Abstract
Direct oral anticoagulants (DOACs) are currently the preferred oral anticoagulant treatment for most of the patients with deep vein thrombosis of the lower extremities and/or pulmonary embolism. DOACs have several advantages over vitamin K antagonists, such as availability of fixed dosages, fewer drug interactions, faster onset of action, shorter half‐life, and lower risk of major and intracranial bleeding. Although the evidence on the use of DOACs in patients with unusual‐site venous thromboembolism (VTE) is limited to a few, small randomized controlled trials, these drugs are increasingly used in clinical practice, and several observational cohort studies have been published recently. This narrative review will describe the latest evidence for the use of the DOACs in patients with thrombosis in atypical locations (splanchnic, cerebral, upper extremity, ovarian, and renal vein thrombosis) and will provide some practical advice for their use in patients with unusual‐site VTE.
Keywords: Budd‐Chiari syndrome, cranial sinus thrombosis, direct‐acting oral anticoagulants, portal vein, upper extremity deep vein thrombosis, venous thromboembolism
Essentials.
Venous thromboembolism (VTE) may involve atypical locations.
There is limited evidence on direct oral anticoagulants (DOACs) for unusual‐site VTE.
Published studies reported heterogeneity of dosages and starting time of DOACs.
In selected patients with unusual‐site VTE, DOACs showed good efficacy and safety profile.
1. INTRODUCTION
Unusual‐site venous thromboembolism (VTE) refers to thrombosis occurring in venous districts outside the veins of the lower limbs and the pulmonary arteries. Examples of atypical locations include abdominal veins (eg, splanchnic, renal, and ovarian), cerebral veins and venous dural sinuses, and the upper extremity venous system. 1
Direct oral anticoagulants (DOACs) are the preferred treatment for the majority of patients with deep vein thrombosis (DVT) of the lower extremities and/or pulmonary embolism (PE), 2 , 3 and their advantages over vitamin K antagonists (VKAs) can also be relevant for patients with unusual‐site VTE. For instance, DOACs have a more predictable anticoagulant response and fewer drug interactions, and thus can be administered at fixed doses. 4 They have a faster onset of action and a shorter half‐life, which can simplify the management of the acute phase of VTE and preoperative interruptions, respectively. 4 Furthermore, they are associated with a lower risk of major bleeding (MB), particularly intracranial hemorrhages (ICHs). 5 , 6 Although DOACs simplify the management of anticoagulated patients, specific laboratory assays may be useful in some situations where there is a need to assess the anticoagulant plasma concentrations (eg, recurrent VTE on treatment) and there is still limited experience with the use of specific antidotes. 4 Additionally, other types of bleeding might be increased, at least with certain DOACs, such as gastrointestinal (GI) 6 and menstrual bleeding. 7
DOACs were not extensively evaluated in patients with VTE in atypical locations, since these patients were not included in the pivotal phase III randomized controlled trials (RCT). 5 Thus, the recommended anticoagulant treatment for unusual‐site VTE still includes parenteral anticoagulation with unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH), eventually followed by VKAs. 8 , 9 However, in the past 5 years, some evidence has emerged from a few small RCTs and a number of observational studies assessing the safety and effectiveness of DOACs in patients with unusual VTE. 10 , 11 , 12 We also recently conducted a vignette‐based survey showing that DOACs were considered by 23%‐28% of physicians for patients with thrombosis of the splanchnic or cerebral veins and low bleeding risk. 13
The aim of this narrative review is to provide an update on the latest evidence for the use of the DOACs in the most common sites of unusual VTE, namely, splanchnic, cerebral, upper extremity, ovarian, and renal venous thrombosis.
2. SPLANCHNIC VEIN THROMBOSIS
2.1. Overview
Splanchnic vein thrombosis (SVT) is a heterogeneous disorder that includes thrombosis of the portal, mesenteric, and splenic veins and Budd‐Chiari syndrome (BCS). The most common risk factors for SVT are liver cirrhosis and solid cancer, which together account for ~50% of cases. 14 , 15 Other risk factors include myeloproliferative neoplasm (MPN) or JAK2V617F mutation, abdominal surgery, abdominal inflammatory disorders or infections, and thrombophilia, while in 15%‐27% of cases SVT is unprovoked. 14 , 15 SVT can have different clinical presentations, including abdominal pain (48%‐55%), GI bleeding (9%‐26%), and ascites (10%‐29%) 14 , 15 ; however, up to a third of cases are incidentally detected at abdominal imaging tests performed for other reasons.
SVT prognosis can also be challenging. These patients have a higher risk of bleeding events compared to those with usual‐site VTE, 16 but at the same time the risk of recurrent thrombosis is not negligible. 15 In particular, patients with cirrhosis have the highest risk of both thrombotic and bleeding complications. 15 Mesenteric vein thrombosis (MVT) can evolve into acute intestinal infarction and can be life‐threatening, with very high short‐term mortality rates (63% at 30‐day). 17 BCS is a particularly severe entity that can present with or rapidly progress to liver failure, and requires liver transplantation in >10% of patients. 18
2.2. General principles of treatment
In the absence of absolute contraindications, anticoagulation should be started early after SVT diagnosis, together with adequate prophylaxis for gastroesophageal varices bleeding, if needed, 19 to improve recanalization rates and reduce the risk of GI bleeding. 20 A recent guidance document from the International Society on Thrombosis and Haemostasis (ISTH) suggested the possibility of prescribing DOACs for non‐cirrhotic patients with acute symptomatic SVT and considering standard treatment with LMWH/VKAs if contraindications to DOACs are present. 19 In cancer‐associated SVT, LMWH/DOACs were recommended over VKAs, with a preference for LMWH if luminal GI cancer, genitourinary neoplasm at high bleeding risk, or concomitant chemotherapy interacted with the DOACs. 19 Finally, LMWH was suggested for cirrhotic SVT, with a switch to VKAs/DOACs if not contraindicated by severe liver dysfunction. 19 Conversely, the recent guidelines of the American College of Gastroenterology suggested starting with parenteral anticoagulation and continuing with standard treatment (LMWH/VKAs), giving more importance to the limited experience with DOACs and the potential malabsorption due to intestinal edema. 21
It has been debated whether patients with asymptomatic, incidentally detected SVT need the same therapeutic approach. While the 2012 guidelines of the American College of Chest Physicians (ACCP) suggested no anticoagulation, 22 several studies published afterwards have shown that symptomatic and incidentally detected SVT share a similar risk of recurrent VTE. 23 , 24 Thus, in the 2020 ISTH guidance, we suggested that the same therapeutic approach as for patients with symptomatic SVT should be followed. 19
While general antithrombotic guidelines advocated an anticoagulant treatment duration of at least 3 months, 22 gastroenterological guidelines indicated a minimum of 6 months. 8 , 21 Factors associated with indefinite anticoagulant treatment duration included persistent risk factors (eg, MPN, severe thrombophilia), unprovoked SVT, or VTE recurrence. 8 , 19 , 21
Due to its severity, BCS often requires a stepwise approach starting with medical treatment (anticoagulation, diuretics), followed by interventional procedures (angioplasty, stenting, thrombolysis, transjugular intrahepatic portosystemic shunt), up to orthotopic liver transplantation. 8 There is consensus that BCS patients require indefinite anticoagulation. 8 , 19
2.3. Evidence regarding DOACs
There is only one RCT that evaluated one of the DOACs in the treatment of SVT. Hanafy et al 11 enrolled 80 patients with acute non‐neoplastic portal vein thrombosis (PVT) in the context of hepatitis C virus–related compensated liver cirrhosis (Child‐Pugh classes A‐B) from two tertiary referral centers in Egypt. Patients were treated with enoxaparin 1 mg/kg twice daily for 3 days, then randomized to rivaroxaban 10 mg twice daily or warfarin (target international normalized ratio [INR] range 2.0‐2.5). During the 1‐year follow‐up, complete or partial recanalization was obtained by all patients in the rivaroxaban groups versus 45% in the warfarin group. There was no severe GI bleeding or recurrent PVT in the rivaroxaban group, whereas these events occurred in 17 and 4 patients, respectively, in the warfarin group. 11 While the results of this study suggested that rivaroxaban could be safe and effective in cirrhotic PVT, several limitations should be considered, such as the small sample size, the low dose of rivaroxaban tested during the acute phase of PVT, and the unclear transition from parenteral to oral anticoagulation in the control group. Furthermore, despite the randomization process, baseline characteristics of the two cohorts were not completely balanced. For instance, there was a higher prevalence of grade 3‐4 esophageal varices in the rivaroxaban group, 11 which might have resulted in different therapeutic approaches, since varices at high risk of bleeding were managed with endoscopic band ligation and there was no mention of prophylactic beta‐blockers. In addition, the fact that other outcome events (such as ascites, hepatic encephalopathy, and mortality) occurred only in patients on VKA suggested that also the degree of hepatic dysfunction might have been different, despite similar Child‐Pugh and Model for End‐Stage Liver Disease scores at baseline.
