Abstract
Background & Objective:
Our patient cohort revealed that obesity is strongly associated with steroid-5α reductase type 2 (SRD5A2) promoter methylation and reduced protein expression. The underlying mechanism of prostatic growth in this population is poorly understood. Here we addressed the question of how obesity, inflammation and steroid hormones affect the development of benign prostatic hyperplasia (BPH).
Material and Methods:
We used pre-adipocytes, macrophages, primary human prostatic stromal cells, prostate tissues from high fat diet induced obese mice, and 35 prostate specimens that collected from patients who underwent transurethral resection of the prostate (TURP). RNA was isolated and quantified with RT-PCR. Genome DNA was extracted and SRD5A2 promoter methylation was determined. Sex hormones were determined by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC/MS). Protein was extracted and determined by ELISA test.
Results:
In prostatic tissues with obesity, the levels of inflammatory mediators were elevated. SRD5A2 promoter methylation was promoted, but SRD5A2 expression was inhibited. Inflammatory mediators and saturated fatty acid synergistically regulated aromatase activity. Obesity promoted an androgenic to estrogenic switch in the prostate.
Conclusions:
Our findings suggest that obesity-associated inflammation induces androgenic to estrogenic switch in the prostate gland, which may serve as an effective strategy for alternative therapies for management of lower urinary tract symptoms associated with BPH in select individuals.
Keywords: Methylation, SRD5A2, Obesity, Inflammation, Androgenic to estrogenic switch, Prostate
Introduction
BPH accounts for the majority of lower urinary tract symptoms (LUTS) due to bladder outlet obstruction in elderly men. While all men experience prostate growth during adulthood, the rate of growth differs significantly among different men. The factors that regulate this differential growth pattern are unknown. Each year management of BPH/LUTS accounts for 7.8 million doctors’ office visits with an associated $4 billion in healthcare expenditures in the US and over $40 billion dollars worldwide1. The steroid-5α reductase type 2 (SRD5A2) gene and protein play a significant role in the development and growth of prostate tissue, and strategies to block SRD5A2 is one of the main approaches to treating BPH/LUTS2. However, at least 30% of patients are resistant or fail to respond to medical management, and it is therefore critically important to elucidate the underlying mechanism. Previously we found that in prostatic samples with SRD5A2 promoter methylation and silencing of the gene expression, estradiol is dramatically elevated, concomitant with significant upregulation of estrogen response genes3. We further found that tumor necrosis factor α (TNF- α) suppresses SRD5A2 mRNA and protein expression, and simultaneously promotes expression of aromatase, the enzyme responsible for conversion of testosterone to estradiol. Our previous work suggests that, in the absence of prostatic SRD5A2, there is an androgenic to estrogenic switch3.
In the US, it is approximated that more than 1 in 3 adults are considered to be obese4,5. The estimated annual medical cost of obesity ranges from $147 billion to nearly $210 billion per year6. In the last decade, metabolic syndrome and markers of metabolism have been identified as potential risk factors for BPH/LUTS7. A recent meta-analysis found that patients with metabolic syndrome had significantly higher total prostate volume than those without metabolic syndrome (P<0.001)8. BPH/LUTS accounts for the major unrecognized urological complications of obesity. Patients eligible for transurethral resection of the prostate were significantly more likely to have higher abdominal circumference, higher serum levels of insulin and Insulin-like growth factor 1 (IGF-1), and lower levels of free testosterone than control subjects with BPH4. Additionally, metabolic disturbances may promote the pathogenesis of prostatic hyperplasia and steroid hormone imbalance. In the Framingham Heart Study, men in the highest quintile of estradiol and estrone levels had significantly higher age and Body Mass Index (BMI) than others9. Data analysis of the Prostate Cancer Prevention Trial (PCPT), Health Professionals Follow-up cohort, and Olmstead County Study and the Baltimore Longitudinal Study of Aging all suggest that obese men had a significantly larger prostate size, and increased BMI was significantly associated with the severity of LUTS10. While obesity’s link to lower urinary tract symptoms has been widely recognized, the underlying mechanism of prostatic growth is poorly understood in this population. Our patient cohort has revealed that aging and obesity are strongly associated with SRD5A2 promoter methylation and reduced protein expression11. In this study, we further addressed the question of whether obesity-associated inflammation promotes the methylation of SRD5A2 gene which would suppress the expression of SRD5A2 protein and promote an androgenic to estrogenic pathway in prostatic tissue of obese men.
Materials and Methods
Patient Specimens
With institutional review board approval, thirty-five prostate specimens were collected from patients who underwent transurethral resection of the prostate at Massachusetts General Hospital with Institutional Review Board approval. Informed consent was obtained from all subjects. The clinical characteristics of the subjects have been summarized previously3. All prostatic samples were from the transition zone and were collected post-surgically in cold saline after pathological examination to exclude malignancy. One portion was homogenized for steroid sex hormone determination and aromatase evaluation. Another portion was frozen in liquid nitrogen and stored at −80°C for protein extraction and RNA extraction. Two prostate specimens were collected from patients who underwent simple prostatectomy for symptomatic BPH at the University of Texas Southwestern Medical Center with Institutional Review Board approval. Fresh samples were processed for cell digestion and cell sorting to obtain stromal cells as described previously12–14.
Full experimental details of animals, regents, cell culture, RNA quantification, SRD5A2 promoter methylation, protein and sex hormone determination are in Supplementary Materials and Methods. The primers used for qRT-PCR are listed in Supplementary Table.
Statistical Analysis
Data from in vitro experiments and animal experiments were presented as means of average determinants ± standard error of the mean (SEM), and the statistical significance of differences was evaluated with unpaired Student’s t-tests. For clinical data, sample size of each group was calculated with a power of 80 %. Continuous variables were presented as median (IQR) and compared using the Mann–Whitney U-test. The association between two different parameters was assessed using Spearman rank correlation. All tests were 2-tailed and p <0.05 was considered statistically significant. The statistical analyses of clinical data were performed with Stata14 (College Station, TX, USA).
Results:
Obesity induces SRD5A2 promoter hyper-methylation and suppressed SRD5A2 expression.
Previously, we assessed the methylation of CpG islands in SRD5A2 promoter. We found that increased BMI was associated with SRD5A2 promoter methylation and decreased SRD5A2 expression11. To further confirm this finding, we used MethylCollector kit to determine prostatic SRD5A2 promoter methylation in 16 men with symptomatic BPH. Similar with previous findings11, we found that in patients with high BMI (BMI≥25), SRD5A2 promoter methylation was significantly higher than that in lean individuals (BMI<25) (Supplementary fig. 1 A, P<0.0001). Accordingly, SRD5A2 expression in increased BMI individuals was significantly lower than that of the lean control group (Supplementary fig. 2 and Supplementary fig. 1 B, P<0.0001).
