Abstract
Background
The use of direct oral anticoagulants (DOACs) is a convenient therapeutic option for patients at risk of thrombosis. DOACs interfere with clot‐based testing for the identification of lupus anticoagulant antibodies (LACs) in patients with antiphospholipid syndrome (APS), a common cause of acquired thrombotic disease.
Objectives
To evaluate a commercially available reagent DOAC‐Stop for the removal of DOAC interference encountered in LAC testing.
Patients/Methods
We collected a cohort of 73 test samples from patients on DOAC therapy identified at a large institutional coagulation laboratory from March to December 2019, along with samples from 40 LAC positive and negative control patients not on therapy. Samples were treated with DOAC‐Stop and tested for anti‐Xa activity and thrombin time for the removal of apixaban, rivaroxaban, argatroban, and dabigatran activity from patient samples. Treated and untreated samples were tested using the activated partial thromboplastin time, silica clotting time, and dilute Russell’s viper venom time to evaluate the reliability and utility of DOAC‐Stop.
Results
DOAC‐Stop markedly reduced DOAC interference from test samples (P < .05). DOAC‐Stop had no effect on LAC testing in the absence of DOAC therapy, permitting the identification of all LAC positive and negative controls. DOAC‐Stop removed false positives and false negatives resulting from DOAC interference and allows the identification of patients meeting criteria for the diagnosis of APS by LAC testing, as well as the detection of patients on rivaroxaban who are triple positive for APS.
Conclusions
DOAC‐Stop is an effective adjunct for the clinical laboratory faced with DOAC interference in LAC testing.
Keywords: antibodies, antiphospholipid, antiphospholipid syndrome, apixaban, lupus coagulation inhibitor, partial thromboplastin time, rivaroxaban
Essentials.
Direct oral anticoagulant (DOAC) therapy is an increasingly common cause of interference in LAC testing.
DOAC‐Stop removes DOAC interference encountered during LAC testing in the clinical lab.
DOAC‐Stop allows for the identification of LACs in patients on DOACs.
Testing with DOAC‐Stop aids in the choice of therapy for patients with venous thromboembolism.
1. INTRODUCTION
Clot‐based lupus anticoagulant (LAC) testing remains an essential tool in the diagnosis of antiphospholipid syndrome (APS). 1 Guidelines communicated by the International Society on Thrombosis and Haemostasis (ISTH) have outlined the criteria for the diagnosis of definite APS. 2 , 3 This includes the laboratory demonstration of antiphospholipid antibodies (aPLs) by either a solid‐phase assay 4 or prolongation of phospholipid‐dependent coagulation assays including the dilute Russell’s viper venom time (dRVVT)‐ and/or activated partial thromboplastin time (aPTT)‐based assays, including the silica clotting time (SCT). 3 , 5 Although the sensitivity and specificity for the detection of aPL varies by the reagents used and cutoffs employed, both solid‐phase and clot‐based assays are independently diagnostic, 6 , 7 , 8 , 9 and in many cases clinically significant antibodies can be detected only by one of these testing modalities. 10 , 11 , 12 Furthermore, patients with “triple‐positive” APS defined as the presence of a LAC as well as anticardiolipin (ACA) and anti‐β2‐glycoprotein I (aβ2GPI) antibodies detected by a solid‐phase system are at higher risk for thrombosis and have important therapeutic requirements based on recent randomized control trial evidence and guidance from the ISTH. 13 , 14 , 15 As such, the reliable detection of clot‐based inhibitory antibodies remains a vital component in the workup for APS.
Patients being evaluated for APS are often on anticoagulant therapy. In patients with venous thromboembolism, traditionally this has consisted of vitamin K antagonists (VKAs), low‐molecular‐weight heparin (LMWH) or fondaparinux and, in certain settings, unfractionated heparin (UFH). 16 These agents present an obstacle to LAC testing due to interference with the clotting cascade. 17 For UFH, a three‐step procedure can exclude false‐positive screening results, and false positives due to LMWH occur only at supratherapeutic concentrations. 18 These interferences can be overcome by the use of heparin neutralizers when these medications are present below the threshold recommended by the manufacturer, which are commonly included in dRVVT but not aPTT reagents. 19 , 20 Factor deficiency in the setting of VKA therapy can be complemented by mixing 3 however, this can produce false negatives in the setting of a weak LAC, 21 as well as false positives depending on the reagents used. 22 Although dilution with normal pooled plasma is widely employed, it is not always reliable in patients on VKAs, and interpretation requires caution. 17 , 22 A potential alternative to mixing studies includes the ratio of the Taipan snake venom time (TSVT) to the ecarin clotting time (ECT), 23 but this method has yet to be widely adopted. With the introduction of direct oral anticoagulants (DOACs), the number of patients being screened for aPLs on these medications has risen rapidly. 24 The presence of a DOAC also interferes with clot‐based LAC testing. 25 , 26 Currently, there is no standardized method to remove these inhibitors from test plasma, resulting in a growing number of assays being rejected. Ultimately, this leads to potential difficulties in making diagnoses and delays in proper therapy for patients on these medications. 27
To remove DOAC interference, a commercially available activated charcoal–based reagent sold under the name DOAC‐Stop (Haematex Research, Hornsby, Australia) has recently been evaluated. 28 Previous studies indicate that DOAC‐Stop is capable of removing DOAC interference, 29 , 30 , 31 , 32 and a recent statement from the ISTH has called for continued investigation into reagents of this type. 17 We have therefore evaluated DOAC‐Stop in a diverse number of clinical samples encountered during routine LAC testing and placed the results of this testing in the context of patient management.
2. METHODS
2.1. Sample selection
This study was carried out with oversight from the Stanford University Institutional Review Board under protocol IRB‐53627. Between March and December 2019, routine patient plasma was collected from orders to the Stanford Special Coagulation Laboratory for LAC testing. Samples were obtained by phlebotomy into plastic or siliconized glass blue‐top vacutainer tubes with buffered 3.2% (0.105 M) sodium citrate, received, and processed within 1 hour of collection. Platelet‐poor plasma was prepared by double centrifugation at 1500 g for 15 minutes (10‐20°C). Plasma was either stored at 2‐8°C and tested within 4 hours or frozen at −80°C before a quick thaw at 37°C and tested immediately after. We screened these samples for potential DOAC interference using the HemosIL Thrombin Time (TT; Instrumentation Laboratory, Bedford, MA, USA) for direct thrombin inhibitors 33 and HemosIL Liquid Anti‐Xa (Instrumentation Laboratory) for direct Xa inhibitors. Samples with potential DOAC interference were identified by a TT >20 seconds or an anti‐Xa activity > 0.1 IU/mL. For these samples, staff and medical directors reviewed the medical chart to identify whether the patient was currently on DOAC therapy, the indication for therapy, and the indication for LAC testing. After completing ordered testing, if sufficient sample remained, two aliquots of plasma were removed. One aliquot was left untreated, and the second was treated with DOAC‐Stop according to the manufacturer’s directions and as described below. We set out to collect 70 total samples representative of specimens encountered by our laboratory, and although argatroban is given exclusively intravenously, 34 we also analyzed the effect of DOAC‐Stop on argatroban alongside the other DOAC‐treated specimens.