Several small observational cohort studies 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 described the use of DOACs in patients with SVT. However, most of them had a retrospective design or used radiological (eg, vein recanalization) instead of clinical outcomes; thus, high‐quality evidence on this topic is limited. The largest cohort, so far, was recently published by Naymagon et al 30 and included 93 patients with non‐cirrhotic PVT treated with apixaban (n = 20), dabigatran (n = 8), or rivaroxaban (n = 65) in the years 2000‐2019. This study also included two groups of patients receiving standard treatment (70 enoxaparin, 108 warfarin). The primary outcome was complete resolution of the thrombosis at imaging and was achieved by 66% of patients in the DOAC group. This result was similar to the LMWH group (57%) and significantly better than the VKA group (31%, corresponding to hazard ratio [HR] 2.91; 95% confidence interval [CI], 1.87‐4.52, for DOACs vs VKAs). 30 Among the secondary outcomes, MB occurred in 2% of patients receiving DOACs, 14% receiving enoxaparin, and 24% receiving warfarin (corresponding to HR, 0.20; 95% CI, 0.05‐0.86, for DOACs vs VKAs). 30 Given the retrospective design, these results could be partly explained by a selection bias in the choice of which patients were treated with DOACs and a temporal bias, since the DOACs were more commonly prescribed in recent years, resulting in a shorter follow‐up duration. However, a potential advantage of DOACs is the more stable anticoagulant levels obtained during the early phase of treatment, when proper anticoagulation is crucial to prevent the development of portal hypertension and VKA levels are generally unstable. The same authors also analyzed a subset of 23 patients with PVT and inflammatory bowel disease and similarly reported good safety and effectiveness of DOACs. 29
Some studies focused on patients with liver cirrhosis treated with DOACs for different indications and highlighted that a broad range of daily dosages were used (eg, apixaban 2.5‐10 mg; rivaroxaban 5‐20 mg; dabigatran 110‐220 mg), 33 , 34 while another study reported that patients with gastroesophageal varices were more likely to receive VKAs. 35
Among those studies specifically considering patients with cirrhosis and SVT, Nagaoki et al 25 evaluated 50 patients with PVT who were initially treated with parenteral danaparoid for 2 weeks. From 2011 to November 2014 they were subsequently switched to warfarin with a target INR range of 1.5‐2.0 (n = 30), while from December 2014 to 2016 they were subsequently switched to edoxaban 60 mg once daily, eventually reduced to 30 mg once daily as per edoxaban’s posology (n = 20). At 6‐month follow‐up, PVT volume was decreased in the edoxaban group, while it was increased in the warfarin group; however, warfarin was targeted at a lower range than usually recommended, and the rates of clinically significant GI bleeding were nonsignificantly higher in the edoxaban group (15% vs 7%). 25
Ai et al 31 performed a prospective cohort study of patients with cirrhosis and chronic PVT, defined as a diagnosis >1 month before and absence of symptoms of portal hypertension or abdominal pain. Using propensity‐score matching, they matched 40 patients receiving DOACs (rivaroxaban 20 mg once daily as first choice, or dabigatran 150 mg twice daily if contraindications to rivaroxaban existed, such as Child‐Pugh classes B‐C), with 40 patients receiving no anticoagulant treatment. The rates of partial or complete recanalization were higher in the DOAC group both at 3‐month (12.8% vs 0%) and 6‐month (28.2% vs 2.6%) follow‐up, with low and not significantly different rates of overall bleeding (7.7% vs 2.5%). 31
Salim et al 26 considered patients with acute symptomatic MVT, 22 treated with different DOACs and 56 with VKAs. During a median follow‐up of 4 years, the two groups showed similar rates of bowel resection (9% vs 23%) and vessel recanalization (69% vs 71%), and no recurrent VTE on treatment. However, it should be noted that MB rates in this study were relatively high in both groups (9.1% vs 14.3%, respectively), although with different patterns (more GI bleeding with DOACs vs more ICH with VKAs). 26
Sharma et al 28 evaluated a cohort of patients with BCS after endovascular intervention, 36 treated with dabigatran and 62 with VKAs, matched by age, sex, and site of obstruction. Dabigatran resulted in similar rates of stent patency compared to VKAs (91% vs 93% at 1‐year follow‐up), without increasing MB (3.5% vs 6.5%). 28
Finally, in a recently published meta‐analysis, the proportion of SVT patients receiving DOACs and experiencing recurrent VTE (8%; 95% CI, 2‐28) or MB (7%; 95% CI, 2‐23) was similar to patients receiving VKAs (recurrent VTE, 8%; 95% CI, 4‐13; MB, 11%; 95% CI, 7‐18). 36
3. CEREBRAL VEIN THROMBOSIS
3.1. Overview
Cerebral vein thrombosis (CVT) includes thrombosis of the dural venous sinuses and thrombosis of the cortical or deep cerebral veins. The superior sagittal sinus and the lateral sinuses are the most frequently involved (37%‐62% and 31%‐44%, respectively). 37 , 38
A number of different underlying risk factors may be present in CVT, the most common being pregnancy/puerperium, estrogen‐containing oral contraceptives, and thrombophilia. 39 Other risk factors include central nervous system (CNS) infections, malignancies, trauma, and surgery. 39 Finally, in 13%‐21% of cases, CVT is unprovoked. 37
CVT has different clinical presentations, varying from headache (89%) to seizures (20%‐30%) to stupor or coma (14%). 37 A concomitant ICH at CVT onset is reported in a third of patients. 37 , 38 CVT has generally a good prognosis, with an overall mortality rate < 10%, and 5%‐10% of patients maintaining a certain level of dependency. 39 Approximately 80% of patients achieve recanalization, which is associated with favorable outcome. 40
3.2. General principles of treatment
The latest guidelines on the treatment of CVT were published by the European Stroke Organization (ESO) in 2017 and recommended standard treatment with parenteral anticoagulation followed by VKAs. 9 LMWH was suggested as first choice over UFH, except in patients with renal failure or other contraindications to LMWH, or in cases that might require rapid reversal of anticoagulation. 9 DOACs were not recommended in these guidelines; however, it was a weak recommendation based on the very low quality of evidence available at the time of publication. 9
The presence of ICH at CVT onset is not a contraindication to anticoagulation, because the hemorrhagic transformation of a venous infarct is likely due to the venous outflow blockage with increased venous pressure and rupture of venules. 41 For this reason, therapeutic doses of heparin were also recommended for patients presenting with ICH. 9
The 2017 ESO guidelines suggested an anticoagulant treatment duration of 3‐12 months for the majority of patients with CVT, except those with recurrent VTE or persistent prothrombotic risk factors that might necessitate lifelong treatment. 9 Previous guidelines published by the European Federation of Neurological Societies in 2010 42 and the American Heart Association/American Stroke Association in 2011 43 suggested 3‐6 months for CVT secondary to transient risk factors, 6‐12 months for unprovoked CVT, and indefinitely for recurrent VTE or severe thrombophilic status.
