Abstract
Background
Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are the malaria control interventions primarily responsible for reductions in transmission intensity across sub-Saharan Africa. These interventions, however, may have differential impact on Anopheles species composition and density. This study examined the changing pattern of Anopheles species in three areas of Uganda with markedly different transmission intensities and different levels of vector control.
Methods
From October 2011 to June 2016 mosquitoes were collected monthly using CDC light traps from 100 randomly selected households in three areas: Walukuba (low transmission), Kihihi (moderate transmission) and Nagongera (high transmission). LLINs were distributed in November 2013 in Walukuba and Nagongera and in June 2014 in Kihihi. IRS was implemented only in Nagongera, with three rounds of bendiocarb delivered between December 2014 and June 2015. Mosquito species were identified morphologically and by PCR (Polymerase Chain Reaction).
Results
In Walukuba, LLIN distribution was associated with a decline in Anopheles funestus vector density (0.07 vs 0.02 mosquitoes per house per night, density ratio [DR] 0.34, 95% CI: 0.18–0.65, p = 0.001), but not Anopheles gambiae sensu stricto (s.s.) nor Anopheles arabiensis. In Kihihi, over 98% of mosquitoes were An. gambiae s.s. and LLIN distribution was associated with a decline in An. gambiae s.s. vector density (4.00 vs 2.46, DR 0.68, 95% CI: 0.49–0.94, p = 0.02). In Nagongera, the combination of LLINs and multiple rounds of IRS was associated with almost complete elimination of An. gambiae s.s. (28.0 vs 0.17, DR 0.004, 95% CI: 0.002–0.009, p < 0.001), and An. funestus sensu lato (s.l.) (3.90 vs 0.006, DR 0.001, 95% CI: 0.0005–0.004, p < 0.001), with a less pronounced decline in An. arabiensis (9.18 vs 2.00, DR 0.15 95% CI: 0.07–0.33, p < 0.001).
Conclusions
LLIN distribution was associated with reductions in An. funestus s.l. in the lowest transmission site and An. gambiae s.s. in the moderate transmission site. In the highest transmission site, a combination of LLINs and multiple rounds of IRS was associated with the near collapse of An. gambiae s.s. and An. funestus s.l. Following IRS, An. arabiensis, a behaviourally resilient vector, became the predominant species, which may have implications for malaria vector control activities. Development of interventions targeted at outdoor biting remains a priority.
Keywords: Seasonality, Malaria control, Interventions, Anopheles density, Species composition
Background
Over the past two decades, improved funding and intensive malaria control efforts have increased coverage of vector control interventions worldwide, chiefly long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [1–3]. Within this period, a significant decline in the burden of malaria has been reported across sub-Saharan Africa, with most of this reduction attributed to LLINs (68%), and to a lesser extent, use of IRS (13%) [1]. Global progress toward reducing the incidence of malaria and related deaths, however, has stalled recently [3]. In response, the World Health Organization (WHO) has called for a locally-tailored approach to malaria control rather than a ‘one size fits all’ policy [3].
In Uganda, focused efforts to ensure universal coverage of LLINs through mass distribution campaigns have increased household ownership of LLINs, from 47% in 2009 to over 80% in 2015 and 2019 [4–6]. IRS has also been implemented, beginning with 10 districts from 2007 to 2014, and moving to 14 new districts in 2014 [5, 7–9]. Concomitantly, malaria prevalence has declined in children under five years old, from 40% in 2009, to 19% in 2015 [5], and, further, to 9% in 2019 [6]. In Uganda [10], Kenya [11] and elsewhere [12], sustained vector control has not only resulted in reductions in transmission intensity, but also changes in Anopheles species composition, their behaviour [13, 14], and density [15].
Anopheles gambiae sensu lato (s.l.) and Anopheles funestus s.l. are the primary malaria vector groups in Uganda [4, 16], and elsewhere in East Africa [17, 18]. Both groups are species complexes, comprising of genetically distinct but morphologically indistinguishable sibling species [19–23]. In the An. gambiae complex, An. gambiae sensu stricto (s.s.) and An. arabiensis differ in several aspects, including breeding environment, host preference, biting behaviours, malaria infection rates, and insecticide resistance patterns [14, 17, 24]. Anopheles gambiae s.s. prefer to feed on humans and rest indoors [17]. In contrast, An. arabiensis is less anthropophilic [25, 26]; feeding preferences vary with host availability across the species range [27, 28], with exophilic tendencies [29, 30]. In some mosquito populations, An. gambiae s.s. has higher Plasmodium falciparum infection rates [31], and higher levels of pyrethroid resistance [32], than An. arabiensis. Hybrids between An. gambiae s.s. and An. arabiensis have also been identified [33, 34], with evidence of gene flow between the two species [34]. The implication of hybrids for malaria control is still poorly understood, although in some populations adaptive introgression of insecticide resistance genes coincident with LLIN distribution has been observed [35]. In contrast, An. funestus s.l. breeds year-round in stable environments, such as marshland [20, 36], and may engage in early-morning biting [37]. Anopheles funestus s.l. remains an important vector in dry seasons as a result of its breeding habits [38, 39].
With the expansion of vector control, changes in Anopheles species composition and mosquito density have been observed in Uganda [10, 15], and elsewhere in sub-Saharan Africa [26, 40, 41]. Changes in malaria vector species composition in response to vector control interventions are not a new phenomenon and have been described previously [42]. Recent studies have demonstrated an increase in the relative abundance of An. arabiensis, when compared to sympatric An. gambiae s.s. following deployment of LLINs and/or IRS [10, 11, 14]. Similarly, the apparent replacement of highly anthropophilic An. funestus s.s. by less anthropophilic (zoophilic) and more exophilic Anopheles rivulorum in response to IRS in neighbouring Tanzania, was observed in the An. funestus s.l. complex in the 1960s [42]. Due to their more zoophilic and exophilic behaviour, vector control interventions have been less effective in controlling certain malaria vector species, such as An. arabiensis [41, 43], and An. rivolurum [42]. To further explore the species-specific impact of vector control interventions, the impact of LLINs and IRS on sympatric An. gambiae s.s., An. arabiensis and An. funestus s.l. was examined on mosquito density in areas with differing malaria endemicity in Uganda.
