Abstract
The incidence of venous thrombosis, mostly pulmonary embolism (PE), ranging from local immunothrombosis to central emboli, but also deep vein thrombosis (DVT) in people with coronavirus disease 2019 (COVID‐19) is reported to be remarkably high. The relevance of better understanding, predicting, treating, and preventing COVID‐19–associated venous thrombosis meets broad support, as can be concluded from the high number of research, review, and guideline papers that have been published on this topic. The Dutch COVID & Thrombosis Coalition (DCTC) is a multidisciplinary team involving a large number of Dutch experts in the broad area of venous thrombosis and hemostasis research, combined with experts on virology, critically ill patients, pulmonary diseases, and community medicine, across all university hospitals and many community hospitals in the Netherlands. Within the consortium, clinical data of at least 5000 admitted COVID‐19–infected individuals are available, including substantial collections of biobanked materials in an estimated 3000 people. In addition to considerable experience in preclinical and clinical thrombosis research, the consortium embeds virology‐hemostasis research models within unique biosafety facilities to address fundamental questions on the interaction of virus with epithelial and vascular cells, in relation to the coagulation and inflammatory system. The DCTC has initiated a comprehensive research program to answer many of the current questions on the pathophysiology and best anticoagulant treatment of COVID‐19–associated thrombotic complications. The research program was funded by grants of the Netherlands Thrombosis Foundation and the Netherlands Organization for Health Research and Development. Here, we summarize the design and main aims of the research program.
Keywords: anticoagulants, COVID‐19, pulmonary embolism, severe acute respiratory syndrome coronavirus 2, thrombosis, venous thrombosis
Essentials.
COVID‐19 patients have an increased risk of thrombotic complications.
The risk increases with disease severity; ICU patients have the highest risk.
Underlying pathophysiological mechanisms are unclear.
There is a need to improve prediction, treatment and prevention of COVID‐19–associated venous thrombosis.
The Dutch COVID & Thrombosis Coalition initiated a comprehensive research program to answer questions on pathophysiology and best anticoagulant treatment of COVID‐19–associated thrombotic complications.
1. INTRODUCTION
The incidence of venous thromboembolic events (VTE), mostly pulmonary embolism (PE) but also deep vein thrombosis (DVT) in people with COVID‐19 has been reported to be remarkably high. The first studies report VTE in up to 48% of people with COVID‐19 admitted to intensive care units (ICUs) and up to 10% on hospital wards, although the reported incidences range widely because of differences in patient selection and threshold for diagnostic testing. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 This may be higher than in other viral infections and critically ill patients with acute respiratory distress syndrome. 4 , 10 Furthermore, VTE in people with COVID‐19 may occur despite thromboprophylaxis with a standard dose of low‐molecular‐weight heparin (LMWH), suggesting a profound procoagulant state. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 11 , 12 In people with COVID‐19, VTE is hypothesized to be part of a systemic coagulopathy that occurs locally in the pulmonary circulation, at least in those with severe infection, referred to as “immunothrombosis.” This phenomenon suggests a strong link between excessive inflammation and immunothrombosis that may not be adequately prevented or counteracted with standard anticoagulation alone. 13 , 14 , 15 , 16 , 17 , 18
Although the etiology of COVID‐19–associated VTE is unknown, several mechanisms have been suggested. 15 , 16 , 19 , 20 Presumably, prothrombotic triggers include a massive immune response, involving recruitment of inflammatory cells, release of proinflammatory chemokines (in severe cases a “cytokine storm”) and expression of microvascular tissue factor. A thromboinflammatory condition is suggested to occur, aggravated by platelet‐leukocyte interaction, activation of the contact system, and potentially by neutrophil extracellular trap production (NETosis). 21 , 22 Bradykinin and kallikrein generated in this process amplify the intrinsic coagulation. Subsequently, massive endothelial cell activation and inflammation, accompanied by high plasma levels of coagulation factors, further provides a substrate for firm clots. However, it is completely unknown if all of these suggested mechanisms are operational, in what sequence they occur, and which triggers are most important for clot formation and occurrence of VTE.
The occurrence of microthrombi in the pulmonary vascular bed is suggested to be a significant part of COVID‐19 pathogenesis: the remarkably high d‐dimer levels in COVID‐19 may relate to specific mechanisms by which the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) invades the body. 13 , 14 , 15 , 16 It is currently unknown whether the hypercoagulable state in people with COVID‐19 is driven by the systemic or local effect of the host immune response to infection or by direct infection of endothelial cells. SARS‐CoV‐2–induced thrombosis can be the result of activation of endothelial cells by circulating activated monocytes, while induction of microthrombi in the pulmonary vascular bed is due to infection of respiratory epithelial cells, which activate endothelial cells of the respiratory tract. Insight in the mechanisms resulting in the procoagulant state is necessary to substantiate therapeutic intervention and monitoring. Notably, influenza virus infections have also been shown to be associated with high incidences of thrombotic complications. 23 , 24 , 25 It remains to be proven that the high incidence of VTE in COVID‐19 is truly a COVID‐19–specific complication.
In addition to short‐term morbidity and mortality due to impaired gas exchange, thrombotic complications may aggravate the chronic complications of COVID‐19. In the general (non‐COVID) population, the post‐PE syndrome and postthrombotic syndrome (PTS) have been reported to occur in 50% of people with VTE despite adequate anticoagulation therapy. 26 , 27 These long‐term complications have a major impact on quality of life and are associated with higher risks of depression, unemployment, social isolation, and excess health care costs. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 However, the long‐term outcomes of COVID‐19–associated VTE are currently unknown.
To find the best possible management of the procoagulant state of people with COVID‐19, that is, to prevent and to optimally treat thrombotic complications, there is a pivotal need to better understand its pathogenesis, natural course, and risk factors. Precise knowledge of the safety and efficacy of currently prescribed thromboprophylaxis and treatments of thrombosis is necessary to guide optimal pharmacological prevention and treatment of COVID‐19–associated VTE.
2. THE DUTCH COVID & THROMBOSIS COALITION
The Dutch COVID & Thrombosis Coalition (DCTC) is a multidisciplinary team involving a large number of Dutch experts in the broad area of thrombosis and hemostasis research, combined with experts on virology, critical care medicine, pulmonary diseases, and community medicine, across all university hospitals and many community hospitals in the Netherlands. Within the consortium, clinical data of at least 5000 admitted COVID‐19–infected people are available, including substantial collections of biobanked materials in an estimated 3000 patients. These samples were prospectively collected from the beginning of the outbreak on, in different health care settings. Due to the emerging outbreak situation, samples were collected as part of ongoing research and biobanked in the participating institutions. Due to the emergency, there was not an overarching SARS‐CoV‐2 study protocol at that time. The use of biomaterial for the specific study questions on SARS‐CoV‐2 as described in this article was approved by the relevant local medical ethical committees. In addition to considerable experience in preclinical and clinical thrombosis research, the consortium embeds virology‐hemostasis research models within unique biosafety facilities to address fundamental questions on the interaction of virus with epithelial and vascular cells, in relation to the coagulation and inflammatory system.