3.3. Evidence regarding DOACs
There is only one RCT that evaluated one of the DOACs in CVT. RE‐SPECT CVT was a multicenter, parallel‐group, open‐label with blinded‐endpoint adjudication clinical trial that enrolled 120 patients with acute CVT. 12 After 5‐15 days of parenteral UFH/LMWH, patients were randomly assigned to dabigatran 150 mg twice daily or warfarin (target INR range, 2.0‐3.0) for 24 weeks. 12 The primary outcome was a composite of MB and recurrent VTE and occurred in one (1.7%) patient in the dabigatran group versus two (3.3%) patients in the warfarin group. Since there was no recurrent VTE on treatment, the outcome events were all bleeding and involved different sites: GI bleeding in the dabigatran group and ICH in the warfarin group. 12 Recanalization occurred in 60.0% and 67.3% of patients in the two groups, while excellent functional outcome at 24 weeks, defined as modified Rankin scale 0‐1 points, in 91.5% and 91.4%, respectively. 12
The RE‐SPECT CVT trial showed with a rigorous study design that dabigatran has a safety and efficacy profile similar to warfarin in these patients. However, the long list of exclusion criteria (such as CNS infections, major head trauma, active malignancy, recent MB, inability to swallow, planned surgical procedures for CVT) 12 allows generalizability of the results only to patients with CVT of mild/moderate severity. Furthermore, due to the relatively small sample size and relatively short follow‐up duration, this trial could not provide information on the risk of recurrent VTE and on the long‐term safety of dabigatran for patients necessitating indefinite anticoagulation. This information, however, could be derived from the studies conducted for the treatment of DVT and PE, where dabigatran showed similar rates of VTE recurrence and a nonsignificant trend toward lower MB events than warfarin, 44 even during the extended treatment period. 45
A number of small observational cohort studies on the use of the DOACs in patients with CVT have been published in recent years, 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 mostly with a retrospective design. Some of these studies included a control group of patients receiving standard treatment, 46 , 50 , 51 , 52 , 53 , 56 , 57 although a selection bias cannot be excluded due to the observational design. In addition, DOACs were usually started after an initial treatment with UFH/LMWH 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 56 to achieve stable clinical conditions. There was only one study that evaluated rivaroxaban directly initiated as a single‐drug approach. 54 Shankar Iyer et al 54 prospectively enrolled 20 patients with CVT who were treated with rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg once daily. At 6‐month follow‐up, all patients obtained either complete (12 patients; 60%) or partial recanalization (8 patients; 40%), without any MB. Furthermore, 19 (95%) patients obtained excellent neurological outcome. 54 The other two prospective studies evaluated different DOACs after approximately a week of heparin lead‐in, and highlighted the variety of dosages used (eg, apixaban 5 mg twice daily, dabigatran 75‐150 mg twice daily, rivaroxaban 15‐20 mg once daily). 55 , 56 Of note, the study by Wasay et al 56 included 45 patients treated with DOACs and 66 patients treated with VKAs (target INR range, 2.0‐3.0) and showed similar rates of excellent functional outcome in the two groups at 6‐month follow‐up (64.1% vs 62.5%, respectively), with low rates of MB. Rusin et al 55 enrolled 36 patients with CVT treated with DOACs and reported similar efficacy outcomes (vessel recanalization in 94.4% of patients and excellent neurological outcome in 66.7%). However, in this study the rates of MB during a median treatment duration of 8.5 months was high (three patients; 8.3%), even though none of the events was life threatening. 55 A recently published meta‐analysis of six studies comparing DOACs and VKAs highlighted similar rates of excellent functional outcome (81.8% vs 76.1%, respectively; risk ratio [RR], 1.02; 95% CI, 0.93‐1.13) and a nonsignificant trend toward lower MB in the DOAC group (1.32% vs 3.45%, respectively; RR, 0.44; 95% CI, 0.12‐1.59). 58
Since in previous studies DOACs were associated with a risk of ICH at least halved compared to VKAs, 5 , 6 their safety benefit was expected to be particularly relevant in patients with CVT. In the RE‐SPECT CVT study, randomization was stratified by the presence/absence of ICH, thus resulting in 20 patients in each group with ICH at baseline. 12 In this subgroup of patients, there was one new ICH during treatment in the warfarin group and one enlargement of the baseline ICH in the dabigatran group, 12 thus confirming the similar safety profile of these two oral anticoagulants. In addition, the multicenter prospective observational study by Wasay et al highlighted that the presence of ICH at baseline was more common in patients receiving DOACs (either rivaroxaban or dabigatran), than in those receiving warfarin (25/45 [55.6%] vs 20/66 [30.3%]; P = .01). 56 This finding suggested that physicians feel more comfortable prescribing DOACs for patients with CVT and concomitant ICH.
4. UPPER EXTREMITY DEEP VEIN THROMBOSIS
4.1. Overview
Upper extremity deep vein thrombosis (UEDVT) includes thrombosis of the axillary, subclavian, internal jugular, or brachiocephalic veins (proximal veins) and thrombosis of the brachial, radial, or ulnar veins (distal veins) and represents around 5%‐10% of all DVTs. 59 , 60 UEDVT can be classified as primary (~25% of cases) or secondary (~75%), based on the pathophysiology. 22 Primary UEDVT refers to effort‐related thrombosis (Paget‐von Schrötter syndrome), thoracic outlet syndrome, and unprovoked thrombotic events. 59 The most common risk factors in secondary UEDVT are malignancy (22%‐64%) and central venous catheters (CVC) (10%‐93%). 59 Other risk factors include implantable pacemakers, recent surgery or trauma, and thrombophilia.
Among the possible complications, PE and postthrombotic syndrome (PTS) were less frequently reported in patients with UEDVT compared to those with lower extremity DVT (LEDVT). In fact, PE was detected in 3% of UEDVT vs 16% of LEDVT (P < .001), 61 while PTS was described in 15%‐25% of cases after UEDVT versus 20%‐50% after LEDVT. 62 Mortality was higher in patients with UEDVT versus LEDVT (9.7 vs 6.7 per 100 person‐years), 63 which might reflect the higher prevalence and the greater severity of underlying cancer.
4.2. General principles of treatment
Guidelines for the treatment of UEDVT are scarce. The 2012 ACCP guidelines recommended parenteral anticoagulation (UFH, LMWH, or fondaparinux) in patients with acute UEDVT involving the axillary vein or more proximal venous districts. 22 While a minimum anticoagulant duration of 3 months was suggested, 22 some underlying factors may favor definite (such as unprovoked UEDVT or removed CVC) or indefinite (such as malignancy or persistent CVC) treatment duration. In clinical practice, the majority of patients are usually treated for 3‐6 months and switched to VKAs after the initial heparin treatment, except those with active malignancy. 1
In CVC‐associated UEDVT, there is no need to remove the catheter if well positioned, functional, and required; however, anticoagulation has to be continued as long as the CVC is in place. 22 , 64 If the CVC is nonfunctional or infected, a short course of anticoagulation (3‐7 days) is suggested before removal, if not contraindicated by high bleeding risk. 64 , 65 However, the results of a large retrospective study conducted in the United States showed that early catheter removal (<48 hours) did not increase the risk of developing PE. 66
4.3. Evidence regarding DOACs
There are no RCTs evaluating the DOACs for the treatment of UEDVT; however, several prospective 67 , 68 , 69 , 70 or retrospective 71 , 72 , 73 cohort studies were published in the past few years. Houghton et al 70 prospectively assessed a cohort of 210 patients with acute UEDVT evaluated at the Mayo Clinic in the years 2013‐2019: 102 treated with DOACs (63 apixaban, 39 rivaroxaban) and 108 treated with LMWH/VKAs. At 3‐month follow‐up, the DOAC group showed similar outcomes compared to the LMWH/VKA group in terms of VTE recurrence (1.0% vs 0.9%), MB (0% vs 2.8%) and mortality (2.0% vs 4.6%). 70 However, the presence of active cancer was more common in the standard treatment group. In this study, there was also a group of 843 patients with LEDVT treated with DOACs, who showed similar outcome rates (VTE recurrence, 0.7%; MB, 1.7%; mortality, 2.4%). 70 Montiel et al 72 described another unselected cohort of UEDVT consisting of 55 patients from the Swedish national anticoagulation registry and similarly reported low rates of event at 6‐month follow‐up (VTE recurrence on treatment, 2%; MB, 0%; mortality, 0%).
