Abstract
Background
Patients diagnosed with pulmonary embolism (PE) are reported to experience symptoms of posttraumatic stress disorder (PTSD) and existential anxiety following their diagnosis. They may also experience negative changes in perspective and hypervigilance of PE symptoms.
Objective
The aim of this study was to document the mental and emotional experience associated with PE diagnosis through the lens of PTSD, to better understand the factors involved in psychological distress following receipt of a PE diagnosis.
Patients/Methods
This was a mixed‐methods study in two parts: (i) measurement of self‐reported PTSD symptoms among patients attending thrombosis clinic and (ii) semistructured interviews with patients about their experience of receiving a diagnosis of PE and its psychological aftermath.
Results
Of 72 patients who participated in the survey, two met the criteria for a tentative diagnosis of PTSD. The semistructured interviews with 37 patients suggested that around half of respondents experienced some degree of ongoing psychological distress. Those with psychological distress often recalled painful symptoms, recalled diagnosis delivery as stressful, worried about PE recurrence, and had anxieties about stopping their anticoagulant medication. Few patients reported inclination to seek support from professional mental health services.
Conclusions
We found ongoing and untreated psychological distress among people who were previously diagnosed with PE.
Keywords: anxiety, posttraumatic, psychological distress, pulmonary embolism, stress disorders, thrombosis
Essentials.
Psychological symptoms can be seen after pulmonary embolism (PE).
Among 72 patients with PE, we identified posttraumatic stress disorder in 3%.
Around half experienced other ongoing psychological distress.
Few patients had accessed mental health services.
1. INTRODUCTION
Venous thromboembolism (VTE) presents as deep vein thrombosis (DVT) or pulmonary embolism (PE) and affects approximately 1 to 2 per 1000 people per year. 1 , 2 While untreated PE can be fatal, the risk of death decreases with treatment, and mortality rates have declined over the past 2 decades. 3 , 4 Today, PE is a treatable and manageable condition with a low mortality rate, given timely diagnosis and appropriate anticoagulation therapy. 5
While extensive research has been done regarding physiological treatment for PE, many gaps remain in our understanding of patients’ psychological and emotional well‐being following PE diagnoses. Patients with PE have been shown to have higher depression and anxiety scores compared to controls. 6 Patients with PE also experience poor mental health as measured by health‐related quality of life. 7 , 8 , 9 For some, PE is regarded as a life‐changing event that leads to a loss of identity, intrusive thoughts and memories, and a need to live a different lifestyle as a result of adapting to physical restraints. 10 Impaired mental well‐being in young adults with VTE has been linked largely to uncertainty regarding their long‐term health and fear of recurrence. 11 , 12 Other common themes reported by patients with VTE include insecurity in symptom management, perceived loss of immortality, negative changes in self‐perception, and living in a constant state of alarm. 13 Despite this evidence, there is little understanding of a mental health prognosis following PE, and we have yet to determine how treatment might improve patient mental health outcomes.
“Postthrombotic panic syndrome,” described by Hunter et al, 14 is a state of hypervigilance of physical reminders and sensations with cyclically triggered emotional and psychological distress. Existing literature of the psychological symptoms of PE, including fear of recurrence, hypervigilance, flashbacks, and intrusive thoughts, all reveal symptoms suggestive of posttraumatic stress disorder (PTSD). 10 , 14 , 15 This study explores the incidence of PTSD among patients with a prior diagnosis of PE and considers what factors might contribute to mental and emotional distress following receipt of a PE diagnosis.
2. METHODS
Mixed‐methods studies allow researchers to combine quantitative and qualitative research findings to further understand a condition. We chose this research approach because there is limited prior research on psychological distress after a diagnosis of PE and very little on PTSD. We used mixed methods whereby we measured self‐reported symptoms relating to PTSD and then asked patients about the psychological effects of having a PE diagnosis during in‐depth interviews. Use of qualitative research methods allowed participants to tell us about their personal experience. We did not test a research hypothesis. Instead, we used all the information recorded in these interviews to summarize, categorize, and look for patterns in patient experience.