Methods
Study sites
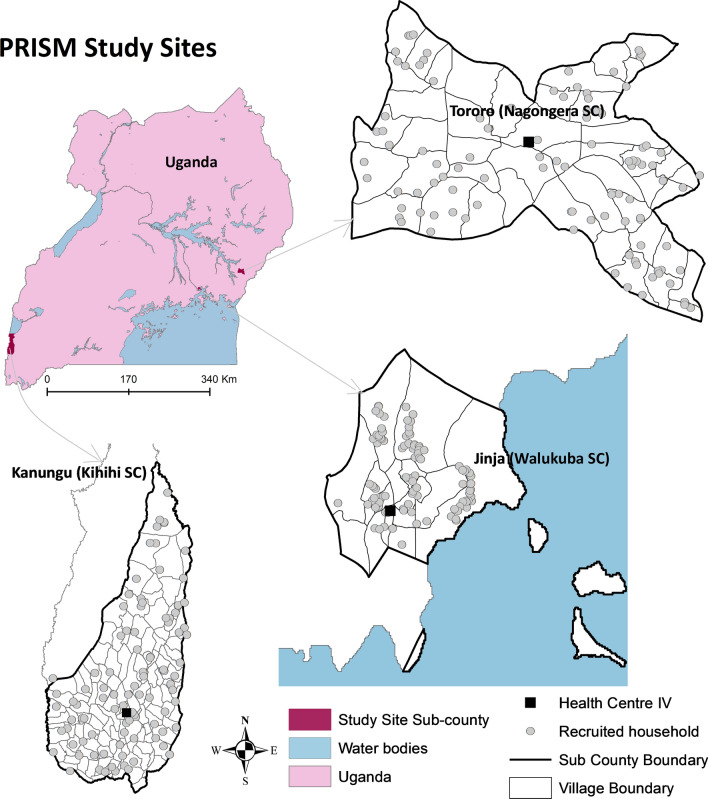
This study was conducted from October 2011 to June 2016 in three sites with differing malaria endemicity, within Walukuba, Kihihi and Nagongera sub-counties (Fig. 1), as part of the PRISM1 (Programme for Resistance, Immunology, Surveillance and Modelling of Malaria) project [10, 44, 45] [46]. Walukuba sub-county (00°26′33.2″N, 33°13′32.3″E), located on the fringes of Lake Victoria in Jinja District, eastern Uganda is a peri-urban area at an elevation of 1,215 m with low malaria transmission [baseline annual human biting rate of 537 and P. falciparum entomological inoculation rate (EIR) of 3.2 infective bites per person per year] [46, 47]. Anopheles arabiensis has been the predominant malaria vector species in this area [46, 48]. Kihihi sub-county (00°45′03.1″S, 29°42′03.6″E), located in Kanungu District, southwestern Uganda, is a rural and hilly area 1,310 m above sea level, with moderate malaria transmission (baseline annual human biting rate of 1,337 and P. falciparum EIR of 14.2 infective bites per person per year) [46]. Anopheles gambiae s.s. has been the main malaria vector species in Kihihi [46, 48]. Nagongera sub-county (00°46′10.6″ N, 34°01′34.1″ E), located in Tororo District, eastern Uganda, is a rural area bordering Kenya with an elevation of 1,185 m with high malaria transmission (baseline annual human biting rate of 16,606 reported in 2014 and P. falciparum EIR of 310 infective bites per person per year) [46]. Anopheles gambiae s.s. has been described as the main malaria vector in Tororo [48], however, in 2014 increasing proportions of An. arabiensis were documented [46]. Seasonality in Uganda is characterized by alternating rainy and dry seasons and a bimodal rainfall pattern. The longer rainy season occurs between July and November and the shorter rainy season between February and May [33].
Fig. 1.
Map of Uganda showing study site location. Grey dots show location of households sampled for CDC light trap collections in the PRISM cohort (Programme for Resistance, Immunology, Surveillance and Modelling of Malaria). Image from Kigozi et al. [45]
During 2011–2016, the primary malaria control interventions deployed in Uganda included artemisinin-based combination therapy for treatment of uncomplicated malaria, distribution of LLINs through mass campaigns, and IRS in select districts [5]. LLINs were delivered to Walukuba and Nagongera in November 2013, and to Kihihi in June 2014. In Nagongera, three rounds of IRS with a carbamate insecticide (bendiocarb) were implemented between December 2014 and June 2015 (1st: December 2014 to Feb 2015, 2nd: June-July 2015, and 3rd: November–December 2015).
Household selection
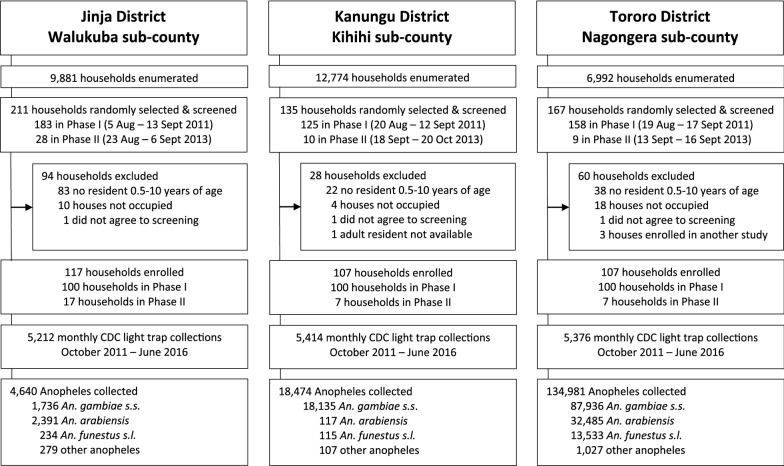
During the initial enrollment period in 2011, 100 households per site were randomly selected from a list of enumerated of households, as previously described [44]. In 2013, additional households were enrolled to replace households that had dropped out of the study to increase the number of enrolled households back to 100 per site (Fig. 2).
Fig. 2.
