Abstract
The apoplast is the extracellular space for signalling, nutrient transport, and plant–microbe interactions, but little is known about how plant viruses use the foliar apoplast. Proteomic analysis of the apoplasts isolated from potato virus X (PVX)‐infected Nicotiana benthamiana plants showed that the coat protein (CP) is the dominant viral component. The presence of the CP in the apoplast was confirmed by western blot, viral nucleic acid was detected by reverse transcription‐PCR and northern blot, and viral particles were observed by transmission electron microscopy (TEM). The apoplast from infected leaves was infectious if rubbed onto healthy leaves but not when infiltrated into them. The exosomes were separated from the apoplast fluid by high‐speed centrifugation and TEM showed that PVX particles were not associated with the exosomes. These results suggest that PVX virions are released to the N. benthamiana apoplast in a one‐way manner and do not share the bidirectional transport of exosomes.
Keywords: apoplast, coat protein, exosomes, potato virus X, viral particles
The presence of potato virus X (PVX) viral particles was identified in the leaf apoplast isolated from PVX‐infected Nicotiana benthamiana. This apoplast was infectious onto healthy leaves by rubbing them but not by infiltration.
1. INTRODUCTION
The apoplastic fluid, the soluble fraction of the extracellular space of plant tissue, contains many substances including nutrients, polysaccharides, secondary metabolites, and secreted proteins. It therefore plays a physiological role in the plants and becomes the battleground for the interaction between plant and pathogens. On the one hand, plant proteins in apoplasts (the secretome) are involved in different physiological and biological processes related to growth regulation, cell wall maintenance, and response to abiotic stresses (Alexandersson et al., 2013; Delaunois et al., 2014; Doehlemann & Hemetsberger, 2013). On the other hand, in order to survive under the harsh conditions of plant apoplasts, foliar pathogens respond to various immune responses to colonize the leaf apoplast. During these processes, plant pathogens secrete effector proteins into the apoplast environment to favour successful infection (Boller & Felix, 2009; Cai et al., 2018; Dong et al., 2011; Liu et al., 2014).
Plant viruses are obligate intracellular parasitic pathogens. A recent study by Movahed et al. using confocal microscopy found that the 6K2 protein of turnip mosaic virus (TuMV), which is a marker for viral replication, localizes in the extracellular space of infected Nicotiana benthamiana leaves. Proteomic analysis of purified extracellular vesicles also verified that TuMV proteins were present in the extracellular space of plant leaves (Movahed et al., 2019). This discovery challenges the view that all the important functions of the viral life cycle take place within cells and highlights the significance of the extracellular space in viral infection.
Potato virus X (PVX) is the type species of the genus Potexvirus and is among the top 10 plant viruses for studies in molecular plant pathology (Scholthof et al., 2011). The genome encodes five multifunctional proteins: open reading frame (ORF) 1 encodes the viral replicase (165 kDa), which is the only viral protein absolutely required for PVX RNA synthesis. The function of viral cell‐to‐cell movement is associated with the partly overlapping triple gene block (TGB), ORFs 2–4, which encode proteins of 25, 12, and 8 kDa, respectively (Argos et al., 1980). ORF5 encodes the viral coat protein (CP) of 25 kDa, which is required for encapsidation and viral cell‐to‐cell movement. Previous reports indicate that the PVX CP can be glycosylated and is a strain‐specific elicitor of Rx1‐mediated virus resistance in potato (Baratova et al., 2004; Bendahmane et al., 1995; Chapman, Hills, et al., 1992; Chapman, Kavanagh, et al., 1992; Goulden et al., 1993; Tozzini et al., 1994).
In this study, we isolated the apoplast from healthy and PVX‐infected N. benthamiana leaves and used proteomic analysis to show that PVX CP was the majority viral component in the apoplast of infected leaves. The specific presence of CP in the apoplast of infected leaves was confirmed by western blot, and transmission electron microscopy (TEM) showed that viral particles were also present but were not associated with the exosomes. The apoplast fluid of PVX‐infected plants was infectious to N. benthamiana plants by mechanical inoculation, but not by infiltration. The biological significance of the extracellular space in viral infection needs to be further investigated.
2. RESULTS
2.1. Proteomic analysis of the apoplast
In our study, leaf apoplasts were extracted from N. benthamiana plants by vacuum infiltration centrifugation (VIC), which is widely used for extraction of leaf apoplast fluid (Delaunois et al., 2016; Joosten, 2012; Movahed et al., 2019; Nouchi et al., 2012; O’Leary et al., 2014; Regente et al., 2009; Rutter & Innes, 2017). To further confirm cellular integrity during the VIC, electrolyte leakage was measured (Gentzel et al., 2019) and statistical analysis showed that there was no significant difference in electrical conductivity between VIC‐treated and mock‐treated samples (Figure S1).
After apoplast isolation, tandem mass spectrometry (MS/MS), and proteomic analysis, a total of 74 and 79 host proteins were identified in the apoplasts of mock‐ and PVX‐infected N. benthamiana, respectively, based on at least two tryptic peptide matches with the N. benthamiana proteome (the data have been deposited at http://www.peptideatlas.org/, PASS01647). Sixty‐one percent of the proteins were present in both samples (Figure 1a) while 19 proteins were only present in the PVX‐infected apoplast, suggesting that they might be related to virus infection or plant immunity.
FIGURE 1.