2.1. Study population
Patients were recruited from a hospital thrombosis clinic in Hamilton, Ontario, over the months of May and June 2018. Patients were eligible for recruitment if they had a prior diagnosis of PE, were attending a scheduled appointment and were aged 18 years or older. Patients were excluded if they had a diagnosis of DVT without PE, could not speak English, or had cognitive impairment. Patients deemed eligible were invited to complete a brief survey about their emotional experiences after being diagnosed with PE, and if they were willing and had time, a short semistructured interview about their experiences following PE diagnosis. Ethics board approval for the research was obtained from the Hamilton Integrated Research Ethics Board.
2.2. Participant survey
The survey (see Appendix S1) opened with four statements on anxiety, low mood, coping, and worry. Respondents were asked to choose on a visual analogue scale (VAS) (0‐100) the degree to which they agreed with each statement. A priori, we defined “agreement” with these statements as a VAS response of between 75 and 100. The remainder of the survey was based on the PTSD Checklist for the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (PCL‐5), available from the National Centre for Post‐Traumatic Stress Disorder. 16 Analysis of the PCL‐5 involved summing the scores from the 20 items to reach a total score ranging between 0 and 80. 17 , 18 Our cut point for a tentative diagnosis of PTSD was 33. 17 , 18 We also considered an alternative interpretation of the PCL‐5 by reviewing clusters of symptoms within criteria B, C, D, and E. The survey was designed to take 5‐10 minutes to complete and was self‐administered using a tablet computer. Formal consent was obtained through the tablet, and participants individually completed the survey within the clinic’s patient rooms. After giving consent and before completing the survey, demographic data including any comorbidities were also collected. Most patients who were eligible agreed to participate in the survey; however, we did not record the number of eligible patients who declined to participate.
2.3. Semistructured interviews
Patients who completed the survey were asked if they were willing to be interviewed about their experience of having PE; consent was obtained for the interviews through a paper consent form. The semistructured interviews were designed to last around 30 minutes and were conducted in private patient rooms. Participants could have a friend or family member present if they wished. The interviews had open‐ended questions covering five main areas of interest (see Appendix S2): experience of receiving a PE diagnosis, psychological impact following PE diagnosis, lifestyle changes following diagnosis, changes in outlook on life, and past or current treatment. The interviews were audio recorded on a tablet computer and subsequently transcribed verbatim. Each participant was given a pseudonym.
Each of the interview questions was treated as an interpretative theme 19 such that the interviews were coded and summarized question by question. This process of summarizing was supported by the use of Microsoft Word, rather than specialist software designed to code qualitative data. Validated through cross‐coding, 20 these summaries were used to build up an image of what it was like, emotionally, for these patients to receive a PE diagnosis and why some of them might subsequently have stressful PE‐related experiences. This analysis process, essentially a content analysis, 21 takes participants’ accounts at face value. 22 No attempt was made to explore how respondents rhetorically constructed their answers 23 or how those answers might have been influenced by the interaction between interviewer and interviewee. 24 We used anonymized extracts from the semistructured interviews (with pseudonyms) to illustrate our thematic findings. We followed the consolidated criteria for reporting qualitative studies. 25
The interviews were conducted by AT, an undergraduate student with no prior medical knowledge and no prior relationship with the participants. She was guided in her analysis of the resulting data by MR, a male postdoctoral social scientist, and supported by KdW, a female emergency and thrombosis physician researcher who provided clinical knowledge of PE. At the outset, the authors acknowledged their inherent biases and stances regarding the study topic. Neither AT nor MR had prior knowledge about venous thrombosis, however, KdW diagnosed PE in the emergency department and managed patients with PE in clinic. KdW came to this research study with the notion that some patients experienced more psychological distress than others after PE.
The data collection period, May to June 2018, was determined by AT’s availability to work full time on the research project. The sample was preset as all patients who accepted an invite to the survey and interview during this period.
3. RESULTS
Divided into two parts, we first present findings from the participant questionnaire and, second, from the semistructured interviews.