The DCTC has initiated a comprehensive research program to answer many of the current questions on the pathophysiology and best treatment of COVID‐19–associated thrombotic complications. The main objective of DCTC’s research is to understand and prevent VTE in people with COVID‐19, and to optimize acute treatment and long‐term health of people with COVID‐19 who are diagnosed with VTE. This objective concerns the overall Dutch population but also important subgroups, including age categories, sex, and ethnic groups. The subobjectives of the five involved work packages (WPs) are summarized in Table 1, and the integration of the WPs is illustrated in Figure 1. The foundation and strength of this research is the enormous amount of collected data of people with COVID‐19 combined with locally collected and biobanked samples and materials. In addition, the consortium combines wide‐ranging expertise on topics in any way related to the current project. The research program was funded by grants of the Netherlands Thrombosis Foundation and the Netherlands Organization for Health Research and Development.
TABLE 1.
Objectives of the research program
| PATHOGENESIS (WP1&2) |
| HEMOSTASIS IN VIVO |
| Main objective: to unravel pathophysiological mechanisms that cause COVID‐19–associated coagulopathy. |
| 1. Investigate the presence of prothrombotic autoantibodies. |
| 2. Investigate the status of the fibrinolytic system. |
| 3. Evaluate the balance of the coagulation system. |
| 4. Analyze the composition of thrombi in tissues specimens. |
| 5. Identify molecular players and biomarkers for thrombosis. |
| VIROLOGY‐HEMOSTASIS IN VITRO |
| Main objective: to identify the molecular mechanisms of SARS‐CoV‐2–induced thrombosis using in vitro infection models of the pulmonary vascular bed. Tropism of SARS‐CoV‐2–infected endothelial cells will be compared to other respiratory viruses that cause VTE. |
| 1. Determine the role of respiratory epithelial cells in inducing a procoagulant state in pulmonary microvascular endothelial cells. |
| 2. Determine the role of activated monocytes from patients with COVID‐19 in inducing a procoagulant state in endothelial cells. |
| 3. Correlate data from in vitro experiments to pathology observations in autopsies. |
| 4. Determine the possible effect of nonneutralizing antibodies on SARS‐CoV‐2 infection kinetics and dynamics in respiratory endothelial cells, epithelial cells, and monocytes. |
| THROMBOPROPHYLAXIS AND TREATMENT OF THROMBOSIS (WP3) |
| Main objective: to define the optimal strategies for prevention and treatment of VTE in patients with COVID‐19. |
| 1. Investigate efficacy and safety of different doses of thromboprophylaxis. |
| 2. Investigate if therapeutic‐dose LMWH is superior to prophylaxis |
| 3. Investigate efficacy and safety of VTE treatment |
| BIOMARKERS AND PREDICTION OF THROMBOSIS (WP4) |
| Main objective: to predict the risk of VTE in admitted patients with COVID‐19 |
| 1. Develop and validate a dynamic prediction model to estimate individual VTE risks. |
| 2. Compare the predictive value between different mechanistic pathways, for different VTE phenotypes, and for the ward and ICU separately. |
| LONG‐TERM CONSEQUENCES OF VTE (WP5) |
| Main objective: to assess the impact and long‐term consequences of VTE in patients with COVID‐19. |
| 1. Evaluate the incidence of COVID‐19 associated VTE after hospital discharge. |
| 2. Assess patient‐reported outcome measures and pulmonary and cardiac function. |
| 3. Establish the rate of thrombus resolution and the incidence of post‐VTE syndromes. |
COVID‐19, coronavirus disease 2019; ICU, intensive care unit; LMWH, low‐molecular‐weight heparin; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; VTE, venous thromboembolism; WP, work package.
FIGURE 1.
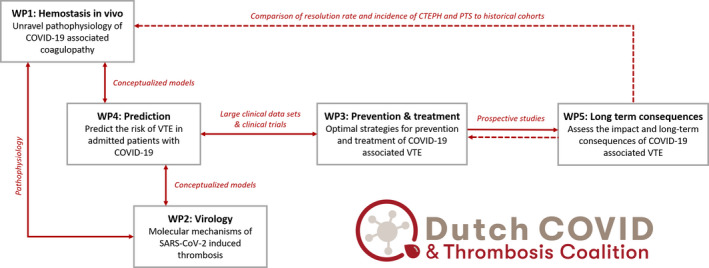
Integration of the five work packages
The goal of the paper is to present our aims and study design and invite other research consortia to collaborate with us in our pursuit to solve the major issue of COVID‐19–associated thrombosis. In particular, we hope to connect the research in WP1 and WP2 to international studies on the specific interaction of viruses and the inflammatory and coagulation system. For WP3 and WP4, we aim to externally validate constructed models and identified associations. Increasing the number of people with COVID‐19 associated VTE who were dedicatedly followed for the occurrence of long‐term complications will help to accurately estimate the incidence of chronic thromboembolic pulmonary hypertension (CTEPH) and PTS (WP5).
3. PATHOGENESIS OF COVID‐19–ASSOCIATED VENOUS THROMBOSIS (WP1 AND WP2)
3.1. Hemostasis in vivo (WP1)
This WP has the overarching aim to obtain mechanistic insight in COVID‐19–associated thrombosis. Five focus areas have been selected: (i) antiphospholipid antibodies, (ii) fibrinolysis, (iii) coagulation and anticoagulants, (iv) thrombus composition, and (5) omics. For each area, multicenter expert teams are in place to investigate responsible mechanisms in plasma samples and tissue specimens that are collected in other WPs in a collaborative manner. Most areas contain hypothesis‐driven research with existing technologies, but the omics approach will be used to identify possible new targets by profiling the plasma proteome of people with COVID‐19 with and without VTE in an unbiased manner. The proposed WPs make use of existing technologies that in some cases require adaptation for investigation of plasma samples from patients who are treated with anticoagulant therapy.
3.1.1. AIM 1: Investigate the presence of prothrombotic autoantibodies in people with COVID‐19
The presence of antiphosholipid antibodies and lupus anticoagulans (LAC) has been described in people with COVID‐19 and linked to unfavorable outcomes. 22 We will investigate whether the LAC phenomenon in people with COVID‐19 is caused by (auto)antibodies, through heat‐inactivation of LAC‐positive plasma samples from COVID‐19 patients. We will subsequently focus on the identification of the causal antibody population using solid‐phase binding assays with immobilized phospholipids and phospholipid‐binding proteins, including β2‐glycoprotein I and prothrombin, the known antigens for LAC‐inducing autoantibodies. To investigate the link between LAC and SARS‐CoV‐2 infections, we will examine cross‐reactivity with SARS‐CoV‐2 capsid proteins and study plasma levels of auto‐antibody targets. We will evaluate their effects on coagulation using adapted calibrated automated thrombography, with special attention to resistance to activated protein C, as well on platelet activation.
3.1.2. AIM 2: Investigate the status of the fibrinolytic system in people with COVID‐19
The working hypothesis is that a deregulated fibrinolytic system contributes to VTE in people with COVID‐19. We will estimate plasma fibrinolytic potential using a clot lysis assay: This functional assay is sensitive for all plasma components known to be involved in clot breakdown. 36 In brief, the clot lysis assay determines the lysis of a tissue factor–induced clot (generated from 50 μL of patient plasma in 96‐well microtiter plates) by exogenous tissue‐type plasminogen activator by monitoring changes in turbidity during clot formation and subsequent lysis. Large epidemiological studies have shown a decreased fibrinolytic capacity assessed with this assay to strongly predict thrombotic risk. 37 In addition, strongly decreased fibrinolytic potential has been demonstrated to predict multiorgan failure and outcome in patients admitted to the ICU with and without underlying liver disease. 38 A hypofibrinolytic state is strongly related to plasma levels of plasminogen activator inhibitor type 1 (PAI‐1) and thrombin‐activatable fibrinolysis inhibitor (TAFI). 36 We therefore will assess plasma levels of both proteins. Interestingly, PAI‐1 and TAFI levels increase in obesity (which is common in critically ill people with COVID‐19) and have both been suggested to be drivers of progression of organ failure in severe infection. We will therefore assess whether PAI‐1 and TAFI levels are independent predictors of adverse outcome of COVID‐19 pneumonia.