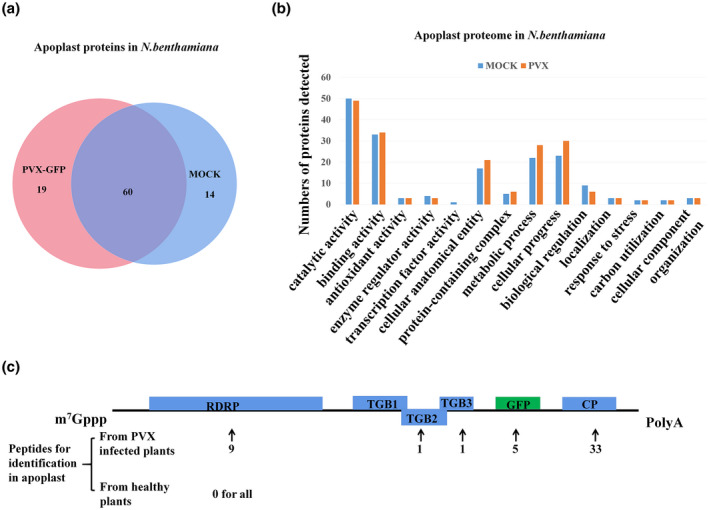
Proteomic analysis of apoplast isolated from mock‐ and potato virus X‐green fluorescent protein (PVX‐GFP)‐infected Nicotiana benthamiana leaves. (a) Venn diagram representation of proteins identified in mock and PVX‐GFP‐infected N. benthamiana leaves. (b) Gene ontology analysis by biological processes, molecular function, and cellular component of apoplast proteome from mock‐ and PVX‐GFP‐infected N. benthamiana leaves. (c) Distribution of peptides on PVX proteins. The number below the arrow indicates the number of peptides identified for a given protein in the proteome analysis of apoplast isolated from PVX‐GFP‐infected N. benthamiana. RDRP, RNA‐dependent RNA polymerase; TGB1, triple gene block protein 1 (P25); TGB2, triple gene block protein 2; TGB3, triple gene block protein 3; CP, coat protein
Gene ontology (GO) classification using the Uniprot “Retrieve/ID mapping” bioinformatics tool showed that host factors involved in cellular anatomical entity, in cellular processes, and in metabolic processes were more represented in the infected sample (Figure 1b). Peptides from each of the viral proteins except triple gene block protein 1 (P25), but especially the viral CP, were also identified in the apoplast of plants infected with PVX (Figure 1c).
2.2. Verification of the presence of PVX CP and its RNA in the apoplast of PVX‐infected N. benthamiana
Because the MS results showed that PVX CP was the dominant viral protein detected in the apoplast isolated from PVX‐infected N. benthamiana (Figure 1c), this was then verified by western blot. The total proteins from leaves of PVX‐infected N. benthamiana and healthy plants were used as controls and the presence of green fluorescent protein (GFP), PVX P25 (the TGB1 protein that had not been identified in the apoplast), and actin was also assayed to test the samples for intracellular contamination. As expected, PVX CP, GFP, P25, and actin were all detected in the total proteins from leaves of PVX‐infected plants and healthy plants had no viral proteins. In the apoplast samples from PVX‐infected N. benthamiana only PVX CP was detected (Figure 2). After extraction of the total RNA from apoplast samples of infected and healthy plants, the CP region of the PVX genome was amplified from the infected sample by reverse transcription (RT)‐PCR with specific primer pairs (Figure 3a) and its identity confirmed by northern blot (Figure 3b).
FIGURE 2.
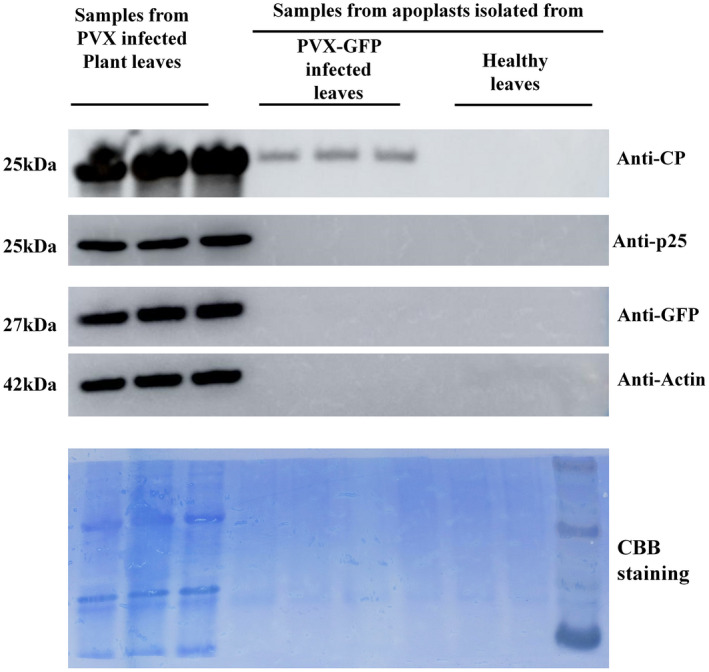
Coat protein (CP) of potato virus X (PVX) is detected in the apoplast isolated from PVX‐green fluorescent protein (PVX‐GFP)‐infected leaves. Western blot showing that PVX CP is specifically detected in the apoplast isolated from PVX‐GFP‐infected Nicotiana benthamiana. GFP, P25 (triple gene block protein 1), and actin were detected in the total proteins of PVX‐GFP‐infected leaves, but not in the apoplast, showing that there was no cytoplasmic contamination of the apoplast sample. Equal amounts of proteins were loaded in each case and Coomassie brilliant blue (CBB) staining of an equal sample was used as loading control. (Overexposed images are shown in Figure S2)
FIGURE 3.