2.2. DOAC‐Stop treatment
One milliliter of citrated plasma was removed to a plastic centrifuge tube. One DOAC‐Stop tablet was added and mixed gently for 5 minutes by intermittent hand inversion at room temperature. Samples were then centrifuged at 2700 g for 6 minutes at 20‐22°C to pellet the DOAC‐Stop reagent. The treated plasma supernatant was transferred to a new centrifuge tube and used for subsequent testing.
2.3. Sample testing
Both DOAC‐Stop treated and untreated aliquots were screened with a battery of tests used in the analysis of a suspected LAC. To assess the reduction of DOAC activity, we used the anti‐Xa and TT assays, given these tests are highly sensitive for direct Xa inhibitors and direct thrombin inhibitors, respectively. 35 We employed a heparin calibrated anti‐Xa assay to monitor reductions in apixaban and rivaroxaban due to regular use of this assay in our laboratory’s workflow. By titrating the STA‐Apixaban Calibrator and STA‐Rivaroxaban Calibrator (Diagnostica Stago, Asnières‐sur‐Seine, France), we computed the residual amount of Xa inhibitors present at below our cutoff (0.1 IU/mL) to be <15.2 ng/mL (95% CI, 0‐45.8 ng/mL) for apixaban and <13.9 ng/mL (95% CI, 0‐54.3 ng/mL) for rivaroxaban (Figure S1). If a sufficient quantity was available, plasma samples were assayed with the HemosIL aPTT‐SP, SCT, and dRVVT (Instrumentation Laboratory) both neat and after a 1:1 mix with CRYOcheck Pooled Normal Plasma (NP; Precision Biologic Inc, Dartmouth, Canada), which satisfies the criteria described in Pengo et al. 3 For the SCT and dRVVT, samples were assayed both by screen (with dilute phospholipid) and confirm (with concentrated phospholipid) reagents according to the manufacturer’s instructions. Interpretation was performed on the aggregated results from the aPTT‐SP and SCT or dRVVT guided by the recommendations of the Scientific and Standardization Committee of the ISTH, the Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology, and the Clinical & Laboratory Standards Institute (CLSI). 3 , 5 , 16 , 36 For aPTT‐based detection, samples were first screened for a prolongation of the aPTT‐SP with a + 2 standard deviation (SD) from the mean cutoff. If this was prolonged, the aPTT‐SP was measured after a 1:1 mix with NP. Results were then interpreted in conjunction with SCT testing using a so‐called integrated approach. 37 , 38 The SCT screen and confirm were determined for both neat plasma and after a 1:1 mix with NP. A normalized screen to confirm ratio for the SCT with a positive cutoff of 1.16 was determined from our local population of controls to be + 2 SD above the mean, as recommended by the manufacturer and in accordance with standards from the CLSI. 36 , 39 . The SCT was used for detection of phospholipid dependence downstream of the aPTT‐SP because the SCT is commercially available from HemosIL as a screen/confirm pair. Prolongation of the SCT screen time alone was not required for confirmatory testing; rather, only phospholipid dependence based on the screen/confirm ratio was judged using the SCT. A sample was deemed to be LAC positive if the aPTT‐SP was prolonged, it persisted after mixing (although we do consider the possibility of a weak LAC), and there was evidence for phospholipid‐dependent correction based on the SCT ratio in neat and 1:1 mixed plasma. Similarly, for the dRVVT, we also used an integrated approach by looking for phospholipid‐dependent prolongation of the dRVVT screen time with an elevated screen to confirm ratio using a positive cutoff of + 3 SD above the mean both in neat and 1:1 mixed plasma. The cutoff was determined to be 1.20, as recommended by the manufacturer and verified for our local population. 39 The normalized screen to confirm ratios were calculated by dividing the patients SCT or dRVVT by the mean for the reference interval derived from 25 normal control plasma samples and then dividing the screen ratio by the confirm ratio. We refer here to these calculated values as the SCT ratio and dRVVT ratio, respectively. Clot‐based assays were run on an ACL TOP 500 analyzer (Instrumentation Laboratory).
When ordered for the patient along with LAC testing, ACA and aβ2GPI were measured using QUANTA Lite ELISA kits (INOVA Diagnostics, San Diego, CA, USA). A cutoff at the 99th percentile for local normal controls was used to call positives.
2.4. LAC positive and negative controls
During the same period, we also collected 20 positive and 20 negative control plasma samples from patients not on DOAC therapy. These were tested before and after treatment (as described above) to assess whether DOAC‐Stop interferes with true‐positive and true‐negative results independent of DOAC presence. Positive controls for LAC were chosen by chart review and phospholipid correction of the SCT and, in all but 3 cases, the dRVVT as well, both in neat plasma and after a 1:1 mix with NP. Of the 20 positive controls, 6 were actively receiving VKAs, 2 were on LMWH, 1 was on UFH, and 11 were not on any anticoagulation. The diagnostic status of positive controls in our study included six patients who carried a prior diagnosis of APS including a history of repeat positive LACs; two were first‐time positives that were later reconfirmed, and the patients were ultimately diagnosed with APS; three were first‐time positive LACs that were reconfirmed later, but the patients were ultimately not diagnosed with APS due to the absence of clinical criteria; two were repeat positives resulting in a diagnosis of APS; three were second‐time positive LACs, but the patients were not diagnosed with APS due to the absence of clinical criteria; and four were first‐time positives that were not retested at our laboratory. Negative control samples were selected based on the presence of a normal value for both the SCT and dRVVT ratio, as well as the absence of any anticoagulation therapy per chart review.
2.5. Statistical analysis
Data for each assay were compared as described in the text and figure legends using R software version 3.5.0. with a threshold of P < .05 set for significance.
3. RESULTS
We collected a cohort of 73 LAC test samples from 71 patients (Figure 1A). Of these samples, 38 were from patients treated with apixaban, 26 with rivaroxaban, 6 with argatroban, and 3 with dabigatran. Sixty‐two percent of samples were from male patients and 38% from females. The mean age of patients was 57.8 years (range, 18‐86 years), and the vast majority of samples were derived from outpatients (86%), while 13% were sourced from Stanford Hospital inpatients. Hematology was the most common ordering service, accounting for 73% of tests. Overlap in the indication for testing and DOAC therapy was exceedingly common (Figure 1A and 1B), concordant in 82% of cases.
Figure 1.