3.1.3. AIM 3: Evaluate the balance of the coagulation system in people with COVID‐19
Hemostasis is a delicately regulated biological system. We will assess the relative contribution of the extrinsic and intrinsic pathways of coagulation and the balance between the pro‐ and anticoagulant pathways. 39 , 40 This aim will be addressed employing a staggered approach from global overall coagulation assays to more specific assessment of individual activation markers of coagulation. Specifically designed thrombin generation assays will be applied to determine the extent of hypercoagulability and the contribution of the protein C pathway (activated protein C sensitivity ratio [APCsr], thrombomodulin). Quantification of coagulation proteases including enzyme‐inhibitor complexes will allow for detailed insight into the pathways involved. Due to potential limitations in sample transport, all assays will be optimized for use in each participating institute with shared standardized operating procedures.
3.1.4. AIM 4. Analyze the composition of COVID‐19–associated thrombi in tissues specimens
Autopsies of people with COVID‐19 are, and will be, performed at the Leiden and Rotterdam medical centers. In the thrombi tissue specimens achieved during autopsy, we will investigate thrombus structure and composition in relation to plasma biomarkers associated with thrombosis and formation neutrophil extracellular traps, age, sex, medication, duration and severity of SARS‐CoV‐2, and investigate if thrombus architecture predicts the early outcome of disease in people with COVID‐19. The biobank of samples in the participating centers includes collected thrombi and plasma that are available for this study, in which we will expand on the thrombus analysis. The composition of the collected thrombi and of in vitro plasma clots will be characterized using state‐of‐the‐art imaging techniques, such as scanning electron microscopy (SEM), routine immunocytochemistry, and advanced fluorescence superresolution confocal microscopy. Thrombus histology will focus on fibrin structure and (inflammatory) cellular composition. Specific immunohistological stainings for fibrin, neutrophils, and markers of NETosis will help in understanding the pathophysiology of thrombosis in people with COVID‐19 and may be extended, with more in‐depth approaches such as RNA sequencing.
The structural characteristics of fibrin fibers that determine permeability will be analyzed by SEM. These analyses will be compared to functional analyses of fibrinolysis rates (ie, the time that is required to degrade the fibrin matrix in vitro). These characteristics are determined by the structural properties of the matrix and by the adherence of cells and proteins during clot formation.
For the longitudinal analysis of internal thrombus heterogeneity, sections will be obtained serially, which allows for three‐dimensional reconstruction. In these sections, we will determine the amount and characteristics of the fibrin matrix, DNA extracellular traps, the number and type of cells and bound proteins, and their location within the thrombus by immunocytochemistry and proteomics. Findings will be linked to the data of patients with COVID‐19 to also consider a relationship with other data available from the case (including clinical data and blood and plasma values).
3.1.5. AIM 5: Identify molecular players and biomarkers for COVID‐19–associated thrombosis
We will use proteomic and genetic analyses to identify molecular players and possible biomarkers for thrombosis in COVID‐19. Recently, an automated, robust, and reproducible workflow for plasma profiling was implemented. 41 This technology provides a “hemostatic and inflammatory signature” by quantifying hundreds of plasma proteins simultaneously, including plasma proteins related to the coagulation and complement system. Importantly, this technology also allows for the detection of releasable contents of platelets, neutrophils, and endothelial cells that are intrinsically linked to thromboinflammatory events.
To study the underlying pathogenesis of VTE in people with COVID‐19, we will apply RNA‐sequencing autopsy material (specifically from the lungs) from deceased people with COVID‐19. Lung material from non‐COVID patients will be used as a control. RNA sequencing from formalin‐fixed paraffin‐embedded material is very feasible using protocols from the Pathology Department at LUMC. Gene signatures (top 200 upregulated and downregulated genes compared to controls) will be analyzed with online prediction tools such as Ingenuity Pathway Analysis and Enrichr to determine biologically relevant pathways underlying COVID‐19–associated thrombotic events, as done before for cancer‐associated thrombotic events. 42 We expect genes and biological pathways associated with inflammation, complement and platelet activation, contact activation, and the intrinsic coagulation pathway to be upregulated.
3.2. Virology‐hemostasis in vitro (WP2)
The main objective in this WP is to identify the molecular mechanisms of SARS‐CoV‐2–induced thrombosis using in vitro infection models of the pulmonary vascular bed and correlate findings to the results from the in vivo studies (WP1) in a translational approach. Tropism of SARS‐CoV‐2–infected endothelial cells will be compared to other respiratory viruses that cause VTE. To fully understand coagulopathy seen in people with COVID‐19 in vitro models studying both the virus and the host response are essential. WP2 will focus on in vitro models studying SARS‐CoV‐2 kinetics and dynamics and their effect on endothelial cells and coculture models. By using infection models, differentiation between direct effects of SARS‐CoV‐2 infection on endothelial cell function and other known causes of endothelitis that might contribute to COVID‐19 pathogenesis can be made. In these primary isolates and coculture models, it can be defined if the procoagulant changes are the result of infection or actual activation of endothelial cells, for instance, by circulating activated monocytes or a damaged basolateral pulmonary epithelium. The latter have proven to play an important role in the pathogenesis of lower respiratory tract influenza virus infection. 43 Furthermore, the relation between microthrombi in the pulmonary vascular bed 44 and subsequent damage in respiratory epithelial cells will be studied. In WP2, the following four specific aims are pursued.
3.2.1. AIM 1: Determine the role of respiratory epithelial cells in inducing a procoagulant state in pulmonary microvascular endothelial cells
The working hypothesis is that SARS‐CoV‐2 infection of respiratory epithelial cells results in alveolar barrier damage and induction of a procoagulant state in endothelial cells. We will coculture SARS‐CoV‐2–infected human respiratory cells with lung microvascular endothelial cells and (i) determine virus kinetics and dynamics, (ii) quantify levels of key inflammatory and procoagulant mediators, (iii) measure epithelial and endothelial barrier function, and (iv) quantify endothelial cell hemostatic function by adapted cell surface thrombin generation tests and tissue factor quantification.
3.2.2. AIM 2: Determine the role of activated monocytes from people with COVID‐19 in inducing a procoagulant state in endothelial cells
The postulation that activated monocytes in people with COVID‐19 induce a procoagulant state in endothelial cells will be investigated. We will analyze monocytes from people with COVID‐19 on tissue factor activity. Furthermore, these monocytes will be cocultured with human umbilical vein endothelial cells and (i) quantify levels of key inflammatory and procoagulant mediators, (ii) measure endothelial barrier function, and (iii) quantify endothelial cell hemostatic function.