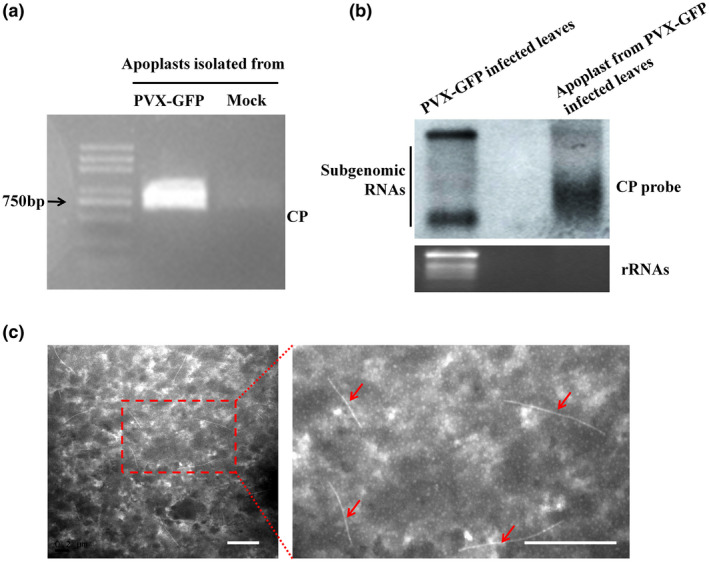
Viral RNA and viral particles are present in the apoplast isolated from potato virus X (PVX)‐infected Nicotiana benthamiana leaves. (a) Agarose gel electrophoresis showing the reverse transcription PCR product of the coat protein (CP) region. (b) Northern blot using CP probe showing that PVX genomic RNA was present in the apoplast isolated from PVX‐green fluorescent protein (PVX‐GFP)‐infected N. benthamiana leaves, with total RNA isolated from PVX‐GFP‐infected leaves as control. (c) Transmission electron microscopy showing that PVX particles of the expected size were observed in the apoplast. The red arrow points to PVX viral particles. Scale bar represents 500 nm
2.3. Identification of viral particles in the apoplast of PVX‐infected N. benthamiana
In negatively stained samples of the apoplast from PVX‐infected N. benthamiana, TEM showed the presence of curved rod‐shaped viral particles of the expected size (Figure 3c). N. benthamiana plants were then mechanically inoculated with fresh apoplast fluid isolated from PVX‐GFP‐infected plants and observed daily under UV light. GFP fluorescence began to appear on newly emerged leaves from 5 days postinoculation (dpi) onwards and western blot with anti‐CP antibody confirmed the presence of PVX (Figure S3a,b). Control plants remained virus‐free. Thus, the results demonstrate that infectious PVX particles are present in the apoplast of PVX‐infected plants.
2.4. PVX‐CP cannot be released alone into the apoplast
As shown above, PVX‐CP can be detected in the apoplast of N. benthamiana infected by PVX, raising the possibility that CP alone might be released into the apoplast. This was tested by expressing either CP:GFP or untagged CP transiently in leaves. Samples were observed by confocal microscopy 3 days after infiltration of Agrobacterium containing the CP:GFP vector and with FM4‐64 staining of the plasma membrane. As shown in Figure 4a, GFP‐labelled CP was not released into the apoplast space under the conditions of plasmolysis (treated with 5% NaCl). Furthermore, western blot results confirmed that neither CP:GFP nor unfused CP could be detected in the apoplast, when transiently expressed alone in N. benthamiana leaves (Figure 4b,c). These data suggest that the CP was not exported alone to the leaf apoplast.
FIGURE 4.
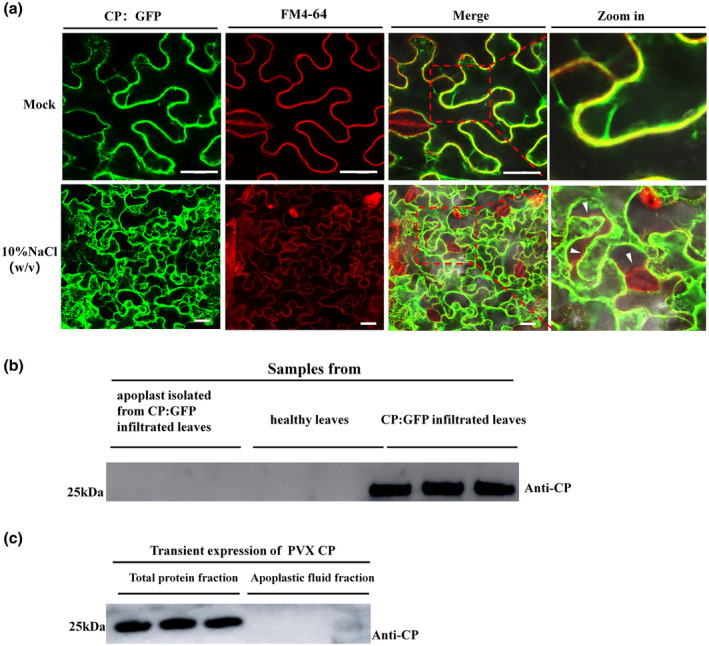
Potato virus X (PVX) coat protein (CP) is not released to the foliar apoplast by transient expression of CP:green fluorescent protein (GFP) or unfused CP. (a) CP:GFP colocalized with the plasma membrane with or without plasmolysis (FM4‐64 staining, red fluorescence). White arrowhead indicates the space after plasmolysis. Bar represents 50 μm. (b) In Nicotiana benthamiana leaves with transient expression of CP:GFP, western blot showed that CP:GFP could not be detected in the apoplast but only in the total protein isolated from the leaves. Coomassie brilliant blue (CBB) staining of equal volume was used in sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) as loading control (Figure S4a). (c) Western blot result showing the transient expression of CP in both the total protein and apoplastic fluid fractions. CBB staining of equal volume loaded SDS‐PAGE was used as loading control (Figure S4b)
2.5. Viral particles are independent of exosomes in the apoplast
The results above suggested to us that viral particles of PVX may have been secreted to the apoplast by some pathway or unidentified mechanism. To test whether the viral particles in the apoplast could be taken up into cells, healthy leaves of N. benthamiana were inoculated with apoplast samples either by rubbing (mechanical inoculation) or by infiltration. The two methods were applied to different halves of the same leaf and at 4 dpi fluorescent spots (infected foci) were detected on the rubbed half and the presence of PVX was confirmed by western blot (Figure 5a). The infiltrated half of the leaf remained free of symptoms, suggesting that viral particles cannot be taken up into cells from the apoplast. To provide a further and prolonged test to determine whether apoplastic fluid from PVX‐GFP‐infected plants could initiate systemic infection, fresh apoplast was used either to infiltrate leaves of N. benthamiana plants or to mechanically inoculate them as controls. The plants were then examined daily under UV light. Green fluorescence began to appear on the noninoculated leaves of the control plants at 5 dpi, but the plants infiltrated with fluid remained without fluorescence or viral symptoms even at 10 dpi, excluding the possibility that infection was merely delayed on the infiltrated plants (Figure S5).
