Abstract
MicroRNAs (miRNAs) are critical modulators of endothelial homeostasis, which highlights their involvement in vascular diseases, including those caused by virus infections. Our main objective was to identify miRNAs involved in the endothelial function and determine their expression in post-mortem lung biopsies of COVID-19 patients with severe respiratory injuries and thrombotic events. Based on functional enrichment analysis, miR-26a-5p, miR-29b-3p, and miR-34a-5p were identified as regulators of mRNA targets involved in endothelial and inflammatory signaling pathways, as well as viral diseases. A miRNA/mRNA network, constructed based on protein–protein interactions of the miRNA targets and the inflammatory biomarkers characterized in the patients, revealed a close interconnection of these miRNAs in association to the endothelial activation/dysfunction. Reduced expression levels of selected miRNAs were observed in the lung biopsies of COVID-19 patients (n = 9) compared to the Controls (n = 10) (P < 0.01-0.0001). MiR-26a-5p and miR-29b-3p presented the best power to discriminate these groups (area under the curve (AUC) = 0.8286, and AUC = 0.8125, respectively). The correlation analysis of the miRNAs with inflammatory biomarkers in the COVID-19 patients was significant for miR-26a-5p [IL-6 (r2 = 0.5414), and ICAM-1 (r2 = 0.5624)], and miR-29b-3p [IL-4 (r2 = 0.8332) and IL-8 (r2 = 0.2654)]. Altogether, these findings demonstrate the relevance and the non-random involvement of miR-26a-5p, miR-29b-3p, and miR-34a-5p in endothelial dysfunction and inflammatory response in patients with SARS-CoV-2 infection and the occurrence of severe lung injury and immunothrombosis.
Keywords: COVID-19, endothelial dysfunction, lung injuries, microRNA, SARS-CoV-2
INTRODUCTION
The coronavirus disease 2019 (COVID-19), which represents a worldwide pandemic disease with high mortality rates, is caused by severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2). SARS–CoV-2 infections mainly target the lungs causing acute respiratory distress syndrome (ARDS) with diffuse alveolar damage (DAD) (1, 2). The infection causes significant changes in endothelial cells functions and morphology, which are evidenced by endothelial activation, disruption of intercellular junctions, cell swelling, and loss of contact with the basal membrane (3, 4).
The angiotensin-converting enzyme (ACE) 2 protein is one of the critical proteins that mediate the SARS–CoV-2 insertion in human cells through the interaction with the spike glycoprotein (protein S) (5). This receptor is abundantly present in the endothelial cells, which supports the data that SARS–CoV-2 infection is associated with endothelial dysfunction and could confer to the patients increased risk of endotheliitis and thrombotic events (4, 6, 7).
The proinflammatory status described in COVID-19 patients can trigger the so-called cytokine storm, with the release of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, IL-8, IL-2R and tumor necrosis factor-α (TNF-α) (8, 9). Moreover, the hyperinflammatory effects of the cytokine storm can also contribute to endothelial activation/dysfunction (10) and cell death by pyroptosis (11), which can result in the aforementioned severe systemic immunothrombotic events.
MicroRNAs (miRNAs) are endogenous small RNA molecules that regulate several physiological processes in the cells, including endothelial homeostasis, which highlights their involvement in vascular diseases (12). Deregulated miRNA expression has been associated with respiratory syndromes and pulmonary diseases caused by viral infections (13, 14). In SARS–CoV-2 infection, the majority of the miRNA reports in association to COVID-19 have been conducted by computational miRNA prediction analysis (15–19). To our knowledge, the expression analysis of miRNAs in lung biopsies of COVID-19 patients has not been performed. Therefore, the main aim of this study was to identify miRNAs involved in the endothelial function and determine their expression in post-mortem lung biopsies of COVID-19 patients that developed severe respiratory injuries and thrombotic events. Three miRNAs were selected, miR-26a-5p, miR-29b-3p, and miR-34a-5p, based on functional enrichment analysis showing their involvement in signaling pathways directly associated with endothelial dysfunction and virus infectious diseases including: the cell-adhesion and adherens junctions, lysine degradation, viral carcinogenesis, hepatitis B, and HTLV1 virus infection. In addition, the expression of miRNAs was correlated with the expression of inflammatory biomarkers associated with the cytokine storm and endothelial dysfunction, known to be critically involved in the pathogenesis of COVID-19.
