Abstract
Background
The majority of patients with hemophilia A with inhibitors who undergo immune tolerance induction (ITI) achieve successful tolerance and transition to factor VIII (FVIII) prophylaxis. A portion of these patients have switched to emicizumab for bleeding prevention. However, the risk of inhibitor relapse on emicizumab is unclear.
Objective
To evaluate the inhibitor status of patients with hemophilia A and inhibitors who achieved successful/partial tolerance after ITI and transitioned from FVIII prophylaxis to emicizumab.
Methods
This is a single‐institution, retrospective review of pediatric patients with severe hemophilia A who have completed ITI with FVIII and switched to emicizumab.
Results/Conclusions
Seven successfully tolerized and five partially tolerized patients were identified. Three patients continued intermittent FVIII infusions on emicizumab at 50‐70 IU/kg twice weekly, once weekly, or every other week due to concerns for inhibitor relapse or loss of recent FVIII tolerance by the treating provider. Eleven of 12 patients (92%) maintained negative inhibitor titers at a mean follow‐up of 14.2 ± 6.1 months. One individual had an inhibitor relapse with a peak titer of 2.5 BU/mL. Five of the 11 patients (45%) with negative inhibitor titers had detectable nonneutralizing anti‐FVIII IgG4 antibodies, but none of the patients had detectable IgG1 antibodies. There were no inhibitor recurrences in a subset of six patients after FVIII re‐exposure for bleeding events or surgery. Given that the presence of an inhibitor significantly impacts factor product choice for bleeding management, ongoing inhibitor monitoring in tolerized patients with hemophilia A who transition to emicizumab is strongly recommended.
Keywords: antibodies, factor VIII, hemophilia A, immune tolerance, pediatrics
Essentials.
The risk of inhibitor recurrence in tolerized patients who switch to emicizumab is not known.
We report the inhibitor status of 12 tolerized patients with hemophilia A on emicizumab.
One individual in the cohort had an inhibitor recurrence with a low‐titer inhibitor.
The majority of tolerized patients in this cohort had negative inhibitor titers on emicizumab.
1. INTRODUCTION
The formation of factor VIII (FVIII)‐neutralizing antibodies, referred to as inhibitors, is a significant complication of FVIII replacement therapy in individuals with hemophilia A. However, approximately 70% of patients with inhibitors who undergo immune tolerance induction (ITI) with FVIII for inhibitor eradication achieve successful or partial tolerance. 1 , 2 Individuals who achieve tolerance after ITI are able to administer FVIII infusions for bleeding prevention and treatment without inhibitor relapse or an anamnestic rise in the inhibitor titer with continued FVIII exposure.
Emicizumab is a humanized bispecific IgG4 monoclonal antibody administered subcutaneously that mimics activated FVIII activity through recognition of factors IXa/IX and X/Xa enabling thrombin generation. 3 In the United States and multiple countries internationally, emicizumab is approved for bleeding prevention in adult and pediatric patients with hemophilia A with and without inhibitors. The reduced dosing frequency, subcutaneous route of administration, and significantly reduced annualized bleeding rates of emicizumab reported in the HAVEN 1‐3 phase 3 trials 4 , 5 , 6 has allowed a variety of individuals with hemophilia A to switch to emicizumab to prevent bleeds. In previously tolerized patients transitioning from FVIII prophylaxis to emicizumab, the absence of regularly scheduled FVIII infusions raises concerns for the loss of inhibitor eradication and FVIII tolerance achieved through ITI, particularly in the pediatric population. However, the risk of inhibitor relapse or anamnesis with FVIII reexposure for bleeds or surgical procedures in tolerized patients on emicizumab alone is not known. Additionally, the optimal post‐ITI FVIII infusion regimen to maintain FVIII tolerance is not well understood.
Due to the mechanism of action of nonfactor therapies such as emicizumab, individuals on these therapies require infusions of FVIII or a bypassing agent (BPA) for bleeding or surgical intervention. 5 , 6 , 7 , 8 , 9 Thus, the presence of an inhibitor and one’s response to FVIII remains the primary determinant of whether FVIII or BPAs are administered in these settings. The primary objective of this study is to evaluate the inhibitor status of a cohort of pediatric patients followed in our hemophilia treatment center with severe hemophilia A and a history of inhibitors who achieved successful or partial tolerance after ITI and transitioned from FVIII prophylaxis to emicizumab.