The Italian multicenter retrospective study by Porfidia et al 73 enrolled 61 patients with non–cancer non–CVC‐related UEDVT treated with the four different DOACs. The DOACs were usually preceded by LMWH/fondaparinux; however, a single‐drug approach was used in 9 of 37 (24.3%) patients receiving rivaroxaban and in 4 of 11 (36.4%) patients receiving apixaban. In two‐thirds of cases, a treatment duration of 3‐6 months was chosen, while the remaining patients were treated for >6 months. Partial or complete recanalization was obtained in all patients, and there were no episodes of VTE recurrence, MB, or mortality. 73
Cancer and CVC‐related UEDVT have been the focus of specific studies. The retrospective single‐institution study by Laube et al 71 analyzed 83 patients treated with rivaroxaban and highlighted a nonnegligible rate of line dysfunction (3.6%) and high rate of mortality (7.2%). In a Canadian prospective multicenter study (CATHETER‐2), 70 patients received rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg once daily, in 51% of cases preceded by LMWH. 68 While there were no episodes of line dysfunction and there was only one episode of recurrent VTE (fatal PE), this trial raised concern with regard to the risk of MB and clinically relevant nonmajor bleeding, which occurred in 13% of patients during the 3‐month follow‐up. 68 This finding could be partly explained by the loading dose of rivaroxaban, since most of the events occurred in the first month, and partly by the severity of the underlying active malignancy. 68
5. OVARIAN VEIN THROMBOSIS
5.1. Overview
Ovarian vein thrombosis (OVT) is around 60 times less frequent than lower extremity DVT. 74 OVT can complicate 0.01%‐0.18% of pregnancies and occurs typically in the postpartum period, with a peak 2‐6 days after delivery. 75 Pregnancy‐related OVT involves the right ovarian vein in 70%‐80% of cases, due to differences in venous drainage. 76 Other risk factors for OVT include estrogen‐containing oral contraceptives, malignancies, recent abdominopelvic surgery, pelvic inflammatory diseases, and infections (septic pelvic thrombophlebitis), 77 while 4%‐16% of cases are classified as unprovoked. 74 , 75
OVT usually manifests with lower abdominal pain, sometimes associated with abdominal tenderness. A palpable cordlike abdominal mass is a specific finding, although reported in < 50% of cases. 78 In addition, nonspecific GI symptoms (eg, anorexia, nausea, vomiting, ileus) were reported. 75 In patients with septic pelvic thrombophlebitis, the classic symptom triad includes spiking fever, pelvic pain, and palpable mass. 79 OVT can also be an incidental finding in women with gynecological malignancies undergoing abdominal imaging as part of routine follow‐up. 80 OVT has nowadays a good prognosis, with mortality rates <5%. 78 In a recent study, at the time of OVT diagnosis 25% of patients showed thrombus extension into the inferior vena cava or the left renal vein, while <10% presented with PE. 75 In another study, the risk of recurrent VTE in OVT was 2.3 per 100 patient‐years, similar to lower extremity DVT; however, recurrences were more frequently located in atypical venous locations. 74
5.2. General principles of treatment
Antibiotics and anticoagulation represent the main treatment options for OVT. In pregnancy‐related OVT, the 2014 guidelines of the Society of Obstetricians and Gynaecologists of Canada recommended parenteral broad‐spectrum antibiotic treatment until at least 48 hours after defervescence and anticoagulation for 1‐3 months. 81 The 2012 Guidelines of the British Committee for Standards in Haematology recommended anticoagulation for 3‐6 months for patients with postpartum OVT and suggested no anticoagulation for incidentally detected cancer‐associated isolated OVT. 82
The important role of anticoagulation in OVT is nowadays well recognized, 1 , 83 despite the limited evidence available. A small clinical trial conducted in the 1990s, in which 14 women with puerperal septic pelvic thrombophlebitis and OVT were randomly assigned to antibiotic treatment alone or with the adjunct of UFH, did not show any difference in the rates of recurrent VTE or in the length of hospitalization. 84 The study by Lenz et al 74 showed that despite OVT being less frequently anticoagulated than lower limb DVT (54% vs 98%, P < .001), VTE recurrences were similar (2.3 vs 1.8 per 100 patient‐years, respectively; P = .49). Assal et al 85 reported a nonsignificant trend toward reduction of VTE recurrence rates with anticoagulation in patients with OVT (5.9% in anticoagulated patients vs 9.9% in patients who were not anticoagulated; P = .59). In general, data from observational studies suggest that the antithrombotic management of OVT is similar to usual‐site VTE, with parenteral anticoagulation during the acute phase, followed by oral anticoagulants (mainly VKAs) in approximately two‐thirds of patients. 74 , 85
5.3. Evidence regarding DOACs
Eight patients with OVT treated with DOACs were included in the study by Janczak et al assessing the treatment of VTE in atypical locations, 10 while 5% of 219 women with OVT included in the study by Lenz et al 74 received direct factor Xa inhibitors; however, separate outcome data were not provided. There are 3 published case reports evaluating the use of rivaroxaban 86 , 87 or apixaban 88 in young women with OVT, with promising results. Finally, Covut et al 89 retrospectively assessed the treatment of 36 women with OVT evaluated at the Cleveland Clinic (United States) in the years 2012‐2018. They found that 10 women received DOACs (rivaroxaban or apixaban), 11 VKAs and 15 enoxaparin. 89 During follow‐up, recanalization was achieved in 70% of women in the DOAC group versus 55% in the VKA group versus 93% in the LMWH group. The 1‐year cumulative incidence of clinically significant bleeding events was 10% versus 18% versus 25% in the three groups, respectively. 89
6. RENAL VEIN THROMBOSIS
6.1. Overview
Renal vein thrombosis (RVT) is around 30 times less frequent than lower limb DVT 90 but is the most frequent non–catheter‐related thrombosis in neonates. 91 , 92 The most common risk factor for RVT is nephrotic syndrome, especially membranous glomerulonephritis. 93 Other risk factors include malignancies (particularly renal cell carcinoma), surgery (including urological procedures and kidney transplantation), and abdominal trauma or infections. 90 , 94 , 95 Finally, approximately 12% of RVTs are classified as unprovoked. 90 , 94
Typical manifestations of RVT include flank pain and tenderness, microscopic or gross hematuria, proteinuria and worsening of renal function. 95 In infants, a painful palpable mass corresponding to the enlarged kidney can be seen. However, acute manifestations are rare, and RVT has usually a chronic onset or can be an incidental finding. 96 Possible complications of RVT include progression into the inferior vena cava (43%‐65% of cases), which increases the risk of embolization. 90 , 93 , 94 Long‐term renal damage and hypertension have also been described. 91 , 97 Bilateral RVT can lead to acute renal failure and can be life threatening, especially in small infants. 95 , 97 Prognosis of RVT depends on the etiology, being favorable in patients with nephrotic syndrome and poor in those with underlying malignancies. 90 , 94
6.2. General principles of treatment
There is little evidence on the treatment of RVT. In line with the management of usual‐site VTE, acute RVT is usually treated with parenteral anticoagulation (UFH/LMWH), eventually followed by VKAs. 82 Recent studies reported a definite anticoagulant treatment duration of 3‐12 months in patients with RVT provoked by a transient risk factor and a longer indefinite duration in those with permanent hypercoagulable states (including persistent nephrotic syndrome) or unprovoked RVT. 93 , 94 , 96
There are two guidelines mentioning the treatment of pediatric RVT. The 2018 Guidelines of the American Society of Hematology suggested anticoagulation in neonates with RVT and considered thrombolysis followed by anticoagulation for life‐threatening RVT (eg, bilateral thrombosis). 97 Previously, the 2012 ACCP guidelines considered either anticoagulation or radiologic monitoring for unilateral RVT without renal impairment or involvement of the inferior vena cava, and suggested either anticoagulation or thrombolysis followed by anticoagulation in bilateral RVT with renal impairment. 98
6.3. Evidence regarding DOACs
Three patients with RVT treated with the DOACs were included in the study by Janczak et al 10 evaluating the use of the DOACs in unusual site VTE. Furthermore, there are four published case reports evaluating the use of rivaroxaban, 99 , 100 apixaban 101 or edoxaban 102 in patients with RVT with good clinical outcomes. However, the DOACs should be used with caution in patients with RVT due to their risk of accumulation in renal insufficiency.
7. PRACTICAL ADVICE REGARDING DOACS IN UNUSUAL‐SITE VTE
Based on the available evidence, DOACs seem to be a promising option for selected SVT patients, especially those with non‐malignant, non‐cirrhotic thrombosis. Given the high hemorrhagic risk of these patients, factor Xa inhibitors might be preferred over thrombin inhibitors, due to their lower risk of GI bleeding. 103 However, there are some conditions frequently associated with SVT in which DOACs are contraindicated or caution is needed (Figure 1). First, since liver failure can affect drug metabolism, all the DOACs are contraindicated in patients with Child‐Pugh class C cirrhosis, and rivaroxaban has been reported to accumulate also in patients with Child‐Pugh class B. 104 Second, since the DOACs have mainly renal excretion, they are contraindicated in severe renal failure (creatine clearance [CrCl] <30 mL/min for dabigatran; CrCl < 15 mL/min for the factor Xa inhibitors). Recent data from pharmacokinetic and retrospective studies suggest that the safety profile of apixaban might be confirmed also in patients with severe chronic kidney disease, 105 , 106 , 107 and several RCTs are assessing this population (RENAL‐AF, NCT02942407; AXADIA, NCT02933697; SAFE‐HD; NCT03987711). Of note, also LMWH is not recommended if CrCl is < 15 mL/min and requires anti‐Xa monitoring or dose reduction if CrCl is 15‐29 mL/min. 104 Third, liver cirrhosis and hypersplenism are frequently associated with thrombocytopenia. Both DOACs and VKAs are not recommended if platelet count is <50 × 109/L, and in this situation LMWH would be the treatment of choice because the dosage can be modulated. 104 There are some concerns regarding the use of DOACs in patients with luminal GI cancer, due to local mucosal damage, which can be associated with poor absorption and higher risk of GI bleeding. Thus, LMWH is usually preferred in these patients. 19
FIGURE 1.