MATERIALS AND METHODS
Sample Population
Nine samples of formalin-fixed paraffin-embedded (FFPE) post-mortem lung biopsies were obtained from patients with COVID-19, treated at Hospital Marcelino Champagnat in Curitiba-Brazil. The cause of death was ARDS, DAD, and multiple organs failure by SARS–CoV-2. A Control group of FFPE post-mortem lung samples was collected, at Hospital de Clínicas, Curitiba-Brazil, of ten patients, who died due to other causes, not involving lung injuries. The major causes of death of this group were due to cancer (gastric, hepatic, laryngeal, and neuroendocrine carcinoma, and lymphoma), acute myocardial infarction (AMI), dementia/cachexia, and peritonitis. This Control selection allowed us to determine specificity of the miRNA deregulation due to SARS–CoV-2 infection, and to rule out the variation of miRNA expression due to distinct tissue types (15, 20).
All the samples were collected under family consent after approval of the ethics committee of the hospitals [Hospital Marcelino Champagnat (REC: 2.550.445/2018) and Hospital de Clínicas (REC: 3.944.734/2020)] and the National Research Ethics Committee (CONEP).
Patients’ Clinical Data Characteristics and Inflammatory Biomarkers of Tissue Expression
The clinical characteristics of the patients evaluated in this study included gender, age, types of comorbidities, and time of hospitalization. Eight of the nine COVID-19 patients were submitted to mechanical ventilation in a period that ranged from eight to 21 days (Table 1).
Table 1.
Comparison between the controls and COVID-19 patients according to the clinical and immunohistochemical information
| Data | Control (n = 10) | COVID-19 (n = 9) |
|---|---|---|
| Sex | Male 7 (70%) | Male 6 (66.7%) |
| Female 3 (30%) | Female 3 (33.3%) | |
| P = 0.793# | ||
| Age, yr* | 42.3/45 (18-60) | 73.4/80 (46-87) |
| P = 0.003# | ||
| Comorbidities(number of cases) | Hypertension (5/9)Cancer (2/9)Chronic kidney disease (2/9)Class II obesity (2/9)Coronary disease (2/9)Dementia (2/9)Diabetes mellitus (2/9)Dyslipidemia (2/9)Giant cells arteritis (1/9)Hypothyroidism (1/9)Liver transplantation (1/9) | |
| Time from hospitalization to death (days)* | 16.3/13 (6–38) | |
| Mechanical ventilation* | 8.5/8 (8–21) | |
| IL-4 expression*†‡ | 2.84/2.26 (0.23–7.41) | 8.26/9.37 (0.71–13.39) |
| P = 0.0509# | ||
| IL-6 expression*†‡ | 0.0/0.6 (0.0–5.6) | 3.9/4.0 (0.3–7.6) |
| P = 0.0001# | ||
| IL-8 expression*†‡ | 0.00/0.00001 (0-0.0001) | 0.79/0.19 (0.004–3.62) |
| P = 0.0008# | ||
| IL-13 expression*†‡ | 0.13/0.02 (0.00–0.76) | 0.39/0.28 (0.02–1.34) |
| P = 0.070# | ||
| TNF-α expression*†‡ | 2.2/1.9 (0.2–4.1) | 5.8/12.3 (3.2–35.3) |
| P = 0.0048# | ||
| ICAM-1 expression*†‡ | 0.4/0.8 (0.1–2.6) | 4.3/7.4 (1.4–20.6) |
| P = 0.0034# | ||
| CASP-1 Allred score*‡ | 2.0/2.4 (2.0–4.0) | 8.0/7.8 (7.2–8.0) |
| P = 0.0009# |
Average/median (Min-Max);
lung tissue expression in percentage per high power field (HPF);
patients from 1 to 6 for COVID-19 group.
= P values from the comparison between COVID-19 and control groups (Mann-Whitney test (P < 0.05)).
The inflammatory protein expression was previously performed by immunohistochemistry (IHC) in the lung biopsies for the Control (n = 10) and COVID-19 (n = 6) groups by our collaborators (3, 21, 22), for the following seven biomarkers: IL-4, IL-6, IL-8, IL-13, TNF-α, intercellular adhesion molecule 1 (ICAM-1), and Caspase-1 (CASP-1). All of these biomarkers presented significant increase in tissue expression in the COVID-19 group compared to the Control group (Table 1).