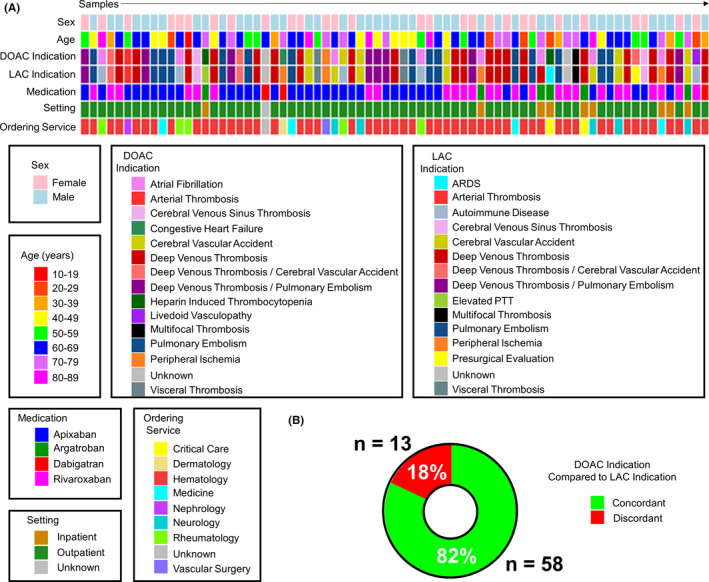
Sample characteristics of the unknown study cohort. (A) Heat map displaying each patient contributing a sample to our test cohort, their sex, age (by decade), the indication for anticoagulation therapy (DOAC indication), the indication for the patient to be tested for a LAC, the direct factor inhibitor the patient was receiving when the sample was drawn (medication), whether the sample was collected while the patient was in‐hospital or an outpatient, and the service ordering the test for each patient (n = 73 samples). The color code for each category is depicted below. (B) A plot showing the number and percent of samples where the indication for DOAC therapy and LAC testing were the same (concordant, plotted in green) or different (discordant, plotted in red). Cases where the indication was unknown (n = 2 samples) were excluded from analysis. ARDS, acute respiratory distress syndrome; DOAC, direct oral anticoagulant; LAC, lupus anticoagulant antibody; PTT, partial thromboplastin time
DOAC‐Stop reduced the anti‐Xa activity to below our laboratory’s cutoff (0.1 IU/mL) in all patients treated with apixaban and rivaroxaban (Figure 2A). Patients on argatroban and dabigatran exhibited anti‐Xa activity below our cutoff (Figure 2B). Similarly, apixaban and rivaroxaban TTs were within the reference range (Figure 2C). Argatroban and dabigatran prolonged the TT in all patients (Figure 2D). Both of their effects were significantly reduced by DOAC‐Stop, returning to within the reference range for all but two specimens containing argatroban.
Figure 2.
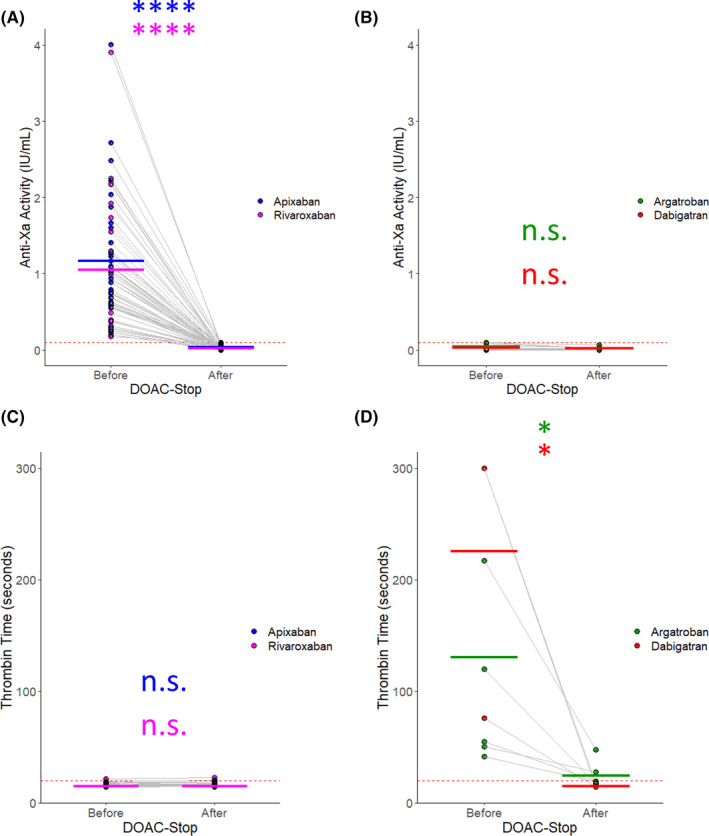
Anti‐Xa and TT results for samples treated with DOAC‐Stop. The samples from our test cohort were split into two aliquots; one was left untreated (“before”) and the other was treated with DOAC‐Stop per the manufacturer’s recommendation (“after”). The data are plotted for each sample tested. Each point represents an individual sample. The light gray lines connect the same sample Before and After treatment. The thick bars indicate the mean Before and After DOAC‐Stop treatment for each assay, colored by medication. The red dotted line indicates the upper threshold of the reference range/cut‐off for each assay. (A) The measured anti‐Xa activity before and after treatment with DOAC‐Stop in samples containing apixaban, blue, or rivaroxaban, purple, is plotted. (B) The measured anti‐Xa activity before and after treatment with DOAC‐Stop in samples containing argatroban, green, or dabigatran, red. (C) The measured TT for each sample containing apixaban or rivaroxaban colored as in (A). (D) The measured TT for each sample containing argatroban or dabigatran colored as in (B). n = 38 for apixaban. n = 26 for rivaroxaban. n = 6 for argatroban. n = 3 for dabigatran. * indicates P < .05; **** indicates P < .0001; n.s. indicates not significant by Student’s t test. DOAC, direct oral anticoagulant; TT, thrombin time
For all LAC‐positive and ‐negative control samples from patients not on DOACs, the anti‐Xa activity was below the cutoff, with the exception of two LAC‐positive samples (anti‐Xa 0.27 and 0.93 IU/mL). These patients were on therapeutic UFH and LMWH, respectively. In both of these specimens and the remaining samples, there was no significant effect of DOAC‐Stop on the anti‐Xa activity (Figure 3A). The patient on UFH had two previously positive LAC panels, suggesting persistence of a true‐positive result. Interestingly, the amount of heparin in her sample was not sufficient to prolong the TT above the cutoff likely due to the presence of hyperfibrinogenemia at the time of sample collection. The patient receiving LMWH likewise had a previously positive LAC collected 24 months earlier while also receiving LMWH; in both instances, his anti‐Xa was within the therapeutic range and below the threshold recommended by the manufacturer for neutralization by the included heparin inhibitor in our reagents, suggesting a true positive. There was no significant effect of DOAC‐Stop on the TT for either positive or negative controls (Figure 3B). All specimens were within the TT reference range (<20 seconds), except for a single patient with an elevated TT (35.9 seconds) that was reduced after DOAC‐Stop (29.8 seconds) to a level likely reflecting an underlying abnormality. This specimen was from a patient with autoimmune disease (drug reaction with eosinophilia and systemic symptoms syndrome) and suspected antifibrinogen antibodies. The LAC‐positive controls generally had a highly elevated aPTT‐SP, whereas the negative controls were on average borderline normal (Figure 3C). On a 1:1 mix with NP, the LAC‐positive samples remained elevated. In contrast, the mean for the LAC‐negative samples returned to normal, suggesting that the mildly elevated aPTT‐SP occasionally seen in this group could be attributed to a factor deficiency, analytical or stochastic variation, or potentially a weak LAC (Figure 3D). None of the LAC‐negative samples exhibited an elevated SCT ratio or dRVVT ratio in neat plasma, making a weak LAC a less likely explanation. Importantly, none of these features were significantly affected by DOAC‐Stop. The SCT ratio was elevated in all LAC‐positive samples and normal in LAC‐negative samples (Figure 3E and 3F). The dRVVT ratio was elevated in all LAC‐positive samples and normal in LAC‐negative samples in neat plasma (Figure 3G). After a 1:1 mix with NP (Figure 3H), three of the dRVVT ratios for LAC‐positive controls fell below the cutoff, suggesting the presence of a weak LAC. Altogether, DOAC‐Stop had no significant effect on the population mean for these assays; however, individual variation before and after treatment could be observed. The diagnostic call determined by the SCT and dRVVT ratio was never changed by DOAC‐Stop (Figure 3E‐H). Furthermore, DOAC‐Stop had no significant effect on the mean SCT or dRVVT time in the isolated screen and confirm assays (Figure 3I‐N). Individual variation within a specimen could be seen, most commonly in samples with prolonged clotting times (see Figure 3I and 3L).