2. METHODS
This is a single‐center retrospective review of electronic medical records of patients followed at the Hemophilia of Georgia Center for Bleeding and Clotting Disorders of Emory University at the Pediatric Hemophilia Treatment Center in the Aflac Cancer and Blood Disorders Center at Children’s Healthcare of Atlanta in Atlanta, Georgia. All pediatric patients < 21 years of age with severe hemophilia A (FVIII activity < 1%) with a history of an inhibitor who achieved successful or partial tolerance status after undergoing ITI with recombinant or plasma‐derived FVIII were included in the study. Successful tolerance was defined by a negative inhibitor titer (<0.6 Bethesda Units (BU)/mL), FVIII recovery ≥ 66% of expected, and an FVIII half‐life >6 hours. 10 Partial tolerance was defined by a negative inhibitor titer but abnormal FVIII recovery or half‐life after 33 months of ITI. Inhibitor titers <5 BU/mL and ≥5 BU/mL were classified as low‐titer inhibitors (LTI) and high‐titer inhibitors (HTIs), respectively. Approval for this study was obtained by the Children’s Healthcare of Atlanta Institutional Review Board (protocol no. 18‐143). All research was conducted according to the principles outlined in the Declaration of Helsinki. Descriptive statistics were primarily used for data analysis due to the nature of the study and small sample size. Fisher’s exact test was used to compare differences in inhibitor relapse based on exposure to intermittent FVIII infusions on emicizumab. A P value <0.05 was considered statistically significant.
2.1. FVIII Inhibitor Assays
Inhibitor testing results reported at routine clinic visits were extracted from electronic medical records. FVIII inhibitor titers before emicizumab were performed by the Nijmegen Bethesda assay in the hospital coagulation laboratory. Inhibitor testing on emicizumab consisted of the chromogenic Bethesda assay using bovine reagents and anti‐FVIII IgG isotyping (ie, IgG1 and IgG4) by fluorescence‐based immunoassay (FLI) performed by the Centers for Disease Control and Prevention. 11 FVIII antibody detection is not available in the hospital coagulation laboratory; thus, inhibitor assessment before emicizumab initiation is limited to the inhibitor titer.
3. RESULTS/DISCUSSION
Twelve male patients with severe hemophilia A between 2 and 19 years old with a history of an inhibitor and successful (seven patients) or partial (five patients) tolerance status were identified (Table 1). All five patients with a partial tolerance status after ITI were categorized as such due to shortened half‐lives despite negative inhibitor titers, normal FVIII recovery, and clinical response to FVIII without anamnesis. Four patients in the cohort had a HTI for their peak historical inhibitor titer before initiation of ITI. All patients transitioned to FVIII prophylaxis after ITI at varying FVIII regimens of 25‐200 international units per kilogram (IU/kg) every other day or three times weekly. All patients were compliant with post‐ITI FVIII prophylaxis for 0.2‐11 years before the start of emicizumab. A negative inhibitor titer was confirmed before initiation of emicizumab. Nine of the 12 patients (75%) transitioned to emicizumab alone administered every 1‐2 weeks. The remaining three patients, two partially tolerized and one successfully tolerized, were maintained on intermittent FVIII infusions with recombinant FVIII at 50‐70 IU/kg twice weekly, once weekly, or every other week on emicizumab at the discretion of the treating provider due to concerns for loss of recently achieved FVIII tolerance (<12 months) in two patients and concerns for a high risk of inhibitor relapse without ongoing FVIII exposure in a partially tolerized patient with >10 years of uninterrupted post‐ITI FVIII prophylaxis.
TABLE 1.