Next, to investigate how obesity affects SRD5A2 promoter methylation in the prostate, we fed two groups of 6-week-old C57BL/6 male mice with high fat diet (HFD) that provides 42% of calories from fat and regular fat diet (RFD) that provides 13.4% of calories from fat, respectively, for 4 months. As expected, the average body weight of mice on HFD was significantly heavier than that of the mice on RFD after 4 months. Meanwhile, the average prostate weight of mice on HFD was 1.53-fold higher than that of the mice on RFD15. Similar to observations in humans, HFD animals had a significantly higher level of SRD5A2 promoter methylation and significantly lower expression of SRD5A2, as well (Supplementary fig. 1 C&D). Furthermore, we observed that in in vitro cultured primary human prostatic stromal cells, adipocyte (Supplementary fig. 1 E–G) conditioned media (Adi-CM) promoted SRD5A2 promoter methylation (Supplementary fig. 1 H, P<0.0001), and inhibited SRD5A2 expression at the transcription level (Supplementary fig. 2 I, P<0.001).
The levels of inflammatory mediators are elevated in prostatic tissues with obesity.
We next sought to investigate the mechanism of obesity inducing SRD5A2 promoter hypermethylation. Measuring inflammatory mediator levels in mouse prostatic tissues and human prostatic tissues, we found that the mRNA expressions of interleukin 1 α (IL1A), interleukin 6 (IL6), and TNF-α in HFD-induced obese mice were significantly increased compared with RFD control animals (Fig. 1 A–C). Similarly, the the mRNA expressions of IL1A, IL6 and TNF-α in overweight (BMI≥25) BPH patients were also significantly elevated compared with the lean controls (BMI<25) (Fig. 1 D–F).
Fig. 1. Increased level of inflammatory mediators in prostatic tissues with obesity.
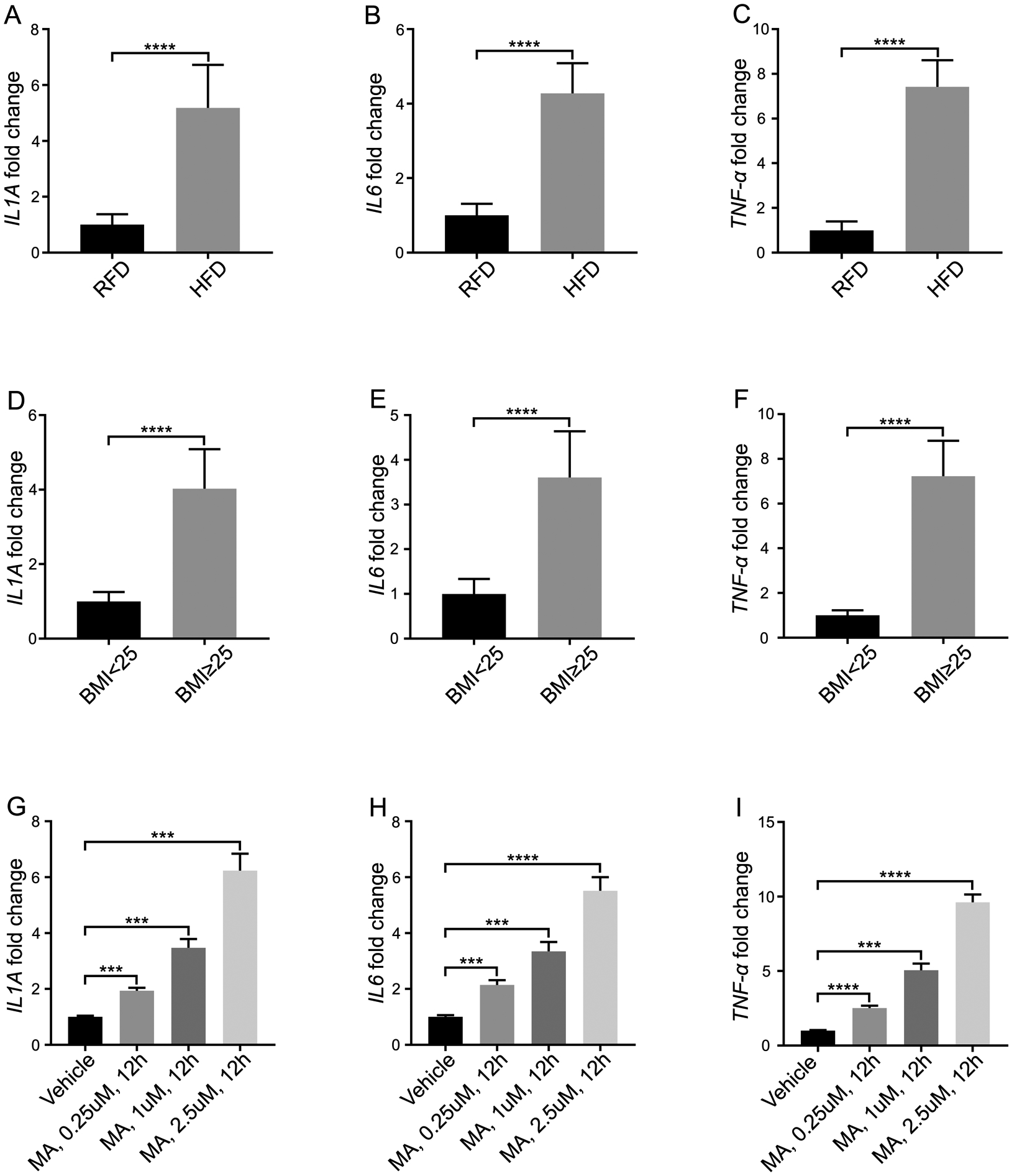
A–C: The mRNA expressions of IL1A, IL6 and TNF-α in mouse prostatic tissues. Mice were fed with high fat diet (HFD) or regular fat diet (RFD), respectively for 4 months. The prostatic tissues were collected, and mRNA was extracted for qPCR. D–F: The mRNA expressions of IL1A, IL6 and TNF-α in human prostatic tissues from overweight patients (BMI≥25) and normal weight control (BMI<25). G–I: The mRNA expressions of IL1A, IL6 and TNF-α in macrophages. Macrophages were activated, followed by administration of myristic acid (MA) at the concentration of 0.25 μM, 1 μM and 2.5 μM for 12 hours. The data represent means of average determinants ± SEM. All experiments were repeated independently at least three times, with similar results. ***P < 0.001, ****P< 0.0001, compared with RFD group in mice tissues), compared with BMI<25 group in human tissues, and compared with vehicle in stroma cells.