Study profile of Walukuba, Kihihi and Nagongera sub counties
Mosquito collection
Mosquitoes were collected monthly from cohort study households using miniature CDC light traps (Model 512; John W. Hock Company, Gainesville, FL, USA) set at 19:00 h and collected the following morning at 07:00 h. One trap was set per household each month from October 2011 to June 2016. Light traps were positioned indoors, 1 m above the ground at the foot end of the bed, next to a study participant, sleeping under a LLIN [46]. Data were excluded from analysis if the target occupant did not sleep in the selected room or if the light trap was faulty.
Mosquito species identification
All anophelines collected were scored morphologically under dissecting microscopes at the study sites using taxonomic keys [21, 49]. A subset of 30–50 mosquitoes was randomly selected per month per site for the entire study period for purposes of identifying members of the An. gambiae species complex using PCR [50]. The An. funestus species complex was not processed beyond morphological identification due to resource limitations (henceforth referred to as An. funestus). Results from the species identification were extrapolated to the total dataset to establish the species composition of all Anopheles collected at each site every month. Approximately, 10% of the Anopheles collected were non-malaria transmitting Anopheles christyi, classified as ‘other Anopheles species’ and were not processed further.
Data management and analysis
Field entomologists recorded CDC light trap data on standardized forms. The data collection forms were double-entered into a Microsoft Access database and checked for discrepancies. Any subsequent inconsistencies were resolved using original data entry forms. Statistical analysis was done using Stata (version 14.2, Stata Corp, College Station, TX, USA).
The primary independent variables investigated were; seasonality (dry versus wet season) and the combined vector control interventions (pre-intervention versus post-intervention). The outcomes of interest were vector density and species composition. Seasonality, denoted by rainy and dry seasons was generated for each site independently. For each site, the same consecutive months were divided into 2 rainy seasons and 2 dry seasons over 1 calendar year. Months with rainfall above and below the median value for the entire observation period were classified as rainy or dry season, respectively, after including a 1-month lag period. Vector density was determined by the number of mosquitoes collected per household per month per site and stratified by seasonality and the period before intervention implementation versus the period after intervention implementation. Simple proportions were compared using a log-binomial regression model with generalized estimating equations to adjust for repeated measures from the same house.
Here, we expand on the PRISM1 results previously reported by Kilama et al. [46] from observations carried out over 12 months (October 2011 to September 2012), by describing species-specific changes in response to vector control interventions carried out over 57 months (October 2011 to June 2016). Musiime et al. also used PRISM1 data to examine the impact of vector control interventions on Anopheles mosquito composition in Nagongera only, as measured using indoor and outdoor human landing catches [10]. This study analyses mosquitoes collected indoors using CDC light traps using longitudinal sampling in the three study sites. The PRISM1 dataset can be accessed at https://clinepidb.org/ce/app/record/dataset/DS_0ad509829e.
Ethical approval and consent
In each study site, the head of household or adult representative was approached for consenting before household recruitment. A written informed consent was obtained as permission to conduct CDC light trap collections within the household. The study was approved by the Uganda National Council for Science and Technology (HS-119ES), Makerere University School of Medicine Research and Ethics Committee (2017-099), the University of California, San Francisco Committee on Human Research (17-22544) and London School of Hygiene and Tropical Medicine Ethics Comittee (14266-6).
Results
Total Anopheles mosquitoes collected
From October 2011 to June 2016, 16,002 light trap collections were performed monthly across the three study sites. Overall, 158,095 Anopheles mosquitoes were collected, including 4,640 (3%) from Walukuba, 18,474 (12%) from Kihihi, and 134,981 (85%) from Nagongera (Table 1, Fig. 2). The number of Anopheles mosquitoes collected per household per night (vector density) varied across the sites from 0.89 in Walukuba to 25.11 in Nagongera (Table 1). Overall, An. arabiensis (n = 2,391) was the predominant malaria vector species in Walukuba accounting for 52% of all collections. In Kihihi, nearly all Anopheles collected (98%) were An. gambiae s.s. (n = 18,135), while in Nagongera, 65% were An. gambiae s.s. (n = 87,936) (Table 1). Of the 1,413 ‘other’ Anopheles species collected in the sites, 1,385 (98%) were identified morphologically as An. christyi, which is classified as a non-malaria vector [51]. There is historical evidence that An. christyi has the ability to transmit malaria parasites [52], however, subsequent reports argue that this ability was either lost or suppressed independently [51] and is thus now considered to be a non-malaria vector. As expected, more Anopheles mosquitoes were collected during rainy seasons, compared to the dry seasons (Table 2).
Table 1.
Characteristics of sites and collections
| Walukuba | Kihihi | Nagongera | |
|---|---|---|---|
| District | Jinja | Kanungu | Tororo |
| Entomological Inoculation Rate (EIR)a | 3.2 | 14.2 | 310.0 |
| Transmission intensity at baseline | Low | Medium | High |
| Households sampled (N) | 5212 | 5414 | 5376 |
| Total Anopheles collected (n) | 4.640 | 18,474 | 134,981 |
| Vector density | 0.89 | 3.41 | 25.11 |
| Mosquito collections | |||
| An. gambiae s.s. (n, %) | 1736 (37%) | 18,135 (98%) | 87,936 (65%) |
| An. arabiensis (n, %) | 2391 (52%) | 117 (0.6%) | 32,485 (24.2%) |
| An. funestus s.l. (n, %) | 234 (5%) | 115 (0.6%) | 13,533 (10%) |
| Other Anopheles (n, %) | 279 (6%) | 107 (0.6%) | 1,027 (0.8% |
aInfectious bites per person per year
Table 2.