3.1. Participant questionnaire
Of the 75 patients who completed the survey, three survey participants met exclusion criteria, having been diagnosed with DVT alone and were excluded from the study. A total of 72 patients ranging from 28 to 85 years of age were included in our study; 45 (63%) were female, and the mean age was 65. The age and sex distribution can be seen in Figure 1. Fifteen percent were diabetic, 11% had coronary artery disease, 3% a history of stroke, 10% a history of depression, 49% were also treated for cancer, and 10% had a history of chronic obstructive pulmonary disease. The median time since PE diagnosis was one year (ranging from before 2000 to 2018), and the median lifetime number of VTE events was one (ranging from one to four). Nineteen participants were within six months of PE diagnosis.
FIGURE 1.
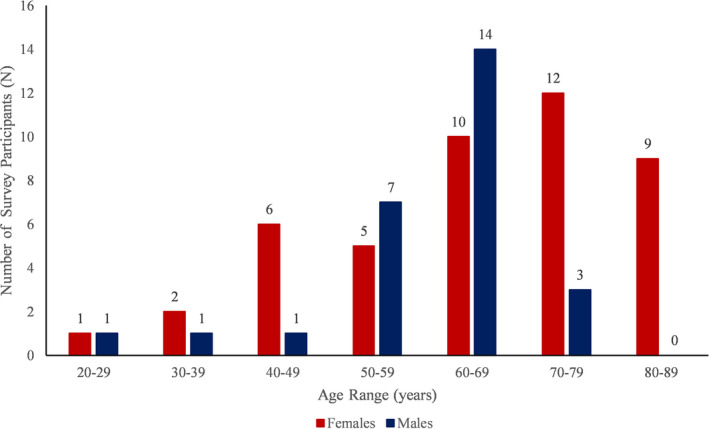
Age and sex distribution of survey respondents
Figure 2 shows the VAS responses for each of the four statements on anxiety, low mood, ability to cope and worry, in a box‐and‐whisker plot. A quarter of people (n = 17) agreed that they felt more anxious since their PE diagnosis (using our predefined definition of 75‐100 on the VAS), but very few people agreed with lower mood, impaired ability to cope, or an increase in worry since their diagnosis. Two of 72 patients (3%) had PCL‐5 scores >33, which was our definition for PTSD diagnosis, 17 of 72 patients (24%) scored a total score of 0 (see Appendix S3). The severity scores within criteria B, C, D, and E did not suggest an alternate psychological pathology. Sex, age, and time since first PE diagnosis did not reveal patterns that suggested an association with PCL‐5 scores.
FIGURE 2.
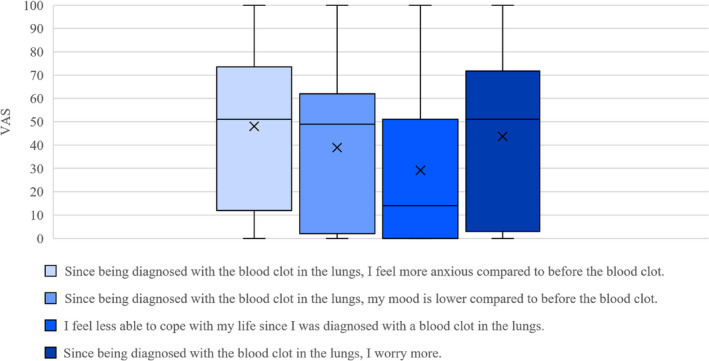
Self‐reported anxiety, mood, coping and worry
3.2. Semistructured interviews
Thirty‐seven participants agreed to be interviewed, all of whom had an interview (see Appendix S4 outlining age and sex of participants). The duration of the interviews ranged from 10 to 75 minutes and the age range of the interviewees was 28‐85 years, with a mean age of 66. All patients were prescribed anticoagulant medications at the time of the interview. This sample was fairly evenly divided between those who reported no psychological distress following PE diagnosis (57%) and those who reported psychological distress (43%).