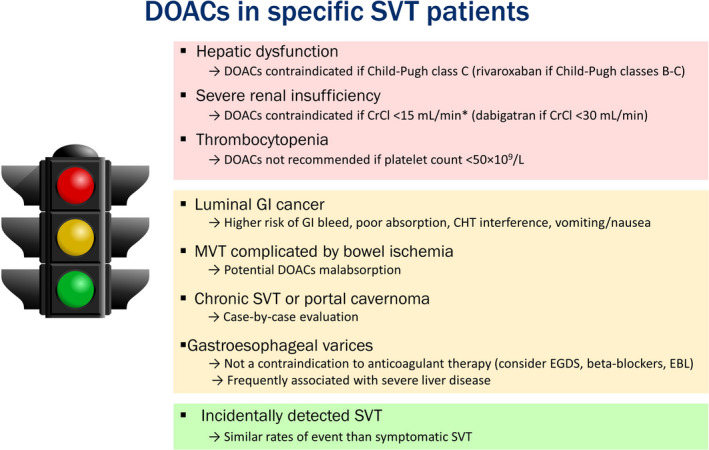
Use of the DOACs in specific patients with SVT. *The US Food and Drug Administration (FDA) removed this contraindication for apixaban in 2019, while as of November 2020 the European Medicine Agency (EMA) still considers apixaban not recommended in this population. The red color on the traffic light refers to situations in which DOACs are contraindicated; yellow refers to debated situations; green refers to situations in which DOACs can be considered. CHT, chemotherapy; CrCl, creatinine clearance; DOACs, direct oral anticoagulants; EBL, endoscopic band ligation; EGDS, esophagogastroduodenoscopy; GI, gastrointestinal; MVT, mesenteric vein thrombosis; SVT, splanchnic vein thrombosis
The presence of esophageal varices should not represent a contraindication to the anticoagulant treatment and to the use of DOACs, especially if adequate prophylaxis is considered (beta‐blockers or endoscopic band ligation). However, since gastroesophageal varices might represent a more advanced liver dysfunction, caution should be applied to these patients.
In severe MVT, the presence of small bowel congestion may result in malabsorption of drugs. 108 With the exception of rivaroxaban, which is primarily absorbed in the stomach, the other DOACs are, at least partially, absorbed in the small intestine. 109 Although retrospective clinical data suggest that DOACs had an efficacy profile similar to VKAs in MVT patients, 26 the potential for malabsorption cannot be excluded.
Given their favorable safety profile, DOACs are a therapeutic option for selected patients with CVT, especially those with mild/moderate clinical manifestations. In this context, dabigatran might be the preferred choice because of its lowest potential for crossing the blood‐brain barrier, compared to the other DOACs. 110 However, more evidence is needed to understand whether these pharmacologic properties translate into different outcomes in clinical practice. Some contraindications to DOACs can also be identified among patients with CVT (Figure 2). First, patients presenting with seizures may receive antiepileptic drugs that interfere with DOACs (eg carbamazepine, phenytoin). 111 Second, the oral administration may be problematic in comatose patients with CVT or those unable to swallow. There is limited evidence for the use of DOACs in CVT associated with CNS malignancies, infections, or trauma; thus, caution should be applied.
FIGURE 2.
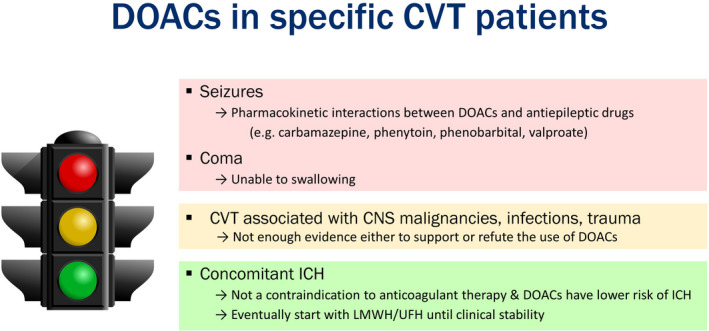
Use of the DOACs in specific CVT patients. The red color on the traffic light refers to situations in which DOACs are contraindicated; yellow refers to debated situations; green refers to situations in which DOACs can be considered. CNS, central nervous system; CVT, cerebral vein thrombosis; DOACs, direct oral anticoagulants; ICH, intracranial hemorrhage; LMWH, low‐molecular‐weight heparin; UFH, unfractionated heparin
DOACs are a promising treatment for selected patients with UEDVT, especially those with non–cancer, non–CVC‐related thrombosis. All four available DOACs might be considered, and the management of UEDVT follows the recommendations for LEDVT. Caution should be applied to patients with cancer‐ and CVC‐related UEDVT, due to the high risk of bleeding. 68 A recently published meta‐analysis of RCTs evaluating cancer‐associated usual‐site VTE showed a (nonsignificant) lower risk of recurrent VTE, balanced by a (nonsignificant) higher risk of MB with the DOACs compared to LMWH, 112 thus supporting the use of the DOACs also in patients with cancer. However, certain conditions (such as GI or genitourinary tumors or CNS metastasis) carry a particularly high bleeding risk, and these patients may not be candidates for DOACs.
Finally, among the different risk factors for unusual‐site VTE, pregnancy and the puerperium represent general contraindications to DOACs because these drugs cross the placenta and are excreted in breast milk. 113 Since warfarin also crosses the placenta, LMWH is the preferred drug antenatally; however, warfarin can be considered during the puerperium. In addition, DOACs are not recommended in patients with severe forms of thrombophilia, such as antiphospholipid syndrome with triple positivity. 114
8. ISTH 2020 CONGRESS REPORT
The results of several studies on the use of the DOACs in unusual‐site VTE were presented during the ISTH 2020 virtual congress. Serrao et al 115 evaluated patients with SVT who were candidates for long‐term anticoagulation and described similar rates of recurrent thrombosis between those shifted to DOACs and those continued on VKAs. De Stefano et al 116 reported that, among patients with MPN treated with DOACs for secondary prevention after different types of VTE, there were also a number of patients with SVT or CVT as primary events.
A case series by Barbar et al 117 confirmed the good safety and efficacy profile of DOACs (mainly dabigatran) in patients with CVT, with no thrombosis recurrence or major or clinically relevant bleeding recorded during treatment. Conversely, the study by Capecchi et al 118 raised some concerns that the risk of bleeding might be higher in patients with CVT treated with anti–factor Xa inhibitors compared to VKAs.
Two abstracts, by Pannu et al 119 and Cohen et al, 120 highlighted that DOACs were used in more than a third of patients with non–cancer‐associated UEDVT, while the collaborative prospective study by Vedovati et al 121 confirmed the safety and effectiveness of the DOACs in these patients, showing low rates of recurrent VTE and MB during follow‐up.
9. FUTURE DIRECTIONS
More evidence will emerge in the next few years, when the results of ongoing studies will become available. There is an ongoing interventional, prospective cohort study evaluating rivaroxaban as a single‐drug approach in patients with acute symptomatic non‐cirrhotic SVT (RIVASVT‐100, NCT02627053) and an open‐label RCT comparing rivaroxaban versus no treatment in patients with chronic non‐cirrhotic PVT without high‐risk thrombophilia (RIPORT, NCT02555111). In acute CVT, there is an ongoing open‐label RCT comparing the use of rivaroxaban versus standard treatment with UFH/LMWH eventually switched to warfarin (SECRET, NCT03178864). There are two ongoing interventional studies assessing the use of the DOACs in patients with UEDVT: the ARM DVT study (NCT02945280) is investigating apixaban as a single‐drug approach in patients with acute symptomatic UEDVT, 122 while CATHETER‐3 is evaluating apixaban after LMWH in patients with cancer and CVC‐related UEDVT (NCT03100071). Finally, we are conducting a collaborative prospective registry on the use of DOACs in unusual‐site VTE (DUST, NCT03778502).
10. CONCLUSIONS
The evidence on the use of DOACs for the treatment of unusual‐site VTE is limited and derived from a few RCTs (one using rivaroxaban in SVT and the other using dabigatran in CVT) and several observational cohort studies. In general, patients enrolled in these studies had unusual‐site VTE of mild/moderate severity and low bleeding risk. The DOACs were prescribed at different dosages and usually started after an initial treatment with parenteral anticoagulation to achieve clinical stability.
Given the limited and low‐quality available evidence on the use of the DOACs in this setting, some authors would still prefer traditional anticoagulant treatment for these patients. 123 However, RCTs in patients with unusual‐site VTE are difficult to conduct, mainly because of the rarity and the severity of these conditions.
We believe that, taken together, the available results suggest that DOACs can be used in selected patients with unusual‐site VTE, since they showed comparable effectiveness and a trend toward better safety than the VKAs. However, caution should be exerted in special categories of patients (such as those with liver cirrhosis, malignancy, CNS infections or trauma, or CVC‐related UEDVT). Ongoing collaborative studies will provide additional data on the safety and efficacy of DOACs in these patients.
RELATIONSHIP DISCLOSURE
NR declares no conflict of interest. WA received research funding from Bayer; and honoraria for advisory boards from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Portola, Janssen, Sanofi, Aspen, and Leo Pharma.
AUTHOR CONTRIBUTIONS
NR reviewed the literature and wrote the first draft of the manuscript. WA critically revised and edited the manuscript. All authors provided final approval of the manuscript.
Riva N, Ageno W. Direct oral anticoagulants for unusual‐site venous thromboembolism. Res Pract Thromb Haemost. 2021;5:265–277. 10.1002/rth2.12480
Handling Editor: Dr Susan Kahn.