Stratified analysis of vector density (density ratio) by seasonality and intervention period
| Study site |
Variable | Categories | An. gambiae s.s | An. arabiensis | An. funestus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vector density | DR (95% CI) | p-value | Vector density | DR (95% CI) | p-value | Vector density | DR (95% CI) | p-value | |||
| Walukuba1 | Seasonality | Dry seasonsa | 0.16 | Reference | 0.22 | Reference | 0.02 | Reference | |||
| Rainy seasonsb | 0.49 | 3.21 (2.15–4.79) | < 0.001 | 0.67 | 2.84 (1.87–4.32) | < 0.001 | 0.06 | 2.57 (1.36–4.88) | 0.004 | ||
|
Intervention Period |
Before LLINsc | 0.34 | Reference | 0.58 | Reference | 0.07 | Reference | ||||
| After LLINsd | 0.33 | 1.29 (0.86–1.93) | 0.21 | 0.35 | 0.85 (0.56–1.30) | 0.45 | 0.02 | 0.34 (0.18–0.65) | 0.001 | ||
| Kihiihi2 | Seasonality | Dry seasonse | 0.78 | Reference | Insufficient number of An. arabiensis collected | Insufficient number of An. funestus s.l collected | |||||
| Rainy seasonsf | 4.50 | 5.56 (3.90–7.92) | < 0.001 | ||||||||
|
Intervention Period |
Before LLINsg | 4.00 | Reference | Insufficient number of An. arabiensis collected | Insufficient number of An. funestus s.l collected | ||||||
| After LLINsh | 2.46 | 0.68 (0.49–0.94) | 0.02 | ||||||||
| Nagongera3 | Seasonality | Dry seasonsi | 2.92 | Reference | 1.54 | Reference | 2.24 | Reference | |||
| Rainy seasonsj | 25.4 | 12.2 (7.05–21.3) | < 0.001 | 9.06 | 7.75 (4.21–14.3) | < 0.001 | 2.70 | 1.61 (0.97–2.66) | 0.06 | ||
|
Intervention Period |
Before LLINsk | 28.0 | Reference | 9.18 | Reference | 3.90 | Reference | ||||
| After LLINsl | 11.2 | 0.40 (0.21–0.73) | < 0.003 | 3.65 | 0.36 (0.18–0.72) | 0.004 | 2.56 | 0.61 (0.36–1.04) | 0.07 | ||
| After 1st Round of IRSm | 2.71 | 0.05 (0.02–0.16) | < 0.001 | 5.44 | 0.33 (0.10–1.09) | 0.07 | 0.10 | 0.02 (0.008–0.06) | < 0.001 | ||
| After 2nd Round of IRSn | 0.17 | 0.004 (0.002–0.009) | < 0.001 | 2.00 | 0.15 (0.07–0.33) | < 0.001 | 0.006 | 0.001 (0.0005–0.004) | < 0.001 | ||
1Low malaria transmission
2Moderate malaria transmission
3High malaria transmission
a February–April, July–September
b May–June, October–January
c Oct 2011–Nov 2013
d Dec 2013–June 2016
e January–February, July–August
f March–June, September–December
g Oct 2011–June 2014
h July 2014–June 2016
i January-March, August–September
j April-July, October–December
k Oct 2011–Nov 2013
l Dec 2013–Feb 2015
m March 2015–June 2015
n July 2015–June 2016
Trends in Anopheles mosquitoes in Walukuba
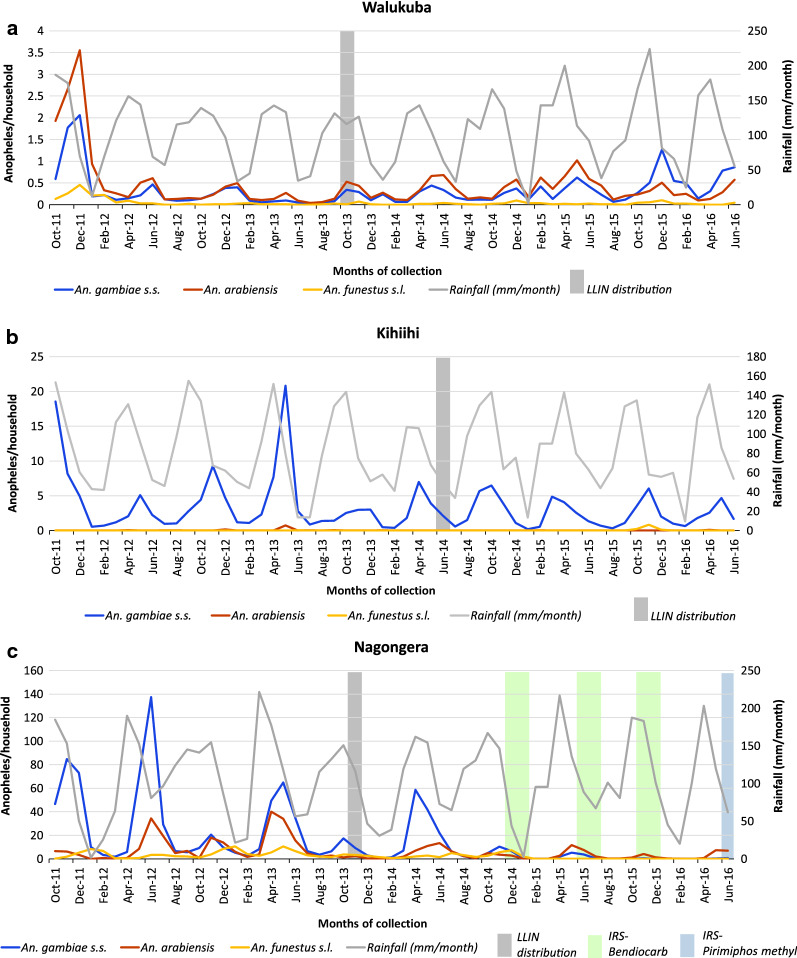
In Walukuba, the rainy season was associated with approximately a three-fold increase in vector density for all three main vectors, including An. gambiae s.s. (density ratio [DR] 3.21, 95% confidence interval [CI]: 2.15–4.79), An. arabiensis (DR 2.84, 95% CI: 1.87–4.32) and An. funestus (DR 2.57, 95% CI: 1.36–4.88; Table 2). Following LLIN distribution, approximately a threefold decline in An. funestus vector density (DR 0.34, 95% CI: 0.18–0.65; Table 2) was observed in Walukuba. The density of An. gambiae s.s. or An. arabiensis following distribution of LLINs was similar to levels before deployment (Table 2). This corresponded with the pattern of distribution observed in the graphical plots examining the absolute numbers of Anopheles collected in Walukuba (Fig. 3a) and the relative proportions (Fig. 4a) of mosquito species.
Fig. 3.