Summary of patient characteristics and treatment regimens
| Pt a | Age, y | Historical peak inhibitor titer, BU/mL | Post‐ITI tolerance status | Last inhibitor titer before emicizumab, BU/mL b | Post‐ITI FVIII prophylaxis regimen before emicizumab | Duration on post‐ITI FVIII regimen, y |
Emicizumab + intermittent FVIII regimen (if applicable) |
Last inhibitor titer on emicizumab, CBU/mL a | Anti‐FVIII antibody detection on emicizumab | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 1.7 | Success | <0.6 | EHL rFVIII‐PEG 80 IU/kg + pdFVIII 20 IU/kg QOD | 10 | Emicizumab 1.5 mg/kg Q1 wk | <0.5 | IgG1 − | IgG4 − |
| 2 | 11 | 256 | Partial | <0.6 | rFVIII 100 IU/kg + pdFVIII 50 IU/kg QOD | 1.3 | Emicizumab 3 mg/kg Q2 wk | 2.1 | IgG1 − | IgG4 + |
| 3 | 19 | 819 | Partial | <0.6 | rFVIII 50 IU/kg QOD | 11 | Emicizumab 3 mg/kg Q2 wk + rFVIII 50 IU/kg Q2 wk | <0.5 | IgG1 − | IgG4 + |
| 4 | 12 | 9.2 | Partial | <0.6 | rFVIII 50 IU/kg QOD | 2 | Emicizumab 3 mg/kg Q2 wk | <0.5 | IgG1 − | IgG4 + |
| 5 | 10 | 4.9 | Partial | <0.6 | rFVIII 100 IU/kg QOD | 1.5 | Emicizumab 1.5 mg/kg Q1 wk | <0.5 | IgG1 − | IgG4 − |
| 6 | 7 | NA | Success | <0.6 | EHL rFVIII‐PEG 120 IU/kg + pdFVIII 30 IU/kg QOD | 3.7 | Emicizumab 3 mg/kg Q2 wk | <0.5 | IgG1 − | IgG4 + |
| 7 | 8 | 1.3 | Success | <0.6 | rFVIII 30/30/60 IU/kg 3x/wk | 4.8 | Emicizumab 3 mg/kg Q2 wk | <0.5 | IgG1 − | IgG4 − |
| 8 | 4 | 2 | Partial | <0.6 | rFVIII 200 IU/kg 3x/wk | 0.2 | Emicizumab 3 mg/kg Q2 wk + rFVIII 70 IU/kg 2x/wk | <0.5 | IgG1 − | IgG4 + |
| 9 | 2 | NA | Success | <0.6 | rFVIII 100 IU/kg QOD | 1.2 | Emicizumab 3 mg/kg Q2 wk | <0.5 | IgG1 − | IgG4 − |
| 10 | 7 | 1.7 | Success | <0.6 | rFVIII 50 IU/kg 3x/wk | 1.4 | Emicizumab 3 mg/kg Q2 wk | <0.5 | IgG1 − | IgG4 − |
| 11 | 15 | 84 | Success | <0.6 | pdFVIII 25 IU/kg QOD | 5 | Emicizumab 1.5 mg/kg Q1 wk | <0.5 | IgG1 − | IgG4 + |
| 12 | 4 | 2 | Success | <0.6 | rFVIII 50 IU/kg 3x/wk | 0.9 | Emicizumab 1.5 mg/kg Q1 wk + rFVIII 50 IU/kg Q1 wk | <0.5 | IgG1 − | IgG4 − |
Abbreviations: BU/mL, Bethesda units per milliliter; CBU, chromogenic Bethesda units; EHL, extended half‐life; FVIII, factor VIII; pdFVIII, plasma‐derived factor VIII; Pt, patient; Q, every; QOD, every other day; rFVIII, recombinant factor VIII; rFVIII‐PEG, pegylated recombinant factor VIII.
Negative chromogenic inhibitor titer < 0.5 CBU/mL.
Negative inhibitor titer < 0.6 BU/mL.