REFERENCES
- 1. Abbattista M, Capecchi M, Martinelli I. Treatment of unusual thrombotic manifestations. Blood. 2020;135(5):326–34. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52. [DOI] [PubMed] [Google Scholar]
- 3. Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riva N, Ageno W. Pros and cons of vitamin K antagonists and non–vitamin K antagonist oral anticoagulants. Semin Thromb Hemost. 2015;41(2):178–87. [DOI] [PubMed] [Google Scholar]
- 5. van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–75. [DOI] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383(9921):955–62. [DOI] [PubMed] [Google Scholar]
- 7. Beyer‐Westendorf J. DOACs in women: pros and cons. Thromb Res. 2019;181(suppl 1):S19–S22. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: vascular diseases of the liver. J Hepatol. 2016;64(1):179–202. [DOI] [PubMed] [Google Scholar]
- 9. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis ‐ endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–13. [DOI] [PubMed] [Google Scholar]
- 10. Janczak DT, Mimier MK, McBane RD, Kamath PS, Simmons BS, Bott‐Kitslaar DM, et al. Rivaroxaban and apixaban for initial treatment of acute venous thromboembolism of atypical location. Mayo Clin Proc. 2018;93(1):40–7. [DOI] [PubMed] [Google Scholar]
- 11. Hanafy AS, Abd‐Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non‐neoplastic portal vein thrombosis. Vascul Pharmacol. 2019;113:86–91. [DOI] [PubMed] [Google Scholar]
- 12. Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, et al. Safety and efficacy of dabigatran etexilate vs dose‐adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019;76(12):1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riva N, Carrier M, Gatt A, Ageno W. Anticoagulation in splanchnic and cerebral vein thrombosis: an international vignette‐based survey. Res Pract Thromb Haemost. 2020;00:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thatipelli MR, McBane RD, Hodge DO, Wysokinski WE. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol. 2010;8(2):200–5. [DOI] [PubMed] [Google Scholar]
- 15. Ageno W, Riva N, Schulman S, Beyer‐Westendorf J, Bang SM, Senzolo M, et al. Long‐term clinical outcomes of splanchnic vein thrombosis: results of an international registry. JAMA Intern Med. 2015;175(9):1474–80. [DOI] [PubMed] [Google Scholar]
- 16. Søgaard KK, Adelborg K, Darvalics B, Horváth‐Puhó E, Beyer‐Westendorf J, Ageno W, et al. Risk of bleeding and arterial cardiovascular events in patients with splanchnic vein thrombosis in Denmark: a population‐based cohort study. Lancet Haematol. 2018;5(10):e441–9. [DOI] [PubMed] [Google Scholar]
- 17. Søgaard KK, Darvalics B, Horváth‐Puhó E, Sørensen HT. Survival after splanchnic vein thrombosis: A 20‐year nationwide cohort study. Thromb Res. 2016;141:1–7. [DOI] [PubMed] [Google Scholar]
- 18. Darwish Murad S, Plessier A, Hernandez‐Guerra M, Fabris F, Eapen CE, Bahr MJ, et al. Etiology, management, and outcome of the Budd‐Chiari syndrome. Ann Intern Med. 2009;151(3):167–75. [DOI] [PubMed] [Google Scholar]
- 19. Di Nisio M, Valeriani E, Riva N, Schulman S, Beyer‐Westendorf J, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J Thromb Haemost. 2020;18(7):1562–8. [DOI] [PubMed] [Google Scholar]
- 20. Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García‐Criado A, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10(7):776–83. [DOI] [PubMed] [Google Scholar]
- 21. Simonetto DA, Singal AK, Garcia‐Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol. 2020;115(1):18–40. [DOI] [PubMed] [Google Scholar]
- 22. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riva N, Ageno W, Schulman S, Beyer‐Westendorf J, Duce R, Malato A, et al. Clinical history and antithrombotic treatment of incidentally detected splanchnic vein thrombosis: a multicentre, international prospective registry. Lancet Haematol. 2016;3(6):e267–e275. [DOI] [PubMed] [Google Scholar]
- 24. Tufano A, Ageno W, Di Micco P, Niglio A, Rosa V, Ballaz A, et al. Outcomes during anticoagulation in patients with symptomatic vs. incidental splanchnic vein thrombosis. Thromb Res. 2018;164:69–74. [DOI] [PubMed] [Google Scholar]
- 25. Nagaoki Y, Aikata H, Daijyo K, Teraoka Y, Shinohara F, Nakamura Y, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. 2018;48(1):51–8. [DOI] [PubMed] [Google Scholar]
- 26. Salim S, Ekberg O, Elf J, Zarrouk M, Gottsäter A, Acosta S. Evaluation of direct oral anticoagulants and vitamin K antagonists in mesenteric venous thrombosis. Phlebology. 2019;34(3):171–8. [DOI] [PubMed] [Google Scholar]
- 27. Scheiner B, Stammet PR, Pokorny S, Bucsics T, Schwabl P, Brichta A, et al. Anticoagulation in non‐malignant portal vein thrombosis is safe and improves hepatic function. Wien Klin Wochenschr. 2018;130(13–14):446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma S, Kumar R, Rout G, Gamanagatti SR, Shalimar. Dabigatran as an oral anticoagulant in patients with Budd–Chiari syndrome post‐percutaneous endovascular intervention. J Gastroenterol Hepatol. 2020;35(4):654–62. 10.1182/blood.2020006827 [DOI] [PubMed] [Google Scholar]
- 29. Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Naymagon S, Mascarenhas J, et al. The natural history, treatments, and outcomes of portal vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Troy K, Schiano T, et al. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020;4(4):655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ai MH, Dong WG, Tan XP, Xu L, Xu C, Zhang Q, et al. Efficacy and safety study of direct‐acting oral anticoagulants for the treatment of chronic portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2020;32(10):1395–400. [DOI] [PubMed] [Google Scholar]
- 32. Rössle M, Bettinger D, Trebicka J, Klinger C, Praktiknjo M, Sturm L, et al. A prospective, multicentre study in acute non‐cirrhotic, non‐malignant portal vein thrombosis: comparison of medical and interventional treatment. Aliment Pharmacol Ther. 2020;52(2):329–39. [DOI] [PubMed] [Google Scholar]
- 33. De Gottardi A, Trebicka J, Klinger C, Plessier A, Seijo S, Terziroli B, et al. Antithrombotic treatment with direct‐acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37(5):694–9. [DOI] [PubMed] [Google Scholar]
- 34. Intagliata NM, Henry ZH, Maitland H, Shah NL, Argo CK, Northup PG, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721–7. [DOI] [PubMed] [Google Scholar]
- 35. Davis KA, Joseph J, Nisly SA. Direct oral anticoagulants and warfarin in patients with cirrhosis: a comparison of outcomes. J Thromb Thrombolysis. 2020;50(2):457–61. [DOI] [PubMed] [Google Scholar]
- 36. Valeriani E, Di Nisio M, Riva N, Cohen O, Garcia‐Pagan J‐C, Magaz M, et al. Anticoagulant therapy for splanchnic vein thrombosis: A systematic review and meta‐analysis. Blood. 2020. [DOI] [PubMed] [Google Scholar]
- 37. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, Investigators ISCVT . Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–70. [DOI] [PubMed] [Google Scholar]
- 38. Dentali F, Poli D, Scoditti U, Di Minno MN, De Stefano V, Siragusa S, et al. Long‐term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297–302. [DOI] [PubMed] [Google Scholar]
- 39. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018;16(10):1918–31. [DOI] [PubMed] [Google Scholar]
- 40. Rezoagli E, Martinelli I, Poli D, Scoditti U, Passamonti SM, Bucciarelli P, et al. The effect of recanalization on long‐term neurological outcome after cerebral venous thrombosis. J Thromb Haemost. 2018;16(4):718–24. [DOI] [PubMed] [Google Scholar]
- 41. Schaller B, Graf R. Cerebral venous infarction: the pathophysiological concept. Cerebrovasc Dis. 2004;18(3):179–88. [DOI] [PubMed] [Google Scholar]
- 42. Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17(10):1229–35. [DOI] [PubMed] [Google Scholar]
- 43. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–92. [DOI] [PubMed] [Google Scholar]
- 44. Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–72. [DOI] [PubMed] [Google Scholar]
- 45. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–18. [DOI] [PubMed] [Google Scholar]