3.2.3. AIM 3: Correlate data from in vitro experiments to pathology observations in autopsies
The postulation that key mediators identified in Aims 1 and 2 can be observed in lung samples from deceased patients (microthrombi and PE) will be investigated. Lung samples from autopsies of fatal SARS‐CoV‐2 infection will be obtained to detect the presence of key procoagulant mediators by immunohistochemistry/in situ hybridization and correlate this with presence of viral antigen and virus‐induced histopathological changes, as has been performed before in influenza virus infection models. 45
3.2.4. AIM 4: Determine the possible effect of nonneutralizing antibodies on SARS‐CoV‐2 infection kinetics and dynamics in respiratory endothelial cells, epithelial cells, and monocytes
Nonneutralizing antibodies may play a role in SARS‐CoV‐2 kinetics and on the extent of the inflammatory response. Serum samples from blood donors who recovered from SARS‐CoV‐2 with different types of antibody responses to SARS‐CoV‐2 as determined by multiantigen profiling of responses have been collected. Using high‐neutralizing and low‐neutralizing binding antibodies, its effect on SARS‐CoV2 kinetics and dynamics in primary cell cultures will be investigated.
4. THROMBOPROPHYLAXIS AND TREATMENT OF COVID‐19–ASSOCIATED VENOUS THROMBOSIS (WP3)
The key aims of WP3 are (i) to investigate the efficacy and safety of different doses of thromboprophylaxis, (ii) to investigate if therapeutic‐dose LMWH is superior to prophylaxis, and (iii) to investigate the efficacy and safety of VTE treatment.
One of the key questions that emerged during the COVID‐19 pandemic relates to the optimal dosing of anticoagulant prophylaxis, most often with LMWH. To address this question, we will combine clinical data on efficacy and safety (bleeding) of anticoagulant strategies derived from many Dutch hospitals, both from established registries (Covidpredict.nl, Isaric, NICE, CAPACITY COVID) and from targeted collection by participating centers. The advantage of the use of multiple sources is enrichment of data to compensate for structural missing data when only one source is used. The Dutch guideline on thromboprohylaxis in people with COVID‐19 recommended to double the doses of LMWH, in particular for patients admitted to the ICU departments. Because the overall choice for a type of treatment per center is predominantly determined by the preference of the treating physicians (ie, the anticoagulant protocol), this will be unrelated to the prognosis of a particular patient and hence be relatively random. This way, and given several additional assumptions, this so‐called instrumental variable (IV) design (using “center” as IV) can mimic a randomized controlled trial (RCT) in which individuals are randomized. Situations when the treatment of an individual patient deviates from this protocol are taken into account in the analysis. 46 Main study end points include all documented VTEs, 47 major bleeding according to International Society on Thrombosis and Haemostasis criteria, 48 and death; follow‐up will be completed at 3 months. Given the need for optimal use of time and resources, the consortium will not start new treatment studies but will coordinate and facilitate participation of Dutch centers in ongoing international RCTs (REMAP‐COVID; https://www.remapcap.org/).
All COVID‐19–infected patients who develop a VTE during their hospital stay will be followed either at the outpatient clinic or, if not possible (living in other region, not able to come to the clinic), by the general practitioner (GP). Linking to the GP and nursing home networks allows for sharing data from hospital and primary care to become optimally informed about all relevant outcomes (recurrent VTE, arterial thromboembolism, major bleeding, readmission, death). Anticipating 5000 patients within the framework of this consortium, data on at least 90% of this population can be accumulated. Incident outcomes will be compared with data from known ongoing cohorts of patients with VTE but without COVID‐19 infection.
While the required dose of thromboprophylaxis is disputed and thus investigated in cohort and RCT studies, there is little debate about optimal treatment of COVID‐19–related VTE. Notably, little is known of the efficacy and safety of anticoagulant treatment of COVID‐19–associated thrombotic complications. We aim to address this issue by implementing existing laboratory assays that monitor in different ways global coagulation activity and can inform physicians about anticoagulant activity of unfractionated heparin and LMWH. To accumulate data on biochemical efficacy of anticoagulant treatment of admitted patients, anti‐Xa, thrombin generation, and rotational thromboelastometry/thromboelastography measurement are performed in a number of centers and related to type and dose of anticoagulant as well as to clinical outcomes. More centers will be engaged to include such assays or, if not available on site, to store plasma samples for analysis.
5. BIOMARKERS AND PREDICTION OF COVID‐19–ASSOCIATED VENOUS THROMBOSIS (WP4)
Currently, the observed high risk of thrombotic complications in admitted patients with COVID‐19 leads to strong preventive measures, that is, administering of double or even therapeutic dosages of LMWH in ICU patients. A high dose of thromboprophylaxis may be effective to prevent thrombosis in patients with a high risk of thrombosis, but it will also needlessly increase the bleeding risk. It is therefore of utmost clinical importance to identify subjects at particular low or high thrombosis risk, to expose only high‐risk individuals to the benefits and risks of thromboprophylaxis. The aim of this WP is therefore to develop and validate a dynamic prediction model to estimate individual VTE risks. In secondary analyses, we plan to compare the predictive value between different mechanistic pathways, for different VTE phenotypes and for the ward and ICU separately. Clinical information of the study population will be supplemented with routine laboratory parameters and imaging data. Furthermore, during the study, more information from the different mechanistic pathways will become available from WP1 and WP2 that can be used for input in the model. Outcome assessment cases with VTE will be identified based on objective radiological tests and different phenotypes of VTE (DVT, PE in the large arteries, [sub]segmental PE) will be distinguished, and their occurrence over time accurately recorded. Candidate predictors will be selected based on the available COVID‐19 literature, supplemented with expert opinion on variables associated with VTE in general. Potential predictors do not have to be causally related to the outcome of interest. Due to different policies between centers, some data may be missing. As we consider these data to be missing at random, we will use multiple imputation techniques.
The association between candidate predictors and VTE will be assessed by Fine‐Gray regression analyses using backward selection procedures (criterion P < .15), applying a time scale starting at hospital admission, and adjusting for competing risk of death. 49 Continuous predictors will not be categorized, and splines will be used to model possible nonlinear relations. The magnitude of discrimination of the model will be quantified with Harrell’s C‐statistic. Regression coefficients will be reduced using a cross‐validated penalized regression approach to correct for optimism. 50 Subgroup analyses will be performed using different subsets of predictors and of outcome phenotypes, first according to the different mechanistic pathways as mentioned under WP1 and WP2.
Second, separate models will be developed and compared for the different VTE phenotypes (characterized as detailed as possible based on clinical and radiological information) as well as separate models limited to ICU patients and starting follow‐up from the day of ICU admission. Internal validation will be taken into account by use of cross‐validated penalized regression. For external validation, we plan to use data from international collaborators.
6. LONG‐TERM CONSEQUENCES OF COVID‐19–ASSOCIATED VENOUS THROMBOSIS (WP5)
A dedicated patient pathway (Figure 2) will be established in all participating hospitals in close collaboration between the departments of pulmonology, internal/vascular medicine, hematology, and cardiology, where all previously admitted patients with COVID‐19 will be followed. At the 3‐month follow‐up visit, all survivors with objective VTE diagnosed will be identified and subjected to state‐of‐the‐art follow‐up procedures as recommended by current Dutch guidelines, aimed at establishing the rate of thrombus resolution and early identification and treatment of long‐term VTE consequences, at efficient use of health care resources. After both 3‐month and 12‐month follow‐up, the recovery of patients will be assessed using patient‐reported outcome measures of quality of life, dyspnea, and functional status. 28 , 51 , 52 , 53 Dedicated diagnostic tests will be applied where appropriate to investigate new or persistent unexplained symptoms or poor functional recovery. 54 By putting the findings (thrombus resolution rates, prevalence of long‐term complications, quality of life, and functional recovery) in patients with COVID‐19 with VTE in the perspectives of two control cohorts, that is, COVID‐19 survivors not diagnosed with VTE and people with VTE from the pre–COVID‐19 era, we will be able to determine the additional impact of VTE in people with COVID‐19, as well as the aggravating effect of the combination of COVID‐19 and VTE compared to VTE without COVID‐19.