Absolute numbers of An. gambiae s.s. (blue line), An. arabiensis (red line) and An. funestus s.l. (yellow line), collected per month in the three study sites. The grey line shows the rainfall pattern, the grey bar depicts LLIN distribution and the green bars depict IRS deployment
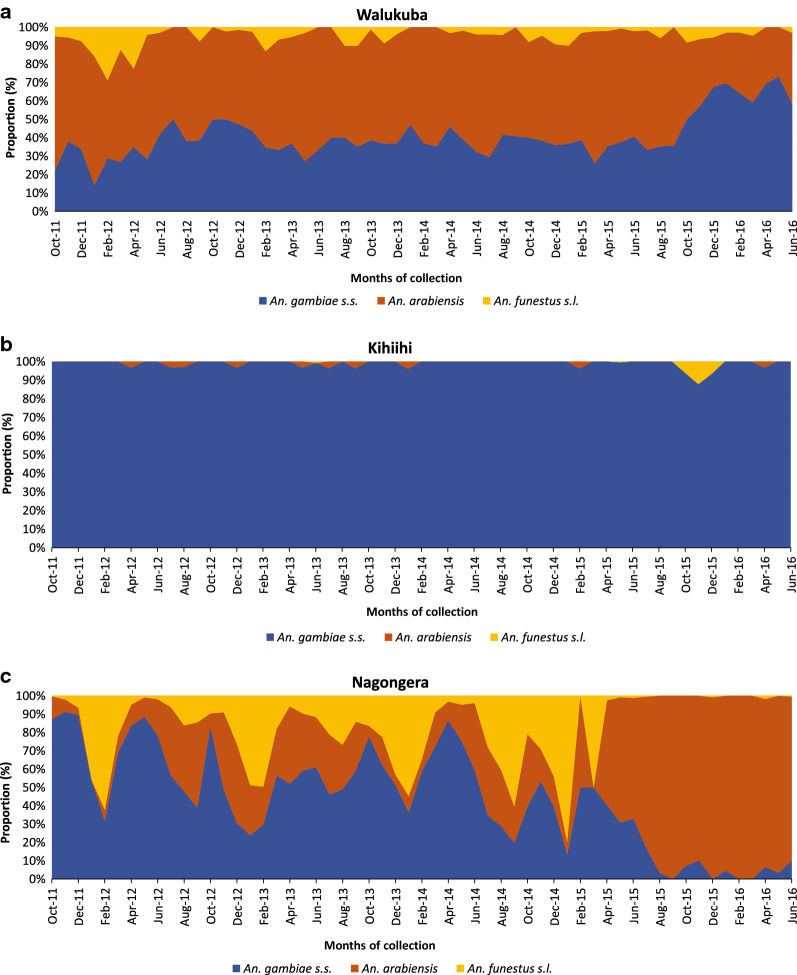
Fig. 4.
Relative numbers / proportion of An. gambiae s.s (blue), An. arabiensis (red) and An. funestus s.l. (yellow), collected per month in the three study sites
Trends in Anopheles mosquitoes in Kihihi
In Kihihi, the rainy season was associated with over a five-fold increase in An. gambiae s.s. density (DR 5.56, 95% CI: 3.90–7.92) compared to the dry season. Insufficient numbers of both An. arabiensis and An. funestus were collected however, precluding further analysis. LLIN distribution in this area was associated with a decrease in A. gambiae s.s. vector density (DR 0.68, 95% CI: 0.49–0.94). This observation is supported by the longitudinal patterns for absolute numbers of Anopheles mosquitoes collected per household (Fig. 3b). When focusing only on trends in relative proportions of Anopheles over time, however, this finding is not obvious (Fig. 4b).
Trends in Anopheles mosquitoes in Nagongera
In Nagongera, there were substantially more An. gambiae s.s. (DR 12.2, 95% CI: 7.05–21.3) and An. arabiensis (DR 7.75, 95% CI 4.21–14.3) during the rainy season, but no significant difference was observed for An. funestus (DR 1.61, 95% CI: 0.97–2.66). LLINs were associated with a significant decrease in vector density for An. gambiae s.s. (DR 0.40, 95% CI: 0.21–0.73) and An. arabiensis (DR 0.36, 95% CI 0.18–0.72), but not An. funestus (DR 0.61, 95% CI: 0.36–1.04). In Nagongera, three rounds of IRS with bendiocarb were delivered following LLIN distribution. The first round of IRS was associated with a 20-fold decline in An. gambiae s.s. vector density compared to the pre-LLIN period (DR 0.05, 95% CI: 0.02–0.16), while the impact on An. funestus was close to elimination (DR 0.02, 95% CI: 0.008–0.06). There was no difference in An. arabiensis densities before and after the first round of IRS (DR 0.33, 95% CI: 0.10–1.09). The 2nd and 3rd rounds of IRS (combined) were associated with further declines in vector density for both An. gambiae s.s. (DR 0.004, 95% CI: 0.002–0.009), and An. funestus (DR 0.001, 95% CI: 0.0005–0.004), but a less pronounced decline was observed in An. arabiensis vector density (DR 0.15, 95% CI: 0.07–0.33). In contrast to Walukuba and Kihihi, substantial reductions in the absolute numbers of An. gambiae s.s. and An. funestus s.l. were observed following the addition of IRS to LLINs (Fig. 3c). The absolute number of An. arabiensis changed less after the introduction of the mass vector control measures, and, as a result, the relative proportion of An. arabiensis increased markedly as the populations of An. gambiae s.s. and An. funestus collapsed, with An. arabiensis left as the predominant species after IRS (Fig. 4c).
Discussion
Over the past 13 years (2007–2020), vector control interventions have been scaled-up substantially across Uganda. Whilst the impact of LLINs and IRS on epidemiological outcomes has been assessed routinely [4, 5, 7, 32, 53, 54], the effect of these interventions on malaria vector species is less commonly investigated. This study characterized vector species composition and density in three epidemiologically diverse settings from 2011 to 2016, while vector control interventions were implemented across the country by the Uganda Ministry of Health (National Malaria Control Division).