Functional Enrichment Pathway Analysis and miRNA/mRNA Target Interaction Network (miRNA Selection)
DIANA tools v.5.0 (http://diana.imis.athena-innovation.gr) was used to identify miRNAs potentially associated with endothelial function and related pathways. Gene targets for the selected miRNAs were queried using DIANA v.5.0 and and miRTarBase v.8.0 (http://mirtarbase.cuhk.edu.cn), based on a prediction score of at least 0.8 and/or in strong validation assays according to miRTarBase. Signaling pathways (Kyoto Encyclopedia of Genes and Genomes (KEGG) were identified and selected by P value (P < 0.05). Cytoscape v.3.8.0 (https://cytoscape.org) was used to construct the network of the selected miRNAs, gene targets, and the inflammatory biomarkers evaluated by IHC. The network interactions were based on the STRING v.11 database (https://www.string-db.org), which was used for mapping protein–protein interaction (PPI), applying the minimum interaction score of 0.9 (highest confidence).
RNA Isolation and RT-qPCR Analysis
RNA was isolated from the 10-μm sections of the lung tissue biopsies from the Control and COVID-19 groups as previously described (23, 24). RNA was reverse transcribed and amplified by RT-quantitative PCR (qPCR) using TaqMan probes for miR-26a-5p (assay number 000405), miR-29b-3p (assay number 000413), and miR-34a-5p (assay number 000426), and RNU48 (assay number 1006) (normalizer) (Applied Biosystems). Samples with threshold cycle (Ct) values of ≥35 were excluded. Each reaction was performed in triplicate, and data were presented as means ± SE and P value ≤ 0.05. MiRNA expression between the groups was calculated using the 2-ΔΔCt method. Mann-Whitney and ANOVA tests were applied to verify miRNAs expression in each group and between the groups, respectively.
Receiver Operating Characteristic (ROC) Curve Analysis
ROC and area under the curve (AUC) analysis were performed to determine the power of the miRNAs to discriminate the Control and COVID-19 groups. Sensitivity was plotted against 1-specificity for the binary classifier (Controls versus COVID-19). AUCs and 95% corresponding confidence intervals were calculated for each miRNA and combinations of miRNAs.
Correlation miRNA and Inflammatory Biomarkers Tissue Expression
The coefficient of determination r square (r2) by Pearson’s test was used to determine the degree of correlation between the miRNAs expression levels and the inflammatory biomarkers expression previously evaluated in the same lung biopsies (1, 21, 25), considering r2 ≥ 0.5 and r ≥ 0.7 as indicative of correlation.
RESULTS
MiRNAs Selection
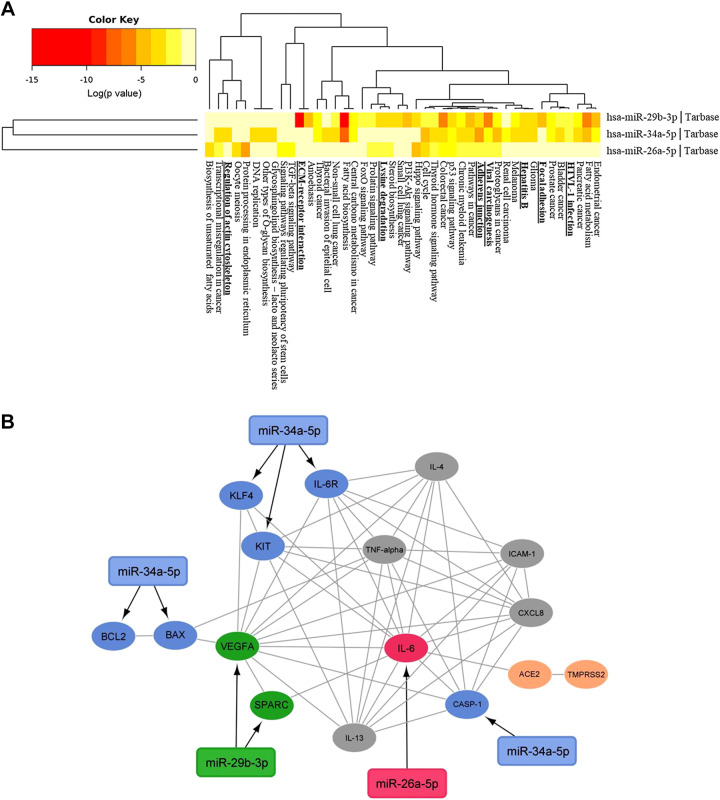