It has been reported that SFAs released from adipocytes can activate macrophages resulting in an inflammatory response16. Therefore we determined whether the elevated levels of inflammatory mediators in the prostate gland of obese versus lean ones might be explained by the effects of saturated fatty acid on macrophages. THP-1 cells, a cell line with properties of human monocyte-derived macrophages were stimulated with saturated fatty acid (SFA) myristic acid (MA) for 12 hours17. We observed that the inflammatory mediators, IL1A, IL6, and TNF-α at the transcriptional level in media were significantly increased with MA stimulation in a dose-dependent manner (Fig. 1 G–I). The conditioned media were collected and used for culturing primary human prostatic stromal cells.
Inflammatory mediators regulate SRD5A2 promoter methylation and expression in human prostatic stromal cells.
Previously, we have found that in prostate epithelial cell lines, inflammatory mediators IL-6 and TNF-α methylate SRD5A2 and silence expression of the gene18. In human prostatic tissue, we found that SRD5A2 only expresses in the stroma but not in the epithelium (Supplementary fig 1), here we used primary cultured human prostatic stromal cells to conduct experiments to determine if MA stimulated the production of pro-inflammatory molecules by macrophages, which in turn may affect the SRD5A2 promoter methylation and expression. While the macrophage conditioned media (Mac-CM) significantly promoted SRD5A2 promoter methylation compared with the control group, culturing with Mac-CM stimulated with MA for 48 hours promoted SRD5A2 promoter methylation in stromal cells in a dose-dependent manner, more significantly (Fig. 2 A). Meanwhile, SRD5A2 expression was significantly suppressed by culturing with this conditioned media (Fig. 2 B).
Fig. 2. SRD5A2 promoter methylation and expression is regulated by inflammatory mediators in human prostatic stromal cells.
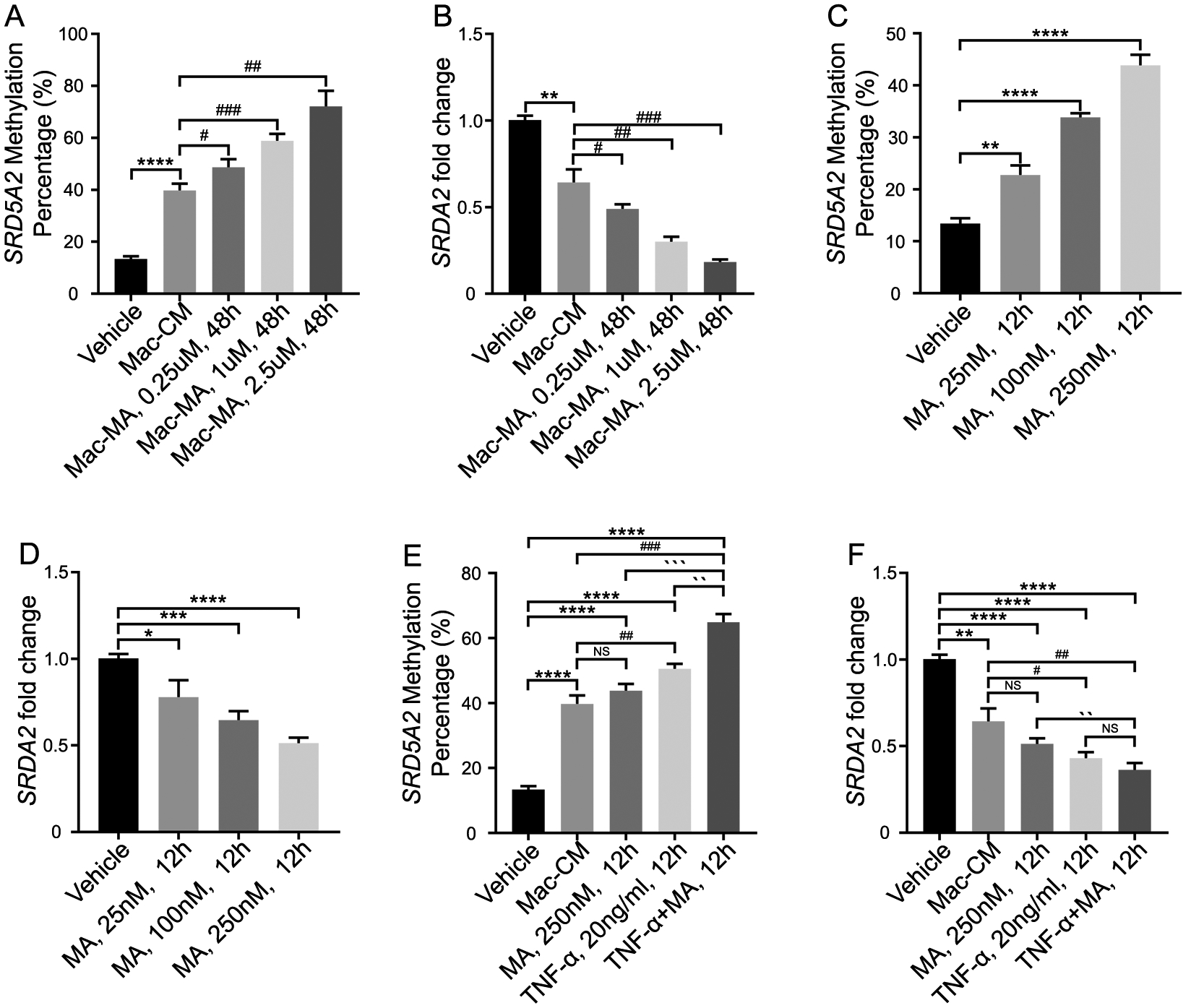
A–B: Stromal cells were treated with macrophage conditioned media. SRD5A2 promoter methylation (A) and SRD5A2 expression levels (B) were measured. C–D: Stromal cells were treated with myristic acid (MA) alone. E–F: Stromal cells were treated with macrophage conditioned media (Mac-CM), MA, TNF-α and combination of MA and TNF-α. The data represent means of average determinants ± SEM. All experiments were repeated independently at least three times, with similar results. *P < 0.05, **P< 0.01, ***P < 0.001, ****P< 0.0001, compared with vehicle. #P < 0.05, ## P< 0.01, ### P < 0.001, compared with Mac-CM. `P < 0.05, ``P< 0.01, ```P < 0.001, ````P< 0.0001, compared with TNF-α +MA, 12h. Mac: macrophage; MA: myristic acid.