As expected, Anopheles densities were higher during the rainy season in all study sites, consistent with other studies [48, 55]. Prior to the widespread implementation of vector control interventions, Anopheles species were sympatric but composition varied between the sites, with An. arabiensis predominant in Walukuba (the lowest transmission site) and An. gambiae s.s. predominant in both Kihihi and Nagongera (the moderate and high transmission sites respectively). Delivery of LLINs was associated with significant declines in vector density for An. funestus in Walukuba, An. gambiae s.s. in Kihihi and in both An. gambiae s.s. and An. arabiensis in Nagongera. Addition of IRS to LLINs in Nagongera was associated with a decline in all vector species, albeit with a greater impact on An. gambiae s.s. and An. funestus, as reported elsewhere [56, 57]. Consequently, An. arabiensis became the predominant species in this area. Understanding the impact of vector control interventions on local malaria vector species is paramount for assessing gaps in current vector control tools.
Malaria vector control interventions, mainly LLINs and IRS have been associated with changes in sympatric Anopheles species composition in Uganda [10], and elsewhere in East Africa [11, 39, 43]. However, a shift in vector species composition and a decline in vector numbers has also been reported in absence of systematic vector control in north-east Tanzania [58, 59], which underscores the possibility of other causes for these changes, such as epidemics of mosquito pathogens, improvements in housing, and changes in climate and land use. Inherent differences in malaria vector ecological characteristics [25], host preference [17], and exophagic and exophilic behavior [29, 60, 61], could be a threat to vector control especially for An. arabiensis [41]. Anopheles arabiensis is considered to have a lower vectorial capacity than An. gambiae s.s. and An. funestus in parts of East Africa [38]. In other settings, however, where An. arabiensis is the principal vector, evidence of strong anthropophagic behaviour and outdoor malaria transmission have been described [60]. The opportunistic feeding behavior of An. arabiensis, enables this species to avoid contact with LLINs and walls sprayed with insecticides which are applied indoors [27, 60, 62, 63]. Empirical evidence shows that highly anthropophilic malaria vectors, such as An. gambiae s.s. and An. funestus s.s., are more responsive to vector control, particularly IRS programmes [10, 39, 42]. A shift in biting patterns of An. funestus, however, including early morning biting [37, 64], and broad daytime biting [65], following introduction of LLINs has been documented.
Current vector control tools target highly anthropophagic and endophilic behaviour [63]. However, there is growing evidence of outdoor biting especially in An. arabiensis [62, 66], which poses a threat to vector control. A similar study, within the study area in Nagongera found a high proportion of An. arabiensis biting outdoors [10]. In this study, the combination of LLIN and IRS had a lower impact on An. arabiensis vector density compared to An. gambiae s.s. and An. funestus, making it the predominant malaria vector post-intervention. The impact of this apparent increase in An. arabiensis vector density on malaria transmission remains unclear, however. A similar study in Nagongera showed limited malaria transmission despite relatively abundant An. arabiensis [10]. In Kenya, there was a decline in malaria transmission following increased LLIN coverage, coincident with the replacement of primary malaria vectors, An. gambiae s.s. and An. funestus by An. arabiensis [39]. It is plausible that An. arabiensis may maintain residual transmission until the primary malaria vectors An. gambiae s.s. or An. funestus ‘bounce back’. This occurred in western Kenya, where previously dominant An. funestus was suppressed following long term use of LLINs, but then recovered, becoming the predominant vector again within a period of almost 20 years, possibly due to high levels of pyrethroid resistance in this species [67]. In a key example of vector control failure in Kwazulu Natal, previously ‘eliminated’ An. funestus was replaced by less endophilic An. arabiensis, but returned after almost 40 years, highly resistant to pyrethroids, and associated with a malaria resurgence in this area [68].
Outdoor biting behaviour of An. arabiensis poses a challenge to malaria vector control. Larval source management with microbial larvicides combined with LLINs has been shown to be protective against malaria infections in rural Kenya [69], and there are several measures including treating cattle with insecticide [60], use of odour-baited traps dispensing spatial repellents [70], and transfluthrin-treated chairs and ribbons [71], which could be deployed as control interventions in the future. In Uganda, there is still an information gap regarding the zoophilic behaviour of An. arabiensis and host choice in the presence of animals and humans. There is need for further research to assess the efficacy of interventions for controlling An. arabiensis.
This study had several limitations. First, the findings presented are from three sub-counties from only three districts. Thus, the study has limited geographical scope and the results may not be generalizable to other settings. Notably, however, the selected sites represented markedly different transmission settings, and all mosquito collections were made from randomly selected households after enumeration. Second, only indoor mosquito collections were done using light traps. Therefore, these results are subject to inherent biases presented by the mosquito trapping method used. Third, species-specific sporozoite data were not collected, therefore, implications to malaria control regarding residual transmission are implied. Within the study area, pyrethroid resistance was documented in both An. gambiae s.s and An. arabiensis [32], with evidence of carbamate resistance observed in An. gambiae s.s. from Nagongera and Kihihi [32]. However, the extent to which insecticide resistance affected mosquito survival under field conditions was not assessed. Study sites were not randomized to receive particular interventions; longitudinal measurements of mosquito density were made alongside vector control interventions delivered by the Uganda Ministry of Health. Whilst monthly rainfall measurements were used in the analysis and interpretation of the results, temperature and humidity data were unavailable for the study period.
Anopheles species composition may change from highly anthropophagic to less anthropophagic malaria vectors in response to vector control. However, the implications of these shifts in species composition on malaria transmission and control programmes are not well understood and require an in-depth examination of Anopheles species specific contribution to local malaria transmission. This study found that LLINs and IRS affected vector densities and species composition differently in different settings. Measuring absolute numbers of mosquitoes to quantify the impact of interventions instead of relying on relative proportions is important in order to understand the full picture.
Conclusions
In areas of low- and moderate- malaria transmission large-scale deployment of LLINs resulted in substantial reductions in An. gambiae s.s. and An. funestus s.l. In the area of intense malaria transmission, the introduction of LLINs and IRS resulted in the near collapse of these main vectors, with An. arabiensis becoming the principal vector, but at lower densities than prior to wide-scale vector control. Measuring the impact of vector control interventions using absolute numbers of mosquitoes collected increased precision. These findings suggest that the impact of LLINs and IRS on the primary malaria vectors (An. gambiae s.s., An. arabiensis and An. funestus) may be affected by the behaviour of these mosquito populations. Current vector control interventions are effective against malaria, but will not lead to elimination of the disease unless additional tools are included as supplementary interventions. Larval source management using chemical or microbial larvicides, combined with environmental management, could be used to improve control, especially in areas of high transmission.