Functional enrichment analysis was performed to identify miRNAs involved in pathways associated with endothelial function. As a result, three miRNAs, miR-26a-5p, miR-29b-3p, and miR-34a-5p, were selected. These miRNAs were demonstrated by pathway enrichment analysis to be involved in cell-adhesion, adherens junctions, and extracellular matrix (ECM) receptor interaction signaling pathways, as well as, in pathways with gene targets that regulate viral diseases, such as those caused by hepatitis B and HTLV1 virus infections (Fig. 1A). From the selected miRNAs, miR-29b-3p and miR-34a-5p were clustered closer and were involved in a higher number of pathways when compared to miR-26a-5p. A search in the miRNA prediction databases revealed 133, 118, and 140 targets for miR-26a-5p, miR-29b-3p, and miR-34a-5p, respectively. Considering only targets involved in the endothelial and associated pathways, a protein–protein interaction (PPI) interaction among them was obtained and a miRNA/mRNA network was constructed, showing the interconnection of the miRNAs selected, corresponding mRNA targets, and the inflammatory biomarkers evaluated in the patients (Fig. 1B). These results formed the base of the miRNA selection of this study, confirming their relevance to the endothelial, and inflammation process, and virus-associated diseases.
Figure 1.
A: heatmap of KEGG pathways for the selected three miRNAs (DIANA tools v.5.0). Black boxes indicate the pathways with relevance to the endothelial function and to viral infection diseases. The color intensities indicate the −log10 (P value) enrichment score of each Gene Ontology (GO) term. B: miRNA/mRNA targets network. In red, green, and blue are the direct targets of miR-26a-5p, 29b-3p, and miR-34a-5p, respectively. In grey, the inflammatory biomarkers analyzed in the group of patients and in orange the SARS–CoV-2 S protein receptors ACE2 and TMPRSS2. Black arrows link each miRNA to its putative targets, and grey lines link the interaction of all the genes (Cytoscape 3.0).
MiRNA Expression Analysis by RT-qPCR
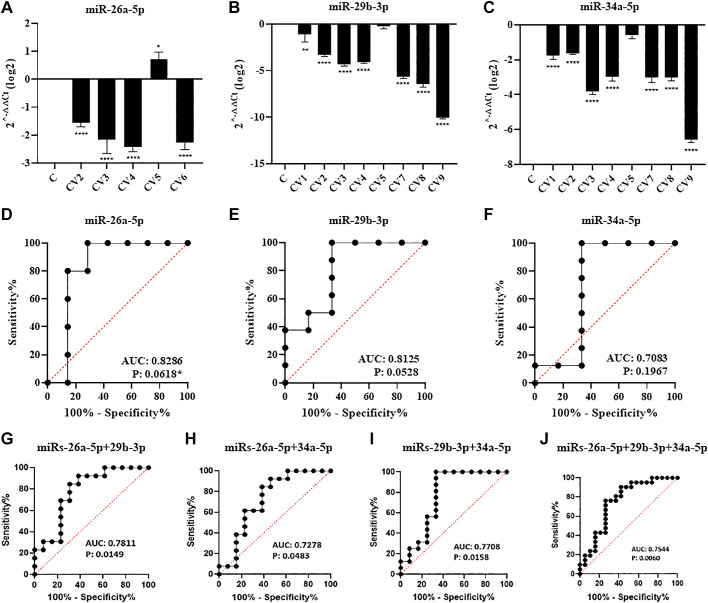
The expression of the miRNAs was evaluated in lung biopsies of the COVID-19 and Control groups. Seven out of nine COVID-19 biopsies were successfully evaluated for the expression of at least one of the miRNAs. The expression of miR-26a-5p was not conclusive (Ct values ≥35) in four COVID-19 biopsies and the miR-29b-3p and miR-34a-5p in one biopsy each. In the Control group, three samples were not conclusive for miR-26a-5p and miR-29b-3, and four for the miR-34a-5p.
The analysis of the relative expression of the miRNAs between the COVID-19 and Control groups demonstrated that they were significantly down-regulated compared to the Control groups (Fig. 2, A–C), except for one sample (CV5) for miR-29b-3p (Fig. 2B) and miR-34a-5p each (Fig. 2C).
