ABSTRACT
Introduction: The novel coronavirus has caused significant mortality worldwide and is primarily associated with severe acute respiratory distress syndrome (ARDS). Apart from ARDS, clinical reports have shown noticeable cardiovascular complications among the patients of COVID-19. Infection from virus, stimulation of cytokine storm, altered immune response, and damage to myocardial tissue are some of the proposed mechanisms of cardiovascular complications in COVID-19.
Areas covered: Based on the clinical reports of CVDs among COVID-19 patients, we have discussed the molecular mechanisms involved in cardiovascular pathogenesis, its prevalence, and association with COVID-19, and various available therapeutic modality for the treatment.
Expert opinion: Seeing the cardiovascular complications in COVID-19 patients and its association with the existing drug, risk–benefit ratio of treatment paradigm, as well as the level of cardiac injury biomarkers must be monitored regularly. Additionally, a well-designed clinical trial should be conducted where head to head comparison can be made with anti-COVID-19 drugs and cardioprotective anti-inflammatory drugs. Nevertheless, vaccines are the best-suited approach, but until then, sanitization, social distancing, and active lifestyle are the best ways to beat this global pandemic situation.
KEYWORDS: Cytokine storm; Pneumocytes; Cardiotoxicity; NLRP3 inflammasome and COVID-19
1. Introduction
The outbreak of novel coronavirus (nCoV-19) in December from Wuhan city of China has shaken the world with its uncontrolled transmission, primarily damaging the lungs, and officially known as coronavirus disease (COVID-19) [1]. Since December, there was an exponential increase in the cases of COVID-19 infection and soon it spread across the globe and WHO considered it as a pandemic [1]. Until now, three strains of coronavirus have been identified, namely, severe acute respiratory stress coronavirus (SARS-CoV), SARS CoV-2, and Middle East respiratory syndrome virus (MERS) [2]. It is important to bring into notice that in 2003, the Guangdong area of China encountered pneumonia-like symptoms with alveolar damage and acute respiratory distress caused by the β coronavirus sub-group (SARS-CoV). As per the records, SARS-CoV spread across 26 nations, infected more than 8098 patients, and caused the death of more than 776 patients [3]. In 2012, MERS was identified in Saudi Arabia, caused damage to upper respiratory tract infection and renal failure and spread across 109 nations and infected more than 120,000 patients and caused the death of more than 3500 patients [3]. Now, SARS-CoV-2 originated from the seafood market of Wuhan city, China, and has spread across the globe. WHO has reported 81.47 million confirmed cases of COVID-19 with 1.79 million deaths globally and more than 215 countries are affected worldwide (Globally, as of 4.11pm CET, 31 December 2020) [4]. The real-time data can be tracked online on the official WHO site and the number of confirmed cases and death are increasing after every second by which the exact figure of incidence and fatality rate of COVID-19 can not be exactly reported. Among all the countries, the U.S.A. alone reported the highest number of confirmed cases, i.e., 19.34 million with mortality of 0.33 million patients (As of 4.11pm CET, 31 December 2020) [1]. Thus, SARS-CoV-2 imposed a serious burden on global health and its mode of transmission and pathogenicity is much more severe than the other two strains. Acute respiratory distress syndrome (ARDS) and cytokine storm are hallmark pathological events and causes of death if not treated timely. It is further hypothesized that COVID-19 uses the ACE-2 receptor for invasion into pneumocytes and from there it gets translocated to different organs and also induces pathological events [5]. Thus, owing to the involvement of ACE-2 receptor, people with coexisting cardiovascular risk are very much susceptible to infection and also, patients with no previous history of cardiac complication are at risk of one or other cardiotoxic events [6,7].
2. COVID-19 and co-existing cardiovascular disorder
Ample literature focuses on the severe acute respiratory tract distress in COVID-19 infected patients, and there are very few reports available on its cardiotoxic manifestations. It has been observed that patients with co-existing cardiovascular disorders (CVDs) are more susceptible to severe cardiac injury and mortality during this infection [8,9]. Based on these observations, we analyzed a large sample-sized study (44,672 patients) reported by Wu et al., where five times higher mortality rate of COVID-19 infected CVD patients was found when compared with non-CVD patients and out of total deceased patients 40% had coexisting hypertension [10]. A meta-analysis of 1527 COVID-19 infected patients (including 6 studies) by Li et al. reported the prevalence of 33.5% of hypertension and other cardiac complications, whereas Guan et al., including 1099 COVID-19 positive cases, showed the requirement of ICU for 36% of patients having underlying cardiovascular complications [11,12]. Tao et al. have reported the findings based on 799 patients where 62% of patients had hypertension and other cardiovascular complications and 77% of patients deceased because of cardiac injury. In deceased patients, significantly elevated levels of lactate dehydrogenase, cardiac troponin T (cTnT) and brain natriuretic peptide (BNP) were found [13]. Two other studies with a sample size of 341 and 150 showed mortality of 45% with elevated cTnT, BNP, venous embolism, hypercoagulation, vascular inflammation, endothelial dysfunction, and myocarditis [14,15]. We further critically analyzed two papers published in JAMA cardiology, by Tao et al. and by Shi et al. Tao et al. have published a report of a retrospective study where, out of 187 confirmed cases of COVID-19 infection, 66 patients, i.e. 35% had co-existing cardiovascular complications, whereas 28%, i.e. 52 patients developed CVD complications during the diseased condition [6]. We also analyzed data of the study and explicated the mortality with the level of cTnT. It was found that out of total fatality reported, 58% was associated with increased cTnT and existing CVD, whereas 19% mortality was associated with normal cTnT and without CVD. However, elevated cTnT and non-CVD correspond for 14%, whereas normal cTnT and non-CVD correspond for 9% fatality [6]. This analysis gives an idea that along with co-existing CVD, cTnT also plays a critical role and its level must be monitored irrespective of the co-existing diseased condition. When the cohort study by Shi et al., 2020 was analyzed, it was found 19% of cases of COVID-19 with CVD and 82% without CVD. We further explored the findings of this study and found that out of the total mortality reported, 74% was associated with CVD and 26% with non CVD [16]. Thus, both the studies have an almost similar trend of percentage mortality associated with co-existing CVD [6,16]. In various other reports, 15–40% of COVID-19 infected patients were reported with severe cardiovascular complications of hypertension, arrhythmia, myocardial injury, and reduced ejection fraction [10,12,14,17–22].