Acknowledgements
We thank Francis Nyangabakye, Uwineza Ernestine, Kyagamba Patrick, Daniel Kabodhogo, Otto Geoffrey, and Salam Musumba who supervised CDC light trap mosquito collection. We also thank the inhabitants of households from which mosquito collections were done.
Abbreviations
- LLIN
Long-lasting insecticidal nets
- IRS
Indoor residual spraying
- PCR
Polymerase chain reaction
- EIR
Entomological inoculation rate
- WHO
World Health Organization
- MoH
Ministry of Health
Authors' contributions
HDM, SGS, JL, GD, and SWL conceived the study. HDM, KM, and AKM participated in data collection. SPK, MK, SGS, SWL, DS, GD and MJD provided critical reviews of the manuscript. GD provided data analysis. All authors participated in the writing of the manuscript. All authors read and approved the final manuscript. HDM and SGS drafted the first version of the manuscript. All authors read and approved the final manuscript.
Funding
This research was made possible through funding from the National Institutes of Allergy and Infectious Diseases (NIAID) as part of the International Centers of Excellence in Malaria Research (ICMER) program (U19AI089674), Fogarty International Center (FIC) of the National Institutes of Health under Award Number D43TW7375 and Award Number D43TW010526. The content presented herein is solely the responsibility of the authors and does not necessarily represent the official views of FIC or NIH.
Availability of data and materials
The data used are available from the corresponding author upon reasonable request. The PRISM1 dataset can also be accessed at https://clinepidb.org/ce/app/record/dataset/DS_0ad509829e.
Declarations
Ethics approval and consent to participate
In each site, the head of household or adult representative was approached for consenting before household recruitment. A written informed consent was obtained as permission to conduct CDC light trap collections within the household. The study was approved by the Uganda National Council for Science and Technology (HS-119ES), Makerere University School of Medicine Research and Ethics Committee (2017-099), the University of California, San Francisco Committee on Human Research (17-22544) and London School of Hygiene and Tropical Medicine (14266-6).
Consent for publication
All authors gave consent for this publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria: global progress 2000–2015 and future challenges. Infect Dis Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. World Malaria Report 2019. Geneva, World Health Organization, 2019.
- 4.Uganda Bureau of Statistics, NMCP, ICF International. Uganda Malaria Indicator Survey . Rockville. USA: Kampala, Uganda; 2009. p. 2010. [Google Scholar]
- 5.Uganda Bureau of Statistics, NMCP, ICF International. Uganda Malaria Indicator Survey 2014–2015. Rockville, USA, Kampala, Uganda, 2015.
- 6.Uganda National Malaria Control Division (NMCD), Uganda Bureau of Statistics, ICF International. 2018–2019 Uganda Malaria Indicator Survey Atlas of Key Indicators. Rockville, USA, Kampala, Uganda, 2019.
- 7.Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE. 2012;7:e42857. doi: 10.1371/journal.pone.0042857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J. 2016;15:146. doi: 10.1186/s12936-016-1201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okia M, Hoel DF, Kirunda J, Rwakimari JB, Mpeka B, Ambayo D, et al. Insecticide resistance status of the malaria mosquitoes: Anopheles gambiae and Anopheles funestus in eastern and northern Uganda. Malar J. 2018;17:157. doi: 10.1186/s12936-018-2293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo. Uganda Malar J. 2019;18:445. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinka ME, Golding N, Massey NC, Wiebe A, Huang Z, Hay SI, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar J. 2016;15:142. doi: 10.1186/s12936-016-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sougoufara S, Harry M, Doucouré S, Sembène P, Sokhna C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo. Senegal Med Vet Entomol. 2016;30:365–368. doi: 10.1111/mve.12171. [DOI] [PubMed] [Google Scholar]
- 15.Alegana VA, Kigozi SP, Nankabirwa J, Arinaitwe E, Kigozi R, Mawejje H, et al. Spatio-temporal analysis of malaria vector density from baseline through intervention in a high transmission setting. Parasit Vectors. 2016;9:637. doi: 10.1186/s13071-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health. The Uganda Malaria Reduction Strategic Plan 2014–2020. Kampala Uganda, 2014.
- 17.Mwangangi JM, Mbogo CM, Nzovu JG, Githure JI, Yan G, Beier JC. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J Am Mosq Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- 18.Ogola EO, Fillinger U, Ondiba IM, Villinger J, Masiga DK, Torto B, et al. Insights into malaria transmission among Anopheles funestus mosquitoes. Kenya Parasit Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies M. A new species of the Anopheles funestus complex (Diptera: Culicidae) from East Africa. Proc R Entomol Soc Lond B. 1962;31:81–86. [Google Scholar]
- 20.Gillies MT, Wilkes TJ. A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Eastern Tanzania. Bull Entomol Res. 1965;56:237–62. [DOI] [PubMed]
- 21.Gillies M, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ South Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- 22.Coetzee M. Distribution of the African malaria vectors of the Anopheles gambiae complex. Am J Trop Med Hyg. 2004;70:103–104. doi: 10.4269/ajtmh.2004.70.103. [DOI] [PubMed] [Google Scholar]
- 23.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 24.Ochomo E, Bayoh NM, Kamau L, Atieli F, Vulule J, Ouma C, et al. Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya. Parasit Vectors. 2014;7:310. doi: 10.1186/1756-3305-7-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White G, Magayuka SA, Boreham P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae): bionomics and vectorial activity of species A and species B at Segera, Tanzania. Bull Entomol Res. 1972;62:295–317.