Since MA-stimulated Mac-CM significantly facilitated SRD5A2 promoter methylation in stromal cells (Fig. 2 A), we then considered assessing the direct effect of MA alone. Unexpectedly, adding MA alone into stromal cell culture media induced SRD5A2 methylation (Fig. 2 C) and inhibited SRD5A2 expression (Fig. 2 D). This result suggests that SFA in prostate tissue may directly regulate SRD5A2 methylation and through activating macrophage to secrete inflammatory mediators. Therefore, we cultured stromal cells with the combination of administrating MA and TNF-α, and found that MA and TNF-α additively regulated SRD5A2 methylation and expression (Fig. 2 E & 2 F). Collectively, these data showed that both inflammatory mediators and saturated fatty acid stimulated macrophages regulated SRD5A2 promoter methylation and expression.
Inflammatory mediators and saturated fatty acid additively regulate aromatase activity in prostatic stromal cells.
It has been reported that inflammation regulates aromatase expression in male adipose tissue19. Our previous study also demonstrated that in the absence of prostatic SRD5A2 there is an androgenic to estrogenic switch in the prostate. Meanwhile, we found that TNF-α promotes expression of aromatase, the enzyme responsible for the conversion of testosterone to estradiol3. Given the link between estrogen biosynthesis and the progression of BPH we sought to determine whether obesity-induced inflammation led to increased aromatase expression in the prostate stroma. We cultured human prostate stromal cells with Adi-CM, and found that aromatase (gene name: CYP19A1) was significantly elevated both at the transcriptional level and protein level (Fig. 3 A & 3 B). Then we asked if inflammatory mediators also promote aromatase levels. Culturing the human prostate stromal cells with Mac-CM increased aromatase activity at the transcriptional and protein levels (Fig. 3 C & 3 D). More importantly, MA-stimulated Mac-CM promoted aromatase activity in a MA dose-dependent manner (Fig. 3 C & 3 D). Similarly, direct addition of MA alone also significantly increased aromatase in stromal cells (Fig. 3 E & 3 F). Combination of TNF-α and MA significantly increased aromatase levels in prostate stromal cells even more compared with TNF-α or MA alone (Fig. 3 G & 3 H). Finally, we tested the aromatase level in BPH human prostatic tissues and in HFD-induced obese mice prostatic tissues. The aromatase level was dramatically elevated both in obese mice and in overweight human (BMI≥25) (Fig. 3 I–K). Together, our data suggest that inflammatory mediators and saturated fatty acid synergistically regulate aromatase activity in the prostate.
Fig. 3. TNF-α and myristic acid synergistically regulate aromatase activity in prostatic stromal cells.
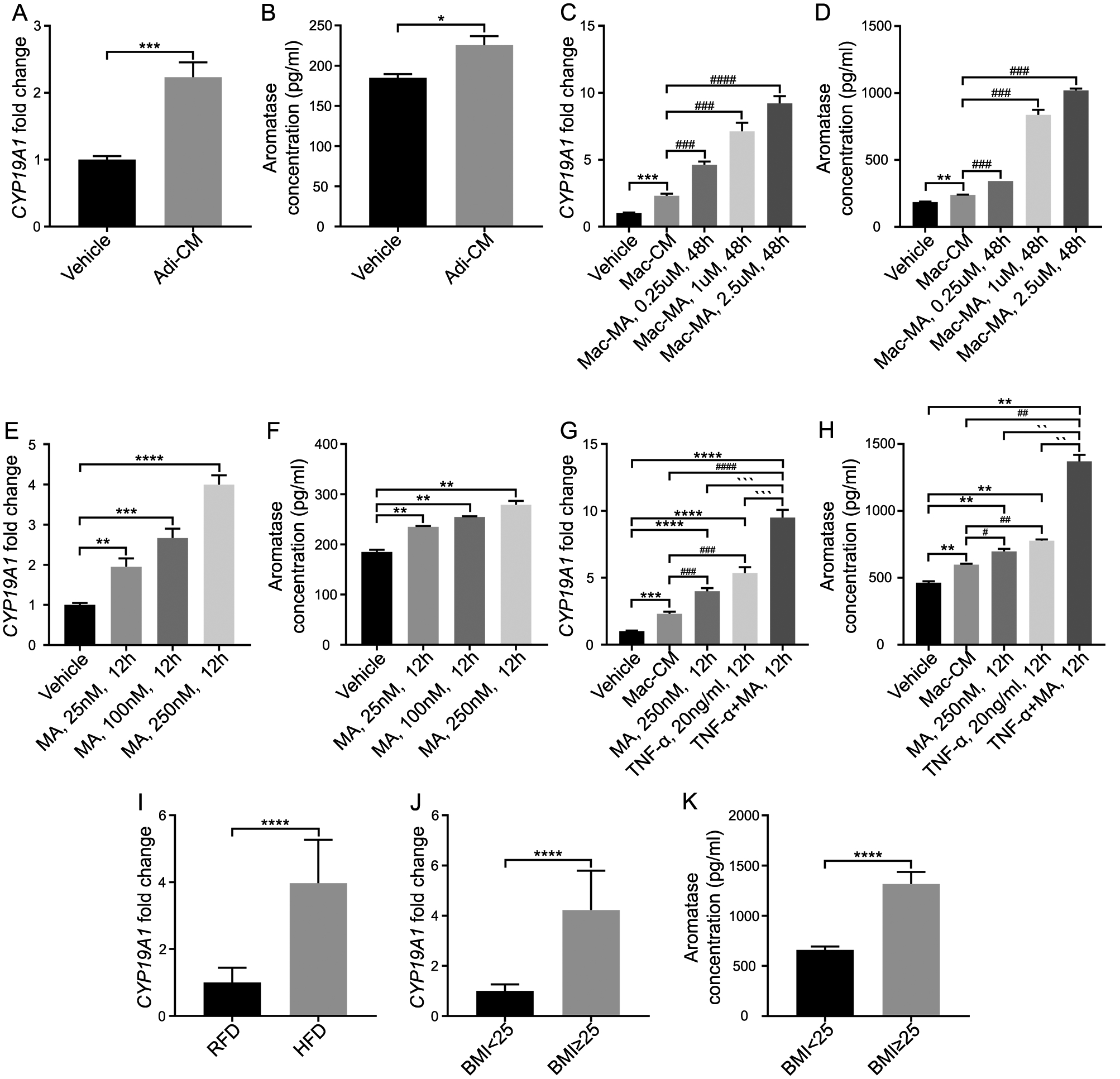
A-B: Stromal cells were treated with adipocyte conditioned media (Adi-CM). Aromatase mRNA (A) and protein levels were measured (B). C–D: Stromal cells were treated with macrophage conditioned media (Mac-CM) and myristic acid-activated macrophage conditioned media (Mac-MA). E–F: Stromal cells were treated with myristic acid (MA) alone. G–H: Stromal cells were treated with macrophage conditioned media (Mac-CM), MA, TNF-α and the combination of MA and TNF-α. Aromatase was measured at the transcriptional level (G) and protein level (H). I: The level of aromatase in HFD-treated mouse prostate tissues. J–K: Aromatase mRNA (J) and protein levels (K) in patient prostate tissues. The data represent means of average determinants ± SEM. All experiments were repeated independently at least three times, with similar results. *P < 0.05, **P< 0.01, ***P < 0.001, ****P< 0.0001, compared with RFD group in mice tissues, compared with BMI<25 group in human tissues, and compared with vehicle group in stroma cells. ## P< 0.01, ### P < 0.001, compared with Mac-CM. `P < 0.05, ``P< 0.01, ```P < 0.001, ````P< 0.0001, compared with TNF-α +MA, 12h. Adi-CM: adipocyte conditioned media; Mac: macrophage conditioned media; MA: myristic acid.