Further, to identify the prevalence and incidence of cardiovascular diseases and its complications in COVID-19 infection, we performed a literature search on databases like PubMed, Google Scholar and EMBASE from December 2019 to 31 December 2020. The following search terms or keywords were used alone or in combination: ‘novel coronavirus,’ ‘COVID-19ʹ ‘cardiovascular disease,’ ‘cardiac injury,’ ‘hypertension,’ ‘myocarditis,’ and ‘heart failure.’ The literature search was restricted to studies published in English. Pre-printed articles were also included. We also included clinical studies. We excluded meta-analysis, systemic analysis, narrative reviews, and those papers which are not having adequate information on cardiovascular complications in COVID-19 infection. We included 16 studies, with a total of 46,379 patients of COVID-19 infection. All the studies were published in English and studies were done in China. Out of 9 16 studies, 12 studies were retrospective, 2 were prospective 1 was a cohort study and 1 was case study. Studies were having different sample sizes from a minimum of 41 to a maximum of 29,692. We also found that CVD associated comorbid patients required a higher number of ICU, ventilators and special care as compared to the normal infected patients and the mortality rate was higher among CVD associated comorbid patients. As per the published evidence, around 30–44% of patients with co-existing CVD and COVID-19 need mechanical ventilation and mortality percentage in such cases was found to be more than 90% [13,18]. Interestingly, our observations were in agreement with several other reports where the prevalence of CVD in COVID-19 was found to be 15–40% [10,12,14,17–22]. However, the above-discussed studies are limited to the Chinese population only. We also studied the status of CVD and COVID-19 in the U.S.A. and Italian populations. At present more than 18.8 million cases and 0.33 million deaths had been reported in the U.S.A, where according to morbidity and mortality report of CDC, U.S.A, 2020, 58.9% patients had hypertension whereas 34.2% of the patients exhibited other cardiovascular complications [21] Further, hospitalization data from the CDC showed that 58.4%, 12.4%, and 11.3% of the patients diagnosed with COVID-19 were admitted in ICU due to hypertension, coronary disease, and heart failure, respectively [23]. According to other reports of CDC, U.S.A out of 8,452 hospitalized COVID-19 positive patients more than 90.7% of patients had one or more comorbidity and cardiovascular complications [24]. As of 18 June 2020, in the U.S.A., 58% of the patients had co-existing hypertension whereas 34.2% of the patients had other cardiovascular complications along with COVID-19 infection [25]. Additionally, as per the case series published by Bhatraju et al. in NEJM, from Washington State, 71% of the patients had hypotension and 38% of the patients exhibited other cardiac complications and the mortality rate was 50% [26]. Further, according to American Heart Association’s annual report, more than half of the US population suffers from one or other cardiovascular or other metabolic disease and thus, the US population are at high risk of COVID-19 infection [27]. In Italy at present 2.08 million cases and 0.073 million mortality have been reported (As of 4.11pm CET, 31 December 2020). According to the report of Abbatecola and Antonelli-Incalzi, out of 7589 patients, 54% of the patients were >70 years and more than 74% of the deaths were related to co-existing cardiovascular complications [28]. Graziano et al. have reported that the case fatality rate in Italy is almost 4 times higher than in China and mortality percentage in COVID 19 was found to be 41% with myocardial ischemia [29]. Similarly, Grasselli et al. have reported that out of 1591 hospitalized in ICU with COVID-19 in northern, Italy 49% of patients had underlying cardiovascular complications, and the mortality was 26% [30]. Inciardi et al. have analyzed 99 COVID-19 hospitalized patients where 53 patients were having co-existing CVD. Major comorbidity was found to be arterial-venous-embolism (15%), coronary artery disease (30%), arterial fibrillation (36%), and heart failure (40%). Mortality associated with CVD was 36% as compared to non-CVD (15%) [31]. According to the official website for COVID-19 (Epimidology for public health, Coronavirus Epicenter), among 32,448 reported cases, 28% patients suffered from myocardial ischemia, 22% from atrial fibrillation, 16% from heart failure, and 67% patients from hypertension [32]. Based on all the studies, we conclude that patients with cardiovascular complications are more likely to be infected by COVID-19, need mechanical ventilation, and are also associated with a higher mortality rate, as shown in Table 1. Further, elevated levels of cardiac troponin T, creatinine kinase (CK-MB), and LDH levels independently predict the severity of cardiovascular complications in COVID-19 infections.
Table 1.
Prevalence of cardiovascular complications in COVID-19 studies in China
| Study | Country | Study type | No. of patients | DM | TD | CVD | %CVD | Reference |
|---|---|---|---|---|---|---|---|---|