We found a number of themes associated with ongoing psychological distress: (i) physical symptoms, (ii) the way the diagnosis was explain or delivered by medical professionals at the time of diagnosis, and (iii) a reliance on anticoagulant medication.
3.2.1. Experience of PE symptoms
Those who did not experience distressing symptoms, feelings, or emotions at the time of their PE event generally considered their PE to be a nontraumatic event and did not commonly express ongoing psychological distress in relation to their PE.
For example, Mr Fitzwilliam was among those with no physical symptoms associated with his PE and places his diagnosis in the context of other, ongoing health problems:
AT: So the cancer diagnosis in your case really overpowered any other diagnosis you had?
Mr Fitzwilliam: Well, it’s more important to me, the cancer prognosis. The blood clot is nothing… it’s been a side issue.
AT: So you haven’t really thought about it at all.
Mr Fitzwilliam: No. (68‐year‐old man, page 2, line 70)
In contrast, Ms Clare describes psychological distress after her diagnosis. She recounted:
Ms Clare: I had very, very tight chest pains […] I was very dizzy and nauseous. The pain was radiating up to my neck and down my arms. I could feel it across my back, and I couldn’t take a deep breath. (49‐year‐old woman, page 2, line 9)
The bodily experiences of having PE and the emotional impact of receiving a diagnosis in which death was only narrowly avoided could, as Ms Pembroke recounts, be vividly relived:
Ms Pembroke: [It’s] something that’s always on the back of your mind. I went to [another country], […] and I flew there, so it was a very long flight, and I was on blood thinners. But still when I got there, I was extremely stressed that I was having another blood clot. I convinced myself that I was having one. And we were looking at hospitals and stuff. It was just in my head. So, I don’t know. I think it’s something that, once you have it, it’s something that’s always in your mind. (28‐year‐old female, page 2, line 57)
3.2.2. Delivery and explanation of the PE diagnosis
Mr Emmanuel initially was not overly concerned when he first learnt about his PE diagnosis from a nurse:
Mr Emmanuel: I’ve had a couple in the leg before, so I wasn’t overly concerned until [the nurse] said, “is there anybody you’d like to call?” (laughs) They drew the curtains, and my wife is there. I’m having one heck of a time breathing. […] [The doctor] told me after, when I was leaving [the hospital], “a lot of people don’t get through this” […] I was one of the lucky ones. […] As the one doctor said, “this is a rare opportunity to talk to someone who made it through.” (laughs) (69‐year‐old man, page 1, line 21)
Mr Emmanuel attributes the change from not “overly concerned” about the PE to the realization that he is “one of the lucky ones,” to the behavior of the clinicians. The nurse asked him if there was anyone he wished to call, and the doctor said to him that a lot of people do not survive PE. Given that many patients have only a hazy knowledge of PE, participants’ recollection of the way in which their diagnosis was delivered could shape their impression and emotional response to PE.
And below, Ms Magdalene describes the emotional impact of receiving her diagnosis of PE:
Ms Magdalene: When [the doctor] said it was a blood clot, […] and it could go any minute, I could have a stroke, I could have a heart attack… […] that was a whole other ball game. […] That has got to be one of the most traumatic things in my life. (63‐year‐old woman, page 3, ln 121)
Among those people we interviewed, the physical, stressful sensations of having PE (Mr Emmanuel’s experience of “having one heck of a time breathing”) coupled with the knowledge that events could have turned out differently (“I was one of the lucky ones”) were twin themes among those respondents who reported ongoing anxieties. Diagnoses that were delivered calmly with explanations for the symptoms and information on PE treatment helped to minimize subsequent anxiety.