Obesity promotes an androgenic to estrogenic switch in prostate.
Our previous study demonstrated that in prostate samples that are methylated at the SRD5A2 promoter locus, there is an “androgenic to estrogenic switch” where the estradiol level is dramatically elevated, concomitant with significantly upregulated estrogen-response genes3. Here we asked if the estrogen signaling pathway is promoted with obesity and obesity-associated inflammation. Recent study demonstrated that there is the differential actions of estrogen receptor α (ESR1) and estrogen receptor β (ESR2) in human prostate stem and progenitor cells20, we then evaluated the gene expression of ESR1 and ESR2, and the concentration of estradiol. As expected, there was a significant increase of ESR1 gene expression and estradiol level in prostate stromal cells when culturing with Adi-CM (Fig. 4 A & 4 B). Then we found that culturing with Mac-MA increased ESR1 gene expression and estradiol concentration (Fig. 4 C & 4 D). In addition, saturated fatty acid MA had a similar effect with TNF-α (Fig. 4 E & 4 F). Combined administration of MA and TNF-α promoted ESR1 mRNA expression and estradiol concentration as well (Fig. 4 G & 4 H).
Fig. 4. Prostatic estrogen receptor-α (ESR1) and estradiol are elevated with adipocyte and inflammatory mediator exposure.
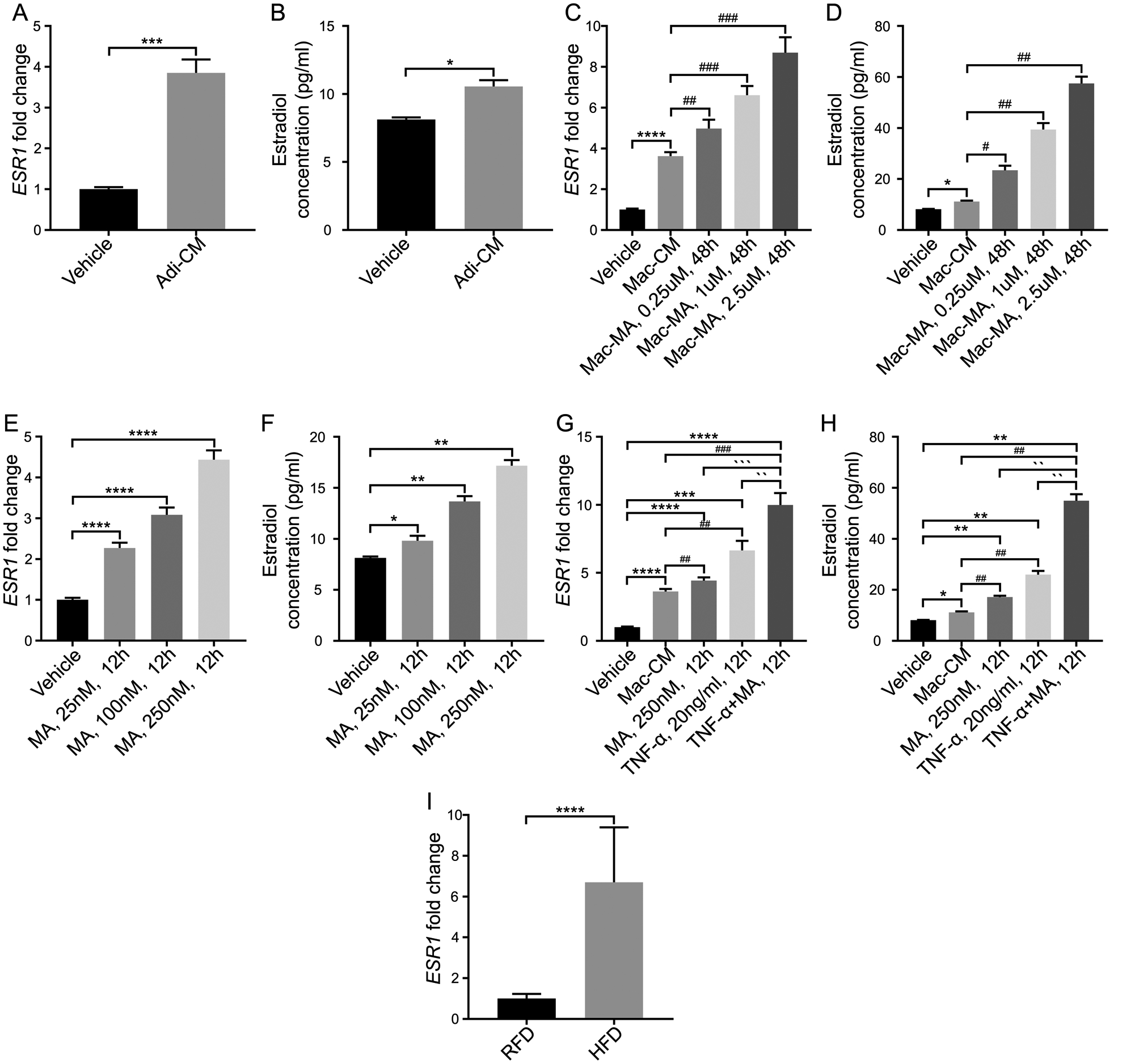
A–B: Stromal cells were treated with adipocyte conditioned media. ESR1 mRNA expressions and estradiol concentration were upregulated after treatment. C–D: Stromal cells were treated with macrophage conditioned media and activated-macrophage conditioned media. ESR1 mRNA expressions and estradiol concentration were upregulated in a dose-dependent manner after treatment. E–F: Stromal cells were treated with myristic acid. ESR1 mRNA expressions and estradiol concentration were upregulated in a dose-dependent manner after treatment. G–H: Stromal cells were treated with macrophage conditioned media (Mac-CM), MA, TNF-α and combination of MA and TNF-α. ESR1 mRNA expressions and estradiol concentration were upregulated after treatment of myristic acid, and the changes were more significant after treatment with TNF-α and combination of myristic acid and TNF- α. I: Prostatic ESR1 mRNA expressions and estradiol concentration were upregulated in mice with HFD exposure. *P < 0.05, **P< 0.01, ***P < 0.001, ****P< 0.0001, compared with RFD group in mice tissues, and compared with vehicle in stroma cells. ## P< 0.01, ### P < 0.001, compared with Mac-CM. `P < 0.05, ``P< 0.01, ```P < 0.001, ````P< 0.0001, compared with TNF-α +MA, 12h. Adi-CM: adipocyte conditioned media; Mac: macrophage conditioned media; MA: myristic acid.