Inhibitor testing was performed at a median of 12.5 months (min‐max: 6‐26 months) after the initiation of emicizumab and the majority of patients had an average of two inhibitor assessments (ranging from one to four assessments) during this time period. All seven patients with successful tolerance status had negative inhibitor titers on emicizumab (Figure 1). Four of the seven patients (57%) had detectable non‐neutralizing anti‐FVIII IgG4 antibodies, but all seven patients had undetectable anti‐FVIII IgG1 antibodies. Among the five partially tolerized patients, one patient with a history of a HTI had an inhibitor recurrence with a peak inhibitor titer of 2.5 chromogenic BU (CBU)/mL. The inhibitor remained a low titer (median, 2.1 CBU/mL; range, 1.2‐2.5 CBU/mL) with detectable IgG4 and undetectable IgG1 on three separate inhibitor assessments. This patient did not have any FVIII or BPA exposure prior to the last assessment of his inhibitor status. It is possible that the discontinuation of his FVIII prophylaxis of 150 IU/kg every other day unmasked an underlying inhibitor that was suppressed by high dose FVIII infusions. It remains to be determined whether this individual will generate a high‐responding inhibitor with FVIII re‐exposure. The four remaining patients with partial tolerance status had negative inhibitor titers. Three patients with negative inhibitor titers had detectable IgG4 antibodies, and no patients had detectable IgG1 antibodies.
FIGURE 1.
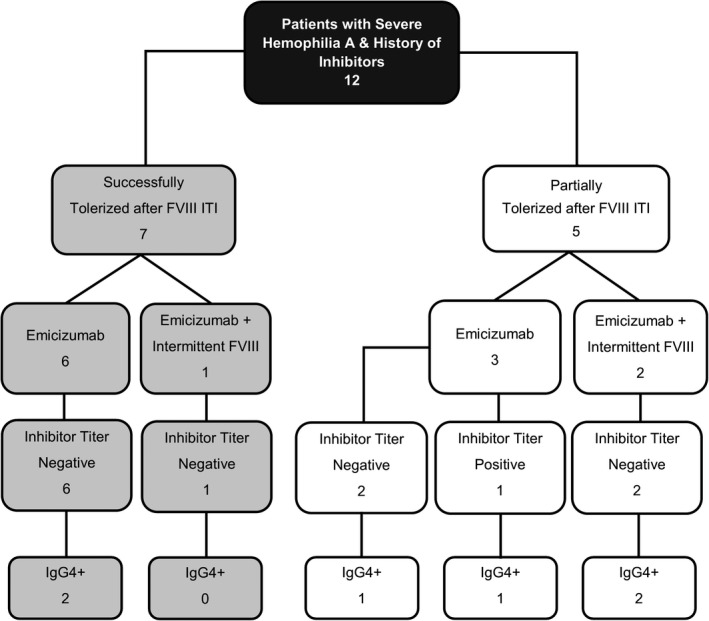
Summary of inhibitor testing outcomes by tolerance status. Flow diagram depicts inhibitor titer and anti–factor VIII (FVIII) IgG4 antibody testing results stratified by tolerance status and emicizumab regimen with or without intermittent FVIII infusions. There were no individuals with detectable anti‐FVIII IgG1 antibodies in this cohort and thus the IgG1 status is not included in the diagram
A subset of six patients on emicizumab without intermittent FVIII infusions had repeat inhibitor testing after FVIII re‐exposure for acute bleeding events or surgical management (Table 2). There were 16 bleeds and three surgeries documented in this subgroup, resulting in 1‐30 total recombinant or plasma‐derived FVIII exposure days per patient. The majority of bleeds were hemarthroses induced by trauma. There were three surgical procedures consisting of port‐a‐cath removals in two patients and neurosurgical intervention for an epidural hematoma resulting from a trauma‐induced back injury in one patient. The last documented inhibitor assessment was performed between 2 and 10 months after the last FVIII exposure for bleeding or surgery. All six patients (100%) had negative inhibitor titers and undetectable IgG1 antibody after FVIII re‐exposure. Two patients (33%) had detectable IgG4 antibodies with new‐onset IgG4 detection in one of the patients 2 months following treatment for an elbow bleed.
TABLE 2.