FIGURE 5.
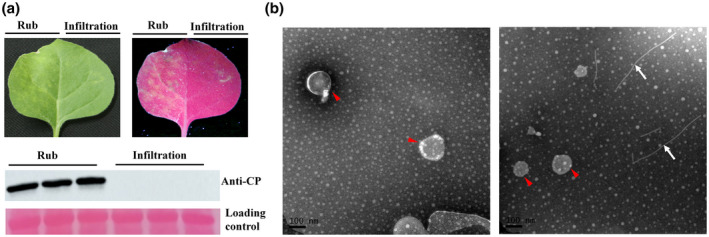
Apoplast with viral particles is infectious by rub inoculation, but not by infiltration. (a) Infected foci and green fluorescent spots were observed on the rub‐inoculated side of the leaf at 4 days postinoculation, but not on the infiltrated side of the leaf. Western blot with anti‐coat protein (CP) antibody confirmed infection by potato virus X (PVX) on the rub‐inoculated side of the leaf. (b) Apoplasts from mock‐inoculated (left) or PVX‐infected (right) leaves were centrifuged at 40,000 × g to extract exosomes and observed by transmission electron microscopy. Exosomes (red arrowhead) were detected in both samples and viral particles (white arrows) in the infected sample but independently of the exosomes. Scale bar = 100 nm
Exosomes are extracellular vesicles in apoplasts (30–150 nm in diameter) that encapsulate various proteins, small RNAs, and other biological information molecules. They are secreted out of cells and taken into cells during various biological processes (Regente et al., 2017; Samuel et al., 2015; Teng et al., 2018). It was previously reported that centrifugation at 40,000 × g was sufficient to isolate exosomes from the plant apoplast (Rutter & Innes, 2017). After centrifuging at 40,000 × g for 1 hr at 4 °C, exosomes (P40) were resuspended in buffer and observed under TEM. Free viral particles were observed in the sample and were not encapsulated in the exosomes (Figure 5b).
3. DISCUSSION
VIC has been widely used to extract apoplastic fluids or extracellular vesicles from plants (Delaunois et al., 2016; Joosten, 2012; Movahed et al., 2019; Nouchi et al., 2012; O’Leary et al., 2014; Regente et al., 2009; Rutter & Innes, 2017). Although this method has proved to be suitable for collecting apoplastic fluids with negligible intracellular contamination (Lohaus et al., 2001), a marker for extracellular vesicles or to detect electrolyte leakage is used to confirm the cellular integrity during this procedure (Gentzel et al., 2019; Movahed et al., 2019; Rutter & Innes, 2017). In our study, tests for electrolyte leakage showed that cellular integrity had been maintained during the process of apoplastic fluid extraction (Figure S1).
The apoplast, the extracellular space of the plant leaf, is often studied as the first battleground between plants and pathogenic microbes (Delaunois et al., 2014; Hammerschmidt, 2010). Although plant viruses are intracellular parasites, they have recently been reported to be involved in the apoplast and to induce extracellular vesicles (Movahed et al., 2019). In this study, we isolated the apoplast from PVX‐infected or mock‐inoculated N. benthamiana plants and characterized their protein components. A total of 97 different proteins were identified in apoplasts from PVX‐infected and mock‐inoculated plants. Some proteins involved in plant defence and immunity were present in both infected and control samples, including pathogenesis‐related protein 1. Previous research has shown that the apoplast is the site of accumulation of many other pathogenesis‐related proteins (van Loon et al., 2006). Another protein in common was heat shock protein Hsp70, which is also found in mammalian exosomes (Lancaster & Febbraio, 2005) and has been proposed as a signal molecule that communicates intercellular stresses through packaging into extracellular vesicles (De Maio, 2011). Moreover, some proteins related to pathogen resistance were exclusively identified in the apoplast of PVX‐infected leaves. For example, adenosyl homocysteinase family protein S‐adenosyl homocysteine hydrolase (SAHH) is reported to participate in the suppression of local RNA silencing by both tomato chlorosis virus CP and potato virus Y HC‐Pro (Canizares et al., 2013). Developmentally regulated plasma membrane polypeptide was also detected in infected leaves. One member of this family, the 22 kDa plasma membrane protein PtBP1, is induced in plants under stress conditions. Virus‐induced gene silencing (VIGS) analysis showed that PtBP1 silencing in N. benthamiana attenuated tobacco mosaic virus resistance compared to the tobacco rattle virus control after PeaT1 treatment (Meng et al., 2018).
Besides the host proteins, we identified viral protein peptides in the MS analysis (Figure 1c). Most of the viral peptides were from the CP and it was then shown by further experiments that this protein was specifically present in the PVX‐infected N. benthamiana apoplast (Figure 2). Confocal observation and western blot results showed that the CP was not released into the apoplast if CP:GFP or unfused CP was transiently expressed in N. benthamiana leaves (Figure 4), suggesting that the CP is exported from cells with other factors or as whole viral particles. Viral CP or particles of some viruses have been shown to be present in the xylem (Betti et al., 2012; Ding et al., 2001; French & Elder, 1999; Manabayeva et al., 2013; Moreno et al., 2004; Opalka et al., 1998; Verchot et al., 2001) and xylem sap is considered to be a part of the apoplast as the extracellular spaces are connected (Ligat et al., 2011; Satoh, 2006). The only other reported specific detection of viruses in the leaf apoplast is the presence of replication complexes of TuMV (Movahed et al., 2019), whereas our results with PVX show the presence of intact, infective, particles.
