these studies have contributed to larger work, including the development of assays to estimate HIV incidence from prevalent samples,38,39 the creation of a repository of reagents of recently transmitted HIV to help researchers and assay developers,40 and to answer questions about HIV and other infectious diseases in the context of European and global cohorts, including issues of host genetics and viral control, differences between European and African cohorts, and co-infections such as Hepatitis C.41–45 The samples and data are being used by many African scientists and post-doctorate investigators to develop translational research capabilities, training opportunities and to strengthen north–south and south–south partnerships within Africa.
What are the main strengths and weaknesses?
These cohorts were set up to both address questions of HIV epidemiology and volunteer retention, as well as recruit volunteers for Protocol C, our early infection cohort. We enrolled a diverse set of cohorts across a diverse epidemic: HIV subtypes included A, C, D and many recombinant viruses. In part because of our strong relationships with each respective community, we had great success enrolling volunteers with incident HIV—∼90% of those diagnosed in Protocol B and the HT study enrolled into Protocol C (Table 1). Retention was high in the Early HIV Infection Cohort, with an annual attrition, on average, of ∼5%. Early timepoints and the collection of PBMCs has enabled us to answer questions related to the events early in infection, from viral dynamics to host immunology. However, because the Early HIV Infection Cohort was set up to answer questions relevant for HIV vaccine trials in an era prior to test and treat (e.g. clinical outcomes that might be amenable to therapeutic vaccines), we did not systematically follow volunteers once they started ART; questions about ART treatment programmes, ART effectiveness and their outcomes may not be well suited to this cohort. Additionally, as costs would have been prohibitive, we did not collect pre-infection PBMCs from all volunteers enrolled into the HIV incidence cohorts and thus have no details on immune status prior to HIV acquisition.
Can I get hold of the data? Where can I find out more?
We are committed to the tenets of Open Data and actively encourage investigators to reach out to us for more information, particularly African scientists from these regions. Data are currently available online and samples can be requested. These data are managed at the IAVI Dataspace, found at https://dataspace.iavi.org/. Data may also be found online with each respective publication that requires adherence to Open Data policies, and HIV sequence data have been submitted to GenBank. More information about IAVI’s epidemiology program and how to obtain data and samples from these studies can be found online at https://www.iavi.org/our-work/iavi-dataspace.
Collaboration and data access
We welcome invitations for collaboration. IAVI maintains an online DataSpace with details on how to contact us and what data and samples are available (https://dataspace.iavi.org/).
Funding
This work (i.e. Protocol B, HT Study and Protocol C) was supported by the United States Agency for International Development (USAID). The following grants also supported the HT Study in Rwanda and Zambia: NIH-NIAID P30 AI050409, NIH-FIC TW001042, NIH-NIAID R01 AI040951, NIH-NIMH R01 MH066767, NIH-NICHD R01 HD040125, NIH-NIAID R01 AI064060, and NIH-NIAID R37 AI51231. Some of the Early HIV Infection Cohort (Protocol C) work done at Emory was supported by the Yerkes National Primate Research Center base grant through the Office of Research Infrastructure Programs (OD P51OD11132). HIV neutralizing antibody work was funded in part by the National Institute Of Allergy And Infectious Diseases (grant number U19AI090970) as well as Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery grants numbered OPP1084519 (2013–2018), OPP1196345 (2019–2021) and OPP1115782 (2015–2019).
Acknowledgements
We gratefully acknowledge the study volunteers and study staff, without whom this work would not be possible. IAVI’s work is made possible by generous support from many donors including: the Bill & Melinda Gates Foundation; the Ministry of Foreign Affairs of Denmark; Irish Aid; the Ministry of Finance of Japan; the Ministry of Foreign Affairs of The Netherlands; the Norwegian Agency for Development Cooperation (NORAD); the United Kingdom Department for International Development (DFID); and the United States Agency for International Development (USAID). The full list of IAVI donors is available at www.iavi.org. This work is made possible by the generous support of the American people through USAID. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Conflict of interest
None declared.
References
- 1. Njai HF, Gombe B, Khamis T. et al. Setting up a standardized peripheral blood mononuclear cells processing laboratory to support multi-center HIV/AIDS vaccine and intervention trials. Lab Med 2011;42:711–18. [Google Scholar]
- 2. Ruzagira E, Wandiembe S, Abaasa A. et al. Prevalence and incidence of HIV in a rural community-based HIV vaccine preparedness cohort in Masaka, Uganda. PLoS One 2011;6:e20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruzagira E, Wandiembe S, Abaasa A. et al. HIV incidence and risk factors for acquisition in HIV discordant couples in Masaka, Uganda: an HIV vaccine preparedness study. PLoS One 2011;6:e24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamali A, Price MA, Lakhi S. et al. Creating an African HIV clinical research and prevention trials network: HIV prevalence, incidence and transmission. PLoS One 2015;10:e0116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fideli US, Allen SA, Musonda R. et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses 2001;17:901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth DL, Stewart KE, Clay OJ, van Der Straten A, Karita E, Allen S.. Sexual practices of HIV discordant and concordant couples in Rwanda: effects of a testing and counselling programme for men. Int J STD AIDS 2001;12:181–88. [DOI] [PubMed] [Google Scholar]
- 7. Allen S, Tice J, Van de Perre P. et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ 1992;304:1605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price MA, Rida W, Mwangome M. et al. Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr 2012;59:185–93. [DOI] [PubMed] [Google Scholar]
- 9. Sanders EJ, Graham SM, Okuku HS. et al. HIV-1 infection in high risk men who have sex with men in Mombasa. AIDS 2007;21:2513–520. [DOI] [PubMed] [Google Scholar]