Figure 3.
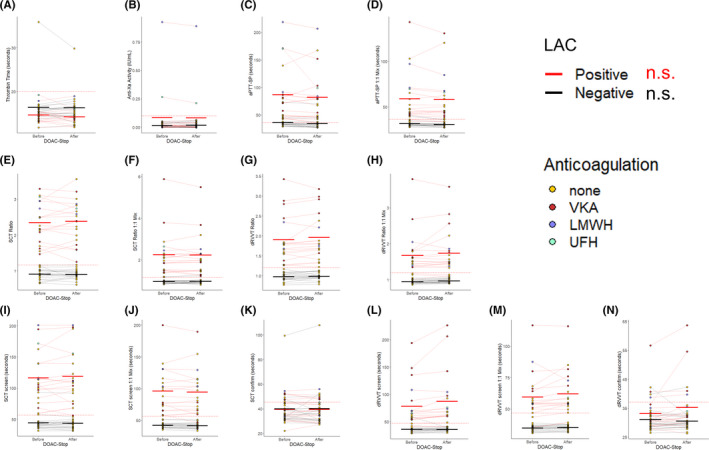
DOAC‐Stop results on LAC testing in the absence of DOAC interference. The samples from our control cohorts, n = 20 LAC positive, red lines, and n = 20 LAC negative, black lines, were split into two aliquots and each aliquot was tested with the indicated assay either “before” or “after” treatment with DOAC‐Stop. Each point represents an individual sample which are colored by the anticoagulation status of the patient from which they were received. The red or black lines connect the same sample Before and After treatment and indicate LAC status. The thick bars indicate the mean before and after DOAC‐Stop treatment for each assay, colored by LAC status. The red dotted line indicates the upper threshold of the reference range/cutoff for each assay. Data are plotted for (A) the anti‐Xa activity; (B) the TT; (C) the aPTT‐SP in neat plasma; (D), the aPTT‐SP after a 1:1 mix with NP; (E), the SCT ratio in neat plasma; (F), the SCT ratio after a 1:1 mix with NP; (G), the dRVVT ratio in neat plasma; (H) the dRVVT ratio after a 1:1 mix with NP; (I) the SCT screen; (J) the SCT screen after a 1:1 mix with NP; (K) the SCT confirm; (L) the dRVVT screen; (M) the dRVVT screen after a 1:1 mix with NP; and (N) the dRVVT confirm. n.s. indicates not significant by Student’s t test for all assays shown. DOAC, direct oral anticoagulant; dRVVT, dilute Russell’s viper venom time; LAC, lupus anticoagulant antibody; NP, normal plasma; SCT, silica clotting time
Argatroban and dabigatran are known to prolong the aPTT, 40 , 41 and consistent with this activity, treatment with DOAC‐Stop shortened the aPTT‐SP in every patient on these medications (Figure 4A and 4B). This effect was significant both in neat plasma and after a 1:1 mix with NP for samples containing argatroban. The effect was consistent for all three samples in the dabigatran group but did not reach significance. Among the patients on direct thrombin inhibitors, there was one patient on dabigatran that was positive for ACA and aβ2GPI antibodies accompanied by a prolonged aPTT‐SP both before and after DOAC‐Stop (see specimen 1, Figure 4A‐L). This patient was deemed likely to have APS and consistent with the presence of a LAC, tested positive by the SCT ratio (Figure 4C and 4D) and the dRVVT ratio (Figure 4E and 4F). The TT for this sample was normalized by DOAC‐Stop (76 seconds before vs 14.2 seconds after treatment), indicating removal of any detectable dabigatran by this assay (see Figure 2D). Scrutinizing the individual screen and confirm times for the SCT and dRVVT (Figure 4G‐L) for this patient revealed highly prolonged screen times before and after DOAC‐Stop, with markedly reduced confirm times after DOAC‐Stop adding another layer of evidence for a true LAC. The remainder of the samples were negative for a LAC by the SCT ratio (Figure 4E and 4F). Two samples, one from a patient on argatroban and the other from a patient on dabigatran, switched from abnormal to normal by the dRVVT ratio in neat plasma after treatment with DOAC‐Stop (Figure 4E). However, the specimens would have been deemed negative for a LAC after a 1:1 mix with NP by the dRVVT ratio (Figure 4F). The elevated dRVVT ratios in these specimens were likely false positives that were removed by DOAC‐Stop; however, the presence of a weak LAC that was rendered undetectable upon mixing or a minor shift in the ratio due to analytical variance cannot be ruled out.
Figure 4.
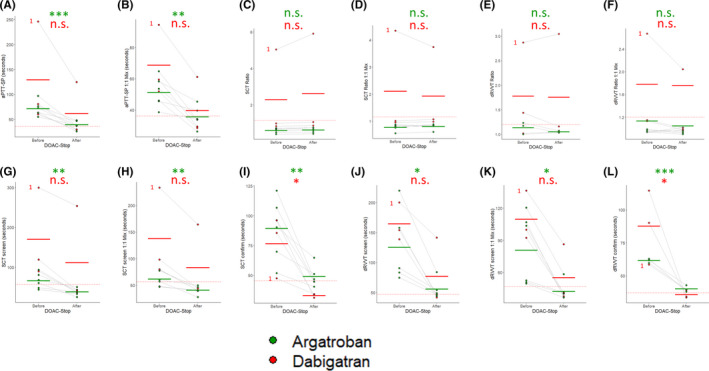
DOAC‐Stop permits LAC testing in patients on direct thrombin inhibitors. The samples from our test cohort containing argatroban n = 6, green, and dabigatran, n = 3, green, were split into two aliquots, and each aliquot was tested with the indicated assay either “before” or “after” treatment with DOAC‐Stop. Each point represents an individual sample. The light gray lines connect the same sample before and after treatment. The thick bars indicate the mean before and after DOAC‐Stop treatment for each assay, colored by medication. The red dotted line indicates the upper threshold of the reference range/cutoff for each assay. Data are plotted for (A) the aPTT‐SP in neat plasma; (B) the aPTT‐SP after a 1:1 mix with NP; (C) the SCT ratio in neat plasma; (D) the SCT ratio after a 1:1 mix with NP; (E) the dRVVT ratio in neat plasma; (F) the dRVVT ratio after a 1:1 mix with NP; (G) the SCT screen; (H) the SCT screen after a 1:1 mix with NP; (I), the SCT confirm; (J), the dRVVT screen; (K) the dRVVT screen after a 1:1 mix with NP; and (L) the dRVVT confirm. A LAC positive sample from a patient receiving dabigatran is indicated for each plot with a red (1). * indicates P < .05; ** indicates P < .01; *** indicates P < .001; n.s. indicates not‐significant by Student’s t test. DOAC, direct oral anticoagulant; dRVVT, dilute Russell’s viper venom time; LAC, lupus anticoagulant antibody; NP, normal plasma; SCT, silica clotting time