In HFD-induced obese mice, ESR1 expression was significantly elevated compared with RFD lean control (Fig. 4 I). Similarly, both ESR1 mRNA expression and estradiol concentrations were upregulated in human prostate tissues of overweight BPH patients (BMI≥25) compared with lean controls (BMI<25) (Fig. 5 C & 5 D). Opposite to this trend, ESR2 and AR mRNA expressions were suppressed in primary cultured stromal cells upon treatment with MA and TNF-α (Fig. 5 A &B, Supplementary Fig. 3 & 4). Together, the data demonstrate that obesity or adipocytes promote an androgenic to estrogenic switch in the prostate tissues.
Fig. 5. Alteration of androgen and estrogen levels in prostate tissue of obese vs lean BPH patients upon treatment with 5-α reductase inhibitors (5ARIs).
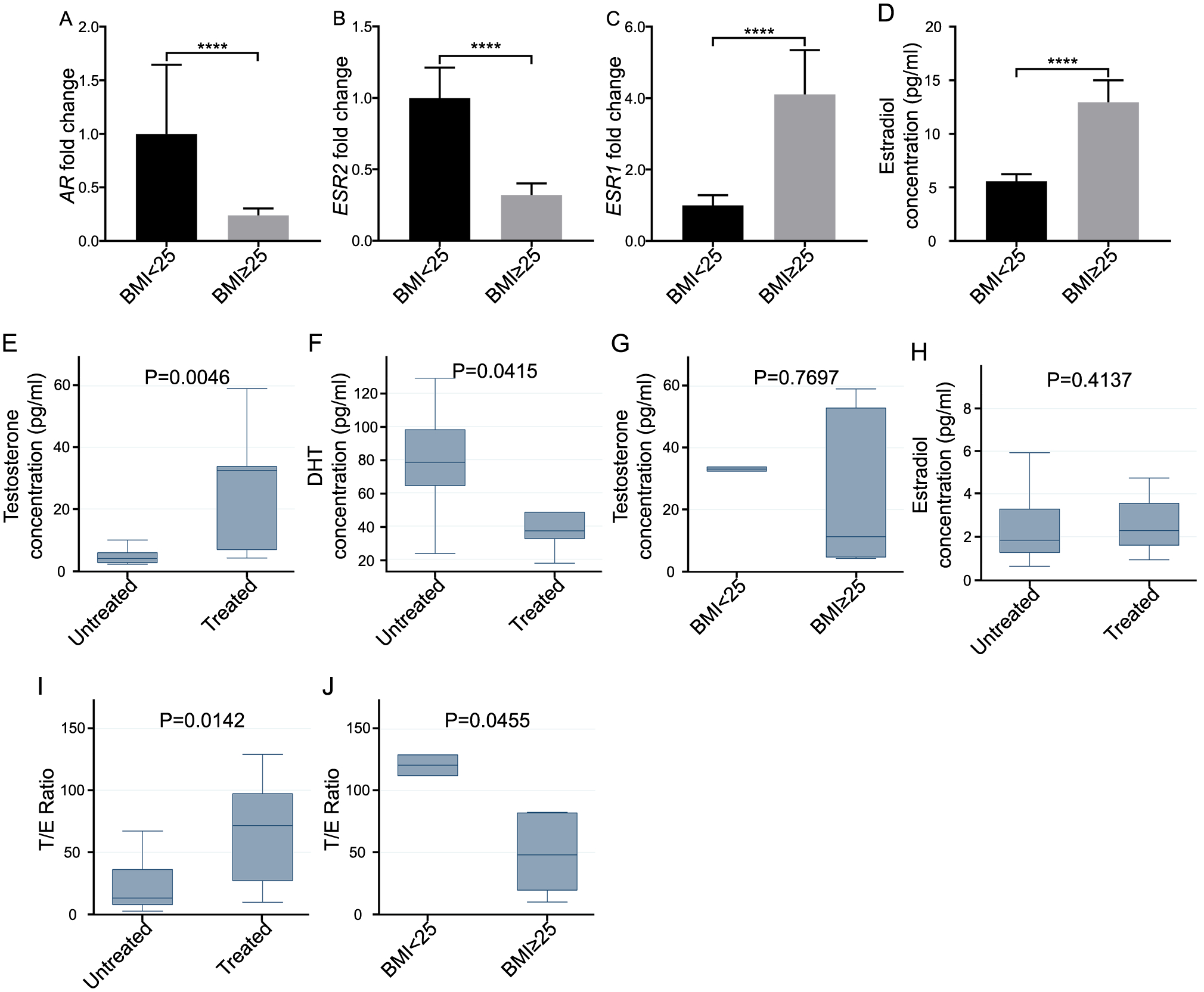
Prostatic androgen receptor (AR) (A), ESR2 (B) and ESR1 (C) expression at mRNA level, and estradiol (D) change in lean and obese men. Prostatic testosterone (E) and DHT (F) in 5ARIs untreated vs. treated men. (G) Prostatic testosterone in lean vs. overweight men. (H) Prostatic estradiol in 5ARIs untreated vs. treated men. (I) The ratio of androgen to estrogen (testosterone/estradiol, T/E) in 5ARIs untreated vs treated groups. (J) The prostatic T/E ratio in lean vs. overweight individuals. *P < 0.05, **P< 0.01, ***P < 0.001, ****P< 0.0001, compared with BMI<25 group or untreated group).