FIGURE 2.
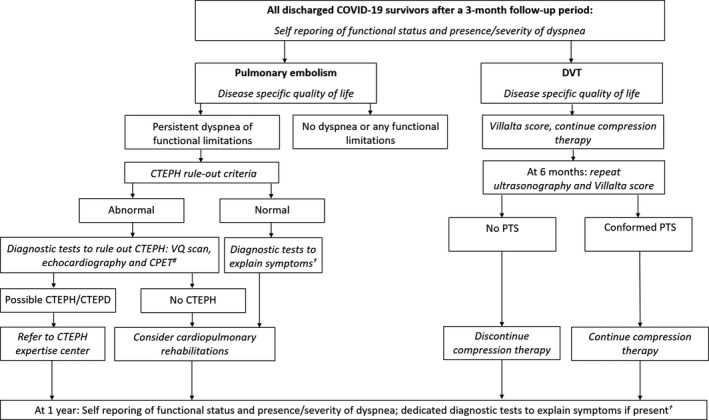
Dedicated patient pathway applied to survivors of COVID‐19–associated VTE according to the Dutch Guideline of care for patients with COVID‐19–associated venous thromboembolism. #CPET is indicated if echocardiography does not show signs of pulmonary hypertension. †VQ scan, pulmonary function tests, CPET, echocardiography depending in individual patient characteristics. In case of Chronic Thromboembolic Pulmonary Disease (CTEPD), patients should be referred to a CTEPH expertise center. CTEPH, chronic thromboembolic pulmonary hypertension; CTEPD, chronic thromboembolic pulmonary disease; VQ scan, ventilation perfusion scintigraphy; PTS, postthrombotic syndrome; CPET, cardiopulmonary exercise test
6.1. Patients with pulmonary embolism
In addition to the standard follow‐up procedures applied to all COVID‐19 survivors, the presence of and risk factors for CTEPH will be assessed in all patients. The pretest probability of CTEPH will be estimated by calculating the “CTEPH prediction score.” 55 , 56 , 57 Only patients with a high pretest probability (>6 points) or those with symptoms that might be associated with CTEPH will be subjected to the CTEPH rule‐out criteria, that is, assessment of the presence of any of the three electrocardiographic criteria of right ventricular (RV) pressure overload, or an abnormal age‐ and gender‐dependent N‐terminal prohormone of brain natriuretic peptide level. 57 , 58 , 59 If at least one of the CTEPH rule‐out criteria could not preclude the presence of RV pressure overload, patients were referred for transthoracic echocardiography, performed according to the 2015 European Society of Cardiology/European Respiratory Society guidelines on pulmonary hypertension. 60 , 61 In case of echocardiographic signs of CTEPH, patients will be subjected to ventilation perfusion scintigraphy. If abnormal, further diagnostic procedures for the final diagnosis of CTEPH will be performed in a CTEPH expertise center. Patients who report persistent functional limitations and/or dyspnea, in whom CTEPH is considered to be ruled out, are referred for perfusion imaging to establish the rate of persistent thrombotic occlusions and perfusion defects. In addition, a complete cardiopulmonary exercise tests will be performed. Based on these results, the cause of the symptoms can be determined, for example, dead space ventilation, breathing abnormalities, deconditioning, pulmonary fibrosis, or otherwise. Patients with persistent functional limitations (Post‐VTE Functional Status scale ≥2 52,62 ), without a strong indication for cardiopulmonary rehabilitation because of post‐ICU syndrome, will be referred for cardiopulmonary rehabilitation aimed at reducing post‐PE syndrome–based functional impairment. The effect of the rehabilitation with regard to functional status, quality of life, and parameters of physical performance will be assessed. At the 1‐year follow‐up visit, patients with persistent symptoms of dyspnea or decreased functional status will be subjected to appropriate diagnostic tests to rule out the presence of late‐onset CTEPH.
6.2. Patients with deep vein thrombosis of the legs
At 6 months, repeat ultrasound imaging of the affected leg will be routinely performed to evaluate the presence and extend of persistent vein obstruction, and the presence of PTS will be established using the Villalta score. 27 , 63 Compression therapy will be tailored on the basis of clinical signs and symptoms scored according to the Villalta PTS scale; patients assigned to individualized therapy with two consecutive Villalta scores of ≤4 will be counseled to stop using the stockings. 64 , 65 All patients will be followed for 1 year for evaluation of the course of PTS symptoms.
7. CHALLENGES
The proposed project is challenging. Mainly, the success of the WPs depends on the actual availability and quality of the biobanks that were built, the possibility to extend and merge large existing databases in the participating hospitals, the strict and independent adjudication of all relevant study outcomes, and the number of patients on whom data (during both admission and long‐term follow‐up) are available for analysis. Much also will depend on the intensity of second or more waves of infection, which will increase the available data/material to work with but also limit the availability of the clinicians to be involved in clinical studies and the possibility to perform preclinical experiments.
8. CONCLUSIONS
The proposed outcome from in vivo and in vitro experiments will give us further insights necessary to understand the pathophysiology of thrombotic complications in people with COVID‐19, and the clinical studies will help predict thrombotic complications as well as optimize therapeutic and preventive strategies. Finally, by focused and rigorous follow‐up of patients with COVID‐19, long‐term complications will be identified early, which will allow for optimal long‐ and short‐term treatment of these patients.
RELATIONSHIP DISCLOSURE
MK has received unrestricted grants paid to the department for research outside this work from Bayer, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Sobi, and has received a speaker’s fee paid to the department from Bayer. FK reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD and Actelion, the Dutch Heart Foundation, and the Dutch Thrombosis Association, all outside the submitted work. EvG received unrestricted [educational] grants paid to the department outside this research from GSK, Pfizer, Gilead, and Janssen.
AUTHOR CONTRIBUTIONS
All authors contributed to the drafting of this manuscript and approved of the final submitted version.
ACKNOWLEDGMENTS
The research program was funded by grants of the Netherlands Thrombosis Foundation (2020_A) and the Netherlands Organization for Health Research and Development (project number 10430012010004).
APPENDIX A.
Albert Schweitzer hospital
Drs. L. F. Te Velde, intensivist
Dr. P. E. Westerweel, haematologist
Amphia hospital
Dr. M. J. J. H. Grootenboers, pulmonologist
Dr. C. van Guldener, vascular medicine specialist
Dr. K. M. Kant, pulmonologist
Amsterdam University Medical Centre
Location AMC
Dr. M. Coppens, vascular medicine specialist
Drs. N. van Es, PhD candidate
Prof. dr. N. P. Juffermans, intensivist
Drs. T. F. van Haaps, PhD‐candidate Department of vascular medicine
Prof. dr. S. Middeldorp, vascular medicine specialist
Dr. A. P. J. Vlaar, intensivist
Location VUMC:
Prof. dr. C. M. P. M. Hertogh, nursing home specialist
Drs. J. G. Hugtenburg, pharmacologist
Dr. J. van Kooten, nursing home specialist
Dr. E. J. Nossent, pulmonologist
Prof. Dr. Y. Smulders, internist
Dr. P. R. Tuinman, intensivist
Dr. A. Vonk Noordegraaf, pulmonologist
Argos zorggroep
Drs. A. Lansbergen, physiotherapist
Bravis Hospital
Drs. J. Leijs, anaesthesiologist‐intensivist
Drs B. Simons, intensivist
Dr. M. Stegenga, internist
Deventer hospital
Dr. G. R. Hajer, vascular medicine specialist
Dr. A. Stemerdink, intensivist
Deaconess hospital Utrecht
Dr. L. E. M. van Lelyveld, intensivist
Dr. C. E. E. van Ofwegen, cardiologist
Erasmus Medical Centre
Drs. J. van den Akker, intensivist
Dr. R. Bierings, cellular biologist, Department Haematology
Dr. H. Endeman, intensivist.