| Guan et al., 2020 | China | Retrospective | 1099 | 81 | 15 | 192 | 17 | [12] |
| Shaobo Shi et al., 2020 |
China | Cohort study | 416 | 60 | 57 | 209 | 50 | [47] |
| Chen T et al., 2020 |
China | Retrospective case series | 274 | 47 | 113 | 116 | 42 | [18] |
| Yuan Yu et al., 2020 |
China | prospective observation | 226 | 47 | 87 | 128 | 57 | [63] |
| Chaomin Wu et al., 2020 |
China | Retrospective cohort study | 201 | 22 | 44 | 47 | 24 | [64] |
| Tao Guo, et al., 2020 |
China | Retrospective single-center case series | 187 | 28 | 43 | 127 | 68 | [6] |
| Fei Zhou et al., 2020 |
China | Retrospective cohort study | 191 | 36 | 54 | 73 | 38 | [14] |
| Dawei Wang et al., 2020 |
China | Retrospective single-center case series | 138 | 14 | 6 | 43 | 46 | [17] |
| Huang et al., 2020 |
China | Prospective observation | 41 | 8 | 6 | 12 | 29 | [65] |
| Prevalence of cardiovascular complications in COVID-19 studies outside China | ||||||||
| Marcello et al. | U.S.A. | Retrospective cohort | 6248 | 1686 | 1874 | 1312 | 21 | [66] |
| Karmen-Tuohy et al. | U.S.A. | Case-control | 63 | 12 | 23 | 17 | 27 | [67] |
| Chhiba et al. | U.S.A. | Retrospective cohort | 1526 | 505 | 401 | 114 | 7 | [68] |
| Grasselli et al. | Italy | Retrospective cohort | 1,591 | 180 | 509 | 223 | 14 | [69] |
| Myers et al., 2020 | Northern California, U,S.A. | Retrospective cohort | 377 | 118 | 164 | 22 | 6 | [70] |
| College of Health et al., 2020 (originally written COVID-19) Surveillance Group | Italy | Retrospective cohort | 29,692 | 870 | 1940 | 2204 | 8 | [71] |
| Richardson et al., 2020 | U.S.A. | Retrospective case series | 5700 | 1808 | 3026 | 996 | 17 | [72] |
DM; Diabetes Mellitus, TD; Total death, CVD cardiovascular disorders
3. Mechanistic insight into the molecular pathogenesis of cardiotoxicity in COVID-19 infection
Until now, there is no substantial mechanism reported for the development of myocardial injury in COVID-19 infection. However, immunopathology and inflammatory mechanisms of COVID-19 have been reported in pneumocytes, which gave the idea to hypothesize the possible mechanism of cardiac inflammation in such patients. It is further hypothesized that COVID-19 uses ACE-2 receptors for invading the host via binding of its spike proteins, and therefore it is speculated that patients taking ACE-2 inhibitors have a higher expression of ACE-2 receptors, which are expressed in the lungs as well as in the heart, thus increasing the risk of infection in patients having cardiovascular complications [33]. After considering various case reports and other published evidences, it also appears that the COVID-19 virus on infection may get disseminated from the respiratory tract via circulatory or lymphatic system into the cardiovascular system (CVS) where it may trigger pro-inflammatory cascade as it does so in the lungs [34]. It has been reported that ssRNA of a virus is recognized by toll-like receptor (TLRs) 3, 7 and 9 with the help of RIG-I (retinoic acid-inducible gene-I) and cGAS (cyclic AMP-GMP synthase) in the cytoplasm and causes recruitment of various adaptors such as MAVS (mitochondrial antiviral signaling protein) and MyD88 (myeloid differentiation primary response protein) [35,36]. MVAS, on one hand, recruit MAPK kinase MKK7 at the mitochondrial surface, where it causes phosphorylation of JNK-2 at thr-183 and also activate p53, leading to initiation of an apoptotic event [37,38], whereas, on the other hand, MAVS directly increases the expression of the NLRP3 inflammasome, followed by the activation of NF-kB and caspase-1 that converts pro-IL-1β into active IL-1β and causes its subsequent release via pyroptosis [39]. Furthermore, MyD88 causes phosphorylation of NF-kB inhibitor (IkB), allowing the nuclear translocation of NF-kB [40,41]. Thus, MAVS, MyD88, activated NF-kB, JNK-2, p53, NLRP-3 inflammasome, and inflammatory cytokines (TNF-α, IL-6, and IL-1β) cumulatively participate in the myocardial inflammation, leading to myocarditis, loss of contractile function, altered ejection fraction, damage to cardiomyocytes and release of cardiac injury markers, such as cTnT and BNP into the blood as shown in Figure 1 [42,43]. Increased production of atherosclerotic plaque, coagulatory process, platelet aggregator process, myocardial hypoxia-ischemia, and thrombosis are some of the other contributing factors in the development of CVD in COVID-19 patients [44]. We further found a high prevalence of hypertension in COVID-19 patients and we speculate the profound role of endothelial dysfunction (ED) in COVID-19 [18]. The vascular endothelium is considered as an important organ for the maintenance of vascular homeostasis and contraction-relaxation mechanism [44]. Any damage to endothelium directly reflects altered vascular tone vasoconstriction leading to ischemia, vascular inflammation, and thrombosis [44]. It was further found that the ACE-2 receptor is also expressed in the endothelial cells and causes vascular derangements [45]. The detailed involvement of ED in COVID-19 was reported in a series of case studies published in Lancet by Varga et al. where they showed the occurrence of hypertension, ST-elevation, reduced left ventricular ejection fraction and myocardial infarction [46]. In the reports, the histopathological analysis showed viral inclusion in the endothelial cells with the accumulation of inflammatory, apoptotic, pyroptotic cells, and sign of endotheliitis [46]. Thus, the study provides substantial evidence for the involvement of ED in COVID-19 patients via inflammation, apoptosis, pyroptosis and thrombosis that causes hypertension and other cardiovascular complications.