Apoplastic fluid contains extracellular vesicles, including exosomes, that are small membrane‐enclosed structures which are known to be involved in the interaction between plant and foliar microbe pathogens (Bleackley et al., 2020; Cai et al., 2018; Hansen & Nielsen, 2018; Hou et al., 2019; Rutter & Innes, 2017; Samuel et al., 2015). Some mammalian viruses are able to use exosomes or transiently acquire membrane structures to transport viral RNA or particles over long distances in the extracellular fluids (Ahsan et al., 2016; Bukong et al., 2014; Canitano et al., 2013; Feng et al., 2013; Masciopinto et al., 2004). While the replication complexes of TuMV are associated with extracellular vesicles in the foliar apoplast (Movahed et al., 2019), our TEM results suggest that PVX particles are not associated with exosomes (Figure 4b). Because no infection was detected on leaves infiltrated with apoplast containing PVX particles, it appears that PVX moves in one direction only from the cell to apoplast, unlike the bidirectional transport of exosomes. Whether the presence of virus in the apoplast increases the chances of its spread within the plant needs to be investigated by further experiments.
4. EXPERIMENTAL PROCEDURES
4.1. Plant materials and inoculation of pathogens
Wild‐type N. benthamiana plants were grown under a 16 hr light/8 hr dark regime at 25 °C. Agrobacterium harbouring an infectious clone of PVX‐GFP (Draghici et al., 2009) was used for infiltration. GFP fluorescence was observed under long‐wavelength UV‐light (Black Ray Model B 100A, Ultra‐Violet Products Ltd) and photographs were taken using a Canon digital camera.
4.2. Agrobacterium infiltration
For Agrobacterium‐mediated transient expression, Agrobacterium tumefaciens GV3101 containing the expression vector was grown at 28 °C overnight, pelleted, resuspended in infiltration buffer (1 M MgCl2, 100 mM acetosyringone, 1 M MES), and incubated at room temperature for 4 hr. The N. benthamiana leaves were infiltrated with A. tumefaciens cultures with an OD600 = 0.1.
4.3. Isolation of apoplasts
Plants grown for 5–6 weeks were inoculated with A. tumefaciens containing the infectious clone PVX‐GFP. Before being harvested, leaves were observed under a UV lamp at 8 dpi. The apoplast was isolated from systemically infected leaves, which had been infiltrated with vesicle isolation buffer (VIB; 20 mM MES, 2 mM CaCl2, 0.1 M NaCl, pH 6) solution as described by Rutter and Innes (2017). Briefly, infiltrated plants were vacuum infiltrated with VIB, and when the leaves were completely wetted, the remaining liquid on the surface was dried with absorbent paper then leaves were placed inside 30‐ml syringes and centrifuged in 50‐ml conical tubes at 700 × g for 1 hr at 4 °C before filtering through a 0.45‐μm membrane.
4.4. Mass spectrometry
Apoplast fluid isolated from mock‐ and PVX‐infected N. benthamiana were sent to the Luming Biological Company for mass spectrometry. Proteins were identified as described previously (Rutter & Innes, 2017). Briefly, they were denatured with 8 M urea, incubated, alkylated, digested with trypsin, desalted, dried, and injected into the mass spectrometer. All proteome analyses were further processed for GO categorization in terms of molecular function, cellular component, and biological processes using Uniprot (https://www.uniprot.org/).
4.5. RNA extraction and RT‐PCR
Total RNA was extracted from the apoplast of mock‐ and PVX‐infected N. benthamiana leaves with chloroform (Invitrogen). Chloroform was mixed with the apoplast in the ratio 1:5 (vol/vol), shaken well, stood until the layers had separated and then centrifuged for 30 min at 13,200 × g and 4 °C. The resulting aqueous phase was mixed with isopropanol in the ratio 1:1 (vol/vol), kept at −20 °C for 1 hr and then centrifuged for 1 hr at 13,200 × g and 4 °C. The pellet obtained was resuspended in 75% ethanol, centrifuged, and dried before being dissolved in ultrapure RNase‐free water. Reverse transcription was performed by PrimeScript RT Enzyme (Takara). PCR was performed on the resulting cDNAs with specific primers targeting the PVX‐CP viral protein encoding sequence (forward sequence 5′‐ATGTCAGCACCAGCTAGCAC‐3′; reverse sequence 5′‐TGGTGGTGGTAGAGTGACAAC‐3′).
4.6. Northern blot
A DNA probe targeting PVX CP was synthesized with primers (5′‐TGGGACTTAGTCAGACACT‐3′; 5′‐ACCTCGAGTGACAGCTGC‐3′) and labelled with digoxigenin (DIG) according to the manufacturer's protocol (DIG Oligonucleotide 3′‐End Labeling Kit; Roche). Prehybridization, hybridization, and signal detection were performed according to the protocol of the DIG High Prime DNA Labelling and Detection Starter Kit II (Roche).
4.7. Western blot
Total proteins of plant samples were extracted with lysis buffer (100 mM Tris.HCl pH 8.8, 60% sodium dodecyl sulphate [SDS], 2% β‐mercaptoethanol). Proteins and apoplast were separated in 12% SDS‐polyacrylamide gel electrophoresis (PAGE) gels after mixing with loading buffer in the ratio 4:1 (vol/vol), then transferred onto nitrocellulose (Amersham) by semidry electroblotting and detected with primary and secondary antibodies (Sigma‐Aldrich). After incubation with secondary antibody, proteins were visualized with the EasySee Western Blot Kit (Transgene Biotech) and imaged with Molecular Imager ChemiDoc Touch (Bio‐Rad). The primary antibodies used in this research were anti‐GFP (Transgene Biotech), and anti‐actin, anti‐PVX P25, and anti‐PVX CP, which had all been prepared in our laboratory.
4.8. Transmission electron microscopy
A Formvar membrane‐coated copper mesh was dipped in apoplast fluid, negatively stained with 2% phosphotungstic acid (pH 6.7), and blotted dry on filter paper. Grids were observed under a Hitachi H‐7650 transmission electron microscope, the acceleration voltage was 80 kV, and the image was recorded with a Gatan 832 CCD camera.