- 26.Molineaux L, Gramiccia G. The Garki project: research on the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva: World Health Organization; 1980. [Google Scholar]
- 27.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, et al. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme. Kenya Malar J. 2008;7:43. doi: 10.1186/1475-2875-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies MT. The problem of exophily in Anopheles gambiae. Bull World Health Organ. 1956;15:437. [PMC free article] [PubMed] [Google Scholar]
- 30.Gordicho V, Vicente JL, Sousa CA, Caputo B, Pombi M, Dinis J, et al. First report of an exophilic Anopheles arabiensis population in Bissau City, Guinea-Bissau: recent introduction or sampling bias? Malar J. 2014;13:423. doi: 10.1186/1475-2875-13-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndiath MO, Cohuet A, Gaye A, Konate L, Mazenot C, Faye O, et al. Comparative susceptibility to Plasmodium falciparum of the molecular forms M and S of Anopheles gambiae and Anopheles arabiensis. Malar J. 2011;10:269. doi: 10.1186/1475-2875-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katureebe A, Zinszer K, Arinaitwe E, Rek J, Kakande E, Charland K, et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med. 2016;13:e1002167. doi: 10.1371/journal.pmed.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013; 27:276–83. [DOI] [PMC free article] [PubMed]
- 34.Weetman D, Steen K, Rippon EJ, Mawejje HD, Donnelly MJ, Wilding CS. Contemporary gene flow between wild An. gambiae s.s. and An. arabiensis. Parasit Vectors. 2014;7:345. [DOI] [PMC free article] [PubMed]
- 35.Norris LC, Main BJ, Lee Y, Collier TC, Fofana A, Cornel AJ, et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc Natl Acad Sci USA. 2015;112:815–820. doi: 10.1073/pnas.1418892112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsdale C, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus. Geneva: World Health Organization; 1970. [Google Scholar]
- 37.Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS ONE. 2014;9:e104967. doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlwood J, Vij R, Billingsley P. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- 39.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akogbeto M, Padonou GG, Bankole HS, Gazard DK, Gbedjissi GL. Dramatic decrease in malaria transmission after large-scale indoor residual spraying with bendiocarb in Benin, an area of high resistance of Anopheles gambiae to pyrethroids. Am J Trop Med Hyg. 2011;85:586–593. doi: 10.4269/ajtmh.2011.10-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS ONE. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillies MT, Smith A. The effect of a residual house-spraying campaign in East Africa on species balance in the Anopheles funestus group. The replacement of A. funestus Giles by A. rivulorum Leeson. Bull Entomol Res. 1960;51:243–52.
- 43.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187. doi: 10.1186/1475-2875-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamya MR, Arinaitwe E, Wanzira H, Katureebe A, Barusya C, Kigozi SP, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 2015;92:903–912. doi: 10.4269/ajtmh.14-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kigozi SP, Pindolia DK, Smith DL, Arinaitwe E, Katureebe A, Kilama M, et al. Associations between urbanicity and malaria at local scales in Uganda. Malar J. 2015;14:374. doi: 10.1186/s12936-015-0865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. [DOI] [PMC free article] [PubMed]
- 47.Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, Rek J, et al. Mind the gap: house structure and the risk of malaria in Uganda. PLoS ONE. 2015;10:e0117396. doi: 10.1371/journal.pone.0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. doi: 10.4269/ajtmh.2006.75.219. [DOI] [PubMed] [Google Scholar]
- 49.Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region) Publ South Afr Inst Med Res. 1968;54:1–343. [Google Scholar]
- 50.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 51.Anthony TG, REH, Kitching IJ. Phylogeny of the pyretophorus series of Anopheles subgenus Cellia (Diptera: Culicidae) Syst Entomol. 1999;24:193–205. doi: 10.1046/j.1365-3113.1999.00092.x. [DOI] [Google Scholar]
- 52.Steyn J. The effect of cultivation of swamps on the Anopheline fauna in Kigezi district. Uganda J Entomol Soc South Afr. 1948;11:76–82. [Google Scholar]
- 53.Nankabirwa JI, Briggs J, Rek J, Arinaitwe E, Nayebare P, Katrak S, et al. Persistent parasitemia despite dramatic reduction in malaria incidence after 3 rounds of indoor residual spraying in Tororo. Uganda J Infect Dis. 2019;219:1104–1111. doi: 10.1093/infdis/jiy628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staedke SG, Gonahasa S, Dorsey G, Kamya MR, Maiteki-Sebuguzi C, Lynd A, et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): a pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet. 2020;95:1292–1303. doi: 10.1016/S0140-6736(20)30214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabbale FG, Akol AM, Kaddu JB, Onapa AW. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District. Uganda Parasit Vectors. 2013;6:340. doi: 10.1186/1756-3305-6-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, Baza D, et al. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am J Trop Med Hyg. 2008;79:12–18. doi: 10.4269/ajtmh.2008.79.12. [DOI] [PubMed] [Google Scholar]
- 57.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJA, et al. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. doi: 10.4269/ajtmh.2009.81.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyrowitsch DW, Pedersen EM, Alifrangis M, Scheike TH, Malecela MN, Magesa SM, et al. Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malar J. 2011;10:188. doi: 10.1186/1475-2875-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11:188. doi: 10.1186/1475-2875-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 61.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yohannes M, Boelee E. Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med Vet Entomol. 2012;26:103–105. doi: 10.1111/j.1365-2915.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 63.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania. PLoS ONE. 2017;12:e0177807. doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 65.Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degefa T, Yewhalaw D, Zhou G, Atieli H, Githeko AK, Yan G. Evaluation of human-baited double net trap and human-odour-baited CDC light trap for outdoor host-seeking malaria vector surveillance in Kenya and Ethiopia. Malar J. 2020;19:174. doi: 10.1186/s12936-020-03244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hargreaves K, Koekemoer L, Brooke B, Hunt R, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 69.Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrés M, Lorenz LM, Mbeleya E, Moore SJ. Modified mosquito landing boxes dispensing transfluthrin provide effective protection against Anopheles arabiensis mosquitoes under simulated outdoor conditions in a semi-field system. Malar J. 2015;14:255. doi: 10.1186/s12936-015-0762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masalu JP, Finda M, Killeen GF, Ngowo HS, Pinda PG, Okumu FO. Creating mosquito-free outdoor spaces using transfluthrin-treated chairs and ribbons. Malar J. 2020;19:109. doi: 10.1186/s12936-020-03180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used are available from the corresponding author upon reasonable request. The PRISM1 dataset can also be accessed at https://clinepidb.org/ce/app/record/dataset/DS_0ad509829e.