Figure 1.
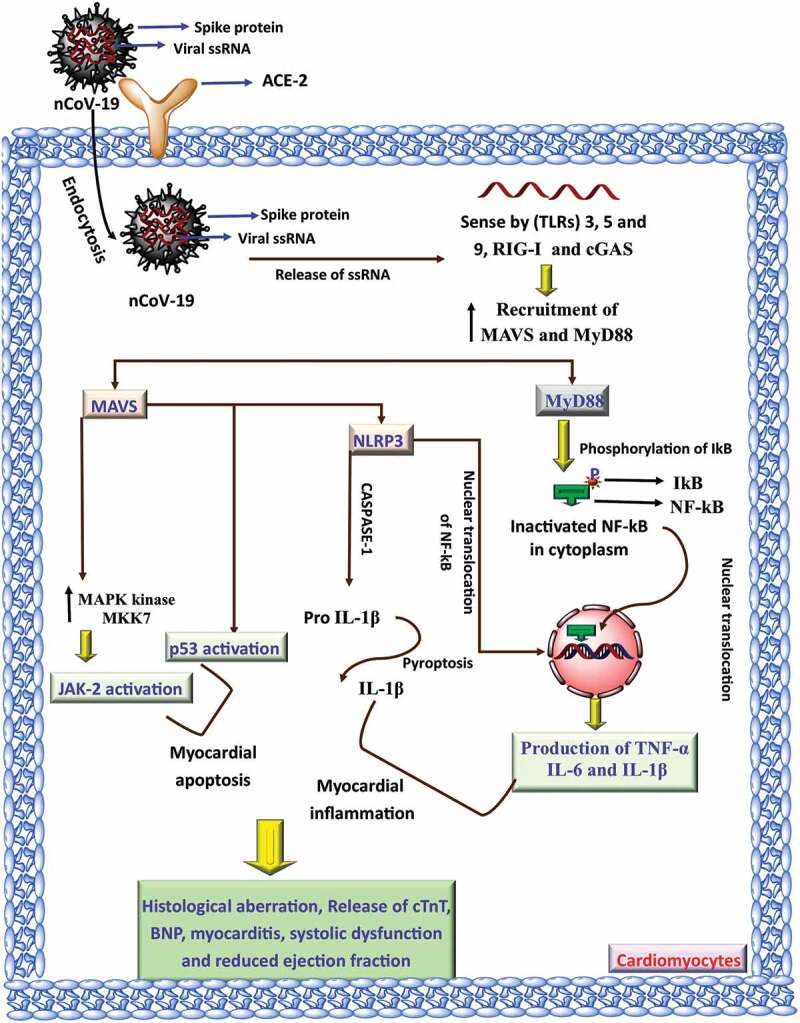
Mechanisms of cardiovascular toxicity by COVID-19 infection. The ssRNA of a virus is recognized in the cytoplasm leading to the recruitment of various adaptors such as MAVS (mitochondrial antiviral signaling protein) and MyD88 (myeloid differentiation primary response protein). MVAS increases the activity of MAPK kinase MKK7 causes phosphorylation of JNK-2 and activates p53 that causes myocardial apoptosis. MAVS and MyD88 also increase the expression of NLRP3. These attributes cumulatively participate in the myocardial inflammation, causes myocarditis., and reduces ejection fraction
4. Cardiovascular complications after recovery from COVID-19
Involvement of cardiovascular pathogenesis in COVID-19 is well established now and clinicians are considering this issue on serious note [6]. Initially, it was found that cardiac complications are acute manifestation of COVID-19 and because this outbreak is new, long-term data on post-COVID-19 complications are not available [47]. However, now in some of the case studies and case series it was found that COVID-19 infection also causes chronic cardiac complications, even when the viral load is normalized [48,49]. In one of the case reports, fulminant myocarditis was reported in patients after weeks of reduced viral load and symptoms [50]. Additionally, it was reported that many patients diagnosed with COVID-19 and mild symptoms have died due to cardiac arrest [50]. Studies have also shown pulmonary as well as ventricular fibrosis among the patients who were discharged from the hospital after full recovery [51,52]. These findings raised a serious concern and thus, a regular long-term follow up is needed for effective therapeutic regime and to prevent the development of cardiovascular complication.
5. Current status of the treatment paradigm for the management of COVID-19 infection and associated risk of cardiotoxicity
For the treatment of COVID-19 infection, various drugs are being investigated, and findings of Raoult et al. have shown hydroxychloroquine and azithromycin to be a potential candidate in reducing the viral load [53]. However, the outcome of this study was heavily criticized due to the methodology of interpretation and also because of the outcome of multiple larger studies that showed a lack of effectiveness of hydroxychloroquine [54–56]. No doubt there are many advantage of hydroxychloroquine (HCQ) over other drugs, such as cost-effectiveness, well-defined pharmacokinetic attributes, established long-term safety profile and multifactorial antiviral mechanism of action but the overall risk–benefit ratio must be considered while using these drugs [57]. Apart from HCQ, Lopinavir, Ritonavir, Ribavirin, Umifenovir, Remdesivir, Favipiravir, corticosteroid, and Taclizumab are being investigated for possible anti-corona viral effect [58]. Lopinavir, Ritonavir, in an open-labeled clinical trial, exhibited no significant viral clearance after 28 days of treatment [59]. Ribavirin, a well-known RNA-dependent RNA polymerase, is a potential candidate, but severe hemolytic anemia in more than 60% cases, teratogenic effect and risk of cardiotoxicity restricted its further investigation. Umifenovir, Remdesivir and Favipiravir, however, showed promising results and being investigated on large sample sized population [58]. Corticosteroid being immunosuppressant has shown promising results in treatment as well as recovery of nCoV infected patients alone or with co-existing cardiovascular complications [6,60]. Amelioration of cytokine storms is speculated to be the primary reason for its therapeutic effect. More recently, anti-cytokines (Tocilizumab and Sarilumab, IL-6 antagonist) and convalescent plasma therapy (approved by the FDA) have emerged as a potential approach for the treatment of nCoV infection [61,62]. However, the use of these repurposed and investigational drugs is associated with an increased risk of cardiotoxicity. Drugs such as Chloroquine/Hydroxychloroquine increases pH, causes electrolyte disbalance to prolong QT interval, direct myocardial toxicity, AV block, arrhythmia and Torsade de Pointes [20]. Lopinavir and Ritonavir, Azithromycin and corticosteroid also cause QT prolongation, interact with anti-coagulant and statins, cause edema, fluid retention, hypertension and Torsade de Pointes [20]. Remedesivir and Interferon have been reported to cause hypotension, arrhythmia cardiac ischemia whereas the use of Tocilizumab causes hypertension [20]. Thus, according to our opinion, the selection of any pharmaco-therapeutics must be considered for its cardiac safety profile and should be monitored throughout the treatment period. Additional cardiovascular complications associated with drugs used in CVD are listed in Table 3.
Table 3.