Figure 2.
A–C: relative expression of miRNAs between the Control (C) and COVID-19 (CV) groups. D–J: ROC/AUC analysis of individual and combined miRNAs between the Control and COVID-19 groups. *P < 0.05, **P < 0.01, ****P < 0.0001.
ROC Analysis
The potential power of the expression of the miRNAs, individually and/or combined, in discriminating the two groups of lung biopsies was determined using the ROC and AUC analysis. MiR-26a-5p and miR-29b-3p were among the miRNAs that showed the best power to discriminate the COVID-19 group from the Controls (AUC = 0.8286, and AUC = 0.8125, respectively) despite presenting a P value slightly above the statistical significance (Fig. 2, D and E). The pairwise analysis of the miRNAs altered their discriminatory power. The miR-26a-5p/miR-29b-3p pair showed an AUC value of 0.7811 in discriminating the COVID-19 group from the Controls (Fig. 2G), although for these groups these miRs alone were more robust. MiR-34a-5p, which alone did not present a significant AUC (Fig. 2F), together with miR-26a-5p and miR-29b-3p, showed a significant AUC value to discriminate the groups (Fig. 2, H and I). Finally, the combination of the three miRNAs did not significantly increase the AUC values among the groups, however, the P value obtained presented with a higher significant level (P = 0.0060) (Fig. 2J).
Correlation of miRNA with Inflammatory Biomarkers Tissue Expression
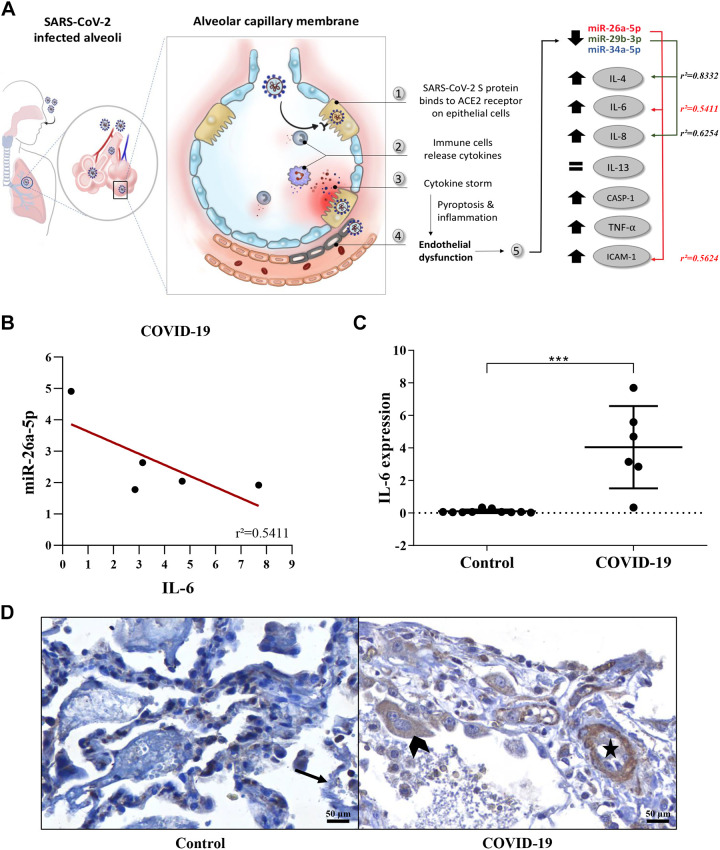
The correlation of the miRNAs expression was performed with the tissue expression of seven inflammatory biomarkers in the two groups of lung biopsies. Significant correlations were observed for miR-26a-5p and miR-29b-3p in the Control and COVID-19 groups (Fig. 3, A and B).
Figure 3.
A: the endothelial dysfunction process in the alveolar capillary membrane of SARS–CoV-2 infected alveoli. B: correlation analysis (r2) of the miR-26a-5p and IL-6 expression levels in the COVID-19 group. C: higher expression of IL-6 levels in the COVID-19 group compared to the Controls (Mann-Whitney test, ***P = 0.0001). D: IL-6 tissue expression (IHC) positivity in the endothelium of the lung vessel (star) and in type II pneumocytes (arrowhead) of a representative tissue section of a COVID-19 sample, compared to the negative expression of lung vessels in a Control sample (black arrow). C and D were constructed based on the data described in Nagashima et al. (3).