The mean aPTT‐SP was prolonged for patients on either apixaban or rivaroxaban, which trended toward shorter times after DOAC‐Stop, reaching significance for apixaban (Figure 5A). The same pattern was observed in the aPTT‐SP after a 1:1 mix with NP (Figure 5B). The SCT ratio in neat plasma and after a 1:1 mix with NP was elevated for three patients on rivaroxaban and two patients on apixaban. This remained unchanged after treatment with DOAC‐Stop, and in aggregate there was no significant effect of DOAC‐Stop in the presence of either apixaban or rivaroxaban in this assay (Figure 5C and 5D). For specimens containing rivaroxaban before DOAC‐Stop treatment, the average dRVVT ratio was above the reference cutoff (1.76 and 1.33 for neat and 1:1 mixed plasma, respectively) (Figure 5E and 5F). DOAC‐Stop markedly reduced the number of positives in rivaroxaban‐treated patient samples from 22 of 26 (85%) down to 4 (15%) in the neat dRVVT ratio (Figure 5E). Similarly, DOAC‐Stop reduced the number of positives in the dRVVT ratio after a 1:1 mix with NP (Figure 5F) from 14 (64%) to 2 (8%). These 2 cases likely represent true positives, as they also had a prolonged aPTT‐SP after mixing with NP and elevated SCT ratios before and after DOAC‐Stop treatment (see specimens 4 and 5, Figure 5A‐L). Using DOAC‐Stop, only three specimens containing rivaroxaban would have been called positive for a LAC by either the SCT ratio or the dRVVT ratio, the two mentioned above and a single specimen that was positive by the SCT ratio alone (see specimen 3, Figure 5A‐L). Indeed, analyzing the individual screen and confirm times for the SCT and dRVVT (Figure 5G‐L) revealed that all three samples had prolonged SCT screen times before and after DOAC‐Stop but were within the reference range after treatment in the SCT confirm assay. The same was true for specimens 4 and 5 when scrutinizing the individual dRVVT screen and confirm times (Figure 5K‐L). Fifteen of the total 26 specimens would have been called positive if interpretation were performed, neglecting the presence of rivaroxaban. Thus, 12 of the 26 samples would have been false positives (46%), which could be eliminated by DOAC‐Stop. Apixaban had the opposite effect on the dRVVT ratio when compared to rivaroxaban. The average dRVVT ratio increased upon treatment with DOAC‐Stop from 0.96 to 1.10 in neat plasma and 0.85 to 1.00 after a 1:1 mix with NP (Figure 5E and 5F). This increase permitted the detection of a sample that would have been called negative by the dRVVT ratio, neglecting the presence of apixaban, to be called positive following DOAC‐Stop treatment (see specimen 1, Figure 5A‐L). This sample is almost certainly a true positive, as the same patient had a prolonged aPTT‐SP after mixing with NP and elevated SCT ratios before and after DOAC‐Stop treatment. Furthermore, tracking the individual screen and confirm times for the SCT and dRVVT (Figure 5G‐L) reveals that this sample had highly prolonged screen times. These times remained prolonged after treatment with DOAC‐Stop. In contrast, the confirm dRVVT after a 1:1 mix with NP switched from prolonged to well within the reference range after DOAC‐Stop (42.6 seconds before to 30.9 seconds after), accounting for the detection of an elevated dRVVT ratio in this case. Thus, DOAC‐Stop removes the tendency of apixaban to cause false negatives by the dRVVT ratio.
Figure 5.
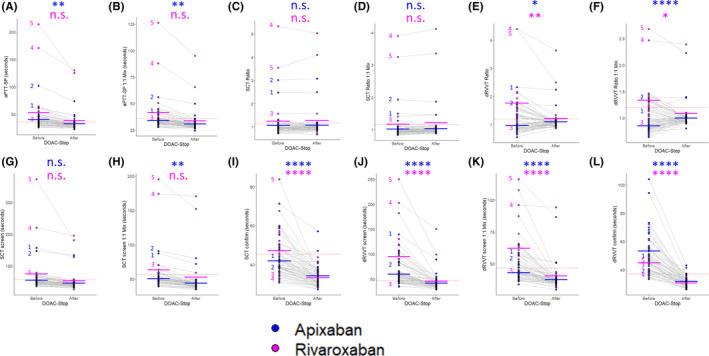
DOAC‐Stop permits LAC testing in patients on apixaban and rivaroxaban. The samples from our test cohort containing apixaban n = 38, blue, and rivaroxaban, n = 26, purple, were split into two aliquots, and each aliquot was tested with the indicated assay either “before” or “after” treatment with DOAC‐Stop. Each point represents an individual sample. The light gray lines connect the same sample before and after treatment. The thick bars indicate the mean Before and After DOAC‐Stop treatment for each assay, colored by medication. The red dotted line indicates the upper threshold of the reference range/cutoff for each assay. Data are plotted for (A), the aPTT‐SP in neat plasma; (B) the aPTT‐SP after a 1:1 mix with NP; (C) the SCT ratio in neat plasma; (D) the SCT ratio after a 1:1 mix with NP; (E) the dRVVT ratio in neat plasma; (F) the dRVVT ratio after a 1:1 mix with NP; (G) the SCT screen; (H) the SCT screen after a 1:1 mix with NP; (I) the SCT confirm; (J) the dRVVT screen; (K) the dRVVT screen after a 1:1 mix with NP; and (L) the dRVVT confirm. LAC‐positive samples are indicated with the numbers (1)‐(5) so they can be followed throughout the battery of assays, colored by the medication the patients were receiving at time of collection. * indicates P < .05; ** indicates P < .01; **** indicates P < .0001; n.s. indicates not significant by Student’s t test. DOAC, direct oral anticoagulant; dRVVT, dilute Russell’s viper venom time; LAC, lupus anticoagulant antibody; NP, normal plasma; SCT, silica clotting time
We evaluated the testing profile of our unknown samples to assess whether DOAC‐Stop affects diagnostic outcomes (Figure 6A). Of the 73 total samples, 14 met the laboratory criteria for presence of aPL (19%). Among these, 8 (11%) were positive by ELISA only, 4 (5%) were positive for a LAC and by ELISA, and 2 samples (3%) were positive for a LAC only. Thus, in 3% of cases, DOAC‐Stop permitted the identification of patients meeting the laboratory criteria for APS by LAC only. We also analyzed the subset of patients on rivaroxaban to identify cases where DOAC‐Stop would allow the identification of triple‐positive patients (Figure 6B). Indeed, 2 of the 26 patients on rivaroxaban (8%) were triple positive.
Figure 6.