Recommendations of various societies of cardiology for the use of ACE inhibitors and ARBs among the patients of cardiovascular disorders
| S. No. | Societies | Recommendations | References |
|---|---|---|---|
| 1 | ESC | ACE inhibitors or ARBs should be used among stable COVID-19 patients as per ESH guidelines 2018 These drugs can only be discontinued on a case-to case basis and on the risk–benefit assessment. |
[73] |
| 2 | ISH | ACE inhibitors or ARBs should be used irrespective of COVID-19 infection. | [74] |
| 3 | ESC Council on Hypertension | Antihypertensive drugs should be continued among all patients. | [101] |
| 4 | CCS | ACE inhibitors or ARBs should be continued unless, found contraindicated because of hyperkalemia, kidney injury, or hypotension. | [75] |
| 5 | Hypertension Canada | Antihypertensive drugs should be continued among all patients. | [76] |
| 6 | ACC/AHA/HFSA | Among the patients of CVDs ACE inhibitors or ARBs should be continued. | [77] |
5.1. Future therapeutic regimen with consideration of cardiovascular protection in COVID-19
Cardiovascular complications are well established in COVID-19 and also the cardiotoxic effect of anti-COVID-19 drugs is known [78]. Hence, there appears an unmet need for a drug that can exhibit a cardioprotective effect along with an anti-COVID-19 effect. If we go for the novel drug development, it will be a time taking and expensive process, and therefore repurposing of existing cardioprotective agents with possible anti-viral and anti-inflammatory effects will be a better strategy [79]. Among various cardioprotective agents, statins are one of the extensively explored drugs with a better safety profile on long-term use [80]. Studies have suggested statins as a potential cardioprotective agent among COVID-19 infected patients, as it exhibits a multifactorial mechanism of action [81–83]. Statins exhibit anti-hyperlipidemic, anti-oxidant, anti-inflammatory, anti-thrombotic, and immunomodulatory effects [83]. Additionally, it was found that nCoV-19 accelerate the MYD88 signaling pathway leading to hyper inflammation as well as cardiotoxicity, and interestingly, statins are known inhibitor of this signaling pathway [83]. Apart from statins, sodium-glucose cotransporter-2 (SGLT-2) inhibitor is a well-known cardioprotective agent which remained a drug of choice in diabetic cardiomyopathy for quite a long time [84]. SGLT-2 inhibitors also found to show cardioprotective effect among non-diabetic patients and reduced the mortality rate and hospitalization frequency among HF patients [85]. Interestingly, SGLT-2 inhibitors have been reported to inhibit the viral entry of nCoV-19 via inhibiting lactate pathways and by increasing the cytosolic pH [86]. Studies have also shown that increased expression level of ACE2 eventually leads to anti-oxidant, vasodilation and anti-fibrosis effect and reduces ARDS, cytokine storm and exhibits cardioprotective effect [87]. Thus, looking into the multifactorial benefit of SGLT-2 inhibitor a metacentric phase III clinical trial is being conducted (DARE-19, NCT04350593) where possible therapeutic effect of Dapagliflozin is being evaluated along with its cardioprotective effect [88]. Additionally, an unwanted invasive procedure such as cardiac catheterization and transthoracic procedures should be avoided unless found necessary. In the case of ST-elevation, patients should be treated with thrombolytic drugs such as tenecteplase, whereas in the case of pulmonary embolism or venous thrombosis, anti-coagulants should be used as per the standard dose regimen.
5.2. Current status of renin-angiotensin-aldosterone system (RAAS) inhibitors in COVID-19
Until now the exact mechanism of cardiovascular pathogenesis in COVID-19 is not clear, however, based on published reports, it is hypothesized that reduced expression of ACE2 is one of the key aspects in cardiotoxicity [33]. In general, S protein of COVID-19 binds with ACE2 and facilitates the entry of virus [21]. ACE2 is one of the critical members of the RAAS and its expression is confirmed in lungs, blood vessels, kidneys and in cardiac tissues [89]. Additionally, the expression level of ACE2 was found higher in the heart as compared to the lungs and therefore it was initially assumed that virus might enter into cardiac tissue and cause cardiotoxicity [89]. However, until now no clinical evidence of virus entry into cardiac cells has been confirmed via ACE2 and thus only indirect but life threatening correlation exists between COVID-19 and cardiotoxicity [90,91]. In various preclinical and clinical studies, reduced expression of ACE2 in the lungs and the heart resulted in the production of inflammatory cytokines, chemokines, aggravated lung damage, caused ARDS, altered cardiac contractility, and hence, increased level of ACE2 showed cardioprotective effect [92,93]. Similar to the protective role of ACE2 in the heart, its protective role in the lungs is also confirmed, as the optimum level of ACE2 regulates the release of surfactant that prevents the collapsing of alveoli [94]. It is also important to highlight that ACE2 is a known inactivator of angiotensin II (cardioprotective) whereas ACE is responsible for activation of angiotensin (cardiotoxic) which indeed is responsible for various cardiotoxic manifestations [95]. Interestingly, when recombinant ACE2 (rACE2) was administered, a significant reduction in COVID-19 infection, as well as cardiac protection, was observed among infected patients and hence, rACE2 acted as a decoy which could be a promising therapeutic against COVID-19 mediated cardiotoxicity [96]. Although, a concern arose for the use of ACE inhibitor or angiotensin receptor blocker (ARBs), as it was assumed that these classes of drugs increased the expression of ACE2 that might potentiate the viral entry [97,98]. Thus, conflict arose worldwide whether ACE inhibitors or ARBs should be used among the patients of CVDs or not, as such patients are more prone to develop COVID-19 infection. Fascinatingly, recent findings from clinical and preclinical studies conducted in Spain, U.S.A., and Italy have clearly shown that the use of these drugs (ACE inhibitors or ARBs) is neither correlated with COVID-19 infection nor worsen the clinical outcomes among patients and thus, various cardiovascular society has issued a recommendation to use ACE inhibitors as well as ARBs among the CVDs patients (Table 2) [99–105].
Table 2.
Mechanisms of action of various repurposed and investigational drugs and their potential cardiovascular complications [20]
| Drugs | Mechanism of action | Cardiovascular complication |
|---|---|---|
| Chloroquine and Hydroxychloroquine | Regulate the pH and inhibit the replication of viral RNA | Myocardial damage, cardiomyopathy, altered nerve conduction, AV block, ventricular Torsades de pointes, and arrhythmia |
| Azithromycin | Inhibit the protein synthesis by COVID-19 | Potential interaction with HMG-CoA inhibitors, aspirin, prolong QT interval, causes arrhythmia and Torsades de pointes |
| Methylprednisolone | Reduce the production of inflammatory mediators | Interact with aspirin, causes fluid retention, edema, and hypertension |
| Remdesivir | Inhibit viral RNA polymerase | It causes hypertension and arrhythmia. |
| Favipiravir | RNA dependent RNA polymerase inhibitor | Interact with statins, aspirin, and anti-arrhythmic drugs. Causes hypertension and hemolytic anemia. |
| Ribavirin | Inhibit viral RNA replication | Interact with aspirin and other anticoagulant and causes anemia. |
| Lopinavir/Ritonavir | Inhibit protease | Interacts with anti-platelet drugs, aspirin, and HMG-CoA inhibitors. Causes hypertension, QT prolongation, alteration in nerve conduction Torsades de pointes, and increases cholesterol level |
| Interferon | Activate the immune system | Causes cardiomyopathy, hypotension, myocardial ischemia, and alter AV conduction |
| Tocilizumab | IL-6 inhibitor | Causes hypertension and interacts with statins |
| Bevacizumb | Reduce pulmonary edema | Potentiate cardiomyopathy, causes hypertension, and coagulation in arteries |
| Anakinra | Reduce inflammation and macrophage activation | Potentiate congestive heart failure |
ESH; European Society of Hypertension, ISH; International Society of Hypertension, CCS Canadian Cardiovascular Society, ACC; American College of Cardiology; AHA, American Heart Association and HFSA; Heart Failure Society of America.