- 46. Geisbüsch C, Richter D, Herweh C, Ringleb PA, Nagel S. Novel factor xa inhibitor for the treatment of cerebral venous and sinus thrombosis: first experience in 7 patients. Stroke. 2014;45(8):2469–71. [DOI] [PubMed] [Google Scholar]
- 47. Mendonça MD, Barbosa R, Cruz‐e‐Silva V, Calado S, Viana‐Baptista M. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: a series of 15 patients. Int J Stroke. 2015;10(7):1115–8. [DOI] [PubMed] [Google Scholar]
- 48. Covut F, Kewan T, Perez O, Flores M, Haddad A, Daw H. Apixaban and rivaroxaban in patients with cerebral venous thrombosis. Thromb Res. 2019;173:77–8. [DOI] [PubMed] [Google Scholar]
- 49. Anticoli S, Pezzella FR, Scifoni G, Ferrari C, Pozzessere C. Treatment of cerebral venous thrombosis with rivaroxaban. J Biomedical Sci. 2016;5:3. [Google Scholar]
- 50. Herweh C, Griebe M, Geisbüsch C, Szabo K, Neumaier‐Probst E, Hennerici MG, et al. Frequency and temporal profile of recanalization after cerebral vein and sinus thrombosis. Eur J Neurol. 2016;23(4):681–7. [DOI] [PubMed] [Google Scholar]
- 51. Hassan WU, Syed MJ, Alamgir W, Awan S, Bell SM, Majid A, et al. Cerebral venous thrombosis at high altitude: analysis of 28 cases. Cerebrovasc Dis. 2019;48(3–6):184–92. [DOI] [PubMed] [Google Scholar]
- 52. Lurkin A, Derex L, Fambrini A, Bertoletti L, Epinat M, Mismetti P, et al. Direct oral anticoagulants for the treatment of cerebral venous thrombosis. Cerebrovasc Dis. 2019;48(1–2):32–7. [DOI] [PubMed] [Google Scholar]
- 53. Powell M, Tremolet de Villers K, Schwarz K, Case D, Trujillo T. A single‐center retrospective evaluation of the use of oral factor Xa inhibitors in patients with cerebral venous thrombosis. Ann Pharmacother. 2020;106002802095274. [DOI] [PubMed] [Google Scholar]
- 54. Shankar Iyer R, Tcr R, Akhtar S, Muthukalathi K, Kumar P, Muthukumar K. Is it safe to treat cerebral venous thrombosis with oral rivaroxaban without heparin? A preliminary study from 20 patients. Clin Neurol Neurosurg. 2018;175:108–11. [DOI] [PubMed] [Google Scholar]
- 55. Rusin G, Wypasek E, Papuga‐Szela E, Żuk J, Undas A. Direct oral anticoagulants in the treatment of cerebral venous sinus thrombosis: a single institution's experience. Neurol Neurochir Pol. 2019;53(5):384–7. [DOI] [PubMed] [Google Scholar]
- 56. Wasay M, Khan M, Rajput HM, Farooq S, Memon MI, AlRukn SA, et al. New oral anticoagulants versus warfarin for cerebral venous thrombosis: a multi‐center. Observational Study. J Stroke. 2019;21(2):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsu A, Mistry H, Lala N, Reagan JL. Preliminary findings regarding the use of direct oral anticoagulants in cerebral venous thrombosis. Clin Neurol Neurosurg. 2020;8(198):106204. [DOI] [PubMed] [Google Scholar]
- 58. Lee GKH, Chen VH, Tan CH, Leow AST, Kong WY, Sia CH, et al. Comparing the efficacy and safety of direct oral anticoagulants with vitamin K antagonist in cerebral venous thrombosis. J Thromb Thromb. 2020;50(3):724–31. [DOI] [PubMed] [Google Scholar]
- 59. Bosch FTM, Di Nisio M, Büller HR, van Es N. Diagnostic and therapeutic management of upper extremity deep vein thrombosis. J Clin Med. 2020;9(7):2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bleker SM, van Es N, van Gils L, Daams JG, Kleinjan A, Büller HR, et al. Clinical course of upper extremity deep vein thrombosis in patients with or without cancer: a systematic review. Thromb Res. 2016;140:S81–S88. [DOI] [PubMed] [Google Scholar]
- 61. Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Deep Vein Thrombosis (DVT) FREE Steering Committee. Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–11. [DOI] [PubMed] [Google Scholar]
- 62. Kahn SR. The post‐thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016(1):413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ageno W, Haas S, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, et al. Upper extremity DVT versus lower extremity DVT: perspectives from the GARFIELD‐VTE registry. Thromb Haemost. 2019;119(8):1365–72. [DOI] [PubMed] [Google Scholar]
- 64. Rajasekhar A, Streiff MB. How I treat central venous access device‐related upper extremity deep vein thrombosis. Blood. 2017;129(20):2727–36. [DOI] [PubMed] [Google Scholar]
- 65. Zwicker JI, Connolly G, Carrier M, Kamphuisen PW, Lee AY. Catheter‐associated deep vein thrombosis of the upper extremity in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12(5):796–800. [DOI] [PubMed] [Google Scholar]
- 66. Houghton DE, Billett HHH, Gaddh M, Onadeko O, George G, Wang T‐F, et al. Optimal timing for removal of an upper extremity central catheter when associated with a deep vein thrombosis: a venous thromboembolism network US multicenter retrospective cohort study. Blood. 2019;134(Suppl 1):325. [Google Scholar]
- 67. Fan F, Zou Y, Zhang S, Zhang Y, Lan B, Song Q, et al. Rivaroxaban in the treatment of PICC‐associated upper extremity venous thrombosis. Clin Ther. 2017;39(9):1882–8. [DOI] [PubMed] [Google Scholar]
- 68. Davies GA, Lazo‐Langner A, Gandara E, Rodger M, Tagalakis V, Louzada M, et al. A prospective study of rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2). Thromb Res. 2018;162:88–92. [DOI] [PubMed] [Google Scholar]
- 69. Schastlivtsev I, Lobastov K, Tsaplin S, Kanzafarova I, Barinov V, Laberko L, et al. Rivaroxaban in the treatment of upper extremity deep vein thrombosis: a single‐center experience and review of the literature. Thromb Res. 2019;181:24–8. [DOI] [PubMed] [Google Scholar]
- 70. Houghton DE, Casanegra AI, Peterson LG, Cochuyt J, Hodge DO, Vlazny D, et al. Treatment of upper extremity deep vein thrombosis with apixaban and rivaroxaban. Am J Hematol. 2020;95(7):817–23. [DOI] [PubMed] [Google Scholar]
- 71. Laube ES, Mantha S, Samedy P, Wills J, Harnicar S, Soff GA. Treatment of central venous catheter‐associated deep venous thrombosis in cancer patients with rivaroxaban. Am J Hematol. 2017;92(1):E9–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Montiel FS, Ghazvinian R, Gottsäter A, Elf J. Treatment with direct oral anticoagulants in patients with upper extremity deep vein thrombosis. Thromb J. 2017;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Porfidia A, Agostini F, Giarretta I, Tonello D, Pastori D, Pignatelli P, et al. Upper extremity deep vein thrombosis treated with direct oral anticoagulants: a multi‐center real world experience. J Thromb Thrombolysis. 2020;50(2):355–60. [DOI] [PubMed] [Google Scholar]
- 74. Lenz CJ, Wysokinski WE, Henkin S, Cohoon KP, Casanegra A, Simmons BS, et al. Ovarian vein thrombosis: incidence of recurrent venous thromboembolism and survival. Obstet Gynecol. 2017;130(5):1127–35. [DOI] [PubMed] [Google Scholar]
- 75. Rottenstreich A, Da'as N, Kleinstern G, Spectre G, Amsalem H, Kalish Y. Pregnancy and non‐pregnancy related ovarian vein thrombosis: clinical course and outcome. Thromb Res. 2016;146:84–8. [DOI] [PubMed] [Google Scholar]
- 76. Riva N, Calleja AJ. Ovarian vein thrombosis: a narrative review. Hamostaseologie. 2020. 10.1055/a-1306-4327 [DOI] [PubMed] [Google Scholar]
- 77. Sharma P, Abdi S. Ovarian vein thrombosis. Clin Radiol. 2012;67(9):893–8. [DOI] [PubMed] [Google Scholar]
- 78. Kominiarek MA, Hibbard JU. Postpartum ovarian vein thrombosis: an update. Obstet Gynecol Surv. 2006;61(5):337–42. [DOI] [PubMed] [Google Scholar]
- 79. Sinha D, Yasmin H, Samra JS. Postpartum inferior vena cava and ovarian vein thrombosis–a case report and literature review. J Obstet Gynaecol. 2005;25(3):312–3. [DOI] [PubMed] [Google Scholar]
- 80. Yassa NA, Ryst E. Ovarian vein thrombosis: a common incidental finding in patients who have undergone total abdominal hysterectomy and bilateral salpingo‐oophorectomy with retroperitoneal lymph node dissection. AJR Am J Roentgenol. 1999;172(1):45–7. [DOI] [PubMed] [Google Scholar]
- 81. Chan W‐S, Rey E, Kent NE, Chan W‐S, Kent NE, Rey E, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstetrics Gynaecol Canada. 2014;36(6):527–53. [DOI] [PubMed] [Google Scholar]
- 82. Tait C, Baglin T, Watson H, Laffan M, Makris M, Perry D, et al. Guidelines on the investigation and management of venous thrombosis at unusual sites. Br J Haematol. 2012;159(1):28–38. [DOI] [PubMed] [Google Scholar]