Dr. M. Goeijenbier, internal medicine, Department Viroscience
Dr. N. G. M. Hunfeld, hospital pharmacist
Prof. dr. E. C. M. van Gorp, infectious diseases specialist, Department Viroscience
Prof. dr. D. A. M. P. J. Gommers, intensivist
Prof. dr. M. P. G. Koopmans, internist, Department Viroscience.
Prof. dr. T. Kuiken, professor of comparative pathology, Department Viroscience
Drs. T. Langerak, PhD‐candidate, Department Viroscience
Dr. M. N. Lauw, haematologist, Department Haematology
Prof. dr. M. P. M. de Maat, head of Biochemistry of Haemostasis and Thrombosis.
Drs. D. Noack, PhD‐candidate Department Viroscience
Drs. M. S. Paats, pulmonologist
Drs. M. P. Raadsen, PhD‐candidate Department Viroscience
Dr. B. Rockx, internist, Department Viroscience
Dr. C. Rokx, infectious diseases specialist
Dr. C. A. M. Schurink, infectious diseases specialist
Drs. K. Tong‐Minh, PhD‐candidate Department Viroscience
Dr. L. van den Toorn, pulmonologist
Dr. C. A. den Uil, Cardiologist‐Intensivist
Drs. C. Visser, PhD‐candidate, Department haematology
Farmadam:
Drs. F. Boutkourt, PhD‐candidate
Drs. T. Roest, pharmacist
Flevohospital Almere
Dr. R. A. Douma, infectious diseases specialist
Dr. M. ten Wolde, internist
Franciscus Gasthuis & Vlietland
Dr. E. J. Wils, intensivist
Hagahospital
Dr. C. van Nieuwkoop, internist
IJsselland hospital
Drs. J. H. Elderman, intensivist
Drs. H. A. Kleinveld, internist
Ikazia Hospital
Dr. S. Stads, intensivist
Jeroen Bosch Hospital
Dr. C. P. C. de Jager, intensivist
Dr. A. P. M. Kerckhoffs, internist
Leids University Medical Center
Drs. M. L. Antoni, cardiologist, Department of cardiology
Dr. M. H. Bos, biochemicus, associate professor, Department of Medicine ‐ Thrombosis and Haemostasis
Drs. J. L. I, Burggraaf, PhD‐candidate , Department of Clinical Epidemiology
Prof. S. C. Cannegieter, clinical epidemiologist, Department of Medicine ‐ Thrombosis and Hemostasis and Department of Department of Clinical Epidemiology
Prof. dr. H. C. J. Eikenboom, haematologist/vascular medicine specialist , Department of Medicine ‐ Thrombosis and Haemostasis.
Dr. P. L. den Exter, vascular medicine specialist, Department of Medicine ‐ Thrombosis and Haemostasis
Dr. J. J. M. Geelhoed, pulmonologist, Department of Pulmonology
Prof. dr. M. V. Huisman, vascular medicine specialist, Department of Medicine ‐ Thrombosis and Haemostasis
Prof. E. de Jonge, internist‐intensivist, Department of Intensive Care Medicine
Drs. F. H. J. Kaptein, PhD‐candidate, Department of Medicine ‐ Thrombosis and Hemostasis
Dr. F. A. Klok, vascular medicine specialist, Department of Medicine ‐ Thrombosis and Haemostasis
Dr. L. J. M. Kroft, radiologist, Department of radiology
Drs. L. Nab, PhD‐candidate, Department of Clinical Epidemiology
Dr. M. K. Ninaber, pulmonologist, Department of Pulmonology
Prof. dr. H. Putter, statistician, Department of Biomedical Data Sciences
Drs. S. R. S. Ramai, pulmonologist, Department of Pulmonology
Dr. A. M. da Rocha Rondon, Postdoctoral researcher, Department of Medicine ‐ Thrombosis and Haemostasis
Dr. A. H. E. Roukens, infectious diseases specialist, Department of infectious diseases
Drs. M. A. M. Stals, PhD‐candidate, Department of Medicine ‐ Thrombosis and Haemostasis
Prof. dr. H .H. Versteeg, cellular biologist, Department of Medicine ‐ Thrombosis and Haemostasis
Dr. H. W. Vliegen, cardiologist, Department of cardiology
Dr. B. J. M. van Vlijmen, cellular biologist, associate professor, Department of Medicine ‐ Thrombosis and Haemostasis
Maasstad hospital
Dr. C. J. van den Bout, intensivist
Drs. A. J. M. van den Enden
Drs. G. Helfrich, pulmonologist
Drs. M. C. van Herwerden, internist in training
Dr. J. G. den Hollander, infectious diseases specialist
Drs. S. Kok
Dr. J. A. M. Labout, intensivist
Dr. E. L. Moussaoui, infectious disease specialist
Dr. E. A. N. Oostdijk, intensivist
Drs. R. Slingerland, pulmonologist
Drs. J. B. van Steenkiste
Maastricht University Medical Centre
Drs. T. van de Berg, PhD‐candidate
Drs. R. Bruggemann, PhD‐candidate
Dr. B. C. T. van Bussel, internist‐intensivist
Prof. dr. H. ten Cate, Internist
Dr. A. Ten Cate‐Hoek, Clinical epidemiologist and medical director of thrombosis service Maastricht
Prof. dr. T. M. Hackeng, biochemist
Dr. ir. Y. Henskens, clinical chemist
Drs. A. Hulshof, PhD‐candidate
Drs. M. Mulder, PhD‐candidate
Prof. dr. L. Schurgers, biochemist
Dr. B. Spaetgens, internist subspecialized in geriatrics
Dr. H. Spronk, biochemist
Prof. dr. M. A. Spruit, executive board member CIRO and Professor in Rehabilitation
Dr. K. Winckers, vascular medicine specialist
Martini Hospital
Dr. R. Hulst, hospital pharmacist
Drs A. C. Reidinga, anaesthesiologist‐intensivist
Drs. R. Sayilir, intensivist
Maxima Medical Centre
Dr. L. Nieuwenhuizen, internist
Medical Centre Leeuwaarden
Drs. B. Franken, haematologist
Dr. I. M. Schrover, vascular medicine specialist
Drs. E. G. M. de Waal, haematologist
Medical Spectrum Twente
Dr. A. Beijshuizen, intensivist
Drs. C. E. Delsing, infectious diseases specialist
Radboud University Medical Centre
Prof. dr. K. Kramer, professor medical safety
Dr. J. Leentjens, vascular medicine specialist
Dr. Q. de Mast, infectious diseases specialist
Noordwest Hospital Group
Dr. W. A. Bax, internist
Dr. H. J. Doodeman, epidemiologist
Dr. S. Simsek, internist
Dr. F. Stam, internist
Onze Lieve Vrouwe Gasthuis hospital
Prof. Dr. N. P. Juffermans, intensivist
Dr. S. van Wissen, vascular medicine specialist
Reinier de Graaf Gasthuis hospital
Dr. R. E. Brouwer, haematologist
Dr. J. L. J. Ellerbroek, infectious diseases specialist
Drs. J. Tijmensen, haematologist
Rijnstate hospital
Dr. M. M. C. Hovens, vascular medicine specialist
Drs. B. D. Westerhof, Anaesthesiologist‐intensivist
Rode Kruis Hospital
Dr. L. M. Faber, Haematologist
Sanquin Research, Amsterdam:
Dr. M. van den Biggelaar, head of Laboratory of Proteomics, Department of Molecular and Cellular Hemostasis
Prof. dr. J. C. M. Meijers, biochemist (and Amsterdam University Medical Centres)
Prof. dr. J. Voorberg, molecular and cellular biologist (and Amsterdam University Medical Centres)
St. Jansdal hospital
Dr. F. N. Croles, Haematologist
Tergooi hospital
Prof. Dr. P. W. Kamphuisen, vascular medicine specialist
Dr. R. Vink, intensivist
Synapse Research Institute:
Dr. B. de Laat, biochemist, director
University Medical Centre Groningen:
Prof. dr. T. Lisman, biochemist
Prof. dr. K. Meijer, haematologist, Department Haematology
Dr. Y. I. G. van Tichelaar, internist
University Medical Centre Utrecht:
Prof. Dr. O. L. Cremer, anaesthesiologist‐intensivist
Dr. G. Geersing, general practitioner, Julius Center, Department primary care
Drs. A. D. Huisman, dietician
Prof dr. H.A.H. Kaasjager, vascular medicine specialist
Dr. N. Kusadasi, haematologist‐intensivist
Dr. M. Nijkeuter, vascular medicine specialist