6. Expert opinion
It is well-known and hypothesized that the cytokine storm is a major cause of cardiac inflammation and various other cardiovascular complications. Therefore, the use of cardioprotective anti-inflammatory drugs would be a better treatment strategy to impart cardiac protection along with the anti-CoV-19 effect. Being more specific, central regulator of inflammation, i.e., IL-1β, TNF-α, and IL-6 are well-established in the pathogenesis of COVID-19 infection and therefore these signaling molecules appear to be a promising target for the treatment of COVID-19 infection [106]. In COVID-19 infection, an increased level of IL-6 is associated with cytokine storm as well as QT-syndrome and torsade de pointes and hence the use of IL-6 inhibitors, such as Tocilizumab will eventually reduce the deleterious effect of increased IL-6 and will also act as a cardioprotective agent [107,108]. To test this fact, a well-designed metacentric clinical trial (ChiCTR2000029765) is being conducted where safety, as well as efficacy of Tocilizumab (IL-6 inhibitor), will be evaluated among COVID-19 infected patients [108]. Similar to Tocilizumab, OXI trial (NCT02648464) is being conducted to find out the cardioprotective effect of HCQ in MI patients [109]. Thus, it seems a promising approach to use the anti-inflammatory drug so that cytokine storm, as well as cardiotoxic manifestations, can be treated simultaneously [110].
Apart from the significant role of various pro-inflammatory cytokines, evidence has also shown altered Th1 and Th2 responses in COVID-19 [111]. Additionally, imbalance between Th1 and Th2 is also found to be responsible for cardiovascular complications in many patients of COVID-19 [111]. Polarization of M2 macrophage was identified to be the pivotal mediator Th1 and Th2 alteration and hence inhibitors of M2 macrophages are being explored for possible anti- COVID-19 effect with better safety profile [112].
Thus, from the aforementioned discussion, it seems persuasive that COVID-19 mediated acute hyper inflammation leads to ARDS and cardiovascular complications. However, apart from the acute phase inflammation, chronic inflammation is also a major clinical issue as it causes pulmonary and cardiac fibrosis and worsens the quality of life in patients [113,114]. Thus, we recommend for a long-term follow-up studies among patients who have recovered from intense and acute phase infection and accordingly, therapeutic regimen can be designed to prevent further damage to vital organs.
Additionally, it was found that, because of the urge of medication to cope with this pandemic, various novel drug candidates have been registered for the clinical trial based on in silico and small preclinical studies, but safety aspects of those drugs were not explored in detail [115]. We propose that it can be an issue of concern for the elderly as well as for the patients who suffer from co-existing cardiovascular complications [116]. Apart from these, some of the novel drugs (synthetic as well as natural bioactive) were found to be effective in the in vitro studies and only small sample-sized clinical trial was conducted but neither their risk–benefit ratio nor long-term safety profile is established [117]. Thus, the transition of newer drugs or repurposed from bench to bedside for COVID-19 infection should be used with utmost precautions and the overall pathological aspect of this disease should be considered [116,118–121]. Thus, it will be wise to consider established cardioprotective agents having significant anti-inflammatory property for the treatment and management of COVID-19 infection, instead of rushing for newer drugs.
Based on these facts we conclude that the mortality rate of co-existing CVDs is much higher than non-CVDs patients. Additionally, a healthy patient is also susceptible to develop various cardiotoxic manifestations, once infected with COVID-19. Nevertheless, further exploration of other confounding factors such as age, sex, genetic heterogeneity, large sample size study, and cardiotoxic effect of medication would substantially give a better picture of cardiotoxic consequences of COVID-19 infection. We, therefore, suggest to the scientific community that cardiovascular complications associated with COVID-19 infection must be taken on a priority basis. Prophylactic use of 4-aminoquinoline, antiretroviral, or anti-cytokines must be monitored for cTnT, BNP, and other cardiac parameters to avoid any cardiovascular complications. We further conclude that, for the safety and long-term efficacy perspective, development of the vaccine is a best-suited approach, but it is a time-taking procedure, hence, till then, social distancing and proper sanitization with an active lifestyle are the best ways to beat this global pandemic situation.
Funding Statement
This paper was not funded.
Article highlights
The novel coronavirus (nCoV-19) began in China and now has taken the shape of a global pandemic.
Acute respiratory distress syndrome (ARDS), pyrexia, body ache, and pulmonary toxicity are some of the common clinical presentations of nCoV-19.
Published evidences also showed significant cardiotoxicity among the patients who were diagnosed with nCoV-19.
A number of published meta-analysis, case studies, and case series have shown that not only does nCoV-19 infection worsen cardiovascular complications, but also makes the patient susceptible to such infection.
Studies have also reported cardiotoxic side effects of several anti-CoV-19 drugs.
To take care of such a complex issue, a more detailed molecular pathogenesis of cardiovascular complication in CoV-19 infection is needed to understand which will help in developing a safer and effective cardioprotective anti-nCoV-19 drugs.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disease WC . WHO COVID-19 update. 2020. 13 April.
- 3.Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus WN . Situation report–22. World Health Organization. 2019. [Cited 2021 Jan 01]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf
- 5.Iqubal A, Syed MA, Najmi AK, et al. Ameliorative effect of nerolidol on cyclophosphamide-induced gonadal toxicity in Swiss Albino mice: biochemical-, histological- and immunohistochemical-based evidences. Andrologia. 2020;52(4):e13535. [DOI] [PubMed] [Google Scholar]
- 6.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). 2020. [DOI] [PMC free article] [PubMed]
- 7.Iqubal A, Syed MA, Haque MM, et al. Effect of nerolidol on cyclophosphamide-induced bone marrow and hematologic toxicity in Swiss albino mice. 2020. [DOI] [PubMed]
- 8.Zheng -Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. 2020;17(5):259–260. [DOI] [PMC free article] [PubMed]
- 9.Iqubal A, Iqubal MK, Sharma S, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sciences. 2019;218:112–131. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. 2020. [DOI] [PMC free article] [PubMed]
- 13.Luo W, Li Y-X, Jiang L-J, et al. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. 2020. [DOI] [PMC free article] [PubMed]
- 15.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology. 2020. [DOI] [PMC free article] [PubMed]
- 17.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. [DOI] [PMC free article] [PubMed]
- 19.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Progress in cardiovascular diseases. 2020. [DOI] [PMC free article] [PubMed]
- 20.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75(18):2352-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal G, Cheruiyot I, Aggarwal S, et al. Association of cardiovascular disease with coronavirus disease 2019 (covid-19) severity: a meta-analysis. Current Problems in Cardiology. 2020;45(8):100617. [DOI] [PMC free article] [PubMed]
- 22.Santoso A, Pranata R, Wibowo A, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020. DOI: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC . COVID-19 CaCOoAPHw. 2020. June 15.