Inhibitor status after FVIII reexposure for bleeds or surgery
| Patient no. | Post‐ITI tolerance status | Emicizumab Regimen |
Bleeding event or surgery (no. of bleeds if > 1) |
FVIII ED at inhibitor testing, d | Inhibitor titer after FVIII reexposure (CBU/mL) a | Anti‐FVIII antibody detection after FVIII reexposure | Interval from last ED to repeat antibody testing, mo | |
|---|---|---|---|---|---|---|---|---|
| 1 | Success | 1.5 mg/kg Q1 wk |
Hemarthroses—Ankle (4), Knee (2), and Elbow Musculoskeletal injuries—back, hip CVL removal |
17 | <0.5 | IgG1 − | IgG4 − | 3 |
| 5 | Partial | 1.5 mg/kg Q1 wk | Epidural hematoma, spinal cord decompression, and hemilaminectomy | 30 | <0.5 | IgG1 − | IgG4 − | 6 |
| 6 | Success | 3 mg/kg Q2 wk |
Hip bleed Triceps muscle hematoma |
3 | <0.5 | IgG1 − | IgG4 + | 4 |
| 9 | Success | 3 mg/kg Q2 wk | CVL‐associated bleed | 1 | <0.5 | IgG1 − | IgG4 − | 10 |
| 10 | Success | 3 mg/kg Q2 wk |
Knee hemarthroses (3) CVL removal |
6 | <0.5 | IgG1 − | IgG4 − | 8 |
| 11 | Success | 1.5 mg/kg Q1 wk | Elbow hemarthrosis | 3 | <0.5 | IgG1 − | IgG4 + | 2 |
Abbreviations: CBU/mL, chromogenic Bethesda units per milliliter; CVL, central venous line; ED, exposure days; FVIII, factor VIII; ITI, immune tolerance induction.
Negative chromogenic inhibitor titer <0.5 CBU/mL.
In this study, we report that 92% (11/12) of patients with successful/partial tolerance status maintained negative inhibitor titers after transitioning to emicizumab. One individual (8%) had an inhibitor recurrence with a LTI. All three patients continued on intermittent FVIII infusions (two partially and one successfully tolerized) maintained negative inhibitor titers on emicizumab. There was no significant difference in the proportion of patients with inhibitor recurrence among the individuals on emicizumab alone compared to those on emicizumab with intermittent FVIII infusions (11% vs 0%; P > .99). Moreover, none of the six patients on emicizumab alone re‐exposed to FVIII for bleeding events or surgeries had inhibitor relapse.
Few large international immune tolerance registries evaluating ITI outcomes report variable but low rates of inhibitor relapse between 2% and 12%. 12 , 13 , 14 Another multicenter, international, retrospective study with an average post‐ITI follow‐up of 9 years reported an inhibitor relapse rate of 6.8% between 13 and 53 months after ITI among 44 patients successfully or partially tolerized with a plasma‐derived FVIII containing von Willebrand factor product. 15 The three individuals with inhibitor relapse (two with complete success and one with partial success after ITI) were all able to reestablish their successful/partial tolerance status after rescue ITI. Conversely, Antun and colleagues 16 reported higher rates of a positive inhibitor titer of 30% on one occasion and 19% on more than one occasion with a 32.5% probability of inhibitor relapse at 5 years. The authors reported that inhibitor recurrence was associated with the use of immunosuppressive therapies such as rituximab and FVIII recovery <85%, but not with adherence to FVIII prophylaxis post‐ITI. Nevertheless, the status of FVIII tolerance in tolerized patients with hemophilia A who transition to a novel nonfactor products in the absence of regular FVIII infusions is unknown. Although larger prospective studies are essential for addressing unanswered questions on FVIII tolerance with novel nonfactor therapies, this study provides important preliminary data suggesting that there may be a low incidence of inhibitor recurrence in successfully and partially tolerized patients with hemophilia A who switch from FVIII prophylaxis to emicizumab.