Ms Clare, for instance, suggested that “if there were more opportunities to have those educational sessions, I think that would go a long way for explaining things to people. […] Now I know why I’m feeling what I’m feeling, it’s not happening again. I’m not going to die today, I’m okay! That just really reassured me.” (page 5, line 205)
3.2.3. Reliance on medication
It is striking that Ms Pembroke says that PE is always “on the back of your mind” or “in your mind.” However, in common with many of our respondents, she did not report making significant lifestyle changes. Rather than improving diet or increasing the amount of exercise they undertook, respondents reported that taking their anticoagulant medication had become an important part of their daily routines. The significance patients can give to taking their medication is illustrated below, in Ms Newnham’s account of why she did not stop her anticoagulated medication following a minor injury:
Ms Newnham: […] you’re so scared in case you miss a pill and your blood is gonna clot. […] you’re told to take a pill at 6 o’clock every day, you’re taking that pill! You don’t think if other things happen to ya. (81‐year‐old woman, page 5, line 178)
Reports of adherence to anticoagulant medication were common in our sample, as were anxieties about stopping this medication. As Ms Kings noted, some patients considered their medication to be “lifesaving” and felt a sense of comfort in continuing with anticoagulation.
Ms Kings: It’s when you’re not treated […] that it can be serious. […] [The risk of bleeding] is a side effect that you put up with to take your life‐saving medication. (45‐year‐old woman, page 2, line 50)
Our results did not suggest that cancer or other more serious health problems were a major factor in psychological distress. Our interview data showed varying levels of psychological distress among cancer patients within our sample. For example, in some cases, the presence of distressing PE symptoms contributed more than cancer to their long‐term anxieties. In addition, neither our survey nor interviews identified patterns by participant sex, age, or time since PE diagnosis.
With respect to patients’ willingness to seek medical help for mental and emotional distress related to their PE diagnosis, our data are quite revealing. The survey data indicates that 64% of patients had not sought medical advice for their mental health. The interview data suggests a number of possible explanations for this: that PE was adequately managed by medication such that there was no need to feel anxious; that emotional distress was not brought up by clinicians, so therefore not discussed; and patients thought that psychological distress was not a topic to discuss with clinicians treating a physical condition. Those respondents who reported speaking to their doctors about their feelings reported concerns about PE recurrence and the possibility that that recurrence could cause a stroke. A minority of respondents turned to family and friends for support.
4. DISCUSSION
Our study identified 2 of 72 people with experience of PE as having a tentative diagnosis of PTSD. Although our sample is small, our findings suggest that PTSD does occur after diagnosis of PE, and our findings of PE‐related anxiety signals that psychological distress occurs and may be going unaddressed. Our semistructured interview data revealed associations between: distressing symptoms and fear of recurrence, diagnosis delivery and psychological distress, and psychological distress and focus on medication. Having psychologically distressing PE symptoms and receiving a diagnosis of PE, which almost inevitably comes with some physiological explanation of PE, can lead some patients to believe that they have narrowly escaped death. This troubling thought, a thought that can be reactivated by physical symptoms, real or imagined, can motivate adherence to regimes of anticoagulant medication.
Prior research suggests that patients experience a great deal of psychological distress following their diagnosis and show symptoms that may be suggestive of PTSD. 10 , 14 , 15 Less than half of our cohort described ongoing psychological distress. The existential anxiety, lost sense of identity, 10 lost immortality, negative self‐perception, and a need to adapt to a different lifestyle was not seen in our study participants. 13 Instead, our participants reported symptom‐related anxiety and fear of recurrent PE with attendant risks. Most felt no need to make significant lifestyle changes and reported simply adding a routine of taking their medications. One thing to consider is the difference in inclusion and exclusion criteria, where the decision to exclude patients with cancer or those with severe comorbidities in other studies may have influenced how participants felt about their PE in relation to other existing health problems. 10 The inclusion of both DVT and PE in other qualitative studies may also have influenced how symptoms presented in these patients, and consequently, how those diagnoses impacted their mental well‐being. 13 , 14 Other studies followed patients at 6 months 14 or 5 years, 10 measuring shorter‐term psychological repercussions, in contrast with our study sample, which included participants who had experienced PE up to 2 decades prior.
Our findings are more in keeping with those described in two recent qualitative studies (from Germany and Denmark). 26 , 27 Like us, they found anxiety and distress, as well as a heightened awareness of their bodily symptoms after being diagnosed with PE. These studies also reported instances where patients felt confusion or discomfort when advised to discontinue their anticoagulant therapy.