- 24.CDC . Key updates for week 23, ending June 6, 2020. June 15 2020
- 25.Centers for Disease Control and Prevention . COVID-NET: COVID-19-associated hospitalization surveillance network, 2020. June 15.
- 26.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao GH. Global syndemic of metabolic diseases: editorial comments. 2019;1(1):02–04. J Diabetes Clin Res. [Google Scholar]
- 28.Abbatecola A, Antonelli-Incalzi R. COVID-19 spiraling of frailty in older Italian patients. J Nutr Health Aging. 2020;1–3. 10.1007/s12603-019-1308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rate C-FOnder G, Rezza G, Brusaferro S. characteristics of patients dying in relation to COVID-19 in Italy. JAMA Published online March. 2020;23. [DOI] [PubMed] [Google Scholar]
- 30.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;1–8:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epicenter C . Epidemiology for public health. 2020. June 15.
- 33.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. 2020. [DOI] [PMC free article] [PubMed]
- 34.Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature. 2001;413(6857):732–738. [DOI] [PubMed] [Google Scholar]
- 36.Seth RB, Sun L, Ea C-K, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122(5):669–682. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Liu H, Li S, et al. MAVS-MKK7-JNK2 defines a novel apoptotic signaling pathway during viral infection. 2014; 10(3):e1004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Gong J, Yang H, et al. The mitochondrial protein mavs stabilizes p53 to suppress tumorigenesis. Cell Reports. 2020;30(3):725–738. e4. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Zhang S, Li F, et al. NLRX1 attenuates apoptosis and inflammatory responses in myocardial ischemia by inhibiting MAVS-dependent NLRP3 inflammasome activation. Molecular Immunology. 2016;76:90–97. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Wang X, Tong W, et al. Umbelliferone alleviates lipopolysaccharide-induced inflammatory responses in acute lung injury by down-regulating TLR4/MyD88/NF-κB signaling. Inflammation. 2019;42(2):440–448. [DOI] [PubMed] [Google Scholar]
- 41.Iqubal A, Sharma S, Najmi AK, et al. Nerolidol ameliorates cyclophosphamide-induced oxidative stress, neuroinflammation and cognitive dysfunction: plausible role of Nrf2 and NF-κB. Life Sciences. 2019;236:116867. [DOI] [PubMed] [Google Scholar]
- 42.Iqubal A, Ahmed M, Ahmad S, et al. Environmental neurotoxic pollutants. 2020:1–24. [DOI] [PubMed]
- 43.Iqubal A, Syed MA, Najmi AK, et al. Nanostructured lipid‐loaded nerolidol ameliorate cyclophosphamide‐induced neuroinflammation and cognitive dysfunction: molecular and cell biology/neuroinflammation. Alzheimer's Dement. 2020;16:e037013. [Google Scholar]
- 44.Colliva A, Braga L, Giacca M, et al. Endothelial cell–cardiomyocyte crosstalk in heart development and disease. J Physiol. 2020;598(14):2923–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 46.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi S, Qin M, Shen B, et al. Cardiac injury in patients with corona virus disease 2019. JAMA Cardiol. 2020.
- 48.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng -Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nature Reviews. Cardiology. 2020;17(5):259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inciardi RM, Solomon SD, Ridker PM, et al. Coronavirus 2019 disease (COVID-19), systemic inflammation, and cardiovascular disease. Journal of the American Heart Association. 2020;9(16):e017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonio GE, Wong K, Hui DS, et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228(3):810–815. [DOI] [PubMed] [Google Scholar]
- 53.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. 2020:105949. [DOI] [PMC free article] [PubMed] [Retracted]
- 54.Linsell L, Bell J. Effect of hydroxychloroquine in hospitalized patients with COVID-19-preliminary report. 2020. [DOI] [PMC free article] [PubMed]
- 55.Mahase EJBBMJ. Covid-19: hydroxychloroquine was ineffective as postexposure prophylaxis, study finds. 2020; 369. [DOI] [PubMed] [Google Scholar]
- 56.Abd-Elsalam S, Esmail ES, Khalaf M, et al. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. The American Journal of Tropical Medicine and Hygiene. 2020;103(4):1635–1639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Lane JCE, Weaver J, Kostka K, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. 2020, 20054551.
- 58.Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). 2020. [DOI] [PMC free article] [PubMed]
- 61.Monteleone G, Sarzi-Puttini PCArdizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020;2(5):e255-e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Y, Xu D, Fu S, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C, Chen X, Cai Y, et al. risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. [Internet]; 2020. [cited 2020 March21]. Online ahead of print; 1807:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020. [DOI] [PMC free article] [PubMed]
- 66.Dolle J, Grami S, Adule R, et al. Characteristics and outcomes of COVID-19 patients in New York city’s public hospital system. 2020. [DOI] [PMC free article] [PubMed]
- 67.Karmen-Tuohy S, Carlucci PM, Zervou FN, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. 2020. [DOI] [PMC free article] [PubMed]
- 68.Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. The Journal of Allergy and Clinical Immunology. 2020;146(2):307–314. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myers LC, Parodi SM, Escobar GJ, et al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. 2020. [DOI] [PMC free article] [PubMed]
- 71.Emmi G, Bettiol A, Mattioli I, et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. 2020:102575. [DOI] [PMC free article] [PubMed]
- 72.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. 2020. [DOI] [PMC free article] [PubMed]
- 73.Iaccarino G, Borghi C, Cicero AF, et al. Renin-angiotensin system inhibition in cardiovascular patients at the time of COVID19: much ado for nothing? A statement of activity from the directors of the board and the scientific directors of the Italian society of hypertension. 2020:1–4. [DOI] [PMC free article] [PubMed]
- 74.Hypertension ISo . A statement from the international society of hypertension on COVID-19. International Society of Hypertension Edinburgh; 2020.