4.9. FM4‐64 staining
FM4‐64 dye was diluted to 5 µg/ml with Hanks' balanced salt solution (HBSS), wrapped with aluminium foil and stored in the dark. N. benthamiana leaves transiently expressing CP:GFP at 3 dpi were stained with FM4‐64 for 1 min and observed by confocal microscopy.
4.10. Inoculation of N. benthamiana with apoplast fluid
Plants at 5 weeks old were chosen to be inoculated with fresh apoplast isolated from PVX‐infected N. benthamiana leaves. An appropriate amount of quartz sand was spread evenly on the leaves, then 30 μl of apoplasts was added and rubbed gently six to eight times by hand. A few minutes later, the excess quartz sand on the leaf surface was gently washed off with water. Plants were observed under a UV lamp daily.
4.11. Electrolyte leakage measurement
Electrolyte leakage detection was performed as previously described (Aguilar et al., 2015), but with some modification. Briefly, the detached leaf samples, which were subjected to the VIC procedure, were floated on 50 ml of double‐distilled water, with untreated leaves as control. After 6 hr at room temperature, the conductivity was recorded (C0) and a final measurement (TC) made after 20 min of boiling. Percentage of electrolyte leakage (EL) was calculated according to the formula: %EL = 100 × C0/TC.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
FIGURE S1 Electrolyte leakage test confirming the integrity of the cell membrane during apoplast isolation. The statistical analysis shows that there was no significant difference in the percentage of electrolyte leakage between the control and experimental groups. A two‐sample unequal variance directional t test was used to test the significance of the difference
FIGURE S2 Original images and overexposed images from Figure 2. Overexposed images were used to confirm that only coat protein could be detected in potato virus X‐infected leaf apoplast
FIGURE S3 Apoplast isolated from potato virus X‐green fluorescent protein (PVX‐GFP)‐infected leaves is infectious by rub inoculation. (a) Nicotiana benthamiana plants rub inoculated with apoplast isolated from PVX‐GFP‐infected leaves showing GFP fluorescence on newly emerged leaves. (b) Western blot with coat protein antibody verifying PVX infection on inoculated plants
FIGURE S4 Coomassie Brilliant Blue (CBB) staining of sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) as loading control. CBB staining of SDS‐PAGE was used as loading control for western blot in Figure 4b. (b) CBB staining of SDS‐PAGE was used as loading control for western blot in Figure 4c
FIGURE S5 Apoplast isolated from potato virus X‐green fluorescent protein (PVX‐GFP)‐infected leaves is not infectious by infiltration. At 10 days postinoculation, N. benthamiana plants rub inoculated with apoplast isolated from PVX‐GFP‐infected leaves had GFP fluorescence on newly emerged leaves but fluid infiltrated plants remained without fluorescence on either local (infiltrated) or top leaves. Western blot to detect PVX coat protein confirmed systemic infection consistent with the above observation
ACKNOWLEDGEMENTS
The authors thank Professor M. J. Adams (Minehead, UK) for manuscript correction and Shanghai Lu Ming Biotech Co., Ltd (Shanghai, China) for assistance with proteomics analysis. This work was financially supported by the Natural Science Foundation of Zhejiang Province (LY20C140001), the Natural Science Foundation of Ningbo city (2019A610409), the State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro‐products (KF20190107), the Chinese Agriculture Research System (CARS‐24‐C‐04), and the K. C. Wong Magna Fund in Ningbo University.
Hu S, Yin Y, Chen B, et al. Identification of viral particles in the apoplast of Nicotiana b enthamiana leaves infected by potato virus X. Mol Plant Pathol. 2021;22:456–464. 10.1111/mpp.13039
Contributor Information
Jianping Chen, Email: chenjianping@nbu.edu.cn.
Yuwen Lu, Email: luyuwen@nbu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aguilar, E. , Almendral, D. , Allende, L. , Pacheco, R. , Chung, B.N. , Canto, T. et al. (2015) The P25 protein of potato virus X (PVX) is the main pathogenicity determinant responsible for systemic necrosis in PVX‐associated synergisms. Journal of Virology, 89, 2090–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan, N.A. , Sampey, G.C. , Lepene, B. , Akpamagbo, Y. , Barclay, R.A. , Iordanskiy, S. et al. (2016) Presence of viral RNA and proteins in exosomes from cellular clones resistant to Rift Valley fever virus infection. Frontiers in Microbiology, 7, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson, E. , Ali, A. , Resjö, S. & Andreasson, E. (2013) Plant secretome proteomics. Frontiers in Plant Science, 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos, P. , Tsukihara, T. & Rossmann, M.G. (1980) A structural comparison of concanavalin A and tomato bushy stunt virus protein. Journal of Molecular Evolution, 15, 169–179. [DOI] [PubMed] [Google Scholar]
- Baratova, L.A. , Fedorova, N.V. , Dobrov, E.N. , Lukashina, E.V. , Kharlanov, A.N. , Nasonov, V.V. et al. (2004) N‐terminal segment of potato virus X coat protein subunits is glycosylated and mediates formation of a bound water shell on the virion surface. European Journal of Biochemistry, 271, 3136–3145. [DOI] [PubMed] [Google Scholar]
- Bendahmane, A. , Köhn, B.A. , Dedi, C. & Baulcombe, D.C. (1995) The coat protein of potato virus X is a strain‐specific elicitor of Rx1‐mediated virus resistance in potato. The Plant Journal, 8, 933–941. [DOI] [PubMed] [Google Scholar]