- 10. Price MA, Rida W, Kilembe W. et al. Control of the HIV-1 load varies by viral subtype in a large cohort of African adults with incident HIV-1 Infection. J Infect Dis 2019;220:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price MA, Wallis CL, Lakhi S. et al. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses 2011;27:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang J, Cormier E, Gilmour J. et al. Human leukocyte antigen variants B*44 and B*57 are consistently favorable during two distinct phases of primary HIV-1 infection in sub-Saharan Africans with several viral subtypes. J Virol 2011;85:8894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders EJ, Okuku HS, Smith AD. et al. High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 2013;27:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Elst EM, Gichuru E, Muraguri N. et al. Strengthening healthcare providers' skills to improve HIV services for MSM in Kenya. AIDS 2015;29 Suppl 3:S237–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Elst EM, Smith AD, Gichuru E. et al. Men who have sex with men sensitivity training reduces homoprejudice and increases knowledge among Kenyan healthcare providers in coastal Kenya. J Int AIDS Soc 2013;16:18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wall KM, Rida W, Haddad LB. et al. Pregnancy and HIV disease progression in an early infection cohort from five African countries. Epidemiology 2017;28:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prentice HA, Porter TR, Price MA. et al. HLA-B*57 versus HLA-B*81 in HIV-1 infection: slow and steady wins the race? J Virol 2013;87:4043–051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prentice HA, Price MA, Porter TR. et al. Dynamics of viremia in primary HIV-1 infection in Africans: insights from analyses of host and viral correlates. Virology 2014;449:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wall KM, Kilembe W, Vwalika B. et al. Sustained effect of couples' HIV counselling and testing on risk reduction among Zambian HIV serodiscordant couples. Sex Transm Infect 2017;93:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wall KM, Inambao M, Kilembe W. et al. HIV testing and counselling couples together for affordable HIV prevention in Africa. Int J Epidemiol 2019;48:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodson E, Goldberg A, Michelo C. et al. HIV transmission in discordant couples in Africa in the context of antiretroviral therapy availability. AIDS 2018;32:1613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Guidance on Couples HIV Testing and Counselling Including Antiretroviral Therapy for Treatment and Prevention in Serodiscordant Couples: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO, 2012. [PubMed] [Google Scholar]
- 23. Amornkul PN, Karita E, Kamali A. et al. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS 2013;27:2775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanders EJ, Price MA, Karita E. et al. Differences in acute retroviral syndrome by HIV-1 subtype in a multicentre cohort study in Africa. AIDS 2017;31:2541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fast PE, Price MA, Rida WN, Kamali A, Karita E.. International Aids Vaccine Initiative (IAVI) African Early Infection Research Group. WHO's new guidelines for antiretroviral treatment. Lancet 2013;382:1778–779. [DOI] [PubMed] [Google Scholar]
- 26. Carlson JM, Schaefer M, Monaco DC. et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 2014;345:1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haaland RE, Hawkins PA, Salazar-Gonzalez J. et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog 2009;5:e1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlson JM, Du VY, Pfeifer N. et al. Impact of pre-adapted HIV transmission. Nat Med 2016;22:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monaco DC, Dilernia DA, Fiore-Gartland A. et al. Balance between transmitted HLA preadapted and nonassociated polymorphisms is a major determinant of HIV-1 disease progression. J Exp Med 2016;213:2049–063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prince JL, Claiborne DT, Carlson JM. et al. Role of transmitted Gag CTL polymorphisms in defining replicative capacity and early HIV-1 pathogenesis. PLoS Pathog 2012;8:e1003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claiborne DT, Prince JL, Scully E. et al. Replicative fitness of transmitted HIV-1 drives acute immune activation, proviral load in memory CD4+ T cells, and disease progression. Proc Natl Acad Sci U S A 2015;112:E1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rong R, Li B, Lynch RM. et al. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 2009;5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rong R, Bibollet-Ruche F, Mulenga J, Allen S, Blackwell JL, Derdeyn CA.. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J Virol 2007;81:1350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landais E, Huang X, Havenar-Daughton C. et al. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog 2016;12:e1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doria-Rose NA, Landais E.. Coevolution of HIV-1 and broadly neutralizing antibodies. Curr Opin HIV AIDS 2019;14:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Landais E, Murrell B, Briney B. et al. HIV envelope glycoform heterogeneity and localized diversity govern the initiation and maturation of a V2 Apex broadly neutralizing antibody lineage. Immunity 2017;47:990–1003 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landais E, Moore PL.. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology 2018;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassanjee R, Pilcher CD, Busch MP. et al. Viral load criteria and threshold optimization to improve HIV incidence assay characteristics. AIDS 2016;30:2361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kassanjee R, Pilcher CD, Keating SM. et al. Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. AIDS 2014;28:2439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez AM, DeMarco CT, Hora B. et al. Development of a contemporary globally diverse HIV viral panel by the EQAPOL program. J Immunol Methods 2014;409:117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Santen DK, van der Helm JJ, Del Amo J. et al. Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990-2014. J Hepatol 2017;67:255–62. [DOI] [PubMed] [Google Scholar]
- 42. Olson AD, Meyer L, Prins M. et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One 2014;9:e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pantazis N, Morrison C, Amornkul PN. et al. Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS One 2012;7:e32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abujaber R, Shea PR, McLaren PJ. et al. No evidence for association of beta-defensin genomic copy number with HIV susceptibility, HIV load during clinical latency, or progression to AIDS. Ann Hum Genet 2017;81:27–34. [DOI] [PubMed] [Google Scholar]
- 45. Ramsuran V, Naranbhai V, Horowitz A. et al. Elevated HLA-A expression impairs HIV control through inhibition of NKG2A-expressing cells. Science 2018;359:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]