IgG1 and IgG4 are the most common subclasses of anti‐FVIII antibodies in patients with hemophilia A with inhibitors; IgG4 tends to correlate with a functional FVIII inhibitor. 17 , 18 , 19 , 20 Interestingly, 45% of patients with negative inhibitor titers in this cohort had detectable non‐neutralizing anti‐FVIII IgG4 antibodies by FLI. Anti‐FVIII antibody profiling is not routinely performed in conjunction with functional inhibitory assays in most clinical laboratories, and the role of antibody subclass identification in inhibitor testing and interpretation remains under investigation. Discordance between the inhibitor titer and antibody detection can also contribute to confusion in the clinical interpretation of antibody subclass identification. Detection of IgG1 antibodies in hemophilia A inhibitors samples by FLI demonstrated that IgG1 antibodies preceded a positive inhibitor titer in five of seven patients who ultimately developed inhibitors, but IgG4 antibodies were detected in patients with negative inhibitor titers and those without an inhibitor history. 11 , 18 Similarly, the prospective observational Hemophilia Inhibitor PUP study evaluating biomarkers in inhibitor development among previously untreated patients with severe hemophilia A detected high‐affinity IgG1 antibodies before detection of an inhibitor along with high‐affinity IgG3 and IgG4 antibodies; however, IgG4 was not detected in subjects with LTIs, FVIII‐specific non‐neutralizing antibodies, or subjects without FVIII inhibitors or antibodies. 21 A limitation of this current study is the lack of routine evaluation of FVIII antibody profiling over time prior to patients switching to emicizumab for comparison. Although IgG4 persistence is concerning for the presence of an underlying anti‐FVIII antibody that could progress to inhibitor recurrence or an anamnestic response in the setting of intense factor exposure with a catastrophic bleeding event or major surgery, the clinical significance of IgG4 detection in this setting remains unclear and warrants further investigation.
Given the number of novel nonfactor therapies in the pipeline for hemophilia A management, 22 further studies evaluating inhibitor risk as well as FVIII tolerance induction and maintenance on these therapies will be critical for guiding management of the different subset of patients with hemophilia A. This is the first study evaluating inhibitor outcomes in previously tolerized pediatric patients who transitioned from post‐ITI FVIII prophylaxis to emicizumab. Future prospective studies evaluating inhibitor outcomes on emicizumab (ie, Emicizumab PUPs and Nuwiq ITI study, clinical trial ID NCT04030052; MOTIVATE [Modern Treatment of Inhibitor‐Positive Patients with Haemophilia A] study, clinical trial ID NCT04023019; and PRIORITY [Preventing Inhibitor Recurrence Indefinitely] study) will provide greater insight into these unanswered questions.
RELATIONSHIP DISCLOSURE
GB has received honoraria for advisory board participation from Bayer, Genentech, and Octapharma. AG has received honoraria for participation in nursing advisory boards for Genentech, Novo Nordisk, and Octapharma. SLM has received honoraria for participation in advisory boards from Bioverativ/Sanofi, Bayer, CSL Behring, Genentech, HEMA Biologics, Takeda/Shire, Spark, Sangamo, Pfizer, Octapharma, Biomarin, and Novo Nordisk. RFS has received honoraria for participation in advisory boards from Genentech, Takeda, Sanofi/Bioverativ, Bayer, Octapharma, Tremeau Pharmaceuticals, Sanofi, Grifols, Pfizer, Biomarin, Uniqure, Catalyst, and Novo Nordisk. RFS has investigator‐initiated grant funding from Octapharma, Grifols, Genentech, and Takeda.
AUTHOR CONTRIBUTIONS
GB collected data, analyzed data, and wrote the manuscript. AG contributed to data collection. SLM and RFS contributed to data analysis and manuscript revision.
ACKNOWLEDGMENTS
AG has funding through the National Hemophilia Foundation (NHF) ‐ Nursing Excellence Fellowship.
Handling Editor: Pantep Angchaisuksiri.
Contributor Information
Glaivy Batsuli, Email: gbatsul@emory.edu.
Shannon L. Meeks, @BloodDoc2.
Robert F. Sidonio, Jr, @Nashgreenie.