- Betti, C. , Lico, C. , Maffi, D. , D'Angeli, S. , Altamura, M.M. , Benvenuto, E. et al. (2012) Potato virus X movement in Nicotiana benthamiana: New details revealed by chimeric coat protein variants. Molecular Plant Pathology, 13, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleackley, M.R. , Samuel, M. , Garcia‐Ceron, D. , McKenna, J.A. , Lowe, R.G.T. , Pathan, M. et al. (2020) Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants. Frontiers in Plant Science, 10, 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. & Felix, G. (2009) A renaissance of elicitors: Perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annual Review of Plant Biology, 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bukong, T.N. , Momen‐Heravi, F. , Kodys, K. , Bala, S. & Szabo, G. (2014) Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2‐miR122‐HSP90. PLoS Pathogens, 10, e1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. , Qiao, L. , Wang, M. , He, B. , Lin, F.‐M. , Palmquist, J. et al. (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science, 360, 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canitano, A. , Venturi, G. , Borghi, M. , Ammendolia, M.G. & Fais, S. (2013) Exosomes released in vitro from Epstein‐Barr virus (EBV)‐infected cells contain EBV‐encoded latent phase mRNAs. Cancer Letters, 337, 193–199. [DOI] [PubMed] [Google Scholar]
- Canizares, M.C. , Lozano‐Duran, R. , Canto, T. , Bejarano, E.R. , Bisaro, D.M. , Navas‐Castillo, J. et al. (2013) Effects of the crinivirus coat protein‐interacting plant protein SAHH on post‐transcriptional RNA silencing and its suppression. Molecular Plant‐Microbe Interactions, 26, 1004–1015. [DOI] [PubMed] [Google Scholar]
- Chapman, S. , Hills, G. , Watts, J. & Baulcombe, D. (1992) Mutational analysis of the coat protein gene of potato virus X: Effects on virion morphology and viral pathogenicity. Virology, 191, 223–230. [DOI] [PubMed] [Google Scholar]
- Chapman, S. , Kavanagh, T. & Baulcombe, D. (1992) Potato virus X as a vector for gene expression in plants. The Plant Journal, 2, 549–557. [DOI] [PubMed] [Google Scholar]
- De Maio, A. (2011) Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go!. Cell Stress and Chaperones, 16, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunois, B. , Baillieul, F. , Clement, C. , Jeandet, P. & Cordelier, S. (2016) Vacuum infiltration‐centrifugation method for apoplastic protein extraction in grapevine. Methods in Molecular Biology, 1459, 249–257. [DOI] [PubMed] [Google Scholar]
- Delaunois, B. , Jeandet, P. , Clément, C. , Baillieul, F. , Dorey, S. & Cordelier, S. (2014) Uncovering plant–pathogen crosstalk through apoplastic proteomic studies. Frontiers in Plant Science, 5, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.S. , Boydston, C.M. & Nelson, R.S. (2001) Presence of brome mosaic virus in barley guttation fluid and its association with localized cell death response. Phytopathology, 91, 440–448. [DOI] [PubMed] [Google Scholar]
- Doehlemann, G. & Hemetsberger, C. (2013) Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytologist, 198, 1001–1016. [DOI] [PubMed] [Google Scholar]
- Dong, S. , Yin, W. , Kong, G. , Yang, X. , Qutob, D. , Chen, Q. et al. (2011) Phytophthora sojae avirulence effector Avr3b is a secreted NADH and ADP‐ribose pyrophosphorylase that modulates plant immunity. PLoS Pathogens, 7, e1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghici, H.K. , Pilot, R. , Thiel, H. & Varrelmann, M. (2009) Functional mapping of PVX RNA‐dependent RNA‐replicase using pentapeptide scanning mutagenesis‐identification of regions essential for replication and subgenomic RNA amplification. Virus Research, 143, 114–124. [DOI] [PubMed] [Google Scholar]
- Feng, Z. , Hensley, L. , McKnight, K.L. , Hu, F. , Madden, V. , Ping, LiFang , et al. (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature, 496, 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, C.J. & Elder, M. (1999) Virus particles in guttate and xylem of infected cucumber (Cucumis sativus L.). Annals of Applied Biology, 134, 81–87. [Google Scholar]
- Gentzel, I. , Giese, L. , Zhao, W. , Alonso, A.P. & Mackey, D. (2019) A simple method for measuring apoplast hydration and collecting apoplast contents. Plant Physiology, 179, 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden, M.G. , Köhm, B.A. , Santa Cruz, S. , Kavanagh, T.A. & Baulcombe, D.C. (1993) A feature of the coat protein of potato virus X affects both induced virus resistance in potato and viral fitness. Virology, 197, 293–302. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt, R. (2010) The dynamic apoplast. Physiological and Molecular Plant Pathology, 74, 199–200. [Google Scholar]
- Hansen, L.L. & Nielsen, M.E. (2018) Plant exosomes: Using an unconventional exit to prevent pathogen entry? Journal of Experimental Botany, 69, 59–68. [DOI] [PubMed] [Google Scholar]
- Hou, Y. , Zhai, Y.I. , Feng, L.I. , Karimi, H.Z. , Rutter, B.D. , Zeng, L. et al. (2019) A Phytophthora effector suppresses trans‐kingdom RNAi to promote disease susceptibility. Cell Host & Microbe, 25, 153‐165.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten, M.H. (2012) Isolation of apoplastic fluid from leaf tissue by the vacuum infiltration‐centrifugation technique. Methods in Molecular Biology, 835, 603–610. [DOI] [PubMed] [Google Scholar]
- Lancaster, G.I. & Febbraio, M.A. (2005) Exosome‐dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. Journal of Biological Chemistry, 280, 23349–23355. [DOI] [PubMed] [Google Scholar]
- Ligat, L. , Lauber, E. , Albenne, C. , Clemente, H.S. , Valot, B. , Zivy, M. et al. (2011) Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics, 11, 1798–1813. [DOI] [PubMed] [Google Scholar]
- Liu, T. , Song, T. , Zhang, X. , Yuan, H. , Su, L. , Li, W. et al. (2014) Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nature Communications, 5, 4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohaus, G. , Pennewiss, K. , Sattelmacher, B. , Hussmann, M. & Hermann Muehling, K. (2001) Is the infiltration‐centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiologia Plantarum, 111, 457–465. [DOI] [PubMed] [Google Scholar]