Androgen and estrogen levels are modified differently in overweight vs. lean human prostate tissue upon the treatment with 5-α reductase inhibitors (5ARIs).
Finally, we investigated if there was a different response to 5ARIs treatment between obese and lean BPH patients. In overweight (BMI≥25) patients, the androgen receptor (AR) and ESR2 levels were suppressed, but ESR1 expression was increased, compared with lean control (BMI<25) (Fig. 5 A–D). When comparing with 5ARIs non-treated group, we also found that 5ARIs treated group had significantly increased testosterone (Fig. 5 E) and decreased dihydrotestosterone (DHT, Fig. 5 F). Furthermore, after 5ARIs treatment, the testosterone level in overweight group was similar to that of the lean group (Fig. 5 G). Although the estradiol level was not significantly different between 5ARIs treated and non-treated group (Fig. 5 H), the ratio of androgen to estrogen (testosterone/estradiol, T/E) in the prostatic tissue was significantly higher in 5ARIs treated group (Fig. 5 I). More importantly, the T/E ratio was significantly lower in the 5ARIs treated overweight group versus the lean control (Fig. 5 J).
Discussion
Epigenetic signature studies for obesity have demonstrated that increasing BMI is associated with increased global methylation of obesity-associated genes in adipose tissue21,22. DNA methylation has a prominent role in regulating gene expression and influencing downstream functional outcomes. In BPH, hypermethylation of specific genes such as MDR1 and RASF1 has been identified in comparison to normal prostate tissues, which may affect the gene expression and development of BPH23. Here, we demonstrated that in overweight humans and mice, SRD5A2 promoter methylation in prostate tissue was significantly higher than that in lean controls. Similarly, SRD5A2 methylation was found both in HFD-induced obese mice and adipocyte conditioned media treated prostatic stromal cells. The data suggest that in obese BPH patients, increased methylation appears not only in the obesity-associated genes, but also in prostate growth regulating genes. Therefore, knowledge about epigenetic modulation of specific genes like SRD5A2 may provide additional insight for selecting better treatment strategies for management of obese patients suffering from lower urinary tract symptoms secondary to BPH.
In many BPH patients who are treated with 5ARI therapy, the hyperplastic prostate continues to grow even though there is an inhibition in the conversion of testosterone to DHT, which can be monitored by decreases in the expression of the androgen dependent protein, PSA24. Continuation of prostatic growth despite the use of 5ARI therapy suggests that there may be alternative mechanisms leading to prostate growth and worsening of bladder outlet symptoms related to BPH. In our study, we found that obesity markedly attenuates the clinical benefits associated with 5ARI therapies for symptomatic BPH25,26. We further demonstrated that, there is an androgenic to estrogenic switch in prostate tissue leading to higher estrogen levels. In males, there is no central organ which produces substantial quantities of estradiol27. Instead, peripheral conversion of estrogen precursors is the main source of estrogen in men. Increased adiposity in obesity can lead to greater aromatization of circulating testosterone into estrogen. When evaluating the role of estrogen receptor status and testosterone levels, we found that in overweight patients, AR, ESR2 levels were suppressed, but estradiol level was increased, which suggests a greater aromatization in prostate tissue. More importantly, the T/E ratio was decreased with 5ARI treatment in the overweight vs. the lean control groups (Fig. 5 H). While the evidence for an association between estrogen concentration and prostate growth is unclear and inconsistent, we agree with the previous study which demonstrated that a lower T/E ratio may stimulate proliferation of normal prostate stromal and epithelial cell lines in vitro28.
Previous studies of adipose tissue indicate that obesity is associated with adipocyte death leading to the accumulation of macrophages. Macrophage-derived cytokines have been suggested to stimulate lipolysis in adipocytes, which lead to increased release of SFA29. SFAs activate TLR4 signaling in macrophages and thereby stimulate the production of proinflammatory mediators30. Here we present evidence that SFA treatment of macrophages led to increased production of TNF-α, IL1A, IL6 (Fig. 1 A–C). Furthermore, both SFA and TNF-α treatments enhanced aromatase expression in adipocytes. The data suggest that, in obesity, a paracrine loop involving macrophages and adipocytes may amplify the inflammatory state and lead to increased aromatase expression31. Collectively, these findings are consistent with the concept that the inflammation associated with obesity in prostate gland leads to increased expression of aromatase, causing increased estrogen biosynthesis.
Given the link that we have established between obesity, inflammation, increased aromatase expression and finally the androgenetic to estrogenic switch, and prior studies that have shown treatment with aromatase inhibitor for 12 months decrease estradiol and reduce prostatic growth rate32, it is possible that in carefully selected patients the estrogen modulators and/or aromatase inhibitors together with 5ARIs will be more efficient to treat BPH in obese/overweight patients.
Obesity is associated with the accumulation of macrophages, the major source of proinflammatory mediators in adipose tissue in obese mice and humans33,34. In the prostate, inflammation and obesity have been associated with the progression of BPH related urinary symptoms and resistance to therapy which may ultimately lead to surgical intervention. Obese men tend to suffer more from urinary obstruction due to an enlarged prostate, and also tend to be more resistant to 5ARI treatment35,36. While our current study could not present direct evidence that obesity led to resistance to 5ARI treatment, future studies will be required to determine whether single agents or combinations of agents that inhibit inflammatory mediators or modulate estrogen/aromatase pathways may be more efficacious in the management of urinary symptoms related to BPH in obese men.
In summary, we took advantage of in vitro cultured primary human prostatic stromal cells, human BPH biopsies and rodent model with HFD induced obesity and obesity-associated sterile prostate inflammation, to address novel research questions on interplaying between obesity, inflammation, steroid hormones and the development of BPH (Supplementary figure 5). While estrogen is closely associated with metabolic syndrome37, and anti-androgen pathways have been a major target for the treatment of patients with BPH38, our findings suggest that estrogenic pathways may serve as an effective treatment strategy for BPH in obese patients and the broader population of men who lack expression of prostatic SRD5A23,39. Developing biomarkers which identify men with prostatic androgenic to estrogenic switch may lead to more effective strategies for helping patients who suffer from bladder outlet obstruction secondary to BPH.
Supplementary Material
Acknowledgements:
AFO gratefully acknowledges financial support from NIH/NIDDK (NIH/R01 DK091353). ZW was supported by the Urology Care Foundation/American Urological Association Research Scholar Award. We gratefully thanks for Dr. Li Xin at University of Washington supplying prostate tissues of mice fed with high fat diet or regular fat diet.