Almost half of our participants recalled receiving their diagnosis of PE as a traumatic event, and these participants tended to experience ongoing psychological distress, often related to worries about possible PE recurrence. Our findings suggest that clinicians delivering a diagnosis of PE should be alert to this psychological impact and avoid unnecessary reference to potential lethality. Practical information about the disease, clarifying that PE is unrelated to strokes and heart attacks, might be helpful. This lack of expert information, poor communication, or disjointed healthcare provision appears in relation to negative psychological effects following PE in almost all prior studies. 10 , 14 , 26 , 27 Rapid access to expert care and advice should be considered a basic requirement for people who experience PE, and a first step toward mitigating the psychological distress.
Our findings raise important questions. What would be the best way to deliver the diagnosis of PE and how could patient follow‐up more effectively alleviate the “back of mind anxieties”? These considerations should lead to further research in this field.
There were limitations in our study design. Our sample size was limited, and our analysis of semistructured interview data took respondents’ accounts at face value. Detailed information was not collected on patients who declined to participate in the study. Selection bias may have influenced the prevalence of psychological distress or anxiety. Furthermore, 19 participants were within 6 months of their diagnosis, which is considered too early to diagnose PTSD. Though our data may be more specifically reflective of PE patients within Canada, and may not necessarily be transferrable to other countries, our mixed‐methods study nonetheless provides useful material for critically reflecting on the mental and emotional distress that some patients experience following receipt of a PE diagnosis.
5. CONCLUSION
In this study, psychological distress was common after PE diagnosis. We found associations between ongoing psychological distress and the following: severe and distressing PE symptoms and fear of recurrent PE, traumatic diagnosis delivery, and emphasis on medication adherence.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to different components related to the study design, data analysis, critical writing, and revision of the intellectual content. All authors have read and approve submission of the manuscript to Research and Practice in Thrombosis and Haemostasis.
Supporting information
Appendix S1. Survey questions
Appendix S2. Interview questions
Appendix S3. PCL‐5 score distribution (n=72)
Appendix S4. Demographic data of interview participants (n=37)
Tran A, Redley M, de Wit K. The psychological impact of pulmonary embolism: A mixed‐methods study. Res Pract Thromb Haemost. 2021;5:301–307. 10.1002/rth2.12484
Handling Editor: Susan Kahn
REFERENCES
- 1. Angchaisuksiri P, Blanco A, Büller H, Gallus A, Hunt B, Hylek E, et al. Thrombosis: a major contributor to global disease burden. Semin Thromb Hemost. 2014;40:724–35. [DOI] [PubMed] [Google Scholar]
- 2. Lazo‐Langner A, Liu K, Shariff S, Garg A, Ray J. Immigration, region of origin, and the epidemiology of venous thromboembolism: a population‐based study. Res Pract Thromb Haemost. 2018;2:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burge A, Freeman K, Klapper P, Haramati L. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 2008;63:381–6. [DOI] [PubMed] [Google Scholar]
- 4. Horlander K, Mannino D, Leeper K. Pulmonary embolism mortality in the United States, 1979–1998. Arch Intern Med. 2003;163:1711. [DOI] [PubMed] [Google Scholar]
- 5. Smith S, Geske J, Maguire J, Zane N, Carter R, Morgenthaler T. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137:1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu C, Li X, Chen H, Cui J, Niu L, He Y, et al. Depression, anxiety and influencing factors in patients with acute pulmonary embolism. Chin Med J. 2011;124:2438–42. [PubMed] [Google Scholar]