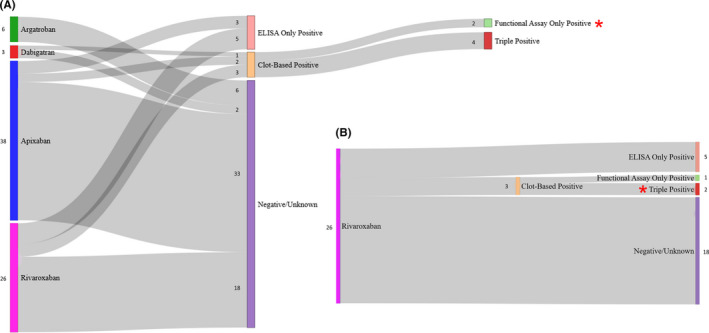
DOAC‐Stop allows the identification of clot‐based‐only positives and triple‐positive patients with APS receiving rivaroxaban. (A) A flow diagram is depicted starting on the left with each of the samples from our test cohort separated by medication (n = 73 samples). The second, or middle, node group indicates whether the patients providing these samples tested positive for a LAC (clot‐based positive) or were positive by ELISA only, or had completely negative/unknown results for both clot‐based and ELISA detectable antiphospholipid antibodies. The third, or right‐most, node group indicates the ELISA results for patients who tested positive for a LAC by clot‐based testing (n = 6). Two were positive only by clot‐based testing (functional assay only Positive, red star), and four were triple positive for LAC, ACA, and aβ2GPI antibodies. Of note, there were no double‐positive cases. (B) The subset of the data presented in (A) highlighting the samples from patients on rivaroxaban. The presence of two triple‐positive cases is indicated by a red star. The number immediately adjacent to each node indicates the quantity of specimens. ACA, anticardiolipin; aβ2GPI, anti‐β2‐glycoprotein I; APS, antiphospholipid syndrome; LAC, lupus anticoagulant antibody
4. DISCUSSION
In this study, we evaluated the utility of DOAC‐Stop for removing DOAC interference during routine LAC testing. We first confirmed that DOAC‐Stop removes the effects of these medications in direct assays for their target: the TT for dabigatran and anti‐Xa for apixaban and rivaroxaban. We then ensured in cases known to be true positives and true negatives for a LAC that DOAC‐Stop did not itself alter the results of LAC testing. Finally, we evaluated this reagent in the setting of routine unknown cases for which LAC testing was ordered while the patients were receiving DOAC therapy. Our results demonstrate that DOAC‐Stop is effective for the removal of most DOAC interference from these tests, does not significantly interfere with clot‐based LAC testing, and permits the evaluation of patients on these medications in the realistic scenario encountered in the clinical laboratory.
DOACs represent a convenient and effective therapeutic choice for patients with thrombosis, used for a growing list of indications. 42 Our current analysis of the reasons clinicians order LAC testing while patients are on DOAC therapy revealed that the underlying indications are highly concordant (82%). DOACs significantly interfere with clot‐based assays, leading the coagulation laboratory to face the difficult choice of rejecting these specimens or interpreting their testing results with caution. 43 The timing at which thrombophilia testing is performed needs clearer guidance, but possible options include cessation of therapy or a two‐staged testing algorithm such as genetic and serologic tests performed while the patient is on anticoagulation, followed by functional testing while off therapy, if warranted. 44
Prior studies have also sought to identify fixes for this problem. Arachchillage et al 45 demonstrated that rivaroxaban induces false‐positive LACs in both the Siemens and HemosIL dRVVT, but not a homemade dRVVT reagent. Interestingly, these commercial reagents did not exhibit false positives in six patients receiving rivaroxaban if samples were collected during a trough (18‐24 hours after dosing). The TSVT and ECT both function by direct activation of prothrombin, the former being LAC sensitive and the latter insensitive. As such, these assays can function as a paired test without interference from direct Xa inhibitors. This was confirmed in the study by Arachchillage et al and has been used successfully by others. 38 However, these reagents would still suffer from direct thrombin inhibitor interference and, as of yet, only a single set of commercial reagents is available (Diagnostic Reagents, Thame, UK). Both Hyphen Biomed and Haematex have developed dRVVT reagents that are less sensitive to DOAC interference, but side‐by‐side comparison with other dRVVT reagents has revealed a reduced sensitivity for true‐positive LACs. 22 , 38 Still, a positive result may provide significant utility in patients on DOAC therapy.
Consistent with the prevailing evidence from prior studies, 28 , 29 , 31 , 32 , 46 we found that DOAC‐Stop markedly reduces the effect of DOACs in LAC testing. This permitted the identification of 5 true‐positive LACs in patients on apixiban/rivaroxaban and 1 on dabigatran. Ząbczyk et al documented that DOAC‐Stop removed 29 presumed false‐positive LACs (of 35 positives before treatment) based on the dRVVT normalized ratio (Siemens). 31 Of these false positives, 27 were from patients on rivaroxaban, similar to our study using the HemosIL dRVVT. Favresse et al 29 evaluated DOAC‐Stop for the removal of apixaban, dabigatran, edoxaban, and rivaroxaban in thrombophilia testing. Their analyses showed that DOAC‐Stop removed interference for all DOACs using the aPTT‐LA and dRVVT (both from Diagnostica Stago, Parsippany, NJ, USA). In contrast, Platton et al found incomplete removal of rivaroxaban and apixaban, as determined by residual anti‐Xa activity above the limit of detection. 32 Unlike most investigators, Slavik et al 30 used the more sensitive method of liquid chromatography–tandem mass spectrometry to assess the effect of this reagent on dabigatran, rivaroxaban, and apixaban. With a limit of quantification <1 ng/mL, this study verified that DOAC‐Stop strongly reduces—but does not eliminate—these medications from patient plasma. Importantly, the residual amount remaining was below the level necessary to interfere with LAC testing as exemplified by the dRVVT. Discrepancies between these studies may be due to the DOAC‐Stop protocol employed, sensitivity of the methodology used, or other forms of interlaboratory variation. We did not evaluate edoxaban in our study, as this medication was approved in the United States in 2015 47 and is less commonly used in our population. 48 Prior studies have indicated similar removal of its interference by DOAC‐Stop 28 , 29 ; therefore, it is reasonable to assume that our results would extend to the removal of edoxaban, but this would need to be explicitly validated.
It is important to note that because we used a heparin calibrated anti‐Xa, reduction of DOAC activity to below our cutoff (0.1 IU/mL) may still represent a quantifiable amount of residual apixaban or rivaroxaban in versions of this assay calibrated to measure these medications. However, we did use calibrators to estimate the expected amount of apixaban and rivaroxaban present below our cutoff (<15.2 and < 13.9 ng/mL, respectively). While the laboratory directly carrying out the experiments in our study uses a heparin calibrated anti‐Xa assay to screen for potential apixaban and rivaroxaban interference, which is a limitation of our study, this scenario likely reflects a real‐world experience applicable to many clinical arenas.
Using our 40 control patients, we investigated the effect of DOAC‐Stop itself on the TT, anti‐Xa activity, aPTT‐SP, SCT, and dRVVT in the absence of DOACs. Indeed, DOAC‐Stop showed no significant effects on any of these assays in terms of their group mean. We note that individual variability before and after DOAC‐Stop treatment was observed. As our control sample size was limited (n = 20 for each cohort), we cannot exclude a subtle effect of DOAC‐Stop, which failed to reach significance for the entire group. Moreover, we included patients anticoagulated with agents other than DOACs in our LAC‐positive controls. DOAC‐Stop may have effects on the anticoagulant effect of these agents or perturb the coagulation cascade in a manner specific to their presence. Further studies aimed at investigating the interaction of DOAC‐Stop with other anticoagulants is warranted. Using a threshold of 1.16 and 1.2 for the SCT and dRVVT ratio, respectively, no single sample crossed these marks upon treatment with DOAC‐Stop. Thus, this reagent appears to permit reasonable diagnostic reproducibility in LAC testing after treatment. Exner et al 28 documented that even though the dRVVT showed essentially no interference induced from DOAC‐Stop itself, a small prolongation of the aPTT (slope 1.323 after/before DOAC‐Stop) was observed in plasma samples in the absence of DOACs. This was attributed to the effect of an additional high‐speed centrifugation step used to pellet the DOAC‐Stop reagent (7000 g). Indeed, we observed no prolongation of the mean aPTT‐SP in our samples consistent with lower centrifugal force applied in our protocol (2700 g).