In the COVID-19 group, among the interleukins (IL-4, IL-6, IL-8, and IL-13) evaluated, an inverse correlation was observed between IL-4 and the expression of miR-29b-3p (r2 = 0.8332, r = −0.9130), and between the IL-6, and miR-26a-5p (r = 0.5411, r = −0.7360). The inverse correlation between the levels of miR-26a-5p and IL-6 (Fig. 3B) corroborates the IHC results in the COVID-19 group (compared to the Controls), showing an increase of IL-6 expression (Fig. 3, C and D). On the other hand, the increased in miR-29b-3p expression was directly correlated to the increase of IL-8 levels (r2 = 0.6254, r = 0.7908). No correlation was observed between IL-13 and miRNAs expression levels.
For the other inflammatory biomarkers analyzed, an inverse correlation of the expression of miR-26a-5p, and ICAM-1 (r2 = 0.5624, r = −0.7499) was observed in the COVID-19 group. For the remaining biomarkers evaluated, no significant correlation with miRNA expression was observed (Fig. 3A).
DISCUSSION
As it is well-known, in the most severe cases of SARS–CoV-2 infection, patients can develop DAD and immunothrombosis, which can eventually lead to multiple organ failure and fatal outcome (1, 2). This clinical scenario is directly associated with the cytokine storm (8, 9). Several studies, including from our collaborators, have shown an increase in the cytokines levels, and CASP-1 in patients with COVID-19 (3, 8, 9, 21, 22). The capillary-alveolar endothelial cells under the COVID-19 cytokines storm are activated and produce NF-κB and adhesion molecules such as ICAM-1 (9). Thus, leukocyte-degranulated enzymes can cause the breakdown of the glycocalyx barrier of the endothelial cells, which can lead to endothelial dysfunction, endotheliitis, and thrombotic events (26). The apoptosis of endothelial cells could be another consequence of SARS–CoV-2 infection and endothelial dysfunction. Endothelial cell death could be mediated by CASP-1 activation with consequent production of cell edema, the release of pro-inflammatory cytokines, and cell fragmentation, characteristics of pyroptosis, which has been described in cells infected by SARS-CoV-2 (3).
MiRNAs have been associated with an inflammatory response due to their role in the regulation of cytokines, such as IL-1, IL-6, and IL-8 (27), as well as, with gene targets that regulate endothelial function (12). The selection of the miRNAs in this study was therefore based on their involvement in signaling pathways associated with the endothelial activation/dysfunction, which is frequently seen in COVID-19 patients that develop DAD and thrombotic events (1, 2). MiR-26a-5p, miR-29b-3p, and miR-34a-5p were observed by KEGG analysis to be interconnected, and acting in common signaling pathways. Among the top 15 pathways observed, the ones directly associated with the endothelial function and virus infectious diseases included: the cell-adhesion and adherens junctions, lysine degradation, viral carcinogenesis, hepatitis B, and HTLV1 virus infection.
The relative expression of miR-26a-5p, miR-29b-3p, and miR-34a-5p was shown to be significantly down-regulated in the lung biopsies of most of the COVID-19 patients compared to the Controls. Roc analysis of individual miRNAs showed that miR-26a-5p and miR-29b-3p presented the best power to discriminate the COVID-19 group from the Controls. Interestingly, the same analysis for the paired and combined miRNAs did not show higher power for all the combinations, except for miR-34a-5p, which alone was not significantly able to discriminate these groups.