- 7. Hogg K, Shaw J, Coyle D, Fallah P, Carreir M, Wells P. Validity of standard gamble estimated quality of life in acute venous thrombosis. Thromb Res. 2014;134:819–25. [DOI] [PubMed] [Google Scholar]
- 8. Kahn S, Akaberi A, Granton J, Anderson D, Wells P, Rodger M, et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med. 2017;130(990):e9–990. [DOI] [PubMed] [Google Scholar]
- 9. Erickson R, Feehan M, Munger M, Tak C, Witt D. Understanding factors associated with quality of life in patients with venous thromboembolism. Thromb Haemost. 2019;119:1869–76. [DOI] [PubMed] [Google Scholar]
- 10. Noble S, Lewis R, Whithers J, Lewis S, Bennett P. Long‐term psychological consequences of symptomatic pulmonary embolism: a qualitative study. BMJ Open. 2014;4:e004561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Højen A, Sørensen E, Dreyer P, Søgaard M, Larsen T. Long‐term mental wellbeing of adolescents and young adults diagnosed with venous thromboembolism: results from a multistage mixed methods study. J Thromb Haemost. 2017;15:2333–43. [DOI] [PubMed] [Google Scholar]
- 12. Hunter R, Noble S, Lewis S, Bennett P. Long‐term psychosocial impact of venous thromboembolism: a qualitative study in the community. BMJ Open. 2019;9:e024805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Højen A, Dreyer P, Lane D, Larsen T, Sørensen E. Adolescents’ and young adults’ lived experiences following venous thromboembolism. Nurs Res. 2016;65:455–64. [DOI] [PubMed] [Google Scholar]
- 14. Hunter R, Lewis S, Noble S, Rance J, Bennett P. “Post‐thrombotic panic syndrome”: a thematic analysis of the experience of venous thromboembolism. Br J Health Psychol. 2016;22:8–25. [DOI] [PubMed] [Google Scholar]
- 15. Bennett P, Patterson K, Noble S. Predicting post‐traumatic stress and health anxiety following a venous thrombotic embolism. J Health Psychol. 2014;21:863–71. [DOI] [PubMed] [Google Scholar]
- 16. Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P.The PTSD Checklist for DSM‐5 (PCL‐5) [Internet]. National Center for PTSD. 2013 [cited 2019 Jan 2]. Scale available from the National Center for PTSD at www.ptsd.va.gov
- 17. Blevins C, Weathers F, Davis M, Witte T, Domino J. The Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5): Development and Initial Psychometric Evaluation. J Trauma Stress. 2015;28:489–98. [DOI] [PubMed] [Google Scholar]
- 18. Verhey R, Chibanda D, Gibson L, Brakarsh J, Seedat S. Validation of the posttraumatic stress disorder checklist – 5 (PCL‐5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cicourel A. Method and measurement in sociology. New York: The Free Press; 1965. [Google Scholar]
- 20. Guest G, MacQueen K, Namey E. Applied thematic analysis. Los Angeles: Sage Publications; 2011. [Google Scholar]
- 21. Hsieh H, Shannon S. Three approaches to qualitative content Analysis. Qual Health Res. 2005;15:1277–88. [DOI] [PubMed] [Google Scholar]
- 22. Silverman D. How was it for you? The Interview Society and the irresistible rise of the (poorly analyzed) interview. Qual Res. 2017;17:144–58. [Google Scholar]
- 23. Wetherell M, Potter J. Discourse analysis and the identification of interpretative repertoires. In: Antaki C, editor. Analysing Everyday Explanation: A Casebook of Methods. Thousand Oaks. CA: Sage Publications, Inc.; 1988. p. 168–83. [Google Scholar]
- 24. Mishler E. Research Interviewing: Context and Narrative. Cambridge: Harvard University Press; 1991. [Google Scholar]
- 25. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. [DOI] [PubMed] [Google Scholar]
- 26. Kirchberger I, Ruile S, Linseisen J, Haberl S, Meisinger C, Berghaus T. The lived experience with pulmonary embolism: a qualitative study using focus groups. Respir Med. 2020;167:105978. [DOI] [PubMed] [Google Scholar]
- 27. Rolving N, Brocki B, Andreasen J. Coping with everyday life and physical activity in the aftermath of an acute pulmonary embolism: a qualitative study exploring patients' perceptions and coping strategies. Thromb Res. 2019;182:185–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Survey questions
Appendix S2. Interview questions
Appendix S3. PCL‐5 score distribution (n=72)
Appendix S4. Demographic data of interview participants (n=37)