REFERENCES
- 1. Mariani G, Ghirardini A, Bellocco R. Immune tolerance in hemophilia‐principal results from the International Registry. Report of the Factor VIII and IX Subcommittee. Thromb Haemost. 1994;72:155–8. [PubMed] [Google Scholar]
- 2. Hay CR, DiMichele DM. International Immune Tolerance S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–44. [DOI] [PubMed] [Google Scholar]
- 3. Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–4. [DOI] [PubMed] [Google Scholar]
- 4. Young G, Liesner R, Chang T, Sidonio R, Oldenburg J, Jiménez‐Yuste V, et al. A multicenter, open‐label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127–38. 10.1182/blood.2019001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahlangu J, Oldenburg J, Paz‐Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811–22. [DOI] [PubMed] [Google Scholar]
- 6. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809–18. [DOI] [PubMed] [Google Scholar]
- 7. McCary I, Guelcher C, Kuhn J, Butler R, Massey G, Guerrera MF, et al. Real‐world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;26:631–6. [DOI] [PubMed] [Google Scholar]
- 8. Barg AA, Livnat T, Budnik I, Avishai E, Brutman‐Barazani T, Tamarin I, et al. Emicizumab treatment and monitoring in a paediatric cohort: real‐world data. Br J Haematol. 2020;191:282–90. 10.1111/bjh.16964 [DOI] [PubMed] [Google Scholar]
- 9. Levy GG, Asikanius E, Kuebler P, Benchikh El Fegoun S, Esbjerg S, Seremetis S. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: Experience from the HAVEN clinical program. J Thromb Haemost. 2019;17:1470–7. [DOI] [PubMed] [Google Scholar]
- 10. Valentino LA, Kempton CL, Kruse‐Jarres R, Mathew P, Meeks SL, Reiss UM. International Immune Tolerance Induction Study I. US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia. 2015;21:559–67. [DOI] [PubMed] [Google Scholar]
- 11. Miller CH, Rice AS, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, et al. Comparison of clot‐based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013;11:1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mariani G, Kroner B; Immune Tolerance Study Group. Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica. 2001;86:1186–93. [PubMed] [Google Scholar]
- 13. Dimichele D. The North American Immune Tolerance Registry: contributions to the thirty‐year experience with immune tolerance therapy. Haemophilia. 2009;15:320–8. [DOI] [PubMed] [Google Scholar]
- 14. Coppola A, Margaglione M, Santagostino E, Rocino A, Grandone E, Mannucci PM, et al. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high‐responding inhibitors. J Thromb Haemost. 2009;7:1809–15. [DOI] [PubMed] [Google Scholar]
- 15. Jimenez‐Yuste V, Oldenburg J, Rangarajan S, Peiro‐Jordan R, Santagostino E. Long‐term outcome of haemophilia A patients after successful immune tolerance induction therapy using a single plasma‐derived FVIII/VWF product: the long‐term ITI study. Haemophilia. 2016;22:859–65. [DOI] [PubMed] [Google Scholar]
- 16. Antun A, Monahan PE, Manco‐Johnson MJ, Callaghan MU, Kanin M, Knoll C, et al. Inhibitor recurrence after immune tolerance induction: a multicenter retrospective cohort study. J Thromb Haemost. 2015;13:1980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121:1039–48. [DOI] [PubMed] [Google Scholar]
- 18. Boylan B, Rice AS, Dunn AL, Tarantino MD, Brettler DB, Barrett JC, et al. Characterization of the anti‐factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence‐based immunoassay. J Thromb Haemost. 2015;13:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilles JG, Arnout J, Vermylen J, Saint‐Remy JM. Anti‐factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993;82:2452–61. [PubMed] [Google Scholar]
- 20. Miller CH. Laboratory testing for factor VIII and IX inhibitors in haemophilia: a review. Haemophilia. 2018;24:186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reipert BM, Gangadharan B, Hofbauer CJ, Berg V, Schweiger H, Bowen J, et al. The prospective Hemophilia Inhibitor PUP Study reveals distinct antibody signatures prior to FVIII inhibitor development. Blood Advances. 2020;4:5785–96. 10.1182/bloodadvances.2020002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weyand AC, Pipe SW. New therapies for hemophilia. Blood. 2019;133:389–98. [DOI] [PubMed] [Google Scholar]