Among the LAC assays we investigated, the presence of DOACs had the most pronounced effects on the aPTT‐SP and dRVVT ratio, the former being more sensitive to dabigatran 33 and the latter to rivaroxaban, which has been shown to be dependent on the composition and concentration phospholipids present, particularly in the screen reagent. 49 , 50 , 51 Indeed, the effect of DOACs are highly impacted by the reagents used. 51 As we have used two aPTT‐based reagents and one dRVVT reagent, our results may not generalize to reagents from different manufacturers. Among patients receiving apixaban or rivaroxaban, 13 samples remained elevated when tested with the aPTT‐SP after treatment with DOAC‐Stop (see Figure 5A). This was reduced to only four samples after a 1:1 mix with NP (see Figure 5B). All four of these specimens were from samples determined to be LAC positive. None of the remaining nine specimens were positive by either the SCT or dRVVT ratio. Thus, they were likely initially prolonged in the aPTT‐SP assay due to factor deficiency, but less likely a weak LAC or stochastic variation could also be responsible.
Importantly, two of the six LAC‐positive patients in our unknown cohort were positive for aPLs by clot‐based testing only and therefore would have been missed without the use of DOAC‐Stop. DOAC‐Stop was highly effective at removing false positives in the dRVVT ratio from samples containing rivaroxaban, accounting for 46% of these samples. Conversely, DOAC‐Stop normalized the slightly lowering effect of apixaban on the dRVVT ratio permitting the identification of a true positive. The differential effect of apixaban on the dRVVT screen and confirm has been documented to lower the dRVVT ratio in spiked plasma samples, 52 supporting our ex vivo results. It should be noted that occasional samples not containing apixaban did exhibit higher SCT or dRVVT ratios after treatment with DOAC‐Stop. This could be seen in both LAC‐positive and ‐negative control specimens (see Figure 3E‐H), suggesting that it is present even in the absence of DOACs. Notably, the level of variation is higher in neat specimens than after a 1:1 mix with NP (compare Figure 3E‐H), indicating likely contributions from increased variability, as the clotting times are prolonged and/or sensitivities of these assays to particularly low levels of any factor that would be complemented upon mixing. It is also possible that the DOAC‐Stop procedure may exacerbate a factor deficiency (eg, by direct binding or denaturation) with slightly stronger effects on the screen time relative to the confirm time resulting in an increased ratio.
DOAC‐Stop is a commercial reagent with associated costs to the clinical laboratory. It is important to emphasize that this method should be applied only to samples from patients with documented ongoing DOAC therapy, both to reduce expenditures and to mitigate the possibility for erroneous LAC results in non–DOAC‐treated samples, as documented by other investigators. 53 Our results demonstrate that DOAC‐Stop is effective at reducing DOAC interference, does not itself significantly interfere with assays commonly employed in LAC testing, and allows for the identification of patients meeting the laboratory criteria for APS including triple‐positive patients on rivaroxaban.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
SAB, JJ, and JLZ designed the study, performed chart review, analyzed the data, and wrote the manuscript. JJ, CP, and TV carried out the experiments, collected data, and processed results.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGMENTS
We are deeply grateful to the staff of the Stanford Hospital Special Coagulation Laboratory for their diligence and scientific excellence while carrying out patient care–related testing that has contributed to this work.
Baker SA, Jin J, Pfaffroth C, Vu T, Zehnder JL. DOAC‐Stop in lupus anticoagulant testing: Direct oral anticoagulant interference removed in most samples. Res Pract Thromb Haemost. 2021;5:314–325. 10.1002/rth2.12472
Handling Editor: Dr Pantep Angchaisuksiri.
REFERENCES
- 1. Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368:1033–44. [DOI] [PubMed] [Google Scholar]
- 2. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 3. Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009;7:1737–40. [DOI] [PubMed] [Google Scholar]
- 4. Devreese KMJ, Pierangeli SS, de Laat B, Tripodi A, Atsumi T, Ortel TL. Testing for antiphospholipid antibodies with solid phase assays: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:792–5. [DOI] [PubMed] [Google Scholar]
- 5. Devreese KMJ, Ortel TL, Pengo V, de Laat B. Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:809–13. [DOI] [PubMed] [Google Scholar]
- 6. Marai I, Gilburd B, Blank M, Shoenfeld Y. Anti‐cardiolipin and anti‐β2‐glycoprotein I (β2GP‐I) antibody assays as screening for anti‐phospholipid syndrome. Hum Antibodies. 2003;12:57–62. [PubMed] [Google Scholar]
- 7. Pengo V, Biasiolo A, Pegoraro C, Cucchini U, Noventa F, Iliceto S. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost. 2017;93:1147–52. [DOI] [PubMed] [Google Scholar]
- 8. Parkpian V, Verasertniyom O, Vanichapuntu M, Totemchokchyakarn K, Nantiruj K, Pisitkul P, et al. Specificity and sensitivity of anti‐β2‐glycoprotein I as compared with anticardiolipin antibody and lupus anticoagulant in Thai systemic lupus erythematosus patients with clinical features of antiphospholipid syndrome. Clin Rheumatol. 2007;26:1663–70. [DOI] [PubMed] [Google Scholar]
- 9. Audrain MAP, Colonna F, Morio F, Hamidou MA, Muller J‐Y. Comparison of different kits in the detection of autoantibodies to cardiolipin and beta2glycoprotein 1. Rheumatology. 2003;43:181–5. [DOI] [PubMed] [Google Scholar]
- 10. Ortel TL. Laboratory diagnosis of the lupus anticoagulant. Curr Rheumatol Rep. 2012;14:64–70. [DOI] [PubMed] [Google Scholar]
- 11. Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–32. [DOI] [PubMed] [Google Scholar]
- 12. Gardiner C, Hills J, Machin S, Cohen H. Diagnosis of antiphospholipid syndrome in routine clinical practice. Lupus. 2012;22:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high‐risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118:4714–8. [DOI] [PubMed] [Google Scholar]
- 14. Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, et al. Rivaroxaban vs warfarin in high‐risk patients with antiphospholipid syndrome. Blood. 2018;132:1365–71. [DOI] [PubMed] [Google Scholar]
- 15. Zuily S, Cohen H, Isenberg D, Woller SC, Crowther M, Dufrost V, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126–37. [DOI] [PubMed] [Google Scholar]
- 16. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47–58. [DOI] [PubMed] [Google Scholar]
- 17. Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:1569–75. [DOI] [PubMed] [Google Scholar]