- 75.Krahn A, Bewick S, Chow C, et al. Guidance from the CCS COVID-19 rapid response team. 2020.
- 76.Bavishi C, Maddox TM, Messerli F. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. 2020. [DOI] [PubMed]
- 77.Flacco ME, Martellucci CA, Bravi F, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart (British Cardiac Society). 2020;106(19):1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nature Reviews. Cardiology. 2020;17(9):543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. 2020. [DOI] [PMC free article] [PubMed]
- 80.Iqubal A, Sharma S, Sharma K, et al. Intranasally administered pitavastatin ameliorates pentylenetetrazol-induced neuroinflammation, oxidative stress and cognitive dysfunction. Life Sciences. 2018;211:172–181. [DOI] [PubMed] [Google Scholar]
- 81.Song SL, Hays SB, Panton CE, et al. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020;9(9):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Butt JH, Gerds TA, Schou M, et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10(12):e044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castiglione V, Chiriacò M, Emdin M, et al. Statin therapy in COVID-19 infection. 2020. [DOI] [PMC free article] [PubMed]
- 84.Li H. Sodium–glucose cotransporter 2 inhibitors: a drug with antidiabetic and cardioprotective properties. 2020. [DOI] [PMC free article] [PubMed]
- 85.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. The New England Journal of Medicine. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 86.Gupta K, Kunal SJH, Lung. SGLT-2 inhibitors as cardioprotective agents in COVID-19. 2020. [DOI] [PMC free article] [PubMed]
- 87.Cure E, Mcjd C, Research MSC, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes & Metabolic Syndrome. Diabetes Metab Syndr. 2020;14(4):349-350. [DOI] [PMC free article] [PubMed]
- 88.Dashora U, Patel DC, Gregory R, et al. Association of British Clinical Diabetologists (ABCD) and diabetes UK joint position statement and recommendations on the use of sodium‐glucose cotransporter inhibitors with insulin for treatment of type 1 diabetes (updated october 2020). 2020:e14458. [DOI] [PubMed]
- 89.Li Y, Zhou W, Yang L, et al. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. 2020:104833. [DOI] [PMC free article] [PubMed]
- 90.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. 2020. [DOI] [PMC free article] [PubMed]
- 91.Genetics C-HGIJEJoH . The COVID-19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. 2020;28(6):715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang F, Yang J, Zhang Y, et al. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11(7):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. [DOI] [PubMed] [Google Scholar]
- 94.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iqubal A, Haque SE, Sharma S, et al. Clinical updates on drug-induced cardiotoxicity. Int J Pharm Sci res. 2018;9:16–26. [Google Scholar]
- 96.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. 2020. [DOI] [PMC free article] [PubMed]
- 97.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 98.Ishiyama Y, Gallagher PE, Averill DB, et al. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension (Dallas, Tex. 1979). 2004;43(5):970–976. [DOI] [PubMed] [Google Scholar]
- 99.Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. The New England Journal of Medicine. 2020;382(17):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26(5):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Simone GJESC. Position statement of the ESC council on hypertension on ACE-inhibitors and angiotensin receptor blockers. 2020;13. [Google Scholar]
- 102.Chinese Society of Cardiology EBJZxxgbzz . Scientific statement on using of renin angiotensin system blockers in patients with cardiovascular disease and COVID-19. 2020;48:E014. [DOI] [PubMed] [Google Scholar]
- 103.De Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. 2020. [DOI] [PMC free article] [PubMed]
- 104.Mancia G, Rea F, Ludergnani M, et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. 2020. [DOI] [PMC free article] [PubMed]
- 105.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. 2020. [DOI] [PMC free article] [PubMed]
- 106.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. 2020. [DOI] [PMC free article] [PubMed]
- 107.Esposito S, Tagliabue C, Bosis S, et al. Levofloxacin for the treatment of mycoplasma pneumoniae-associated meningoencephalitis in childhood. International Journal of Antimicrobial Agents. 2011;37(5):472–475. [DOI] [PubMed] [Google Scholar]
- 108.Aromolaran AS, Srivastava U, Alí A, et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PloS One. 2018;13(12):e0208321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartman O, Kovanen PT, Lehtonen J, et al. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: rationale and design of the OXI trial. European Heart Journal. Cardiovascular Pharmacotherapy. 2017;3(2):92–97. [DOI] [PubMed] [Google Scholar]
- 110.Wang L, Zhang Y, Zhang S. Cardiovascular impairment in COVID-19: learning from current options for cardiovascular anti-inflammatory therapy. Front Cardiovasc Med. 2020;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong C, Lam C, Wu A, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology. 2004;136(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. The Journal of Clinical Investigation. 2016;126(6):2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ngai JC, Ko FW, Ng SS, et al. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology (Carlton, Vic.). 2010;15(3):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Batawi S, Tarazan N, Al-Raddadi R, et al. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS). Health Qual Life Outcomes. 2019;17(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shah B, Modi P, Sagar SRJLS. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. 2020:117652. [DOI] [PMC free article] [PubMed]
- 116.Iqubal MK, Chaudhuri A, Iqubal A, et al. Targeted delivery of natural bioactives and lipid-nanocargos against signaling pathways involved in skin cancer. 2020. [DOI] [PubMed]
- 117.Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. 2020. [DOI] [PMC free article] [PubMed]
- 118.Chen F, Hao Y, Zhang Z, et al. An urgent call for raising the scientific rigorousness of clinical trials on COVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(3):301. [DOI] [PubMed] [Google Scholar]
- 119.Iqubal A, Iqubal MK, Khan A, et al. Gene therapy, a novel therapeutic tool for neurological disorders: current progress, challenges and future prospective. Current Gene Therapy. 2020;20(3):184–194. [DOI] [PubMed] [Google Scholar]
- 120.Iqubal A, Rahman SO, Ahmed M, et al. current quest in natural bioactive compounds for alzheimer’s disease: multi-targeted-designed-ligand based approach with preclinical and clinical based evidence. 2020. [DOI] [PubMed]
- 121.Iqubal MK, Saleem S, Iqubal A, et al. Natural, synthetic and their combinatorial nanocarriers based drug delivery system in the treatment paradigm for wound healing via dermal targeting. Current Pharmaceutical Design. 2020;26(36):4551–4568. [DOI] [PubMed] [Google Scholar]