Footnotes
Conflict of interest: No conflicts of interest are declared by all of our authors.
Reference
- 1.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol 2005; 173(4): 1309–1313. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998; 338(9): 557–563. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Hu L, Salari K, Bechis SK, Ge R, Wu S et al. Androgenic to oestrogenic switch in the human adult prostate gland is regulated by epigenetic silencing of steroid 5alpha-reductase 2. J Pathol 2017; 243(4): 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. The Journal of urology 2002; 168(2): 599–604. [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama 2012; 307(5): 491–497. [DOI] [PubMed] [Google Scholar]
- 6.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012; 31(1): 219–230. [DOI] [PubMed] [Google Scholar]
- 7.Giri A, Edwards TL, Motley SS, Byerly SH, Fowke JH. Genetic Determinants of Metabolism and Benign Prostate Enlargement: Associations with Prostate Volume. PloS one 2015; 10(7): e0132028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vignozzi L, Gacci M, Maggi M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nature reviews Urology 2016; 13(2): 108–119. [DOI] [PubMed] [Google Scholar]
- 9.Jasuja GK, Travison TG, Davda M, Murabito JM, Basaria S, Zhang A et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. The journals of gerontology Series A, Biological sciences and medical sciences 2013; 68(6): 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowke JH, Koyama T, Fadare O, Clark PE. Does Inflammation Mediate the Obesity and BPH Relationship? An Epidemiologic Analysis of Body Composition and Inflammatory Markers in Blood, Urine, and Prostate Tissue, and the Relationship with Prostate Enlargement and Lower Urinary Tract Symptoms. PloS one 2016; 11(6): e0156918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechis SK, Otsetov AG, Ge R, Wang Z, Vangel MG, Wu CL et al. Age and Obesity Promote Methylation and Suppression of 5-Alpha Reductase 2- Implications for Personalized Therapy in Benign Prostatic Hyperplasia. J Urol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein AS, Drake JM, Burnes DL, Finley DS, Zhang H, Reiter RE et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nature protocols 2011; 6(5): 656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strand DW, Aaron L, Henry G, Franco OE, Hayward SW. Isolation and analysis of discreet human prostate cellular populations. Differentiation; research in biological diversity 2016; 91(4–5): 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Kwon OJ, Henry G, Malewska A, Wei X, Zhang L et al. Non-Cell-Autonomous Regulation of Prostate Epithelial Homeostasis by Androgen Receptor. Molecular cell 2016; 63(6): 976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon OJ, Zhang B, Zhang L, Xin L. High fat diet promotes prostatic basal-to-luminal differentiation and accelerates initiation of prostate epithelial hyperplasia originated from basal cells. Stem Cell Res 2016; 16(3): 682–691. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry 2007; 282(48): 35279–35292. [DOI] [PubMed] [Google Scholar]
- 17.Perreault M, Roke K, Badawi A, Nielsen DE, Abdelmagid SA, El-Sohemy A et al. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids 2014; 49(3): 255–263. [DOI] [PubMed] [Google Scholar]
- 18.Ge R, Wang Z, Bechis SK, Otsetov AG, Hua S, Wu S et al. DNA methyl transferase 1 reduces expression of SRD5A2 in the aging adult prostate. Am J Pathol 2015; 185(3): 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polari L, Yatkin E, Martinez Chacon MG, Ahotupa M, Smeds A, Strauss L et al. Weight gain and inflammation regulate aromatase expression in male adipose tissue, as evidenced by reporter gene activity. Mol Cell Endocrinol 2015; 412: 123–130. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar S, Rinaldi JC, Malhotra NR, Xie L, Hu DP, Gauntner TD et al. Differential Actions of Estrogen Receptor alpha and beta via Nongenomic Signaling in Human Prostate Stem and Progenitor Cells. Endocrinology 2019; 160(11): 2692–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benton MC, Johnstone A, Eccles D, Harmon B, Hayes MT, Lea RA et al. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biol 2015; 16: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aissi D, Wahl S et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet 2014; 383(9933): 1990–1998. [DOI] [PubMed] [Google Scholar]
- 23.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol 2007; 177(3): 822–831. [DOI] [PubMed] [Google Scholar]
- 24.Lin-Tsai O, Clark PE, Miller NL, Fowke JH, Hameed O, Hayward SW et al. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate 2014; 74(6): 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller RL, Gerber L, Moreira DM, Andriole G Jr., Hamilton RJ, Fleshner N et al. Obesity is associated with increased prostate growth and attenuated prostate volume reduction by dutasteride. Eur Urol 2013; 63(6): 1115–1121. [DOI] [PubMed] [Google Scholar]
- 26.Parsons JK, Schenk JM, Arnold KB, Messer K, Till C, Thompson IM et al. Finasteride reduces the risk of incident clinical benign prostatic hyperplasia. Eur Urol 2012; 62(2): 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellem SJ, Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer 2007; 7(8): 621–627. [DOI] [PubMed] [Google Scholar]
- 28.King KJ, Nicholson HD, Assinder SJ. Effect of increasing ratio of estrogen: androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate 2006; 66(1): 105–114. [DOI] [PubMed] [Google Scholar]
- 29.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011; 4(3): 329–346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol 2009; 20(5): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007; 27(1): 84–91. [DOI] [PubMed] [Google Scholar]
- 32.Dias JP, Melvin D, Shardell M, Ferrucci L, Chia CW, Gharib M et al. Effects of Transdermal Testosterone Gel or an Aromatase Inhibitor on Prostate Volume in Older Men. J Clin Endocrinol Metab 2016; 101(4): 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 34.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112(12): 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaron-Brooks LM, Sasaki T, Vickman RE, Wei L, Franco OE, Ji Y et al. Hyperglycemia and T Cell infiltration are associated with stromal and epithelial prostatic hyperplasia in the nonobese diabetic mouse. Prostate 2019; 79(9): 980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol 2009; 182(6 Suppl): S27–31. [DOI] [PubMed] [Google Scholar]
- 37.Williams G Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Molecular and cellular endocrinology 2012; 351(2): 269–278. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan SA, Lee JY, Meehan AG, Kusek JW. Time Course of Incident Adverse Experiences Associated with Doxazosin, Finasteride, and Combination Therapy in Men with Benign Prostatic Hyperplasia: the Medical Therapy of Prostatic Symptoms (MTOPS) Trial. J Urol 2015. [DOI] [PubMed] [Google Scholar]
- 39.Roehrborn CG, Spann ME, Myers SL, Serviss CR, Hu L, Jin Y. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis 2015; 18(1): 43–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