Prof. dr. R.E.G. Schutgens, haematologist, Van Creveldkliniek
Dr. R.T. Urbanus, biochemist, Van Creveldkliniek
Drs. J. Westerink, vascular medicine specialist
Wilhelmina hospital Assen
Drs. H. J. Faber, intensivist
Zaans Medical Center
Drs. S. Koster, anaesthesiologist‐intensivist
Hospital Group Gelderse Vallei
Dr. R. H. H. Bemelmans, internist
Dr. B. Festen, intensivist
Zorgsaam Hospital
Dr. M. A. van Dijk, internist
Drs. A. P. Hall, intensivist
Zuyderland Hospital
Dr. D. J. L. van Twist, vascular medicine specialist
Cannegieter SC, ten Cate H, van Gorp ECM, et al; The Dutch COVID Thrombosis Coalition study group . Caging the dragon: Research approach to COVID‐19–related thrombosis. Res Pract Thromb Haemost. 2021;5:278–290. 10.1002/rth2.12470
Handling Editor: Mary Cushman
Contributor Information
Marieke J. H. A. Kruip, Email: m.kruip@erasmusmc.nl, Email: DCTC@erasmusmc.nl.
Frederikus A. Klok, @Erik_Klok_MD.
REFERENCES
- 1. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–7. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148–50. 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thrombosis haemostasis: JTH. 2020;18(8):1995–2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santoliquido A, Porfidia A, Nesci A, De Matteis G, Marrone G, Porceddu E, et al. Incidence of deep vein thrombosis among non‐ICU patients hospitalized for COVID‐19 despite pharmacological thromboprophylaxis. J Thrombosis Haemostasis: JTH. 2020;18(9):2358–2363. 10.1111/jth.14992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren B, Yan F, Deng Z, Zhang S, Xiao L, Wu M, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID‐19 in Wuhan. Circulation. 2020;142:181–3. 10.1161/circulationaha.120.047407 [DOI] [PubMed] [Google Scholar]
- 7. Nahum J, Morichau‐Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID‐19). JAMA Network Open. 2020;3:e2010478. 10.1001/jamanetworkopen.2020.10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Longchamp A, Longchamp J, Manzocchi‐Besson S, Whiting L, Haller C, Jeanneret S, et al. Venous thromboembolism in critically Ill patients with COVID‐19: results of a screening study for deep vein thrombosis. Res Pract Thrombosis Haemostasis. 2020;4:842–7. 10.1002/rth2.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thrombosis Haemostasis. 2020;4(7):1178‐1191. 10.1002/rth2.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D, et al. Deep venous thrombosis in medical‐surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33:1565–71. 10.1097/01.ccm.0000171207.95319.b2 [DOI] [PubMed] [Google Scholar]
- 11. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:2950–73. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230‐1232. 10.1055/s-0040-1712097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, et al. Clinical and computed tomography characteristics of COVID‐19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–9. 10.1016/j.thromres.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respirat Med. 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. New Engl J Med. 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thrombosis Haemostasis: JTH. 2020;18:1559–61. 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levi M, Hunt BJ. Thrombosis and coagulopathy in COVID‐19: an illustrated review. Res Pract Thrombosis Haemostasis. 2020;4:744–51. 10.1002/rth2.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannegieter SC, Klok FA. COVID‐19 associated coagulopathy and thromboembolic disease: commentary on an interim expert guidance. Res Pract Thrombosis Haemostasis. 2020;4:439–45. 10.1002/rth2.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–20. 10.1016/s0140-6736(20)30920-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thrombosis Haemostasis: JTH. 2020;18:1738–42. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID‐19. JCI Insight. 2020;5(11):e138999. 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid‐19. New Engl J Med. 2020;383:288–90. 10.1056/NEJMc2013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. New Engl J Med. 2018;378:345–53. 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 24. Keller TT, van der Sluijs KF, de Kruif MD, Gerdes VE, Meijers JC, Florquin S, et al. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006;99:1261–9. 10.1161/01.RES.0000250834.29108.1a [DOI] [PubMed] [Google Scholar]
- 25. Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–96. 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post‐PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28:221–6. 10.1016/j.blre.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 27. Rabinovich A, Kahn SR. How I treat the postthrombotic syndrome. Blood. 2018;131:2215–22. 10.1182/blood-2018-01-785956 [DOI] [PubMed] [Google Scholar]
- 28. Kahn SR, Ducruet T, Lamping DL, Arsenault L, Miron MJ, Roussin A, et al. Prospective evaluation of health‐related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165:1173–8. 10.1001/archinte.165.10.1173 [DOI] [PubMed] [Google Scholar]
- 29. Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA, et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med. 2017;130(990):e9–e21. 10.1016/j.amjmed.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 30. Akaberi A, Klok FA, Cohn DM, Hirsch A, Granton J, Kahn SR. Determining the minimal clinically important difference for the PEmbQoL questionnaire, a measure of pulmonary embolism‐specific quality of life. J Thrombosis Haemostasis: JTH. 2018;16:2454–61. 10.1111/jth.14302 [DOI] [PubMed] [Google Scholar]
- 31. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. 10.1016/j.thromres.2015.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunter R, Lewis S, Noble S, Rance J, Bennett PD. “Post‐thrombotic panic syndrome”: a thematic analysis of the experience of venous thromboembolism. Br J Health Psychol. 2017;22:8–25. 10.1111/bjhp.12213 [DOI] [PubMed] [Google Scholar]
- 33. Sista AK, Klok FA. Late outcomes of pulmonary embolism: the post‐PE syndrome. Thromb Res. 2018;164:157–62. 10.1016/j.thromres.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 34. Chuang LH, van Hout B, Cohen AT, Gumbs PD, Kroep S, Bauersachs R, et al. Deep‐vein thrombosis in Europe ‐ burden of illness in relationship to healthcare resource utilization and return to work. Thromb Res. 2018;170:165–74. 10.1016/j.thromres.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 35. Hunter R, Noble S, Lewis S, Bennett P. Long‐term psychosocial impact of venous thromboembolism: a qualitative study in the community. BMJ Open. 2019;9:e024805. 10.1136/bmjopen-2018-024805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meltzer ME, Lisman T, de Groot PG, Meijers JC, le Cessie S, Doggen CJ, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI‐1. Blood. 2010;116:113–21. 10.1182/blood-2010-02-267740 [DOI] [PubMed] [Google Scholar]