The miRNAs expression levels were significantly correlated with the expression of four of the seven inflammatory biomarkers previously analyzed (3, 21, 22) in the COVID-19 patients. MiR-26a-5p expression was observed inversely correlated with IL-6 tissue expression, one of the cytokines directly regulated by this miRNA (28). IL-6 is an important biomarker for the severity of COVID-19, supporting our correlation results in the COVID-19 lung biopsies and others studies describing its association with endothelial activation/dysfunction and inflammatory vascular responses (29, 30). The second miRNA selected, miR-29b-3p, was also shown to be associated with an immune and adaptive response (31). In our analysis, the decreased expression of miR-29b-3p was correlated with an increase in the levels of IL-4 and IL-8 in the COVID-19 group compared to the Controls, which was also observed by IHC. Finally, miR-34a-5p was significantly down-regulated in the COVID-19 group compared to the Controls. However, individually, this miRNA was not able to significantly discriminate these groups. Corroborating our findings, Shah et al. (32) demonstrated the involvement of miR-34a-5p in ARDS, in which the reduction of its expression reduced endothelial dysfunction, and lung injury. Interestingly, this miRNA was also among the ones described by Bartoszewski et al. (15) as presenting the highest number of target sites in the SARS–CoV-2 RNA, potentially impacting the CASP-1–mediated cellular processes and also pointing to the sponge/depletion of specific miRNAs mechanisms. Our findings support this in silico data in the lung tissue of COVID-19 patients, where, although not significantly correlated, we showed a reduced expression of miR-34a-5p and a higher expression of CASP-1, its direct gene target.
Altogether, our findings demonstrate the relevance and the non-random involvement of miR-26a-5p, miR-29b-3p, and miR-34a-5p in endothelial dysfunction and inflammatory response in patients with SARS–CoV-2 infection and the occurrence of severe lung injury and immunothrombosis. These clinical events are the most damaging occurrences correlated with fatal outcomes in patients with severe forms of COVID-19.
It is relevant to point out that the controls (non-damaged lung tissue biopsies) of this study were selected to guarantee the tissue specificity of the miRNA expression among the groups, and characterize their basal expression levels. Additional groups of controls, such as COVID-19 negative post-mortem lung biopsies could also be included, however, these biopsies were not available to this study. It is also relevant to note that few of the inflammatory biomarkers evaluated in the patients of this study were direct targets of the miRNAs analyzed, except for the pairings miR-26a-5p/IL-6 and miR-34a-5p/CASP-1. Nonetheless, an interconnected network was observed, which highlights the complex regulation of miRNAs and target genes and the co-synergistic mode of action of miRNAs.
GRANTS
This work was supported by a grant from the National Council for Scientific and Technological Development (CNPq) to L. de Noronha.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C., L.N., and L.R.C. conceived and designed research; A.C., A.S.F., and S.G.S.F. performed experiments; A.C., A.S.F., S.G.S.F., M.L.V.A., C.B.V.P., S.N., C.M.S., A.F.R.S.M., C.P.B., and L.R.C. analyzed data; A.C., A.S.F., S.G.S.F., M.L.V.A., C.B.V.P., S.N., C.M.S., A.F.R.S.M., C.P.B., L.N., and L.R.C. interpreted results of experiments; A.C., A.S.F., and S.G.S.F. prepared figures; A.C., A.S.F., S.G.S.F., and M.L.V.A. drafted manuscript; A.C., A.S.F., S.G.S.F., L.N., and L.R.C. edited and revised manuscript; A.C., L.N., and L.R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Akanksha Mahajan from Northwestern University, Chicago, IL, for the preparation of the figures.
REFERENCES
- 1.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. [Erratum inLancetRespir Med. 8: e26; 2020]. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagashima S, Mendes MCC, Martins AP, Borges NH, Godoy TM, Miggiolaro A, da Silva Dezidério F, Machado-Souza C, de Noronha L. Endothelial dysfunction and thrombosis in patients with COVID-19. Arterioscler Thromb Vasc Biol 40: 2404–2407, 2020. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 135: 2033–2040, 2020. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 18: 1517–1519, 2020. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 93: 250–256, 2020. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815, 2007. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 11.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemecz M, Alexandru N, Tanko G, Georgescu A. Role of microRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep 18: 87, 2016. doi: 10.1007/s11906-016-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruscella P, Bottini S, Baudesson C, Pawlotsky JM, Feray C, Trabucchi M. Viruses and miRNAs: more friends than foes. Front Microbiol 8: 824, 2017. doi: 10.3389/fmicb.2017.