- 18. De Kesel PMM, Devreese KMJ. The effect of unfractionated heparin, enoxaparin, and danaparoid on lupus anticoagulant testing: Can activated carbon eliminate false‐positive results? Res Pract Thromb Haemost. 2020;4:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobsen EM, Trettenes EJ, Wisløff F, Abildgaard U. Detection and quantification of lupus anticoagulants in plasma from heparin treated patients, using addition of polybrene. Thromb J. 2006;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olah Z, Szarvas M, Bereczky Z, Kerenyi A, Kappelmayer J, Boda Z. Direct thrombin inhibitors and factor Xa inhibitors can influence the diluted prothrombin time used as the initial screen for lupus anticoagulant. Arch Pathol Lab Med. 2013;137:967–73. [DOI] [PubMed] [Google Scholar]
- 21. Moore GW, Henley A, Greenwood CK, Rangarajan S. Further evidence of false negative screening for lupus anticoagulants. Thromb Res. 2008;121:477–84. [DOI] [PubMed] [Google Scholar]
- 22. Depreter B, Devreese KMJ. Dilute Russell’s viper venom time reagents in lupus anticoagulant testing: a well‐considered choice. Clin Chem Lab Med. 2017;55:91–101. [DOI] [PubMed] [Google Scholar]
- 23. Moore GW. Combining Taipan snake venom time/ecarin time screening with the mixing studies of conventional assays increases detection rates of lupus anticoagulants in orally anticoagulated patients. Thromb J. 2007;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sindet‐Pedersen C, Pallisgaard JL, Staerk L, Berger JS, Lamberts M, Torp‐Pedersen C, et al. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism ‐ a Danish nationwide cohort study. Sci Rep. 2017;7:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Douxfils J, Ageno W, Samama C‐M, Lessire S, ten Cate H, Verhamme P, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209–19. [DOI] [PubMed] [Google Scholar]
- 26. Hoxha A, Banzato A, Ruffatti A, Pengo V. Detection of lupus anticoagulant in the era of direct oral anticoagulants. Autoimmun Rev. 2017;16:173–8. [DOI] [PubMed] [Google Scholar]
- 27. Undas A, Góralczyk T. Direct oral anticoagulants in patients with thrombophilia: challenges in diagnostic evaluation and treatment. Adv Clin Exp Med. 2016;25:1321–30. [DOI] [PubMed] [Google Scholar]
- 28. Exner T, Michalopoulos N, Pearce J, Xavier R, Ahuja M. Simple method for removing DOACs from plasma samples. Thromb Res. 2018;163:117–22. [DOI] [PubMed] [Google Scholar]
- 29. Favresse J, Lardinois B, Sabor L, Devalet B, Vandepapeliere J, Braibant M, et al. Evaluation of the DOAC‐Stop® procedure to overcome the effect of DOACs on several thrombophilia screening tests. TH Open. 2018;02:e202–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slavik L, Jacova J, Friedecky D, Ulehlova J, Tauber Z, Prochazkova J, et al. Evaluation of the DOAC‐Stop procedure by LC‐MS/MS assays for determining the residual activity of dabigatran, rivaroxaban, and apixaban. Clin Appl Thromb. 2019;25:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michał Z, Magdalena K, Joanna N, Anetta U. The effect of DOAC‐Stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med. 2019;57:1374. [DOI] [PubMed] [Google Scholar]
- 32. Platton S, Hunt C. Influence of DOAC Stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol. 2019;41:227–33. [DOI] [PubMed] [Google Scholar]
- 33. Hapgood G, Butler J, Malan E, Chunilal S, Tran H. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost. 2017;110:308–15. [DOI] [PubMed] [Google Scholar]
- 34. Argatroban: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020883s014lbl.pdf Accessed 3/20/2020
- 35. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clinical and Laboratory Standards Institute. Laboratory Testing for the Lupus Anticoagulant; Approved Guideline. CLSI document H60‐A. 2014.
- 37. Favaloro EJ, Bonar R, Marsden K. Internal quality control and external quality assurance in testing for antiphospholipid antibodies: part II—lupus anticoagulant. Semin Thromb Hemost. 2012;38:404–11. [DOI] [PubMed] [Google Scholar]
- 38. Moore GW, Peyrafitte M, Dunois C, Amiral J. Newly developed dilute Russell’s viper venom reagents for lupus anticoagulant detection with improved specificity. Lupus. 2017;27:95–104. [DOI] [PubMed] [Google Scholar]
- 39. Clinical and Laboratory Standards Institute . Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline (Third edition). CLSI document C28‐A3. 2008.
- 40. Gosselin RC, King JH, Janatpour KA, Dager WE, Larkin EC, Owings JT. Comparing direct thrombin inhibitors using aPTT, ecarin clotting times, and thrombin inhibitor management testing. Ann Pharmacother. 2004;38:1383–8. [DOI] [PubMed] [Google Scholar]
- 41. Lind SE, Boyle ME, Fisher S, Ishimoto J, Trujillo TC, Kiser TH. Comparison of the aPTT with alternative tests for monitoring direct thrombin inhibitors in patient samples. Am J Clin Pathol. 2014;141:665–74. [DOI] [PubMed] [Google Scholar]
- 42. Milling TJ Jr, Frontera J. Exploring indications for the use of direct oral anticoagulants and the associated risks of major bleeding. Am J Manag Care. 2017;23:S67–80. [PMC free article] [PubMed] [Google Scholar]
- 43. Seheult JN, Meyer MP, Bontempo FA, Chibisov I. The effects of indirect‐ and direct‐acting anticoagulants on lupus anticoagulant assays: a large, retrospective study at a coagulation reference laboratory. Am J Clin Pathol. 2017;147:632–40. [DOI] [PubMed] [Google Scholar]
- 44. Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arachchillage DRJ, Mackie IJ, Efthymiou M, Isenberg DA, Machin SJ, Cohen H. Interactions between rivaroxaban and antiphospholipid antibodies in thrombotic antiphospholipid syndrome. J Thromb Haemost. 2015;13:1264–73. [DOI] [PubMed] [Google Scholar]
- 46. Favaloro EJ, Gilmore G, Arunachalam S, Mohammed S, Baker R. Neutralising rivaroxaban induced interference in laboratory testing for lupus anticoagulant (LA): a comparative study using DOAC Stop and andexanet alfa. Thromb Res. 2019;180:10–9. [DOI] [PubMed] [Google Scholar]
- 47. U.S. Food and Drug Administration (FDA) . Drug Approval Package: Savaysa (edoxaban tosylate) Tablets NDA #206316. 2015.
- 48. Alcusky M, McManus DD, Hume AL, Fisher M, Tjia J, Lapane KL. Changes in anticoagulant utilization among United States nursing home residents with atrial fibrillation from 2011 to 2016. J Am Heart Assoc. 2019;8(9): e012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flieder T, Weiser M, Eller TH, Dittrich M, von Bargen K, Alban S, et al. Interference of DOACs in different DRVVT assays for diagnosis of lupus anticoagulants. Thromb Res. 2018;165:101–6. [DOI] [PubMed] [Google Scholar]
- 50. Góralczyk T, Iwaniec T, Wypasek E, Undas A. False‐positive lupus anticoagulant in patients receiving rivaroxaban: 24 h since the last dose are needed to exclude antiphospholipid syndrome. Blood Coagul Fibrinolysis. 2015;26:473–5. [DOI] [PubMed] [Google Scholar]
- 51. Hillarp A, Strandberg K, Gustafsson KM, Lindahl TL. Unveiling the complex effects of direct oral anticoagulants on dilute Russell’s viper venom time assays. J Thromb Haemost. 2020;18:1866–73. [DOI] [PubMed] [Google Scholar]
- 52. Bonar R, Favaloro EJ, Mohammed S, Ahuja M, Pasalic L, Sioufi J, et al. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology (Phila). 2016;48:60–71. [DOI] [PubMed] [Google Scholar]
- 53. De Kesel PM, Devreese KMJ. Direct oral anticoagulant adsorption: Impact on lupus anticoagulant testing—review of the literature and evaluation on spiked and patient samples. J Thromb Haemost. 2020;18:2003–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