- 37. Lisman T. Decreased plasma fibrinolytic potential as a risk for venous and arterial thrombosis. Semin Thromb Hemost. 2017;43:178–84. 10.1055/s-0036-1585081 [DOI] [PubMed] [Google Scholar]
- 38. Blasi A, Patel VC, Adelmeijer J, Azarian S, Hernandez Tejero M, Calvo A, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute‐on‐chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology (Baltimore, MD). 2020;71:1381–90. 10.1002/hep.30915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alshaikh NA, Rosing J, Thomassen M, Castoldi E, Simioni P, Hackeng TM. New functional assays to selectively quantify the activated protein C‐ and tissue factor pathway inhibitor‐cofactor activities of protein S in plasma. J Thrombosis Haemostasis: JTH. 2017;15:950–60. 10.1111/jth.13657 [DOI] [PubMed] [Google Scholar]
- 40. Visser M, Heitmeier S, Ten Cate H, Spronk HMH. Role of factor XIA and plasma kallikrein in arterial and venous thrombosis. Thromb Haemost. 2020;120:883–993. 10.1055/s-0040-1710013 [DOI] [PubMed] [Google Scholar]
- 41. Geyer PE, Holdt LM, Teupser D, Mann M. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol. 2017;13:942. 10.15252/msb.20156297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ünlü B, van Es N, Arindrarto W, Kiełbasa SM, Mei H, Westerga J, et al. Genes associated with venous thromboembolism in colorectal cancer patients. J Thrombosis Haemostasis: JTH. 2018;16:293–302. 10.1111/jth.13926 [DOI] [PubMed] [Google Scholar]
- 43. Short KR, Kasper J, van der Aa S, Andeweg AC, Zaaraoui‐Boutahar F, Goeijenbier M, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Resp J. 2016;47:954–66. 10.1183/13993003.01282-2015 [DOI] [PubMed] [Google Scholar]
- 44. Menter T, Haslbauer JD, Nienhold R, Savic S, Deigendesch H, Frank S, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198‐209 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goeijenbier M, van Gorp EC, Van den Brand JM, Stittelaar K, Bakhtiari K, Roelofs JJ, et al. Activation of coagulation and tissue fibrin deposition in experimental influenza in ferrets. BMC Microbiol. 2014;14:134. 10.1186/1471-2180-14-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short‐term drug effects using a physician‐specific prescribing preference as an instrumental variable. Epidemiology (Cambridge, Mass). 2006;17:268–75. 10.1097/01.ede.0000193606.58671.c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thrombosis Haemostasis: JTH. 2013;11:412–22. 10.1111/jth.12124 [DOI] [PubMed] [Google Scholar]
- 48. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S , Standardization Committee of the International Society on T , Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thrombosis Haemostasis: JTH. 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 49. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi‐state models. Stat Med. 2007;26:2389–430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 50. Goeman JJ. L1 penalized estimation in the Cox proportional hazards model. Biometric J Biometrische Zeitschrift. 2010;52:70–84. 10.1002/bimj.200900028 [DOI] [PubMed] [Google Scholar]
- 51. Klok FA, Boon G, Barco S, Endres M, Geelhoed JJM, Knauss S, et al. The Post‐COVID‐19 Functional Status (PCFS) scale: a tool to measure functional status over time after COVID‐19. Eur Resp Journal. 2020;56:2001494. 10.1183/13993003.01494-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klok FA, Cohn DM, Middeldorp S, Scharloo M, Buller HR, van Kralingen KW, et al. Quality of life after pulmonary embolism: validation of the PEmb‐QoL Questionnaire. J Thrombosis Haemostasis: JTH. 2010;8:523–32. 10.1111/j.1538-7836.2009.03726.x [DOI] [PubMed] [Google Scholar]
- 53. Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028. 10.1038/nrdp.2018.28 [DOI] [PubMed] [Google Scholar]
- 54. Klok FA, Couturaud F, Delcroix M, Humbert M. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Eur Resp J. 2020;55:2000189. 10.1183/13993003.00189-2020 [DOI] [PubMed] [Google Scholar]
- 55. Klok FA, Dzikowska‐Diduch O, Kostrubiec M, Vliegen HW, Pruszczyk P, Hasenfuss G, et al. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thrombosis Haemostasis: JTH. 2016;14:121–8. 10.1111/jth.13175 [DOI] [PubMed] [Google Scholar]
- 56. Ende‐Verhaar YM, Ruigrok D, Bogaard HJ, Huisman MV, Meijboom LJ, Vonk Noordegraaf A, et al. Sensitivity of a simple noninvasive screening algorithm for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. TH Open. 2018;2:e89–e95. 10.1055/s-0038-1636537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boon G, Bogaard HJ, Klok FA. Essential aspects of the follow‐up after acute pulmonary embolism: an illustrated review. Res Pract Thrombosis Haemostasis. 2020;4:958–68. 10.1002/rth2.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klok FA, Surie S, Kempf T, Eikenboom J, van Straalen JP, van Kralingen KW, et al. A simple non‐invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011;128:21–6. 10.1016/j.thromres.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 59. Klok FA, Tesche C, Rappold L, Dellas C, Hasenfuss G, Huisman MV, et al. External validation of a simple non‐invasive algorithm to rule out chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2015;135:796–801. 10.1016/j.thromres.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 60. Ende‐Verhaar YM, Huisman MV, Klok FA. To screen or not to screen for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2017;151:1–7. 10.1016/j.thromres.2016.12.026 [DOI] [PubMed] [Google Scholar]
- 61. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;2016(37):67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 62. Boon G, Barco S, Bertoletti L, Ghanima W, Huisman MV, Kahn SR, et al. Measuring functional limitations after venous thromboembolism: optimization of the Post‐VTE Functional Status (PVFS) Scale. Thromb Res. 2020;190:45–51. 10.1016/j.thromres.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 63. Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, et al. The postthrombotic syndrome: evidence‐based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636–61. 10.1161/CIR.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 64. Ten Cate‐Hoek AJ, Amin EE, Bouman AC, Meijer K, Tick LW, Middeldorp S, et al. Individualised versus standard duration of elastic compression therapy for prevention of post‐thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single‐blind, allocation‐concealed, non‐inferiority trial. Lancet Haemat. 2018;5:e25–e33. 10.1016/S2352-3026(17)30227-2 [DOI] [PubMed] [Google Scholar]
- 65. Mol GC, van de Ree MA, Klok FA, Tegelberg MJ, Sanders FB, Koppen S, et al. One versus two years of elastic compression stockings for prevention of post‐thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ. 2016;353:i2691. 10.1136/bmj.i2691 [DOI] [PMC free article] [PubMed] [Google Scholar]