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolzenburg LR, Harris A. The role of microRNAs in chronic respiratory disease: recent insights. Biol Chem 399: 219–234, 2018. doi: 10.1515/hsz-2017-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartoszewski R, Dabrowski XM, Jakiela B, Matalon S, Harrod KS, Sanak M, Collawn JF. SARS-CoV-2 may regulate cellular responses through depletion of specific host miRNAs. Am J Physiol Lung Cell Mol Physiol 319: L444–L455, 2020. doi: 10.1152/ajplung.00252.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Sany MRU, Islam MS, Islam A. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front Genet 11: 765, 2020. doi: 10.3389/fgene.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nersisyan S, Shkurnikov M, Turchinovich A, Knyazev E, Tonevitsky A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS One 15: e0235987, 2020. doi: 10.1371/journal.pone.0235987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saçar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ 8: e9369, 2020. doi: 10.7717/peerj.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sardar R, Satish D, Birla S, Gupta D. Dataset of mutational analysis, miRNAs targeting SARS-CoV-2 genes and host gene expression in SARS-CoV and SARS-CoV-2 infections. Data Brief 32: 106207, 2020. doi: 10.1016/j.dib.2020.106207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, Rheinheimer S, Meder B, Stähler C, Meese E, Keller A. Distribution of miRNA expression across human tissues. Nucleic Acids Res 44: 3865–3877, 2016. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azevedo MLV, Vaz de Paula CB, Nagashima S, Machado-Souza C, Ribeiro AFMM, Junior JS, Malaquias MAS, Raboni SM, Neto PC, Souza DG, Baena CP, Noronha L. Alveolar neutrophilic recruitment in COVID-19 may not be mediated by Th17 response. Res Square 2020. July. doi: 10.21203/rs.3.rs-36238/v2. [DOI] [Google Scholar]
- 22.Vaz de Paula CB, Azevedo MLV, Nagashima S, Martins APC, Malaquias MAS, Ribeiro AFMM, Junior JS, Avelino G, Carstens LB, Carmo LAP, Noronha L. IL-4/IL-13 remodeling pathway of covid-19 lung injury. Research Square, 2020. June. doi: 10.21203/rs.3.rs-34688/v1. [DOI] [Google Scholar]
- 23.Sugita B, Gill M, Mahajan A, Duttargi A, Kirolikar S, Almeida R, Regis K, Oluwasanmi OL, Marchi F, Marian C, Makambi K, Kallakury B, Sheahan L, Cavalli IJ, Ribeiro EM, Madhavan S, Boca S, Gusev Y, Cavalli LR. Differentially expressed miRNAs in triple negative breast cancer between African-American and non-Hispanic white women. Oncotarget 7: 79274–79291, 2016. doi: 10.18632/oncotarget.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugita BM, Pereira SR, De Almeida RC, Gill M, Mahajan A, Duttargi A, Kirolikar S, Fadda P, de Lima RS, Urban CA, Makambi K, Madhavan S, Boca S, Gusev Y, Cavalli IJ, Ribeiro EMFS, Cavalli LR. Integrated copy number and miRNA expression analysis in triple negative breast cancer of Latin American patients. Oncotarget 10: 6184–6203, 2019. doi: 10.18632/oncotarget.27250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arisan ED, Dart A, Grant GH, Arisan S, Cuhadaroglu S, Lange S, Uysal-Onganer P. The prediction of miRNAs in SARS-CoV-2 genomes: hsa-miR databases identify 7 key miRs linked to host responses and virus pathogenicity-related KEGG pathways significant for comorbidities. Viruses 12: 614, 2020. doi: 10.3390/v12060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, Oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J 29: 3595–3611, 2015. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology 58: 158–170, 2013. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 29.Xing X, Guo S, Zhang G, Liu Y, Bi S, Wang X, Lu Q. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz J Med Biol Res 53: e9106, 2020. [Erratum inBraz J Med Biol Res.02; 53(3): e9106, 2020]. doi: 10.1590/1414-431X20199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong X, Zhang L, Li Y, Li P, Li J, Cheng G. Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of miR-26a-5p via inhibiting TLR4/NF-κB pathway in human endothelial cells. Biomed Pharmacother 108: 1783–1789, 2018. doi: 10.1016/j.biopha.2018.09.175. [DOI] [PubMed] [Google Scholar]
- 31.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol 12: 861–869, 2011. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 32.Shah D, Das P, Alam MA, Mahajan N, Romero F, Shahid M, Singh H, Bhandari V. MicroRNA-34a promotes endothelial dysfunction and mitochondrial-mediated apoptosis in murine models of acute lung injury. Am J Respir Cell Mol Biol 60: 465–477, 2019. doi: 10.1165/rcmb.2018-0194OC. [DOI] [PubMed] [Google Scholar]