- van Loon, L.C. , Rep, M. & Pieterse, C.M. (2006) Significance of inducible defense‐related proteins in infected plants. Annual Review of Phytopathology, 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Manabayeva, S.A. , Shamekova, M. , Park, J.‐W. , Ding, X.S. , Nelson, R.S. , Hsieh, Y.‐C. et al. (2013) Differential requirements for tombusvirus coat protein and P19 in plants following leaf versus root inoculation. Virology, 439, 89–96. [DOI] [PubMed] [Google Scholar]
- Masciopinto, F. , Giovani, C. , Campagnoli, S. , GAlli‐Stampino, L. , Colombatto, P. , Brunetto, M. et al. (2004) Association of hepatitis C virus envelope proteins with exosomes. European Journal of Immunology, 34, 2834–2842. [DOI] [PubMed] [Google Scholar]
- Meng, F. , Xiao, Y. , Guo, L. , Zeng, H. , Yang, X. & Qiu, D. (2018) A DREPP protein interacted with PeaT1 from Alternaria tenuissima and is involved in elicitor‐induced disease resistance in Nicotiana plants. Journal of Plant Research, 131, 827–837. [DOI] [PubMed] [Google Scholar]
- Moreno, I.M. , Thompson, J.R. & Garcia‐Arenal, F. (2004) Analysis of the systemic colonization of cucumber plants by cucumber green mottle mosaic virus. Journal of General Virology, 85, 749–759. [DOI] [PubMed] [Google Scholar]
- Movahed, N. , Cabanillas, D.G. , Wan, J. , Vali, H. , Laliberte, J.F. & Zheng, H. (2019) Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiology, 180, 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi, I. , Hayashi, K. , Hiradate, S. , Ishikawa, S. , Fukuoka, M. , Chen, C.P. et al. (2012) Overcoming the difficulties in collecting apoplastic fluid from rice leaves by the infiltration‐centrifugation method. Plant and Cell Physiology, 53, 1659–1668. [DOI] [PubMed] [Google Scholar]
- O’Leary, B.M. , Rico, A. , McCraw, S. , Fones, H.N. & Preston, G.M. (2014) The infiltration‐centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example. Journal of Visualized Experiments, 94, 52113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalka, N. , Brugidou, C. , Bonneau, C. , Nicole, M. , Beachy, R.N. , Yeager, M. et al. (1998) Movement of rice yellow mottle virus between xylem cells through pit membranes. Proceedings of the National Academy of Sciences of the United States of America, 95, 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regente, M. , Corti‐Monzon, G. , Maldonado, A.M. , Pinedo, M. , Jorrin, J. & de la Canal, L. (2009) Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Letters, 583, 3363–3366. [DOI] [PubMed] [Google Scholar]
- Regente, M. , Pinedo, M. , San Clemente, H. , Balliau, T. , Jamet, E. & de la Canal, L. (2017) Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. Journal of Experimental Botany, 68, 5485–5495. [DOI] [PubMed] [Google Scholar]
- Rutter, B.D. & Innes, R.W. (2017) Extracellular vesicles isolated from the leaf apoplast carry stress‐response proteins. Plant Physiology, 173, 728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, M. , Bleackley, M. , Anderson, M. & Mathivanan, S. (2015) Extracellular vesicles including exosomes in cross kingdom regulation: A viewpoint from plant–fungal interactions. Frontiers in Plant Science, 6, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, S. (2006) Organic substances in xylem sap delivered to above‐ground organs by the roots. Journal of Plant Research, 119, 179–187. [DOI] [PubMed] [Google Scholar]
- Scholthof, K.B. , Adkins, S. , Czosnek, H. , Palukaitis, P. , Jacquot, E. , Hohn, T. et al. (2011) Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology, 12, 938–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, Y. , Ren, Y.I. , Sayed, M. , Hu, X. , Lei, C. , Kumar, A. et al. (2018) Plant‐derived exosomal microRNAs shape the gut microbiota. Cell Host & Microbe, 24, e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzini, A.C. , Ek, B. , Palva, E.T. & Hopp, H.E. (1994) Potato virus X coat protein: A glycoprotein. Virology, 202, 651–658. [DOI] [PubMed] [Google Scholar]
- Verchot, J. , Driskel, B.A. , Zhu, Y. , Hunger, R.M. & Littlefield, L.J. (2001) Evidence that soilborne wheat mosaic virus moves long distance through the xylem in wheat. Protoplasma, 218, 57–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Electrolyte leakage test confirming the integrity of the cell membrane during apoplast isolation. The statistical analysis shows that there was no significant difference in the percentage of electrolyte leakage between the control and experimental groups. A two‐sample unequal variance directional t test was used to test the significance of the difference
FIGURE S2 Original images and overexposed images from Figure 2. Overexposed images were used to confirm that only coat protein could be detected in potato virus X‐infected leaf apoplast
FIGURE S3 Apoplast isolated from potato virus X‐green fluorescent protein (PVX‐GFP)‐infected leaves is infectious by rub inoculation. (a) Nicotiana benthamiana plants rub inoculated with apoplast isolated from PVX‐GFP‐infected leaves showing GFP fluorescence on newly emerged leaves. (b) Western blot with coat protein antibody verifying PVX infection on inoculated plants
FIGURE S4 Coomassie Brilliant Blue (CBB) staining of sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) as loading control. CBB staining of SDS‐PAGE was used as loading control for western blot in Figure 4b. (b) CBB staining of SDS‐PAGE was used as loading control for western blot in Figure 4c
FIGURE S5 Apoplast isolated from potato virus X‐green fluorescent protein (PVX‐GFP)‐infected leaves is not infectious by infiltration. At 10 days postinoculation, N. benthamiana plants rub inoculated with apoplast isolated from PVX‐GFP‐infected leaves had GFP fluorescence on newly emerged leaves but fluid infiltrated plants remained without fluorescence on either local (infiltrated) or top leaves. Western blot to detect PVX coat protein confirmed systemic infection consistent with the above observation
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