- 83. Bannow BTS, Skeith L. Diagnosis and management of postpartum ovarian vein thrombosis. Hematology Am Soc Hematol Educ Program. 2017;2017(1):168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown CE, Stettler RW, Twickler D, Cunningham FG. Puerperal septic pelvic thrombophlebitis: incidence and response to heparin therapy. Am J Obstet Gynecol. 1999;181(1):143–8. [DOI] [PubMed] [Google Scholar]
- 85. Assal A, Kaner JD, Danda N, Cohen HW, Billett HH. Risk factors and prognosis of ovarian vein thrombosis. Blood Coagul Fibrinolysis. 2017;28(6):468–74. [DOI] [PubMed] [Google Scholar]
- 86. Cook RM, Rondina MT, Horton DJ. Rivaroxaban for the long‐term treatment of spontaneous ovarian vein thrombosis caused by factor V Leiden homozygosity. Ann Pharmacother. 2014;48(8):1055–60. [DOI] [PubMed] [Google Scholar]
- 87. Naoum J, Mohsen A, Daher J, Eid T. Novel management of ovarian vein thrombosis: a case report. Saudi Pharm J. 2018;26(5):608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Azhar E, Nguyen T, Waheed A. Left ovarian vein thrombosis presenting as acute postpartum pyelonephritis. Cureus. 2020;12(2):e6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Covut F, Kewan T, Perez O, Thapa B, Babar A, Alomari M, et al. Direct oral anticoagulants versus warfarin and enoxaparin in ovarian vein thrombosis. Am J Ther. 2019. 10.1097/MJT.0000000000001084 [DOI] [PubMed] [Google Scholar]
- 90. Wysokinski WE, Gosk‐Bierska I, Greene EL, Grill D, Wiste H, McBane RD. Clinical characteristics and long‐term follow‐up of patients with renal vein thrombosis. Am J Kidney Dis. 2008;51(2):224–32. [DOI] [PubMed] [Google Scholar]
- 91. Lau KK, Stoffman JM, Williams S, McCusker P, Brandao L, Patel S, et al. Neonatal renal vein thrombosis: review of the English‐language literature between 1992 and 2006. Pediatrics. 2007;120(5):e1278–84. [DOI] [PubMed] [Google Scholar]
- 92. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ross O, Pourmoussa A, Batech M, Sim JJ. Characteristics of patients diagnosed with renal vein thrombosis and glomerulopathy: a case series. Int Urol Nephrol. 2017;49(2):285–93. [DOI] [PubMed] [Google Scholar]
- 94. Rottenstreich A, Barzilai M, Da'as N, Kleinstern G, Varon D, Kalish Y. Active malignancy in patients with renal vein thrombosis: influence upon clinical course and survival. Clin Exp Nephrol. 2017;21(1):49–54. [DOI] [PubMed] [Google Scholar]
- 95. Asghar M, Ahmed K, Shah SS, Siddique MK, Dasgupta P, Khan MS. Renal vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34(2):217–23. [DOI] [PubMed] [Google Scholar]
- 96. De Stefano V, Martinelli I. Abdominal thromboses of splanchnic, renal and ovarian veins. Best Pract Res Clin Haematol. 2012;25(3):253–64. [DOI] [PubMed] [Google Scholar]
- 97. Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak‐Göttl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e737S–e801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dupree LH, Reddy P. Use of rivaroxaban in a patient with history of nephrotic syndrome and hypercoagulability. Ann Pharmacother. 2014;48(12):1655–8. [DOI] [PubMed] [Google Scholar]
- 100. Matta A, Elenizi K, AlHarthi R, Moussallem N, Elhajjaji N, Lhermusier T, et al. A case of isolated unilateral right renal vein thrombosis associated with bilateral pulmonary embolism treated with rivaroxaban a direct‐acting oral anticoagulant. Am J Case Rep. 2019;20:1152–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Date Y, Nagamine H, Hara H, Kawase Y. Renal vein thrombosis after open repair of abdominal aortic aneurysm successfully treated by direct oral anticoagulants. Vasc Endovascular Surg. 2019;53(5):408–10. [DOI] [PubMed] [Google Scholar]
- 102. Shimada Y, Nagaba Y, Nagaba H, Kamata M, Murano J, Kamata F, et al. Edoxaban was effective for treating renal vein thrombosis in a patient with nephrotic syndrome. Intern Med. 2017;56(17):2307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burr N, Lummis K, Sood R, Kane JS, Corp A, Subramanian V. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta‐analysis. Lancet Gastroenterol Hepatol. 2017;2(2):85–93. [DOI] [PubMed] [Google Scholar]
- 104. Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic vein thrombosis: current perspectives. Vasc Health Risk Manag. 2019;15:449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chang M, Yu Z, Shenker A, Wang J, Pursley J, Byon W, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56(5):637–45. [DOI] [PubMed] [Google Scholar]
- 106. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes associated with apixaban use in patients with end‐stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Herndon K, Guidry TJ, Wassell K, Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a Veterans Affairs hospital. Ann Pharmacother. 2020;54(6):554–60. [DOI] [PubMed] [Google Scholar]
- 108. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Martin KA, Lee CR, Farrell TM, Moll S. Oral anticoagulant use after bariatric surgery: a literature review and clinical guidance. Am J Med. 2017;130(5):517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ferro CJ, Solkhon F, Jalal Z, Al‐Hamid AM, Jones AM. Relevance of physicochemical properties and functional pharmacology data to predict the clinical safety profile of direct oral anticoagulants. Pharmacol Res Perspect. 2020;8(3):e00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Galgani A, Palleria C, Iannone LF, De Sarro G, Giorgi FS, Maschio M, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mulder FI, Bosch FTM, Young AM, Marshall A, McBane RD, Zemla TJ, et al. Direct oral anticoagulants for cancer‐associated venous thromboembolism: a systematic review and meta‐analysis. Blood. 2020;136(12):1433–41. [DOI] [PubMed] [Google Scholar]
- 113. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e44S–88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arachchillage DRJ, Laffan M. What is the appropriate anticoagulation strategy for thrombotic antiphospholipid syndrome? Br J Haematol. 2020;189(2):216–27. [DOI] [PubMed] [Google Scholar]
- 115. Serrao A, Merli M, Lucani B, Aprile F, Fiori L, Aprile SM, et al. Long‐term anticoagulant treatment of splanchnic venous thrombosis in high risk patients: direct oral anticoagulants vs vitamin K antagonists. Res Pract Thromb Haemost. 2020;4(S1):PB2464. [Google Scholar]
- 116. De Stefano V, Betti S, Za T, Ciminello A, Bartolomei F, Ceglie S, et al. Effectiveness and safety of direct oral anticoagulants in patients with Ph‐negative myeloproliferative neoplasms and venous thromboembolism. Res Pract Thromb Haemost. 2020;4(S1):PB2166. [Google Scholar]
- 117. Barbar S, Boscaro F, De Bon E, Bozzolin A, Scarano L, Fabris F, et al. Cerebral vein thrombosis treatment with direct oral anticoagulants: a case series. Res Pract Thromb Haemost. 2020;4(S1):PB2468. [Google Scholar]
- 118. Capecchi M, Abbattista M, Gianniello F, Artoni A, Bucciarelli P, Peyvandi F, et al. Direct oral anticoagulants and vitamin K antagonists for treatment of cerebral vein thrombosis. Res Pract Thromb Haemost. 2020;4(S1):PB2414. [Google Scholar]
- 119. Pannu T, Herget E, Suryanarayan D. Retrospective study of patients with upper extremity clots presenting to emergency departments in tertiary care hospitals in a major Canadian city in the last five years. Blood. 2019;134(suppl 1):1163. [Google Scholar]
- 120. Cohen O, Bertú L, Antonucci E, Palareti G, Ageno W. Clinical characteristics and outcomes of non‐cancer, non‐CVC associated upper extremity DVT. Res Pract Thromb Haemost. 2020;4(S1):PB2448. [Google Scholar]
- 121. Vedovati MC, Tratar G, Mavri A, Pierpaoli L, Agnelli G, Becattini C. Upper extremities deep vein thrombosis and DOAC Treatment: a prospective cohort study. Eur Heart J. 2020;4(S1):PB2435. [DOI] [PubMed] [Google Scholar]
- 122. Woller SC, Stevens SM, Johnson SA, Bledsoe JR, Galovic B, Lloyd JF, et al. Apixaban for routine management of upper extremity deep venous thrombosis (ARM‐DVT): methods of a prospective single‐arm management study. Res Pract Thromb Haemost. 2019;3(3):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Klok FA, Barco S. Anticoagulation in splanchnic and cerebral vein thrombosis: still groping in the dark. Res Pract Thromb Haemost. 2020;4(7):1080–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


